 Found 2000 hits with Last Name = 'wen' and Initial = 'l'
Found 2000 hits with Last Name = 'wen' and Initial = 'l' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Dual specificity calcium/calmodulin-dependent 3',5'-cyclic nucleotide phosphodiesterase 1A
(Homo sapiens (Human)) | BDBM168129
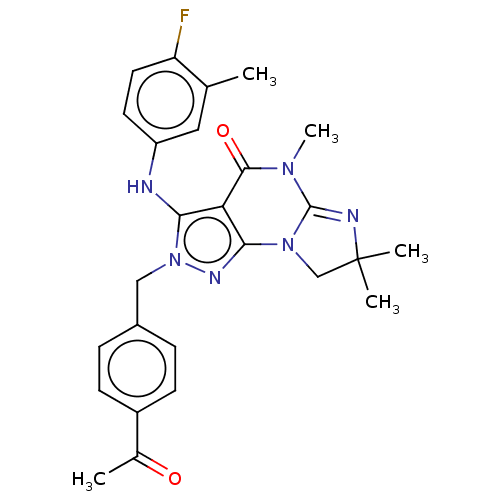
(US9073936, 4 | US9598426, 4)Show SMILES CN1C2=NC(C)(C)CN2c2nn(Cc3ccc(cc3)C(C)=O)c(Nc3ccc(F)c(C)c3)c2C1=O |t:2| Show InChI InChI=1S/C26H27FN6O2/c1-15-12-19(10-11-20(15)27)28-22-21-23(32-14-26(3,4)29-25(32)31(5)24(21)35)30-33(22)13-17-6-8-18(9-7-17)16(2)34/h6-12,28H,13-14H2,1-5H3 | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
INTRA-CELLULAR THERAPIES, INC.
US Patent
| Assay Description
Assay: The following phosphodiesterase enzymes may be used: 3′,5′-cyclic-nucleotide-specific bovine brain phosphodiesterase (Sigma, St. L... |
US Patent US9598426 (2017)
BindingDB Entry DOI: 10.7270/Q29S1T2J |
More data for this
Ligand-Target Pair | |
Dual specificity calcium/calmodulin-dependent 3',5'-cyclic nucleotide phosphodiesterase 1A
(Homo sapiens (Human)) | BDBM168129

(US9073936, 4 | US9598426, 4)Show SMILES CN1C2=NC(C)(C)CN2c2nn(Cc3ccc(cc3)C(C)=O)c(Nc3ccc(F)c(C)c3)c2C1=O |t:2| Show InChI InChI=1S/C26H27FN6O2/c1-15-12-19(10-11-20(15)27)28-22-21-23(32-14-26(3,4)29-25(32)31(5)24(21)35)30-33(22)13-17-6-8-18(9-7-17)16(2)34/h6-12,28H,13-14H2,1-5H3 | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 0.100 | -58.0 | n/a | n/a | n/a | n/a | n/a | 7.5 | 30 |
INTRA-CELLULAR THERAPIES, INC.
US Patent
| Assay Description
Materials: All chemicals are available from Sigma-Aldrich (St. Louis, Mo.) except for IMAP reagents (reaction buffer, binding buffer, FL-GMP and IMAP... |
US Patent US9073936 (2015)
BindingDB Entry DOI: 10.7270/Q2348J5T |
More data for this
Ligand-Target Pair | |
Dual specificity calcium/calmodulin-dependent 3',5'-cyclic nucleotide phosphodiesterase 1A
(Homo sapiens (Human)) | BDBM168126
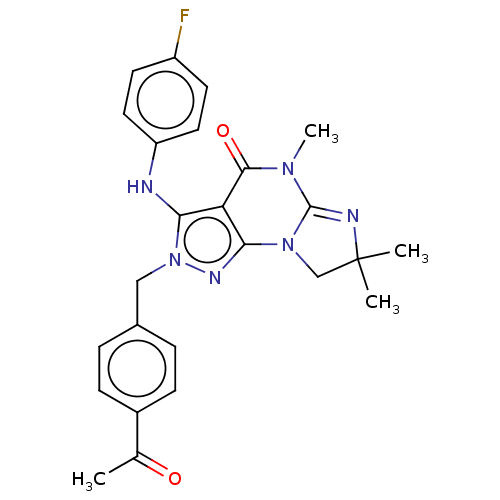
(US9073936, 1 | US9598426, 1)Show SMILES CN1C2=NC(C)(C)CN2c2nn(Cc3ccc(cc3)C(C)=O)c(Nc3ccc(F)cc3)c2C1=O |t:2| Show InChI InChI=1S/C25H25FN6O2/c1-15(33)17-7-5-16(6-8-17)13-32-21(27-19-11-9-18(26)10-12-19)20-22(29-32)31-14-25(2,3)28-24(31)30(4)23(20)34/h5-12,27H,13-14H2,1-4H3 | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 0.200 | -56.3 | n/a | n/a | n/a | n/a | n/a | 7.5 | 30 |
INTRA-CELLULAR THERAPIES, INC.
US Patent
| Assay Description
Materials: All chemicals are available from Sigma-Aldrich (St. Louis, Mo.) except for IMAP reagents (reaction buffer, binding buffer, FL-GMP and IMAP... |
US Patent US9073936 (2015)
BindingDB Entry DOI: 10.7270/Q2348J5T |
More data for this
Ligand-Target Pair | |
Dual specificity calcium/calmodulin-dependent 3',5'-cyclic nucleotide phosphodiesterase 1A
(Homo sapiens (Human)) | BDBM168126

(US9073936, 1 | US9598426, 1)Show SMILES CN1C2=NC(C)(C)CN2c2nn(Cc3ccc(cc3)C(C)=O)c(Nc3ccc(F)cc3)c2C1=O |t:2| Show InChI InChI=1S/C25H25FN6O2/c1-15(33)17-7-5-16(6-8-17)13-32-21(27-19-11-9-18(26)10-12-19)20-22(29-32)31-14-25(2,3)28-24(31)30(4)23(20)34/h5-12,27H,13-14H2,1-4H3 | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
INTRA-CELLULAR THERAPIES, INC.
US Patent
| Assay Description
Assay: The following phosphodiesterase enzymes may be used: 3′,5′-cyclic-nucleotide-specific bovine brain phosphodiesterase (Sigma, St. L... |
US Patent US9598426 (2017)
BindingDB Entry DOI: 10.7270/Q29S1T2J |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Homo sapiens (Human)) | BDBM50048803

(5-(2-(4-(benzo[d]isothiazol-3-yl)piperazin-1-yl)et...)Show SMILES Clc1cc2NC(=O)Cc2cc1CCN1CCN(CC1)c1nsc2ccccc12 Show InChI InChI=1S/C21H21ClN4OS/c22-17-13-18-15(12-20(27)23-18)11-14(17)5-6-25-7-9-26(10-8-25)21-16-3-1-2-4-19(16)28-24-21/h1-4,11,13H,5-10,12H2,(H,23,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| US Patent
| 0.280 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Intra-Cellular Therapies, Inc.
US Patent
| Assay Description
Binding assay using 5-HT2A, Dopamine D2, SERT, αA1, 5-HT2C and H1 Receptors. |
US Patent US8598119 (2013)
BindingDB Entry DOI: 10.7270/Q28K77RC |
More data for this
Ligand-Target Pair | |
Dual specificity calcium/calmodulin-dependent 3',5'-cyclic nucleotide phosphodiesterase 1A
(Homo sapiens (Human)) | BDBM168128
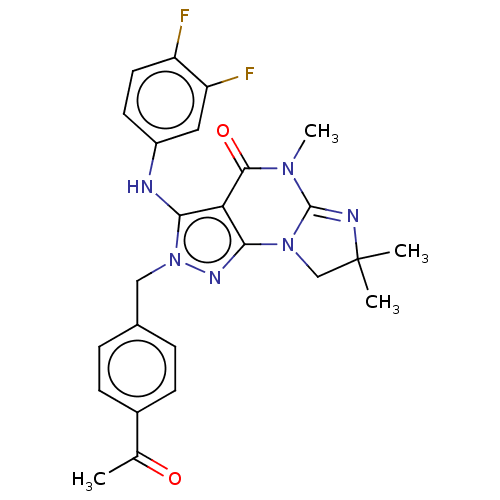
(US9073936, 3 | US9598426, 3)Show SMILES CN1C2=NC(C)(C)CN2c2nn(Cc3ccc(cc3)C(C)=O)c(Nc3ccc(F)c(F)c3)c2C1=O |t:2| Show InChI InChI=1S/C25H24F2N6O2/c1-14(34)16-7-5-15(6-8-16)12-33-21(28-17-9-10-18(26)19(27)11-17)20-22(30-33)32-13-25(2,3)29-24(32)31(4)23(20)35/h5-11,28H,12-13H2,1-4H3 | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
INTRA-CELLULAR THERAPIES, INC.
US Patent
| Assay Description
Assay: The following phosphodiesterase enzymes may be used: 3′,5′-cyclic-nucleotide-specific bovine brain phosphodiesterase (Sigma, St. L... |
US Patent US9598426 (2017)
BindingDB Entry DOI: 10.7270/Q29S1T2J |
More data for this
Ligand-Target Pair | |
Dual specificity calcium/calmodulin-dependent 3',5'-cyclic nucleotide phosphodiesterase 1A
(Homo sapiens (Human)) | BDBM168128

(US9073936, 3 | US9598426, 3)Show SMILES CN1C2=NC(C)(C)CN2c2nn(Cc3ccc(cc3)C(C)=O)c(Nc3ccc(F)c(F)c3)c2C1=O |t:2| Show InChI InChI=1S/C25H24F2N6O2/c1-14(34)16-7-5-15(6-8-16)12-33-21(28-17-9-10-18(26)19(27)11-17)20-22(30-33)32-13-25(2,3)29-24(32)31(4)23(20)35/h5-11,28H,12-13H2,1-4H3 | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 0.300 | -55.3 | n/a | n/a | n/a | n/a | n/a | 7.5 | 30 |
INTRA-CELLULAR THERAPIES, INC.
US Patent
| Assay Description
Materials: All chemicals are available from Sigma-Aldrich (St. Louis, Mo.) except for IMAP reagents (reaction buffer, binding buffer, FL-GMP and IMAP... |
US Patent US9073936 (2015)
BindingDB Entry DOI: 10.7270/Q2348J5T |
More data for this
Ligand-Target Pair | |
Dual specificity calcium/calmodulin-dependent 3',5'-cyclic nucleotide phosphodiesterase 1A
(Homo sapiens (Human)) | BDBM168129

(US9073936, 4 | US9598426, 4)Show SMILES CN1C2=NC(C)(C)CN2c2nn(Cc3ccc(cc3)C(C)=O)c(Nc3ccc(F)c(C)c3)c2C1=O |t:2| Show InChI InChI=1S/C26H27FN6O2/c1-15-12-19(10-11-20(15)27)28-22-21-23(32-14-26(3,4)29-25(32)31(5)24(21)35)30-33(22)13-17-6-8-18(9-7-17)16(2)34/h6-12,28H,13-14H2,1-5H3 | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 0.400 | -54.5 | n/a | n/a | n/a | n/a | n/a | 7.5 | 30 |
INTRA-CELLULAR THERAPIES, INC.
US Patent
| Assay Description
Materials: All chemicals are available from Sigma-Aldrich (St. Louis, Mo.) except for IMAP reagents (reaction buffer, binding buffer, FL-GMP and IMAP... |
US Patent US9073936 (2015)
BindingDB Entry DOI: 10.7270/Q2348J5T |
More data for this
Ligand-Target Pair | |
Dual specificity calcium/calmodulin-dependent 3',5'-cyclic nucleotide phosphodiesterase 1B
(Homo sapiens (Human)) | BDBM168129

(US9073936, 4 | US9598426, 4)Show SMILES CN1C2=NC(C)(C)CN2c2nn(Cc3ccc(cc3)C(C)=O)c(Nc3ccc(F)c(C)c3)c2C1=O |t:2| Show InChI InChI=1S/C26H27FN6O2/c1-15-12-19(10-11-20(15)27)28-22-21-23(32-14-26(3,4)29-25(32)31(5)24(21)35)30-33(22)13-17-6-8-18(9-7-17)16(2)34/h6-12,28H,13-14H2,1-5H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
INTRA-CELLULAR THERAPIES, INC.
US Patent
| |
US Patent US9598426 (2017)
BindingDB Entry DOI: 10.7270/Q29S1T2J |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Homo sapiens (Human)) | BDBM50001885

((risperidone)3-{2-[4-(6-Fluoro-benzo[d]isoxazol-3-...)Show SMILES Cc1nc2CCCCn2c(=O)c1CCN1CCC(CC1)c1noc2cc(F)ccc12 Show InChI InChI=1S/C23H27FN4O2/c1-15-18(23(29)28-10-3-2-4-21(28)25-15)9-13-27-11-7-16(8-12-27)22-19-6-5-17(24)14-20(19)30-26-22/h5-6,14,16H,2-4,7-13H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| US Patent
| 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Intra-Cellular Therapies, Inc.
US Patent
| Assay Description
Binding assay using 5-HT2A, Dopamine D2, SERT, αA1, 5-HT2C and H1 Receptors. |
US Patent US8598119 (2013)
BindingDB Entry DOI: 10.7270/Q28K77RC |
More data for this
Ligand-Target Pair | |
Dual specificity calcium/calmodulin-dependent 3',5'-cyclic nucleotide phosphodiesterase 1A
(Homo sapiens (Human)) | BDBM168127
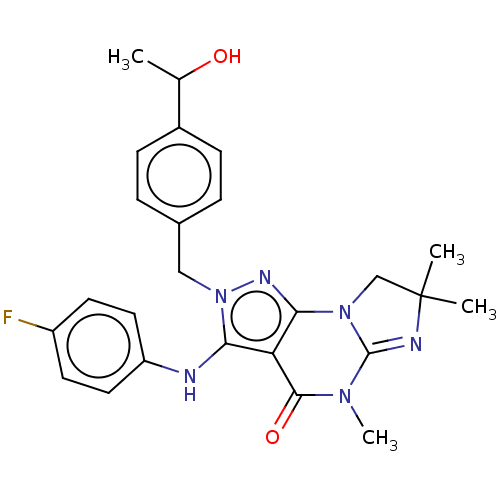
(US9073936, 2 | US9598426, 2)Show SMILES CC(O)c1ccc(Cn2nc3N4CC(C)(C)N=C4N(C)C(=O)c3c2Nc2ccc(F)cc2)cc1 |c:16| Show InChI InChI=1S/C25H27FN6O2/c1-15(33)17-7-5-16(6-8-17)13-32-21(27-19-11-9-18(26)10-12-19)20-22(29-32)31-14-25(2,3)28-24(31)30(4)23(20)34/h5-12,15,27,33H,13-14H2,1-4H3 | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
INTRA-CELLULAR THERAPIES, INC.
US Patent
| Assay Description
Assay: The following phosphodiesterase enzymes may be used: 3′,5′-cyclic-nucleotide-specific bovine brain phosphodiesterase (Sigma, St. L... |
US Patent US9598426 (2017)
BindingDB Entry DOI: 10.7270/Q29S1T2J |
More data for this
Ligand-Target Pair | |
Dual specificity calcium/calmodulin-dependent 3',5'-cyclic nucleotide phosphodiesterase 1A
(Homo sapiens (Human)) | BDBM168127

(US9073936, 2 | US9598426, 2)Show SMILES CC(O)c1ccc(Cn2nc3N4CC(C)(C)N=C4N(C)C(=O)c3c2Nc2ccc(F)cc2)cc1 |c:16| Show InChI InChI=1S/C25H27FN6O2/c1-15(33)17-7-5-16(6-8-17)13-32-21(27-19-11-9-18(26)10-12-19)20-22(29-32)31-14-25(2,3)28-24(31)30(4)23(20)34/h5-12,15,27,33H,13-14H2,1-4H3 | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 0.5 | -54.0 | n/a | n/a | n/a | n/a | n/a | 7.5 | 30 |
INTRA-CELLULAR THERAPIES, INC.
US Patent
| Assay Description
Materials: All chemicals are available from Sigma-Aldrich (St. Louis, Mo.) except for IMAP reagents (reaction buffer, binding buffer, FL-GMP and IMAP... |
US Patent US9073936 (2015)
BindingDB Entry DOI: 10.7270/Q2348J5T |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Homo sapiens (Human)) | BDBM50007659

(CHEMBL3233142)Show SMILES Cc1ccc(cc1)S([O-])(=O)=O.[H][C@]12CC[NH+](CCCC(=O)c3ccc(F)cc3)C[C@@]1([H])c1cccc3N(C)CCN2c13 |r| Show InChI InChI=1S/C24H28FN3O.C7H8O3S/c1-26-14-15-28-21-11-13-27(16-20(21)19-4-2-5-22(26)24(19)28)12-3-6-23(29)17-7-9-18(25)10-8-17;1-6-2-4-7(5-3-6)11(8,9)10/h2,4-5,7-10,20-21H,3,6,11-16H2,1H3;2-5H,1H3,(H,8,9,10)/t20-,21-;/m0./s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.540 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Intra-Cellular Therapies, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [125]DOI from human recombinant full length 5HT2A receptor expressed in HEK293E cells |
J Med Chem 57: 2670-82 (2014)
Article DOI: 10.1021/jm401958n
BindingDB Entry DOI: 10.7270/Q27D2WNZ |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM21398

(4-[4-(4-Chloro-phenyl)-4-hydroxy-piperidin-1-yl]-1...)Show SMILES OC1(CCN(CCCC(=O)c2ccc(F)cc2)CC1)c1ccc(Cl)cc1 Show InChI InChI=1S/C21H23ClFNO2/c22-18-7-5-17(6-8-18)21(26)11-14-24(15-12-21)13-1-2-20(25)16-3-9-19(23)10-4-16/h3-10,26H,1-2,11-15H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
US Patent
| 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Intra-Cellular Therapies, Inc.
US Patent
| Assay Description
Binding assay using 5-HT2A, Dopamine D2, SERT, αA1, 5-HT2C and H1 Receptors. |
US Patent US8598119 (2013)
BindingDB Entry DOI: 10.7270/Q28K77RC |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Homo sapiens (Human)) | BDBM50007666
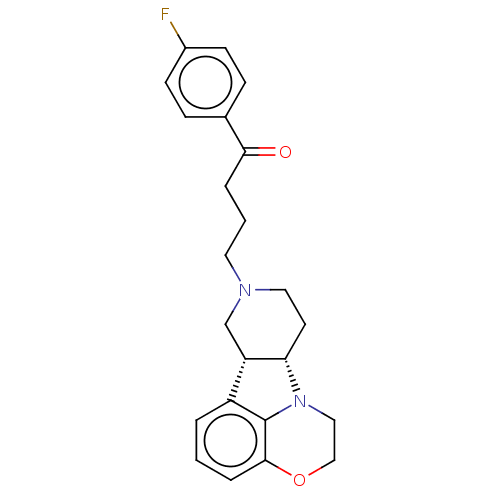
(CHEMBL3233432)Show SMILES [H][C@]12CCN(CCCC(=O)c3ccc(F)cc3)C[C@@]1([H])c1cccc3OCCN2c13 |r| Show InChI InChI=1S/C23H25FN2O2/c24-17-8-6-16(7-9-17)21(27)4-2-11-25-12-10-20-19(15-25)18-3-1-5-22-23(18)26(20)13-14-28-22/h1,3,5-9,19-20H,2,4,10-15H2/t19-,20-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.890 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Intra-Cellular Therapies, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [125]DOI from human recombinant full length 5HT2A receptor expressed in HEK293E cells |
J Med Chem 57: 2670-82 (2014)
Article DOI: 10.1021/jm401958n
BindingDB Entry DOI: 10.7270/Q27D2WNZ |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Homo sapiens (Human)) | BDBM50007667
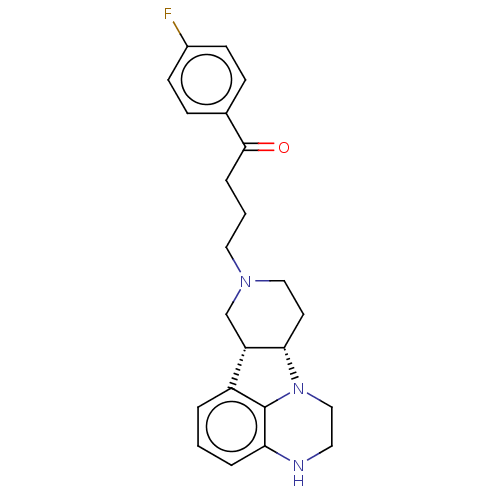
(CHEMBL3233143)Show SMILES [H][C@]12CCN(CCCC(=O)c3ccc(F)cc3)C[C@@]1([H])c1cccc3NCCN2c13 |r| Show InChI InChI=1S/C23H26FN3O/c24-17-8-6-16(7-9-17)22(28)5-2-12-26-13-10-21-19(15-26)18-3-1-4-20-23(18)27(21)14-11-25-20/h1,3-4,6-9,19,21,25H,2,5,10-15H2/t19-,21-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.940 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Intra-Cellular Therapies, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [125]DOI from human recombinant full length 5HT2A receptor expressed in HEK293E cells |
J Med Chem 57: 2670-82 (2014)
Article DOI: 10.1021/jm401958n
BindingDB Entry DOI: 10.7270/Q27D2WNZ |
More data for this
Ligand-Target Pair | |
Histamine H1 receptor
(Homo sapiens (Human)) | BDBM50001884

(2-[4-(4-Methyl-benzyl)-piperazin-1-yl]-1-(2-methyl...)Show SMILES CN1CCN(CC1)C1=Nc2cc(Cl)ccc2Nc2ccccc12 |t:8| Show InChI InChI=1S/C18H19ClN4/c1-22-8-10-23(11-9-22)18-14-4-2-3-5-15(14)20-16-7-6-13(19)12-17(16)21-18/h2-7,12,20H,8-11H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Intra-Cellular Therapies, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of histamine H1 receptor (unknown origin) |
J Med Chem 57: 2670-82 (2014)
Article DOI: 10.1021/jm401958n
BindingDB Entry DOI: 10.7270/Q27D2WNZ |
More data for this
Ligand-Target Pair | |
Dual specificity calcium/calmodulin-dependent 3',5'-cyclic nucleotide phosphodiesterase 1B
(Homo sapiens (Human)) | BDBM168126

(US9073936, 1 | US9598426, 1)Show SMILES CN1C2=NC(C)(C)CN2c2nn(Cc3ccc(cc3)C(C)=O)c(Nc3ccc(F)cc3)c2C1=O |t:2| Show InChI InChI=1S/C25H25FN6O2/c1-15(33)17-7-5-16(6-8-17)13-32-21(27-19-11-9-18(26)10-12-19)20-22(29-32)31-14-25(2,3)28-24(31)30(4)23(20)34/h5-12,27H,13-14H2,1-4H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
INTRA-CELLULAR THERAPIES, INC.
US Patent
| |
US Patent US9598426 (2017)
BindingDB Entry DOI: 10.7270/Q29S1T2J |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Homo sapiens (Human)) | BDBM50007662

(CHEMBL3233427)Show SMILES [H][C@]12CCN(CCCC(=O)c3ccc(F)cc3)C[C@@]1([H])c1cccc3CCCN2c13 |r| Show InChI InChI=1S/C24H27FN2O/c25-19-10-8-17(9-11-19)23(28)7-3-13-26-15-12-22-21(16-26)20-6-1-4-18-5-2-14-27(22)24(18)20/h1,4,6,8-11,21-22H,2-3,5,7,12-16H2/t21-,22-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Intra-Cellular Therapies, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [125]DOI from human recombinant full length 5HT2A receptor expressed in HEK293E cells |
J Med Chem 57: 2670-82 (2014)
Article DOI: 10.1021/jm401958n
BindingDB Entry DOI: 10.7270/Q27D2WNZ |
More data for this
Ligand-Target Pair | |
Dual specificity calcium/calmodulin-dependent 3',5'-cyclic nucleotide phosphodiesterase 1A
(Homo sapiens (Human)) | BDBM168126

(US9073936, 1 | US9598426, 1)Show SMILES CN1C2=NC(C)(C)CN2c2nn(Cc3ccc(cc3)C(C)=O)c(Nc3ccc(F)cc3)c2C1=O |t:2| Show InChI InChI=1S/C25H25FN6O2/c1-15(33)17-7-5-16(6-8-17)13-32-21(27-19-11-9-18(26)10-12-19)20-22(29-32)31-14-25(2,3)28-24(31)30(4)23(20)34/h5-12,27H,13-14H2,1-4H3 | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 1 | -52.2 | n/a | n/a | n/a | n/a | n/a | 7.5 | 30 |
INTRA-CELLULAR THERAPIES, INC.
US Patent
| Assay Description
Materials: All chemicals are available from Sigma-Aldrich (St. Louis, Mo.) except for IMAP reagents (reaction buffer, binding buffer, FL-GMP and IMAP... |
US Patent US9073936 (2015)
BindingDB Entry DOI: 10.7270/Q2348J5T |
More data for this
Ligand-Target Pair | |
Histone H1.0
(Homo sapiens (Human)) | BDBM50001884

(2-[4-(4-Methyl-benzyl)-piperazin-1-yl]-1-(2-methyl...)Show SMILES CN1CCN(CC1)C1=Nc2cc(Cl)ccc2Nc2ccccc12 |t:8| Show InChI InChI=1S/C18H19ClN4/c1-22-8-10-23(11-9-22)18-14-4-2-3-5-15(14)20-16-7-6-13(19)12-17(16)21-18/h2-7,12,20H,8-11H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| US Patent
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Intra-Cellular Therapies, Inc.
US Patent
| Assay Description
Binding assay using 5-HT2A, Dopamine D2, SERT, αA1, 5-HT2C and H1 Receptors. |
US Patent US8598119 (2013)
BindingDB Entry DOI: 10.7270/Q28K77RC |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Homo sapiens (Human)) | BDBM50007718

(CHEMBL3233430)Show SMILES [H][C@]12CCN(CCCC(=O)c3ccc(F)cc3)C[C@@]1([H])c1cccc3CCCCN2c13 |r| Show InChI InChI=1S/C25H29FN2O/c26-20-11-9-18(10-12-20)24(29)8-4-14-27-16-13-23-22(17-27)21-7-3-6-19-5-1-2-15-28(23)25(19)21/h3,6-7,9-12,22-23H,1-2,4-5,8,13-17H2/t22-,23-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Intra-Cellular Therapies, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [125]DOI from human recombinant full length 5HT2A receptor expressed in HEK293E cells |
J Med Chem 57: 2670-82 (2014)
Article DOI: 10.1021/jm401958n
BindingDB Entry DOI: 10.7270/Q27D2WNZ |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Homo sapiens (Human)) | BDBM50007665

(CHEMBL3233431)Show SMILES [H][C@]12CCN(CCCC(=O)c3ccc(F)cc3)C[C@@]1([H])c1cccc3SCCN2c13 |r| Show InChI InChI=1S/C23H25FN2OS/c24-17-8-6-16(7-9-17)21(27)4-2-11-25-12-10-20-19(15-25)18-3-1-5-22-23(18)26(20)13-14-28-22/h1,3,5-9,19-20H,2,4,10-15H2/t19-,20-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Intra-Cellular Therapies, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [125]DOI from human recombinant full length 5HT2A receptor expressed in HEK293E cells |
J Med Chem 57: 2670-82 (2014)
Article DOI: 10.1021/jm401958n
BindingDB Entry DOI: 10.7270/Q27D2WNZ |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM50130293

(7-{4-[4-(2,3-dichlorophenyl)piperazin-1-yl]butoxy}...)Show SMILES Clc1cccc(N2CCN(CCCCOc3ccc4CCC(=O)Nc4c3)CC2)c1Cl Show InChI InChI=1S/C23H27Cl2N3O2/c24-19-4-3-5-21(23(19)25)28-13-11-27(12-14-28)10-1-2-15-30-18-8-6-17-7-9-22(29)26-20(17)16-18/h3-6,8,16H,1-2,7,9-15H2,(H,26,29) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
US Patent
| 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Intra-Cellular Therapies, Inc.
US Patent
| Assay Description
Binding assay using 5-HT2A, Dopamine D2, SERT, αA1, 5-HT2C and H1 Receptors. |
US Patent US8598119 (2013)
BindingDB Entry DOI: 10.7270/Q28K77RC |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Homo sapiens (Human)) | BDBM50007661

(CHEMBL3233423)Show SMILES [H][C@]12CCN(CCCC(=O)c3ccc(F)cc3)CC[C@@]1([H])c1cccc3N(C)CCN2c13 |r| Show InChI InChI=1S/C25H30FN3O/c1-27-16-17-29-22-12-15-28(13-3-6-24(30)18-7-9-19(26)10-8-18)14-11-20(22)21-4-2-5-23(27)25(21)29/h2,4-5,7-10,20,22H,3,6,11-17H2,1H3/t20-,22-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Intra-Cellular Therapies, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [125]DOI from human recombinant full length 5HT2A receptor expressed in HEK293E cells |
J Med Chem 57: 2670-82 (2014)
Article DOI: 10.1021/jm401958n
BindingDB Entry DOI: 10.7270/Q27D2WNZ |
More data for this
Ligand-Target Pair | |
Histamine H1 receptor
(Homo sapiens (Human)) | BDBM35254

(2-methyl-4-(4-methylpiperazin-1-yl)-10H-thieno[2,3...)Show InChI InChI=1S/C17H20N4S/c1-12-11-13-16(21-9-7-20(2)8-10-21)18-14-5-3-4-6-15(14)19-17(13)22-12/h3-6,11,19H,7-10H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Intra-Cellular Therapies, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of histamine H1 receptor (unknown origin) |
J Med Chem 57: 2670-82 (2014)
Article DOI: 10.1021/jm401958n
BindingDB Entry DOI: 10.7270/Q27D2WNZ |
More data for this
Ligand-Target Pair | |
Histone H1.0
(Homo sapiens (Human)) | BDBM35254

(2-methyl-4-(4-methylpiperazin-1-yl)-10H-thieno[2,3...)Show InChI InChI=1S/C17H20N4S/c1-12-11-13-16(21-9-7-20(2)8-10-21)18-14-5-3-4-6-15(14)19-17(13)22-12/h3-6,11,19H,7-10H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| US Patent
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Intra-Cellular Therapies, Inc.
US Patent
| Assay Description
Binding assay using 5-HT2A, Dopamine D2, SERT, αA1, 5-HT2C and H1 Receptors. |
US Patent US8598119 (2013)
BindingDB Entry DOI: 10.7270/Q28K77RC |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Homo sapiens (Human)) | BDBM50007668
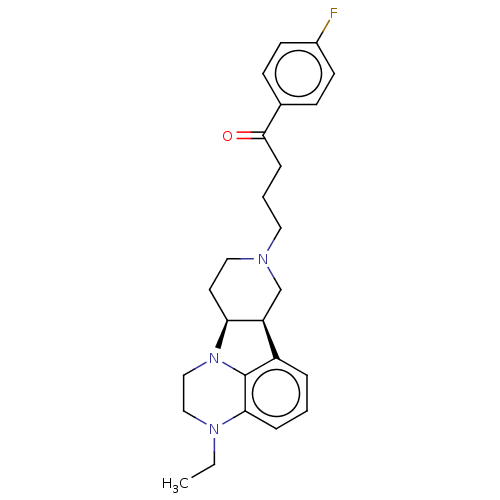
(CHEMBL3233144)Show SMILES [H][C@]12CCN(CCCC(=O)c3ccc(F)cc3)C[C@@]1([H])c1cccc3N(CC)CCN2c13 |r| Show InChI InChI=1S/C25H30FN3O/c1-2-28-15-16-29-22-12-14-27(17-21(22)20-5-3-6-23(28)25(20)29)13-4-7-24(30)18-8-10-19(26)11-9-18/h3,5-6,8-11,21-22H,2,4,7,12-17H2,1H3/t21-,22-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 2.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Intra-Cellular Therapies, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [125]DOI from human recombinant full length 5HT2A receptor expressed in HEK293E cells |
J Med Chem 57: 2670-82 (2014)
Article DOI: 10.1021/jm401958n
BindingDB Entry DOI: 10.7270/Q27D2WNZ |
More data for this
Ligand-Target Pair | |
Alpha-1A adrenergic receptor
(Homo sapiens (Human)) | BDBM50001885

((risperidone)3-{2-[4-(6-Fluoro-benzo[d]isoxazol-3-...)Show SMILES Cc1nc2CCCCn2c(=O)c1CCN1CCC(CC1)c1noc2cc(F)ccc12 Show InChI InChI=1S/C23H27FN4O2/c1-15-18(23(29)28-10-3-2-4-21(28)25-15)9-13-27-11-7-16(8-12-27)22-19-6-5-17(24)14-20(19)30-26-22/h5-6,14,16H,2-4,7-13H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
US Patent
| 2.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Intra-Cellular Therapies, Inc.
US Patent
| Assay Description
Binding assay using 5-HT2A, Dopamine D2, SERT, αA1, 5-HT2C and H1 Receptors. |
US Patent US8598119 (2013)
BindingDB Entry DOI: 10.7270/Q28K77RC |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Homo sapiens (Human)) | BDBM50007680
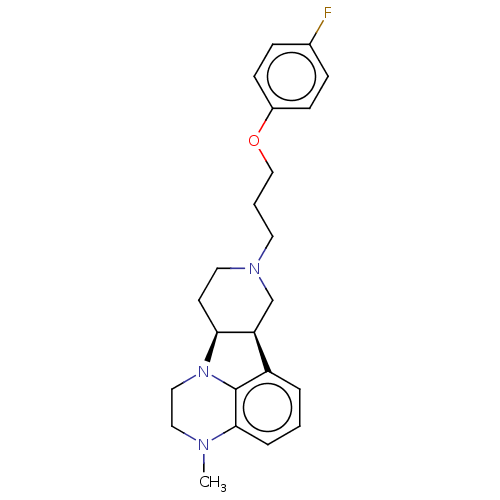
(CHEMBL3233409)Show SMILES [H][C@]12CCN(CCCOc3ccc(F)cc3)C[C@@]1([H])c1cccc3N(C)CCN2c13 |r| Show InChI InChI=1S/C23H28FN3O/c1-25-13-14-27-21-10-12-26(11-3-15-28-18-8-6-17(24)7-9-18)16-20(21)19-4-2-5-22(25)23(19)27/h2,4-9,20-21H,3,10-16H2,1H3/t20-,21-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Intra-Cellular Therapies, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [125]DOI from human recombinant full length 5HT2A receptor expressed in HEK293E cells |
J Med Chem 57: 2670-82 (2014)
Article DOI: 10.1021/jm401958n
BindingDB Entry DOI: 10.7270/Q27D2WNZ |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Homo sapiens (Human)) | BDBM35254

(2-methyl-4-(4-methylpiperazin-1-yl)-10H-thieno[2,3...)Show InChI InChI=1S/C17H20N4S/c1-12-11-13-16(21-9-7-20(2)8-10-21)18-14-5-3-4-6-15(14)19-17(13)22-12/h3-6,11,19H,7-10H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| US Patent
| 2.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Intra-Cellular Therapies, Inc.
US Patent
| Assay Description
Binding assay using 5-HT2A, Dopamine D2, SERT, αA1, 5-HT2C and H1 Receptors. |
US Patent US8598119 (2013)
BindingDB Entry DOI: 10.7270/Q28K77RC |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Homo sapiens (Human)) | BDBM35254

(2-methyl-4-(4-methylpiperazin-1-yl)-10H-thieno[2,3...)Show InChI InChI=1S/C17H20N4S/c1-12-11-13-16(21-9-7-20(2)8-10-21)18-14-5-3-4-6-15(14)19-17(13)22-12/h3-6,11,19H,7-10H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 2.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Intra-Cellular Therapies, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [125]DOI from human recombinant full length 5HT2A receptor expressed in HEK293E cells |
J Med Chem 57: 2670-82 (2014)
Article DOI: 10.1021/jm401958n
BindingDB Entry DOI: 10.7270/Q27D2WNZ |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Homo sapiens (Human)) | BDBM50007683
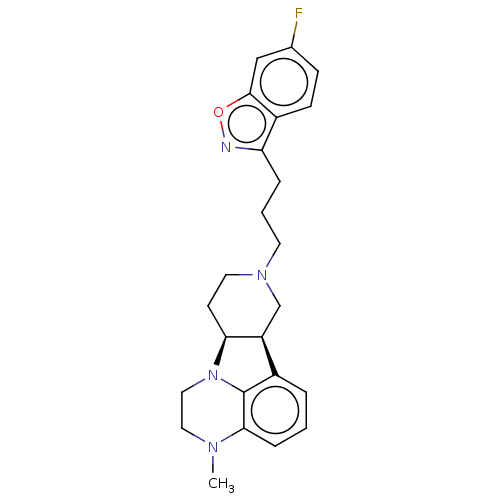
(CHEMBL3233412)Show SMILES [H][C@]12CCN(CCCc3noc4cc(F)ccc34)C[C@@]1([H])c1cccc3N(C)CCN2c13 |r| Show InChI InChI=1S/C24H27FN4O/c1-27-12-13-29-21-9-11-28(15-19(21)17-4-2-6-22(27)24(17)29)10-3-5-20-18-8-7-16(25)14-23(18)30-26-20/h2,4,6-8,14,19,21H,3,5,9-13,15H2,1H3/t19-,21-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Intra-Cellular Therapies, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [125]DOI from human recombinant full length 5HT2A receptor expressed in HEK293E cells |
J Med Chem 57: 2670-82 (2014)
Article DOI: 10.1021/jm401958n
BindingDB Entry DOI: 10.7270/Q27D2WNZ |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Homo sapiens (Human)) | BDBM50007663
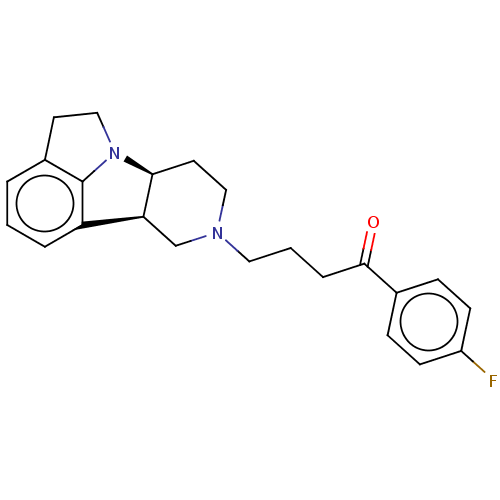
(CHEMBL3233429)Show SMILES [H][C@]12CCN(CCCC(=O)c3ccc(F)cc3)C[C@@]1([H])c1cccc3CCN2c13 |r| Show InChI InChI=1S/C23H25FN2O/c24-18-8-6-16(7-9-18)22(27)5-2-12-25-13-11-21-20(15-25)19-4-1-3-17-10-14-26(21)23(17)19/h1,3-4,6-9,20-21H,2,5,10-15H2/t20-,21-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 2.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Intra-Cellular Therapies, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [125]DOI from human recombinant full length 5HT2A receptor expressed in HEK293E cells |
J Med Chem 57: 2670-82 (2014)
Article DOI: 10.1021/jm401958n
BindingDB Entry DOI: 10.7270/Q27D2WNZ |
More data for this
Ligand-Target Pair | |
Replicase polyprotein 1ab
(Human SARS coronavirus (SARS-CoV) (Severe acute re...) | BDBM429243
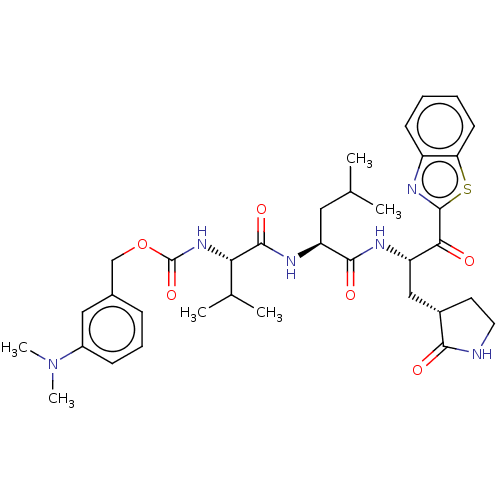
(jm5b01461, Compound 60)Show SMILES CC(C)C[C@H](NC(=O)[C@@H](NC(=O)OCc1cccc(c1)N(C)C)C(C)C)C(=O)N[C@@H](C[C@@H]1CCNC1=O)C(=O)c1nc2ccccc2s1 Show InChI InChI=1S/C35H46N6O6S/c1-20(2)16-27(38-33(45)29(21(3)4)40-35(46)47-19-22-10-9-11-24(17-22)41(5)6)32(44)37-26(18-23-14-15-36-31(23)43)30(42)34-39-25-12-7-8-13-28(25)48-34/h7-13,17,20-21,23,26-27,29H,14-16,18-19H2,1-6H3,(H,36,43)(H,37,44)(H,38,45)(H,40,46)/t23-,26-,27-,29-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bonn
| Assay Description
This is a review article. |
Med Res Rev (2020)
Article DOI: 10.1002/med.21724
BindingDB Entry DOI: 10.7270/Q2JS9ST6 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Homo sapiens (Human)) | BDBM50007682
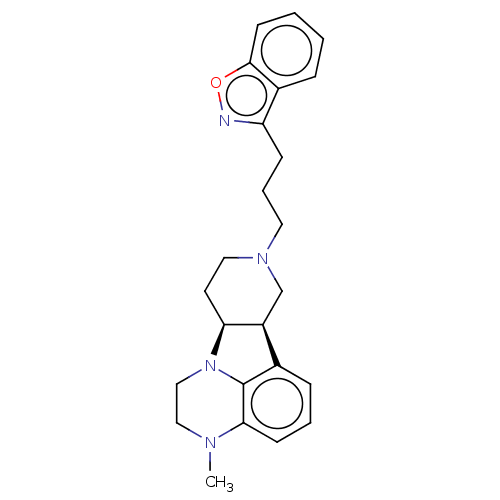
(CHEMBL3233411)Show SMILES [H][C@]12CCN(CCCc3noc4ccccc34)C[C@@]1([H])c1cccc3N(C)CCN2c13 |r| Show InChI InChI=1S/C24H28N4O/c1-26-14-15-28-21-11-13-27(16-19(21)17-7-4-9-22(26)24(17)28)12-5-8-20-18-6-2-3-10-23(18)29-25-20/h2-4,6-7,9-10,19,21H,5,8,11-16H2,1H3/t19-,21-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Intra-Cellular Therapies, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [125]DOI from human recombinant full length 5HT2A receptor expressed in HEK293E cells |
J Med Chem 57: 2670-82 (2014)
Article DOI: 10.1021/jm401958n
BindingDB Entry DOI: 10.7270/Q27D2WNZ |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Homo sapiens (Human)) | BDBM50007673
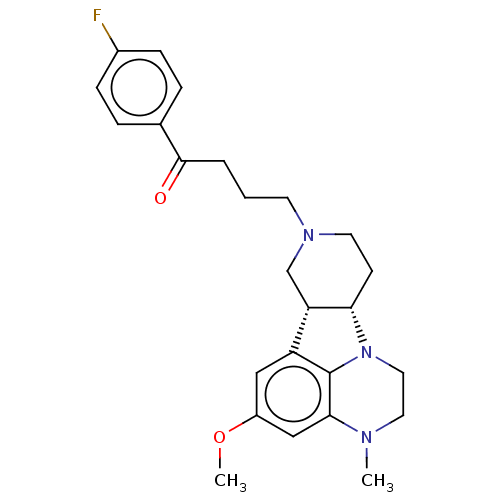
(CHEMBL3233149)Show SMILES [H][C@]12CCN(CCCC(=O)c3ccc(F)cc3)C[C@@]1([H])c1cc(OC)cc3N(C)CCN2c13 |r| Show InChI InChI=1S/C25H30FN3O2/c1-27-12-13-29-22-9-11-28(10-3-4-24(30)17-5-7-18(26)8-6-17)16-21(22)20-14-19(31-2)15-23(27)25(20)29/h5-8,14-15,21-22H,3-4,9-13,16H2,1-2H3/t21-,22-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Intra-Cellular Therapies, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [125]DOI from human recombinant full length 5HT2A receptor expressed in HEK293E cells |
J Med Chem 57: 2670-82 (2014)
Article DOI: 10.1021/jm401958n
BindingDB Entry DOI: 10.7270/Q27D2WNZ |
More data for this
Ligand-Target Pair | |
DNA gyrase subunit A/B
(Escherichia coli (strain K12)) | BDBM24601
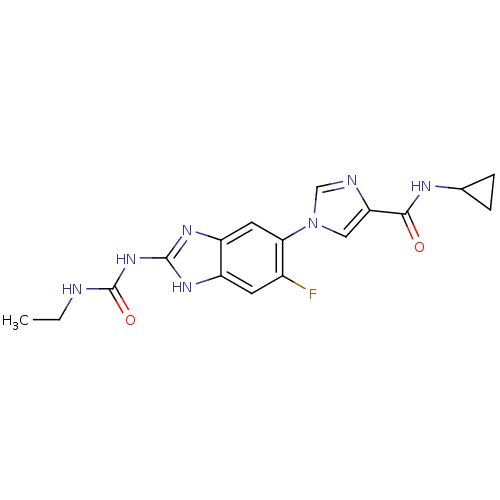
(Benzimidazole urea analogue, 5 | N-cyclopropyl-1-{...)Show SMILES CCNC(=O)Nc1nc2cc(c(F)cc2[nH]1)-n1cnc(c1)C(=O)NC1CC1 Show InChI InChI=1S/C17H18FN7O2/c1-2-19-17(27)24-16-22-11-5-10(18)14(6-12(11)23-16)25-7-13(20-8-25)15(26)21-9-3-4-9/h5-9H,2-4H2,1H3,(H,21,26)(H3,19,22,23,24,27) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
| Article
PubMed
| <4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex
| Assay Description
Enzymatic hydrolysis of ATP to ADP was coupled to the conversion of NADH to NAD+. The decrease in NADH absorbance was monitored at 340 nm for 20 min ... |
J Med Chem 51: 5243-63 (2008)
Article DOI: 10.1021/jm800318d
BindingDB Entry DOI: 10.7270/Q2J67F7T |
More data for this
Ligand-Target Pair | |
DNA gyrase subunit A/B
(Escherichia coli (strain K12)) | BDBM24606
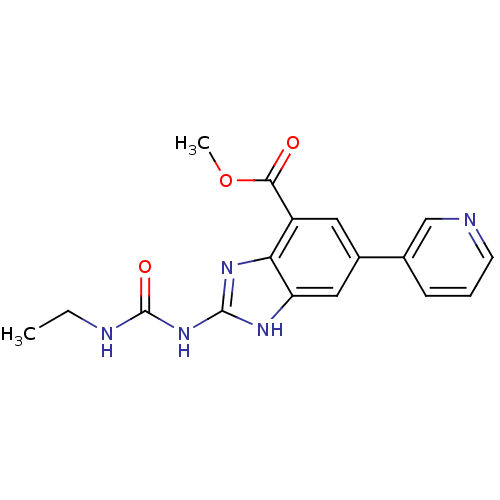
(Benzimidazole urea analogue, 10 | methyl 2-[(ethyl...)Show SMILES CCNC(=O)Nc1nc2c(cc(cc2[nH]1)-c1cccnc1)C(=O)OC Show InChI InChI=1S/C17H17N5O3/c1-3-19-17(24)22-16-20-13-8-11(10-5-4-6-18-9-10)7-12(14(13)21-16)15(23)25-2/h4-9H,3H2,1-2H3,(H3,19,20,21,22,24) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
| Article
PubMed
| <4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex
| Assay Description
Enzymatic hydrolysis of ATP to ADP was coupled to the conversion of NADH to NAD+. The decrease in NADH absorbance was monitored at 340 nm for 20 min ... |
J Med Chem 51: 5243-63 (2008)
Article DOI: 10.1021/jm800318d
BindingDB Entry DOI: 10.7270/Q2J67F7T |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Homo sapiens (Human)) | BDBM50007672

(CHEMBL3233148)Show SMILES [H][C@]12CCN(CCCC(=O)c3ccc(F)cc3)C[C@@]1([H])c1cc(Br)cc3N(C)CCN2c13 |r| Show InChI InChI=1S/C24H27BrFN3O/c1-27-11-12-29-21-8-10-28(9-2-3-23(30)16-4-6-18(26)7-5-16)15-20(21)19-13-17(25)14-22(27)24(19)29/h4-7,13-14,20-21H,2-3,8-12,15H2,1H3/t20-,21-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Intra-Cellular Therapies, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [125]DOI from human recombinant full length 5HT2A receptor expressed in HEK293E cells |
J Med Chem 57: 2670-82 (2014)
Article DOI: 10.1021/jm401958n
BindingDB Entry DOI: 10.7270/Q27D2WNZ |
More data for this
Ligand-Target Pair | |
Dual specificity calcium/calmodulin-dependent 3',5'-cyclic nucleotide phosphodiesterase 1B
(Homo sapiens (Human)) | BDBM168128

(US9073936, 3 | US9598426, 3)Show SMILES CN1C2=NC(C)(C)CN2c2nn(Cc3ccc(cc3)C(C)=O)c(Nc3ccc(F)c(F)c3)c2C1=O |t:2| Show InChI InChI=1S/C25H24F2N6O2/c1-14(34)16-7-5-15(6-8-16)12-33-21(28-17-9-10-18(26)19(27)11-17)20-22(30-33)32-13-25(2,3)29-24(32)31(4)23(20)35/h5-11,28H,12-13H2,1-4H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
INTRA-CELLULAR THERAPIES, INC.
US Patent
| |
US Patent US9598426 (2017)
BindingDB Entry DOI: 10.7270/Q29S1T2J |
More data for this
Ligand-Target Pair | |
DNA gyrase subunit A/B
(Escherichia coli (strain K12)) | BDBM24617
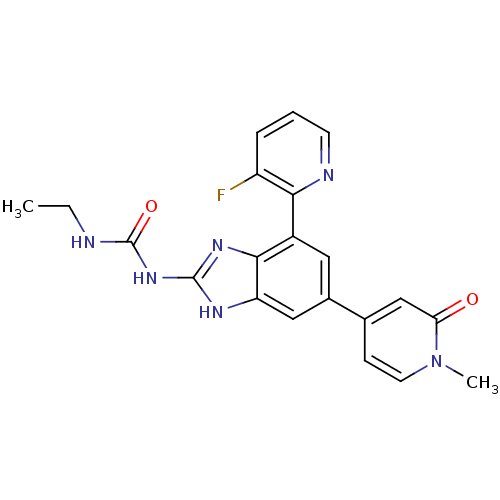
(3-ethyl-1-[7-(3-fluoropyridin-2-yl)-5-(1-methyl-2-...)Show SMILES CCNC(=O)Nc1nc2c(cc(cc2[nH]1)-c1ccn(C)c(=O)c1)-c1ncccc1F Show InChI InChI=1S/C21H19FN6O2/c1-3-23-21(30)27-20-25-16-10-13(12-6-8-28(2)17(29)11-12)9-14(19(16)26-20)18-15(22)5-4-7-24-18/h4-11H,3H2,1-2H3,(H3,23,25,26,27,30) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| <4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex
| Assay Description
Enzymatic hydrolysis of ATP to ADP was coupled to the conversion of NADH to NAD+. The decrease in NADH absorbance was monitored at 340 nm for 20 min ... |
J Med Chem 51: 5243-63 (2008)
Article DOI: 10.1021/jm800318d
BindingDB Entry DOI: 10.7270/Q2J67F7T |
More data for this
Ligand-Target Pair | |
DNA gyrase subunit A/B
(Escherichia coli (strain K12)) | BDBM24608
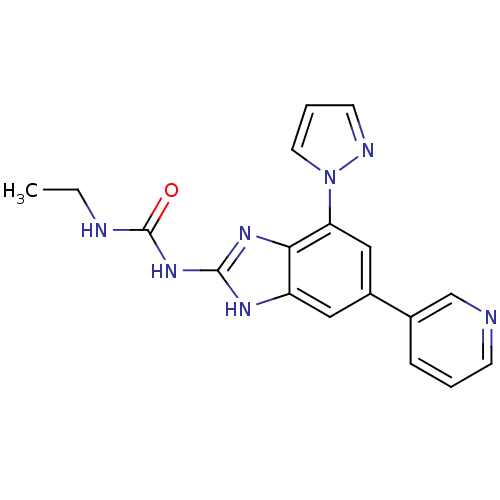
(3-ethyl-1-[7-(1H-pyrazol-1-yl)-5-(pyridin-3-yl)-1H...)Show SMILES CCNC(=O)Nc1nc2c(cc(cc2[nH]1)-c1cccnc1)-n1cccn1 Show InChI InChI=1S/C18H17N7O/c1-2-20-18(26)24-17-22-14-9-13(12-5-3-6-19-11-12)10-15(16(14)23-17)25-8-4-7-21-25/h3-11H,2H2,1H3,(H3,20,22,23,24,26) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| <4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex
| Assay Description
Enzymatic hydrolysis of ATP to ADP was coupled to the conversion of NADH to NAD+. The decrease in NADH absorbance was monitored at 340 nm for 20 min ... |
J Med Chem 51: 5243-63 (2008)
Article DOI: 10.1021/jm800318d
BindingDB Entry DOI: 10.7270/Q2J67F7T |
More data for this
Ligand-Target Pair | |
DNA gyrase subunit A/B
(Escherichia coli (strain K12)) | BDBM24609
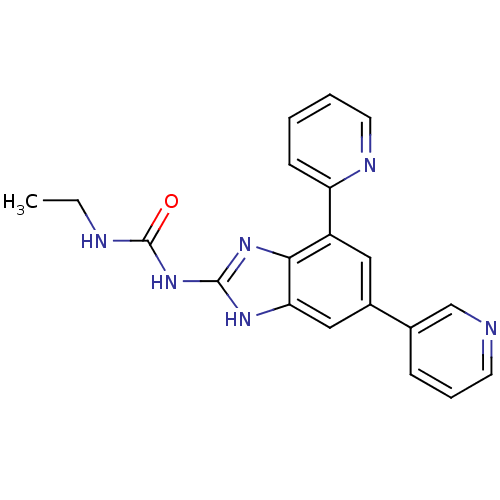
(3-ethyl-1-[7-(pyridin-2-yl)-5-(pyridin-3-yl)-1H-1,...)Show SMILES CCNC(=O)Nc1nc2c(cc(cc2[nH]1)-c1cccnc1)-c1ccccn1 Show InChI InChI=1S/C20H18N6O/c1-2-22-20(27)26-19-24-17-11-14(13-6-5-8-21-12-13)10-15(18(17)25-19)16-7-3-4-9-23-16/h3-12H,2H2,1H3,(H3,22,24,25,26,27) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| <4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex
| Assay Description
Enzymatic hydrolysis of ATP to ADP was coupled to the conversion of NADH to NAD+. The decrease in NADH absorbance was monitored at 340 nm for 20 min ... |
J Med Chem 51: 5243-63 (2008)
Article DOI: 10.1021/jm800318d
BindingDB Entry DOI: 10.7270/Q2J67F7T |
More data for this
Ligand-Target Pair | |
DNA gyrase subunit A/B
(Escherichia coli (strain K12)) | BDBM24614
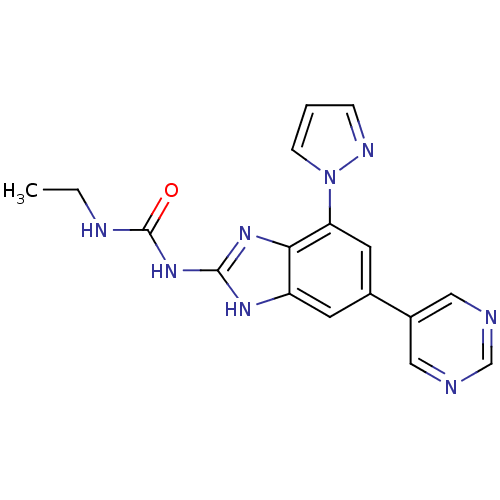
(3-ethyl-1-[7-(1H-pyrazol-1-yl)-5-(pyrimidin-5-yl)-...)Show SMILES CCNC(=O)Nc1nc2c(cc(cc2[nH]1)-c1cncnc1)-n1cccn1 Show InChI InChI=1S/C17H16N8O/c1-2-20-17(26)24-16-22-13-6-11(12-8-18-10-19-9-12)7-14(15(13)23-16)25-5-3-4-21-25/h3-10H,2H2,1H3,(H3,20,22,23,24,26) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| <4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex
| Assay Description
Enzymatic hydrolysis of ATP to ADP was coupled to the conversion of NADH to NAD+. The decrease in NADH absorbance was monitored at 340 nm for 20 min ... |
J Med Chem 51: 5243-63 (2008)
Article DOI: 10.1021/jm800318d
BindingDB Entry DOI: 10.7270/Q2J67F7T |
More data for this
Ligand-Target Pair | |
DNA gyrase subunit A/B
(Escherichia coli (strain K12)) | BDBM24615
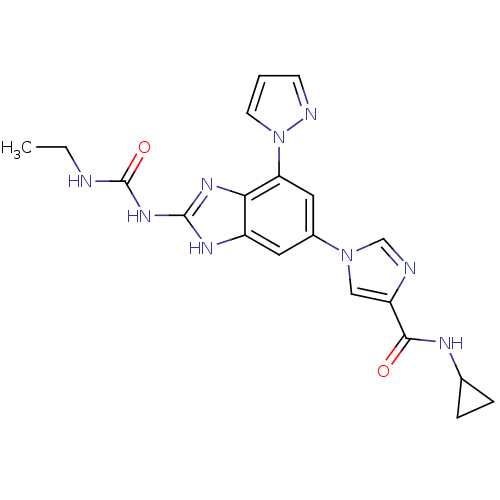
(Benzimidazole urea analogue, 19 | N-cyclopropyl-1-...)Show SMILES CCNC(=O)Nc1nc2c(cc(cc2[nH]1)-n1cnc(c1)C(=O)NC1CC1)-n1cccn1 Show InChI InChI=1S/C20H21N9O2/c1-2-21-20(31)27-19-25-14-8-13(9-16(17(14)26-19)29-7-3-6-23-29)28-10-15(22-11-28)18(30)24-12-4-5-12/h3,6-12H,2,4-5H2,1H3,(H,24,30)(H3,21,25,26,27,31) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
| Article
PubMed
| <4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex
| Assay Description
Enzymatic hydrolysis of ATP to ADP was coupled to the conversion of NADH to NAD+. The decrease in NADH absorbance was monitored at 340 nm for 20 min ... |
J Med Chem 51: 5243-63 (2008)
Article DOI: 10.1021/jm800318d
BindingDB Entry DOI: 10.7270/Q2J67F7T |
More data for this
Ligand-Target Pair | |
DNA gyrase subunit A/B
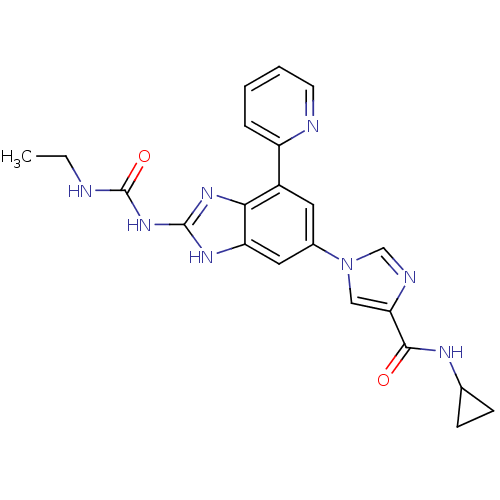
(Escherichia coli (strain K12)) | BDBM24616
(1-(6-(4-(Cyclopropylcarbamoyl)-1H-imidazol-1-yl)-4...)Show SMILES CCNC(=O)Nc1nc2c(cc(cc2[nH]1)-n1cnc(c1)C(=O)NC1CC1)-c1ccccn1 Show InChI InChI=1S/C22H22N8O2/c1-2-23-22(32)29-21-27-17-10-14(9-15(19(17)28-21)16-5-3-4-8-24-16)30-11-18(25-12-30)20(31)26-13-6-7-13/h3-5,8-13H,2,6-7H2,1H3,(H,26,31)(H3,23,27,28,29,32) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
| Article
PubMed
| <4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex
| Assay Description
Enzymatic hydrolysis of ATP to ADP was coupled to the conversion of NADH to NAD+. The decrease in NADH absorbance was monitored at 340 nm for 20 min ... |
J Med Chem 51: 5243-63 (2008)
Article DOI: 10.1021/jm800318d
BindingDB Entry DOI: 10.7270/Q2J67F7T |
More data for this
Ligand-Target Pair | |
Dual specificity calcium/calmodulin-dependent 3',5'-cyclic nucleotide phosphodiesterase 1A
(Homo sapiens (Human)) | BDBM168127

(US9073936, 2 | US9598426, 2)Show SMILES CC(O)c1ccc(Cn2nc3N4CC(C)(C)N=C4N(C)C(=O)c3c2Nc2ccc(F)cc2)cc1 |c:16| Show InChI InChI=1S/C25H27FN6O2/c1-15(33)17-7-5-16(6-8-17)13-32-21(27-19-11-9-18(26)10-12-19)20-22(29-32)31-14-25(2,3)28-24(31)30(4)23(20)34/h5-12,15,27,33H,13-14H2,1-4H3 | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 4 | -48.7 | n/a | n/a | n/a | n/a | n/a | 7.5 | 30 |
INTRA-CELLULAR THERAPIES, INC.
US Patent
| Assay Description
Materials: All chemicals are available from Sigma-Aldrich (St. Louis, Mo.) except for IMAP reagents (reaction buffer, binding buffer, FL-GMP and IMAP... |
US Patent US9073936 (2015)
BindingDB Entry DOI: 10.7270/Q2348J5T |
More data for this
Ligand-Target Pair | |
Dual specificity calcium/calmodulin-dependent 3',5'-cyclic nucleotide phosphodiesterase 1A
(Homo sapiens (Human)) | BDBM168128

(US9073936, 3 | US9598426, 3)Show SMILES CN1C2=NC(C)(C)CN2c2nn(Cc3ccc(cc3)C(C)=O)c(Nc3ccc(F)c(F)c3)c2C1=O |t:2| Show InChI InChI=1S/C25H24F2N6O2/c1-14(34)16-7-5-15(6-8-16)12-33-21(28-17-9-10-18(26)19(27)11-17)20-22(30-33)32-13-25(2,3)29-24(32)31(4)23(20)35/h5-11,28H,12-13H2,1-4H3 | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 4 | -48.7 | n/a | n/a | n/a | n/a | n/a | 7.5 | 30 |
INTRA-CELLULAR THERAPIES, INC.
US Patent
| Assay Description
Materials: All chemicals are available from Sigma-Aldrich (St. Louis, Mo.) except for IMAP reagents (reaction buffer, binding buffer, FL-GMP and IMAP... |
US Patent US9073936 (2015)
BindingDB Entry DOI: 10.7270/Q2348J5T |
More data for this
Ligand-Target Pair | |
Dual specificity calcium/calmodulin-dependent 3',5'-cyclic nucleotide phosphodiesterase 1B
(Homo sapiens (Human)) | BDBM168127

(US9073936, 2 | US9598426, 2)Show SMILES CC(O)c1ccc(Cn2nc3N4CC(C)(C)N=C4N(C)C(=O)c3c2Nc2ccc(F)cc2)cc1 |c:16| Show InChI InChI=1S/C25H27FN6O2/c1-15(33)17-7-5-16(6-8-17)13-32-21(27-19-11-9-18(26)10-12-19)20-22(29-32)31-14-25(2,3)28-24(31)30(4)23(20)34/h5-12,15,27,33H,13-14H2,1-4H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
INTRA-CELLULAR THERAPIES, INC.
US Patent
| |
US Patent US9598426 (2017)
BindingDB Entry DOI: 10.7270/Q29S1T2J |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data