

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Monoglyceride lipase (Homo sapiens (Human)) | BDBM50503336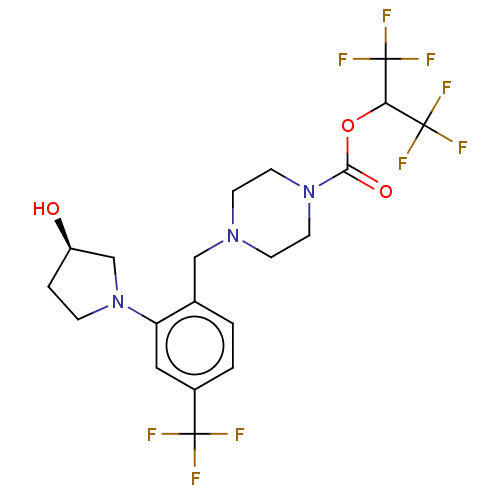 (CHEMBL4437377) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Abide Therapeutics Curated by ChEMBL | Assay Description Inhibition of MGLL derived from human PC3 cell lysates preincubated for 30 mins followed by JW912 addition after 30 mins by gel-based ABPP assay | J Med Chem 61: 9062-9084 (2018) Article DOI: 10.1021/acs.jmedchem.8b00951 BindingDB Entry DOI: 10.7270/Q2TH8QZH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Monoglyceride lipase (Homo sapiens (Human)) | BDBM50503331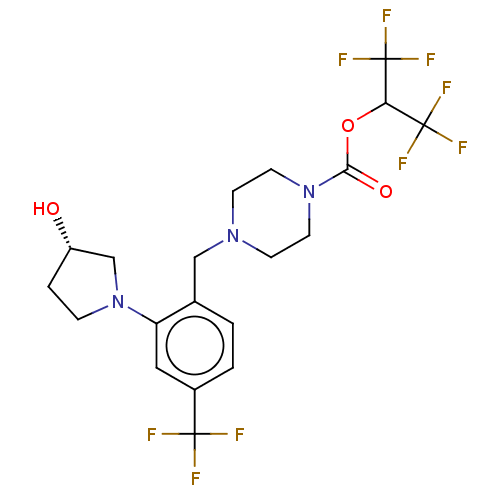 (CHEMBL4522687) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Abide Therapeutics Curated by ChEMBL | Assay Description Inhibition of MGLL derived from human PC3 cell lysates preincubated for 30 mins followed by JW912 addition after 30 mins by gel-based ABPP assay | J Med Chem 61: 9062-9084 (2018) Article DOI: 10.1021/acs.jmedchem.8b00951 BindingDB Entry DOI: 10.7270/Q2TH8QZH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Monoglyceride lipase (Homo sapiens (Human)) | BDBM50503340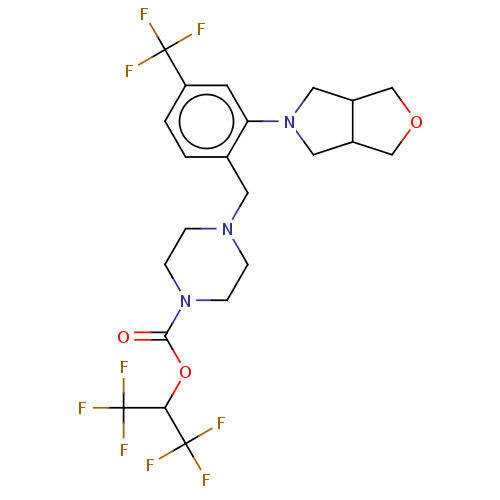 (CHEMBL4468636) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Abide Therapeutics Curated by ChEMBL | Assay Description Inhibition of MGLL derived from human PC3 cell lysates preincubated for 30 mins followed by JW912 addition after 30 mins by gel-based ABPP assay | J Med Chem 61: 9062-9084 (2018) Article DOI: 10.1021/acs.jmedchem.8b00951 BindingDB Entry DOI: 10.7270/Q2TH8QZH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Monoglyceride lipase (Homo sapiens (Human)) | BDBM180052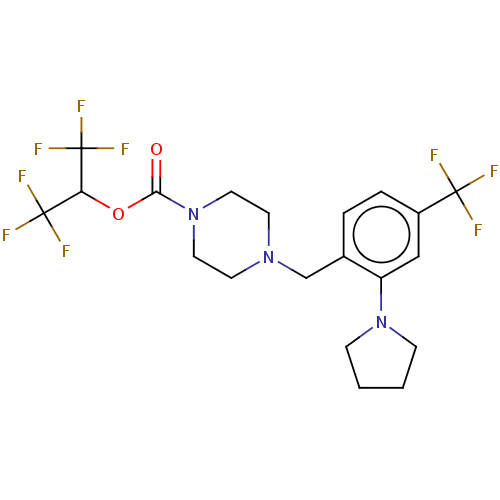 (US9133148, 9aq) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Abide Therapeutics Curated by ChEMBL | Assay Description Inhibition of MGLL in human intact PC3 cells preincubated for 30 mins followed by JW912 addition after 30 mins by gel-based ABPP assay | J Med Chem 61: 9062-9084 (2018) Article DOI: 10.1021/acs.jmedchem.8b00951 BindingDB Entry DOI: 10.7270/Q2TH8QZH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholesteryl ester transfer protein (Homo sapiens (Human)) | BDBM50312718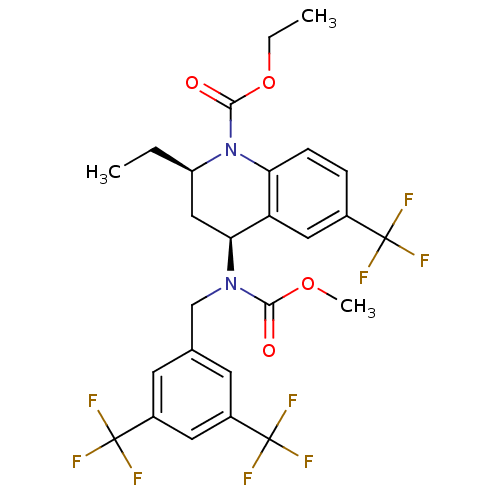 (CHEMBL479527 | torcetrapib) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer HealthCare AG Curated by ChEMBL | Assay Description Inhibition of human plasma CETP assessed as [3H]cholesterol ester transfer after 18 hrs by scintillation proximity assay | Bioorg Med Chem Lett 20: 1740-3 (2010) Article DOI: 10.1016/j.bmcl.2010.01.071 BindingDB Entry DOI: 10.7270/Q28S4Q1P | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Monoglyceride lipase (Homo sapiens (Human)) | BDBM179948 (US9133148, 2l) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Abide Therapeutics Curated by ChEMBL | Assay Description Inhibition of MGLL derived from human PC3 cell lysates preincubated for 30 mins followed by JW912 addition after 30 mins by gel-based ABPP assay | J Med Chem 61: 9062-9084 (2018) Article DOI: 10.1021/acs.jmedchem.8b00951 BindingDB Entry DOI: 10.7270/Q2TH8QZH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Monoglyceride lipase (Homo sapiens (Human)) | BDBM180039 (US9133148, 9ad) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Abide Therapeutics Curated by ChEMBL | Assay Description Inhibition of MGLL derived from human PC3 cell lysates preincubated for 30 mins followed by JW912 addition after 30 mins by gel-based ABPP assay | J Med Chem 61: 9062-9084 (2018) Article DOI: 10.1021/acs.jmedchem.8b00951 BindingDB Entry DOI: 10.7270/Q2TH8QZH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Monoglyceride lipase (Homo sapiens (Human)) | BDBM50503335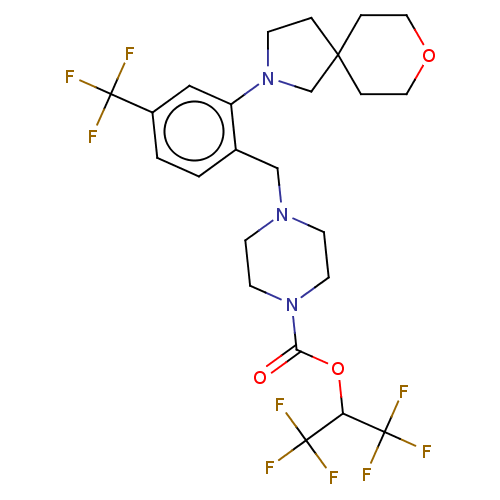 (CHEMBL4450649) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Abide Therapeutics Curated by ChEMBL | Assay Description Inhibition of MGLL derived from human PC3 cell lysates preincubated for 30 mins followed by JW912 addition after 30 mins by gel-based ABPP assay | J Med Chem 61: 9062-9084 (2018) Article DOI: 10.1021/acs.jmedchem.8b00951 BindingDB Entry DOI: 10.7270/Q2TH8QZH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Monoglyceride lipase (Homo sapiens (Human)) | BDBM50503334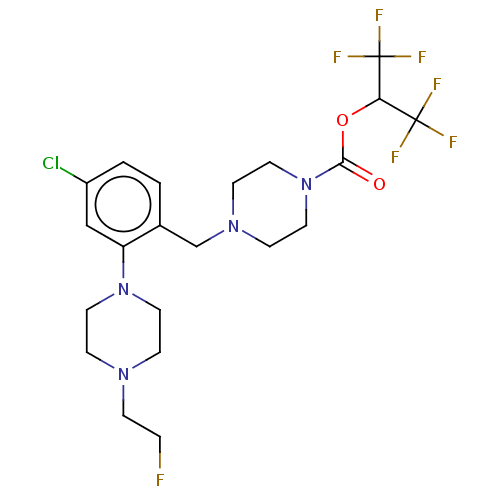 (CHEMBL4468639) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Abide Therapeutics Curated by ChEMBL | Assay Description Inhibition of MGLL derived from human PC3 cell lysates preincubated for 30 mins followed by JW912 addition after 30 mins by gel-based ABPP assay | J Med Chem 61: 9062-9084 (2018) Article DOI: 10.1021/acs.jmedchem.8b00951 BindingDB Entry DOI: 10.7270/Q2TH8QZH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Monoglyceride lipase (Homo sapiens (Human)) | BDBM180078 (US9133148, 10g) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Abide Therapeutics Curated by ChEMBL | Assay Description Inhibition of MGLL derived from human PC3 cell lysates preincubated for 30 mins followed by JW912 addition after 30 mins by gel-based ABPP assay | J Med Chem 61: 9062-9084 (2018) Article DOI: 10.1021/acs.jmedchem.8b00951 BindingDB Entry DOI: 10.7270/Q2TH8QZH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Monoglyceride lipase (Homo sapiens (Human)) | BDBM50503339 (CHEMBL4469632) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Abide Therapeutics Curated by ChEMBL | Assay Description Inhibition of MGLL derived from human PC3 cell lysates preincubated for 30 mins followed by JW912 addition after 30 mins by gel-based ABPP assay | J Med Chem 61: 9062-9084 (2018) Article DOI: 10.1021/acs.jmedchem.8b00951 BindingDB Entry DOI: 10.7270/Q2TH8QZH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholesteryl ester transfer protein (Homo sapiens (Human)) | BDBM50312714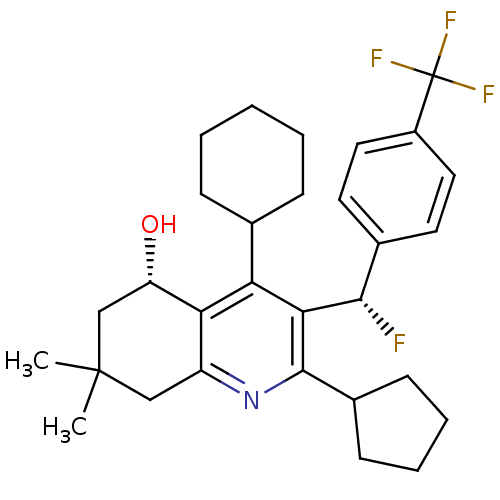 ((S)-4-cyclohexyl-2-cyclopentyl-3-((S)-fluoro(4-(tr...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer HealthCare AG Curated by ChEMBL | Assay Description Inhibition of human plasma CETP assessed as [3H]cholesterol ester transfer after 18 hrs by scintillation proximity assay | Bioorg Med Chem Lett 20: 1740-3 (2010) Article DOI: 10.1016/j.bmcl.2010.01.071 BindingDB Entry DOI: 10.7270/Q28S4Q1P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Monoglyceride lipase (Mus musculus (mouse)) | BDBM50503336 (CHEMBL4437377) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Abide Therapeutics Curated by ChEMBL | Assay Description Inhibition of MGLL derived from mouse brain homogenates preincubated for 30 mins followed by JW912 addition after 30 mins by gel-based ABPP assay | J Med Chem 61: 9062-9084 (2018) Article DOI: 10.1021/acs.jmedchem.8b00951 BindingDB Entry DOI: 10.7270/Q2TH8QZH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Monoglyceride lipase (Homo sapiens (Human)) | BDBM50503333 (CHEMBL4539731) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Abide Therapeutics Curated by ChEMBL | Assay Description Inhibition of MGLL derived from human PC3 cell lysates preincubated for 30 mins followed by JW912 addition after 30 mins by gel-based ABPP assay | J Med Chem 61: 9062-9084 (2018) Article DOI: 10.1021/acs.jmedchem.8b00951 BindingDB Entry DOI: 10.7270/Q2TH8QZH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Monoglyceride lipase (Homo sapiens (Human)) | BDBM50503327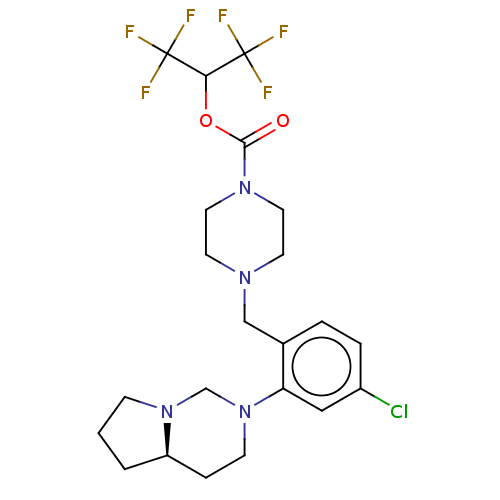 (CHEMBL4435014) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Abide Therapeutics Curated by ChEMBL | Assay Description Inhibition of MGLL derived from human PC3 cell lysates preincubated for 30 mins followed by JW912 addition after 30 mins by gel-based ABPP assay | J Med Chem 61: 9062-9084 (2018) Article DOI: 10.1021/acs.jmedchem.8b00951 BindingDB Entry DOI: 10.7270/Q2TH8QZH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Monoglyceride lipase (Homo sapiens (Human)) | BDBM50503342 (CHEMBL4436074) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Abide Therapeutics Curated by ChEMBL | Assay Description Inhibition of MGLL derived from human PC3 cell lysates preincubated for 30 mins followed by JW912 addition after 30 mins by gel-based ABPP assay | J Med Chem 61: 9062-9084 (2018) Article DOI: 10.1021/acs.jmedchem.8b00951 BindingDB Entry DOI: 10.7270/Q2TH8QZH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Monoglyceride lipase (Homo sapiens (Human)) | BDBM179938 (US9133148, 2b) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Abide Therapeutics Curated by ChEMBL | Assay Description Inhibition of MGLL derived from human PC3 cell lysates preincubated for 30 mins followed by JW912 addition after 30 mins by gel-based ABPP assay | J Med Chem 61: 9062-9084 (2018) Article DOI: 10.1021/acs.jmedchem.8b00951 BindingDB Entry DOI: 10.7270/Q2TH8QZH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Monoglyceride lipase (Homo sapiens (Human)) | BDBM50503344 (CHEMBL4439400) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Abide Therapeutics Curated by ChEMBL | Assay Description Inhibition of MGLL derived from human PC3 cell lysates preincubated for 30 mins followed by JW912 addition after 30 mins by gel-based ABPP assay | J Med Chem 61: 9062-9084 (2018) Article DOI: 10.1021/acs.jmedchem.8b00951 BindingDB Entry DOI: 10.7270/Q2TH8QZH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Monoglyceride lipase (Mus musculus (mouse)) | BDBM50503333 (CHEMBL4539731) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Abide Therapeutics Curated by ChEMBL | Assay Description Inhibition of MGLL derived from mouse brain homogenates preincubated for 30 mins followed by JW912 addition after 30 mins by gel-based ABPP assay | J Med Chem 61: 9062-9084 (2018) Article DOI: 10.1021/acs.jmedchem.8b00951 BindingDB Entry DOI: 10.7270/Q2TH8QZH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Monoglyceride lipase (Homo sapiens (Human)) | BDBM180052 (US9133148, 9aq) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Abide Therapeutics Curated by ChEMBL | Assay Description Inhibition of MGLL derived from human PC3 cell lysates preincubated for 30 mins followed by JW912 addition after 30 mins by gel-based ABPP assay | J Med Chem 61: 9062-9084 (2018) Article DOI: 10.1021/acs.jmedchem.8b00951 BindingDB Entry DOI: 10.7270/Q2TH8QZH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Monoglyceride lipase (Homo sapiens (Human)) | BDBM50503337 (CHEMBL4517565) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Abide Therapeutics Curated by ChEMBL | Assay Description Inhibition of MGLL derived from human PC3 cell lysates preincubated for 30 mins followed by JW912 addition after 30 mins by gel-based ABPP assay | J Med Chem 61: 9062-9084 (2018) Article DOI: 10.1021/acs.jmedchem.8b00951 BindingDB Entry DOI: 10.7270/Q2TH8QZH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Monoglyceride lipase (Mus musculus (mouse)) | BDBM50503331 (CHEMBL4522687) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Abide Therapeutics Curated by ChEMBL | Assay Description Inhibition of MGLL derived from mouse brain homogenates preincubated for 30 mins followed by JW912 addition after 30 mins by gel-based ABPP assay | J Med Chem 61: 9062-9084 (2018) Article DOI: 10.1021/acs.jmedchem.8b00951 BindingDB Entry DOI: 10.7270/Q2TH8QZH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Monoglyceride lipase (Homo sapiens (Human)) | BDBM50503345 (CHEMBL4550205) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
Abide Therapeutics Curated by ChEMBL | Assay Description Inhibition of MGLL derived from human PC3 cell lysates preincubated for 30 mins followed by JW912 addition after 30 mins by gel-based ABPP assay | J Med Chem 61: 9062-9084 (2018) Article DOI: 10.1021/acs.jmedchem.8b00951 BindingDB Entry DOI: 10.7270/Q2TH8QZH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Monoglyceride lipase (Mus musculus (mouse)) | BDBM50503340 (CHEMBL4468636) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
Abide Therapeutics Curated by ChEMBL | Assay Description Inhibition of MGLL derived from mouse brain homogenates preincubated for 30 mins followed by JW912 addition after 30 mins by gel-based ABPP assay | J Med Chem 61: 9062-9084 (2018) Article DOI: 10.1021/acs.jmedchem.8b00951 BindingDB Entry DOI: 10.7270/Q2TH8QZH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Monoglyceride lipase (Mus musculus (mouse)) | BDBM50503334 (CHEMBL4468639) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
Abide Therapeutics Curated by ChEMBL | Assay Description Inhibition of MGLL derived from mouse brain homogenates preincubated for 30 mins followed by JW912 addition after 30 mins by gel-based ABPP assay | J Med Chem 61: 9062-9084 (2018) Article DOI: 10.1021/acs.jmedchem.8b00951 BindingDB Entry DOI: 10.7270/Q2TH8QZH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholesteryl ester transfer protein (Homo sapiens (Human)) | BDBM50312715 ((S)-4'-cyclohexyl-3'-((S)-fluoro(4-(trifluoromethy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer HealthCare AG Curated by ChEMBL | Assay Description Inhibition of CETP assessed as cholesterol ester transfer after 4 hrs by microemulsion based fluorescence assay | Bioorg Med Chem Lett 20: 1740-3 (2010) Article DOI: 10.1016/j.bmcl.2010.01.071 BindingDB Entry DOI: 10.7270/Q28S4Q1P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Monoglyceride lipase (Homo sapiens (Human)) | BDBM180033 (US9133148, 9x) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
Abide Therapeutics Curated by ChEMBL | Assay Description Inhibition of MGLL derived from human PC3 cell lysates preincubated for 30 mins followed by JW912 addition after 30 mins by gel-based ABPP assay | J Med Chem 61: 9062-9084 (2018) Article DOI: 10.1021/acs.jmedchem.8b00951 BindingDB Entry DOI: 10.7270/Q2TH8QZH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholesteryl ester transfer protein (Homo sapiens (Human)) | BDBM50312718 (CHEMBL479527 | torcetrapib) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer HealthCare AG Curated by ChEMBL | Assay Description Inhibition of CETP assessed as cholesterol ester transfer after 4 hrs by microemulsion based fluorescence assay | Bioorg Med Chem Lett 20: 1740-3 (2010) Article DOI: 10.1016/j.bmcl.2010.01.071 BindingDB Entry DOI: 10.7270/Q28S4Q1P | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Monoglyceride lipase (Homo sapiens (Human)) | BDBM180029 (US9133148, 9t) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
Abide Therapeutics Curated by ChEMBL | Assay Description Inhibition of MGLL derived from human PC3 cell lysates preincubated for 30 mins followed by JW912 addition after 30 mins by gel-based ABPP assay | J Med Chem 61: 9062-9084 (2018) Article DOI: 10.1021/acs.jmedchem.8b00951 BindingDB Entry DOI: 10.7270/Q2TH8QZH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Monoglyceride lipase (Homo sapiens (Human)) | BDBM180076 (US9133148, 10e) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Abide Therapeutics Curated by ChEMBL | Assay Description Inhibition of MGLL derived from human PC3 cell lysates preincubated for 30 mins followed by JW912 addition after 30 mins by gel-based ABPP assay | J Med Chem 61: 9062-9084 (2018) Article DOI: 10.1021/acs.jmedchem.8b00951 BindingDB Entry DOI: 10.7270/Q2TH8QZH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholesteryl ester transfer protein (Homo sapiens (Human)) | BDBM50312718 (CHEMBL479527 | torcetrapib) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer HealthCare AG Curated by ChEMBL | Assay Description Inhibition of human plasma CETP assessed as cholesterol ester transfer after 24 hrs by fluorescence assay | Bioorg Med Chem Lett 20: 1740-3 (2010) Article DOI: 10.1016/j.bmcl.2010.01.071 BindingDB Entry DOI: 10.7270/Q28S4Q1P | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Cholesteryl ester transfer protein (Homo sapiens (Human)) | BDBM50312716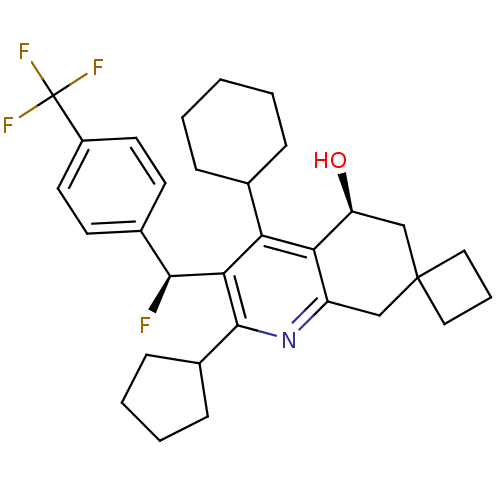 ((S)-4'-cyclohexyl-2'-cyclopentyl-3'-((S)-fluoro(4-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer HealthCare AG Curated by ChEMBL | Assay Description Inhibition of CETP assessed as cholesterol ester transfer after 4 hrs by microemulsion based fluorescence assay | Bioorg Med Chem Lett 20: 1740-3 (2010) Article DOI: 10.1016/j.bmcl.2010.01.071 BindingDB Entry DOI: 10.7270/Q28S4Q1P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholesteryl ester transfer protein (Homo sapiens (Human)) | BDBM50312713 ((S)-4-cyclohexyl-3-((S)-fluoro(4-(trifluoromethyl)...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer HealthCare AG Curated by ChEMBL | Assay Description Inhibition of human plasma CETP assessed as [3H]cholesterol ester transfer after 18 hrs by scintillation proximity assay | Bioorg Med Chem Lett 20: 1740-3 (2010) Article DOI: 10.1016/j.bmcl.2010.01.071 BindingDB Entry DOI: 10.7270/Q28S4Q1P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Monoglyceride lipase (Homo sapiens (Human)) | BDBM50503338 (CHEMBL4584757) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 24 | n/a | n/a | n/a | n/a | n/a | n/a |
Abide Therapeutics Curated by ChEMBL | Assay Description Inhibition of MGLL derived from human PC3 cell lysates preincubated for 30 mins followed by JW912 addition after 30 mins by gel-based ABPP assay | J Med Chem 61: 9062-9084 (2018) Article DOI: 10.1021/acs.jmedchem.8b00951 BindingDB Entry DOI: 10.7270/Q2TH8QZH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholesteryl ester transfer protein (Homo sapiens (Human)) | BDBM50312720 ((S)-3'-((S)-fluoro(4-(trifluoromethyl)phenyl)methy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 24 | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer HealthCare AG Curated by ChEMBL | Assay Description Inhibition of CETP assessed as cholesterol ester transfer after 4 hrs by microemulsion based fluorescence assay | Bioorg Med Chem Lett 20: 1740-3 (2010) Article DOI: 10.1016/j.bmcl.2010.01.071 BindingDB Entry DOI: 10.7270/Q28S4Q1P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Monoglyceride lipase (Mus musculus (mouse)) | BDBM50503335 (CHEMBL4450649) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 24 | n/a | n/a | n/a | n/a | n/a | n/a |
Abide Therapeutics Curated by ChEMBL | Assay Description Inhibition of MGLL derived from mouse brain homogenates preincubated for 30 mins followed by JW912 addition after 30 mins by gel-based ABPP assay | J Med Chem 61: 9062-9084 (2018) Article DOI: 10.1021/acs.jmedchem.8b00951 BindingDB Entry DOI: 10.7270/Q2TH8QZH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Monoglyceride lipase (Mus musculus (mouse)) | BDBM180012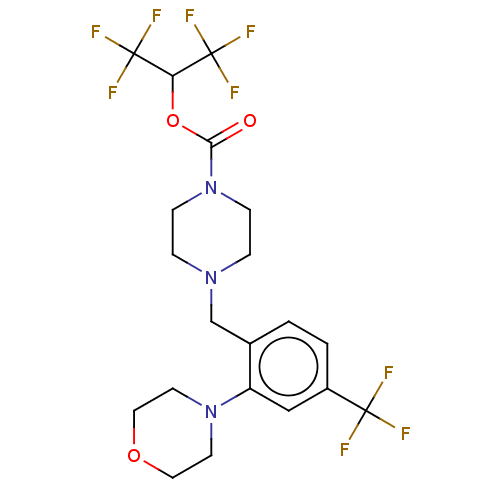 (US9133148, 9c) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
Abide Therapeutics Curated by ChEMBL | Assay Description Inhibition of MGLL derived from mouse brain homogenates preincubated for 30 mins followed by JW912 addition after 30 mins by gel-based ABPP assay | J Med Chem 61: 9062-9084 (2018) Article DOI: 10.1021/acs.jmedchem.8b00951 BindingDB Entry DOI: 10.7270/Q2TH8QZH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholesteryl ester transfer protein (Homo sapiens (Human)) | BDBM50312714 ((S)-4-cyclohexyl-2-cyclopentyl-3-((S)-fluoro(4-(tr...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer HealthCare AG Curated by ChEMBL | Assay Description Inhibition of CETP assessed as cholesterol ester transfer after 4 hrs by microemulsion based fluorescence assay | Bioorg Med Chem Lett 20: 1740-3 (2010) Article DOI: 10.1016/j.bmcl.2010.01.071 BindingDB Entry DOI: 10.7270/Q28S4Q1P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Platelet-activating factor acetylhydrolase (Homo sapiens (Human)) | BDBM179948 (US9133148, 2l) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 26 | n/a | n/a | n/a | n/a | n/a | n/a |
Abide Therapeutics Curated by ChEMBL | Assay Description Inhibition of PLA2G7 derived from human PC3 cell lysates preincubated for 30 mins followed by JW912 addition after 30 mins by gel-based ABPP assay | J Med Chem 61: 9062-9084 (2018) Article DOI: 10.1021/acs.jmedchem.8b00951 BindingDB Entry DOI: 10.7270/Q2TH8QZH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Monoglyceride lipase (Homo sapiens (Human)) | BDBM50503329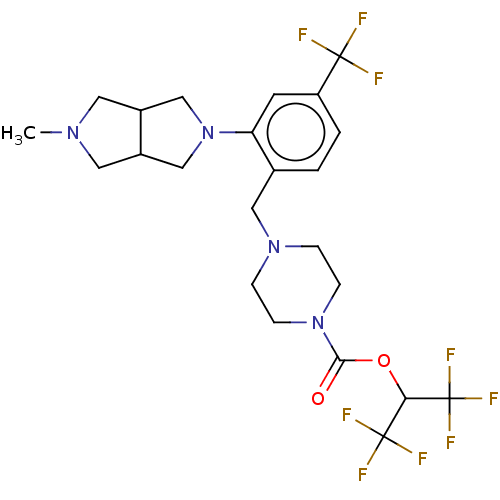 (CHEMBL4546592) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 26 | n/a | n/a | n/a | n/a | n/a | n/a |
Abide Therapeutics Curated by ChEMBL | Assay Description Inhibition of MGLL derived from human PC3 cell lysates preincubated for 30 mins followed by JW912 addition after 30 mins by gel-based ABPP assay | J Med Chem 61: 9062-9084 (2018) Article DOI: 10.1021/acs.jmedchem.8b00951 BindingDB Entry DOI: 10.7270/Q2TH8QZH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Monoglyceride lipase (Mus musculus (mouse)) | BDBM180052 (US9133148, 9aq) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 27 | n/a | n/a | n/a | n/a | n/a | n/a |
Abide Therapeutics Curated by ChEMBL | Assay Description Inhibition of MGLL derived from mouse brain homogenates preincubated for 30 mins followed by JW912 addition after 30 mins by gel-based ABPP assay | J Med Chem 61: 9062-9084 (2018) Article DOI: 10.1021/acs.jmedchem.8b00951 BindingDB Entry DOI: 10.7270/Q2TH8QZH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Monoglyceride lipase (Mus musculus (mouse)) | BDBM180035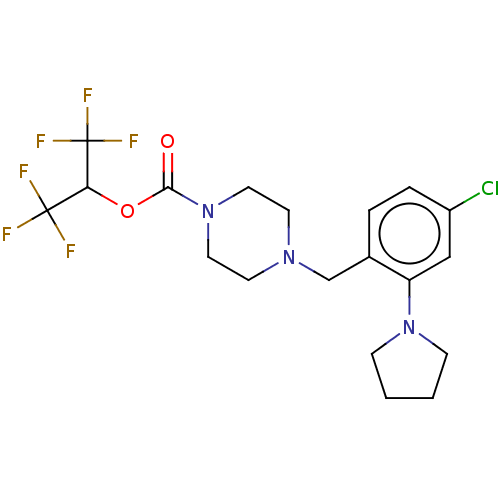 (US9133148, 9z) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 27 | n/a | n/a | n/a | n/a | n/a | n/a |
Abide Therapeutics Curated by ChEMBL | Assay Description Inhibition of MGLL derived from mouse brain homogenates preincubated for 30 mins followed by JW912 addition after 30 mins by gel-based ABPP assay | J Med Chem 61: 9062-9084 (2018) Article DOI: 10.1021/acs.jmedchem.8b00951 BindingDB Entry DOI: 10.7270/Q2TH8QZH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Monoglyceride lipase (Homo sapiens (Human)) | BDBM180075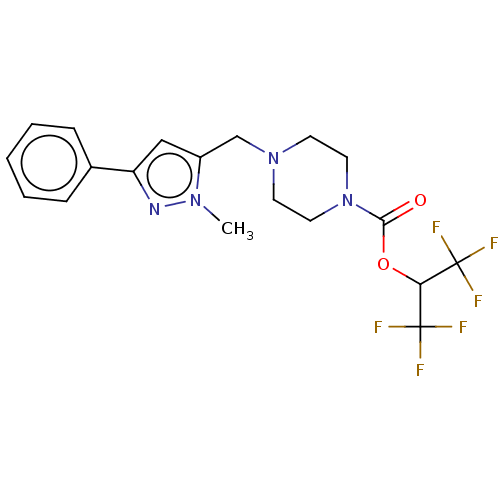 (US9133148, 10d) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 28 | n/a | n/a | n/a | n/a | n/a | n/a |
Abide Therapeutics Curated by ChEMBL | Assay Description Inhibition of MGLL derived from human PC3 cell lysates preincubated for 30 mins followed by JW912 addition after 30 mins by gel-based ABPP assay | J Med Chem 61: 9062-9084 (2018) Article DOI: 10.1021/acs.jmedchem.8b00951 BindingDB Entry DOI: 10.7270/Q2TH8QZH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Monoglyceride lipase (Mus musculus (mouse)) | BDBM50503337 (CHEMBL4517565) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 29 | n/a | n/a | n/a | n/a | n/a | n/a |
Abide Therapeutics Curated by ChEMBL | Assay Description Inhibition of MGLL derived from mouse brain homogenates preincubated for 30 mins followed by JW912 addition after 30 mins by gel-based ABPP assay | J Med Chem 61: 9062-9084 (2018) Article DOI: 10.1021/acs.jmedchem.8b00951 BindingDB Entry DOI: 10.7270/Q2TH8QZH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholesteryl ester transfer protein (Homo sapiens (Human)) | BDBM50312713 ((S)-4-cyclohexyl-3-((S)-fluoro(4-(trifluoromethyl)...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer HealthCare AG Curated by ChEMBL | Assay Description Inhibition of CETP assessed as cholesterol ester transfer after 4 hrs by microemulsion based fluorescence assay | Bioorg Med Chem Lett 20: 1740-3 (2010) Article DOI: 10.1016/j.bmcl.2010.01.071 BindingDB Entry DOI: 10.7270/Q28S4Q1P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholesteryl ester transfer protein (Homo sapiens (Human)) | BDBM50312719 ((S)-2-cyclopentyl-3-((S)-fluoro(4-(trifluoromethyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 31 | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer HealthCare AG Curated by ChEMBL | Assay Description Inhibition of CETP assessed as cholesterol ester transfer after 4 hrs by microemulsion based fluorescence assay | Bioorg Med Chem Lett 20: 1740-3 (2010) Article DOI: 10.1016/j.bmcl.2010.01.071 BindingDB Entry DOI: 10.7270/Q28S4Q1P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholesteryl ester transfer protein (Homo sapiens (Human)) | BDBM50312730 ((S)-(4'-cyclohexyl-5'-hydroxy-2'-isopropyl-6',8'-d...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 33 | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer HealthCare AG Curated by ChEMBL | Assay Description Inhibition of CETP assessed as cholesterol ester transfer after 4 hrs by microemulsion based fluorescence assay | Bioorg Med Chem Lett 20: 1740-3 (2010) Article DOI: 10.1016/j.bmcl.2010.01.071 BindingDB Entry DOI: 10.7270/Q28S4Q1P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholesteryl ester transfer protein (Homo sapiens (Human)) | BDBM50312715 ((S)-4'-cyclohexyl-3'-((S)-fluoro(4-(trifluoromethy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 33 | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer HealthCare AG Curated by ChEMBL | Assay Description Inhibition of human plasma CETP assessed as [3H]cholesterol ester transfer after 18 hrs by scintillation proximity assay | Bioorg Med Chem Lett 20: 1740-3 (2010) Article DOI: 10.1016/j.bmcl.2010.01.071 BindingDB Entry DOI: 10.7270/Q28S4Q1P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Monoglyceride lipase (Homo sapiens (Human)) | BDBM180035 (US9133148, 9z) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 34 | n/a | n/a | n/a | n/a | n/a | n/a |
Abide Therapeutics Curated by ChEMBL | Assay Description Inhibition of MGLL derived from human PC3 cell lysates preincubated for 30 mins followed by JW912 addition after 30 mins by gel-based ABPP assay | J Med Chem 61: 9062-9084 (2018) Article DOI: 10.1021/acs.jmedchem.8b00951 BindingDB Entry DOI: 10.7270/Q2TH8QZH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholesteryl ester transfer protein (Homo sapiens (Human)) | BDBM50312717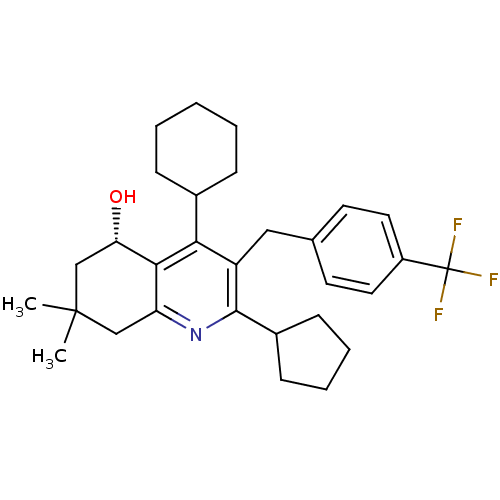 ((S)-4-cyclohexyl-2-cyclopentyl-7,7-dimethyl-3-(4-(...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 39 | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer HealthCare AG Curated by ChEMBL | Assay Description Inhibition of CETP assessed as cholesterol ester transfer after 4 hrs by microemulsion based fluorescence assay | Bioorg Med Chem Lett 20: 1740-3 (2010) Article DOI: 10.1016/j.bmcl.2010.01.071 BindingDB Entry DOI: 10.7270/Q28S4Q1P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 2873 total ) | Next | Last >> |