 Found 533 hits with Last Name = 'wilkinson' and Initial = 's'
Found 533 hits with Last Name = 'wilkinson' and Initial = 's' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Translocator protein
(Rattus norvegicus (rat)) | BDBM50073602
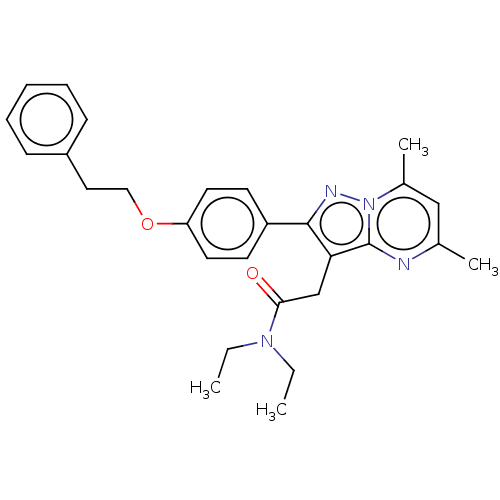
(CHEMBL3408973)Show SMILES CCN(CC)C(=O)Cc1c(nn2c(C)cc(C)nc12)-c1ccc(OCCc2ccccc2)cc1 Show InChI InChI=1S/C28H32N4O2/c1-5-31(6-2)26(33)19-25-27(30-32-21(4)18-20(3)29-28(25)32)23-12-14-24(15-13-23)34-17-16-22-10-8-7-9-11-22/h7-15,18H,5-6,16-17,19H2,1-4H3 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Stanford University School of Medicine
Curated by ChEMBL
| Assay Description
Displacement of [3H]PK11195 from TSPO in Wistar rat kidney mitochondrial fractions incubated for 90 mins by liquid scintillation counting method |
Eur J Med Chem 93: 392-400 (2015)
Article DOI: 10.1016/j.ejmech.2015.02.004
BindingDB Entry DOI: 10.7270/Q2G73GF4 |
More data for this
Ligand-Target Pair | |
Translocator protein
(Homo sapiens (Human)) | BDBM50122293
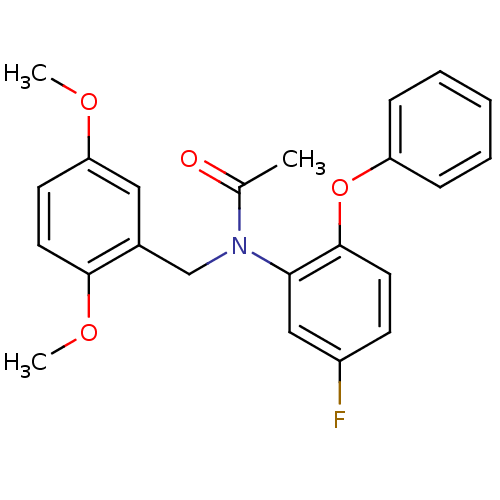
(CHEMBL401000 | CHEMBL63064 | N-(2,5-Dimethoxy-benz...)Show SMILES COc1ccc(OC)c(CN(C(C)=O)c2cc(F)ccc2Oc2ccccc2)c1 Show InChI InChI=1S/C23H22FNO4/c1-16(26)25(15-17-13-20(27-2)10-12-22(17)28-3)21-14-18(24)9-11-23(21)29-19-7-5-4-6-8-19/h4-14H,15H2,1-3H3 | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Stanford University School of Medicine
Curated by ChEMBL
| Assay Description
Inhibition of human TSPO |
Eur J Med Chem 93: 392-400 (2015)
Article DOI: 10.1016/j.ejmech.2015.02.004
BindingDB Entry DOI: 10.7270/Q2G73GF4 |
More data for this
Ligand-Target Pair | |
Translocator protein
(Rattus norvegicus (rat)) | BDBM50073596
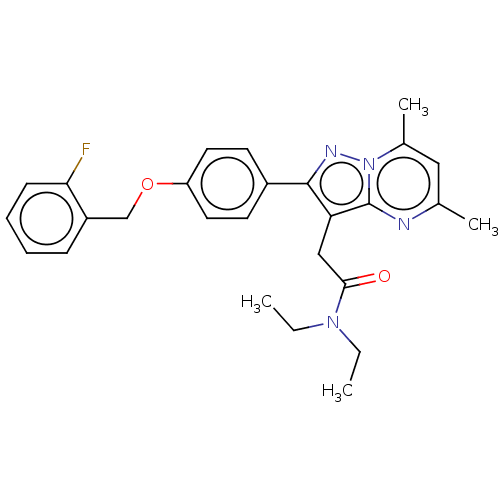
(CHEMBL3408967)Show SMILES CCN(CC)C(=O)Cc1c(nn2c(C)cc(C)nc12)-c1ccc(OCc2ccccc2F)cc1 Show InChI InChI=1S/C27H29FN4O2/c1-5-31(6-2)25(33)16-23-26(30-32-19(4)15-18(3)29-27(23)32)20-11-13-22(14-12-20)34-17-21-9-7-8-10-24(21)28/h7-15H,5-6,16-17H2,1-4H3 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.310 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Stanford University School of Medicine
Curated by ChEMBL
| Assay Description
Displacement of [3H]PK11195 from TSPO in Wistar rat kidney mitochondrial fractions incubated for 90 mins by liquid scintillation counting method |
Eur J Med Chem 93: 392-400 (2015)
Article DOI: 10.1016/j.ejmech.2015.02.004
BindingDB Entry DOI: 10.7270/Q2G73GF4 |
More data for this
Ligand-Target Pair | |
Translocator protein
(Rattus norvegicus (rat)) | BDBM50073597

(CHEMBL3408968)Show SMILES CCN(CC)C(=O)Cc1c(nn2c(C)cc(C)nc12)-c1ccc(OCc2cccc(F)c2)cc1 Show InChI InChI=1S/C27H29FN4O2/c1-5-31(6-2)25(33)16-24-26(30-32-19(4)14-18(3)29-27(24)32)21-10-12-23(13-11-21)34-17-20-8-7-9-22(28)15-20/h7-15H,5-6,16-17H2,1-4H3 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.470 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Stanford University School of Medicine
Curated by ChEMBL
| Assay Description
Displacement of [3H]PK11195 from TSPO in Wistar rat kidney mitochondrial fractions incubated for 90 mins by liquid scintillation counting method |
Eur J Med Chem 93: 392-400 (2015)
Article DOI: 10.1016/j.ejmech.2015.02.004
BindingDB Entry DOI: 10.7270/Q2G73GF4 |
More data for this
Ligand-Target Pair | |
Translocator protein
(Rattus norvegicus (rat)) | BDBM50073600
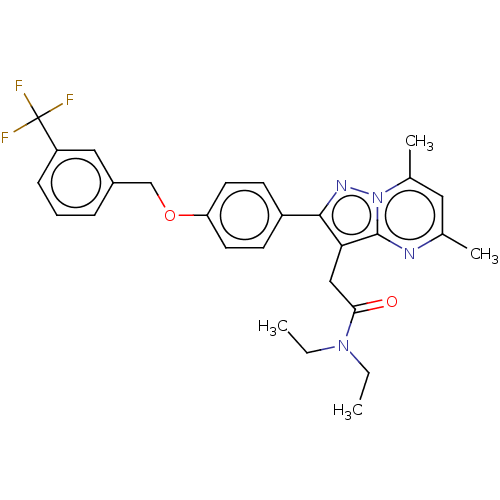
(CHEMBL3408971)Show SMILES CCN(CC)C(=O)Cc1c(nn2c(C)cc(C)nc12)-c1ccc(OCc2cccc(c2)C(F)(F)F)cc1 Show InChI InChI=1S/C28H29F3N4O2/c1-5-34(6-2)25(36)16-24-26(33-35-19(4)14-18(3)32-27(24)35)21-10-12-23(13-11-21)37-17-20-8-7-9-22(15-20)28(29,30)31/h7-15H,5-6,16-17H2,1-4H3 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.670 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Stanford University School of Medicine
Curated by ChEMBL
| Assay Description
Displacement of [3H]PK11195 from TSPO in Wistar rat kidney mitochondrial fractions incubated for 90 mins by liquid scintillation counting method |
Eur J Med Chem 93: 392-400 (2015)
Article DOI: 10.1016/j.ejmech.2015.02.004
BindingDB Entry DOI: 10.7270/Q2G73GF4 |
More data for this
Ligand-Target Pair | |
Translocator protein
(Rattus norvegicus (rat)) | BDBM50229594
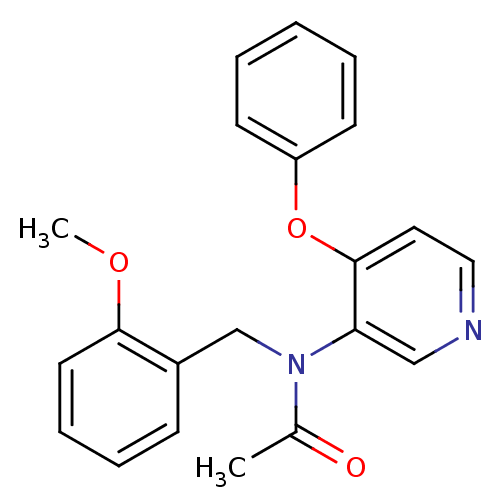
(CHEMBL253597 | N-(2-methoxybenzyl)-N-(4-phenoxypyr...)Show InChI InChI=1S/C21H20N2O3/c1-16(24)23(15-17-8-6-7-11-20(17)25-2)19-14-22-13-12-21(19)26-18-9-4-3-5-10-18/h3-14H,15H2,1-2H3 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Stanford University School of Medicine
Curated by ChEMBL
| Assay Description
Inhibition of rat TSPO |
Eur J Med Chem 93: 392-400 (2015)
Article DOI: 10.1016/j.ejmech.2015.02.004
BindingDB Entry DOI: 10.7270/Q2G73GF4 |
More data for this
Ligand-Target Pair | |
Translocator protein
(Rattus norvegicus (rat)) | BDBM50073598
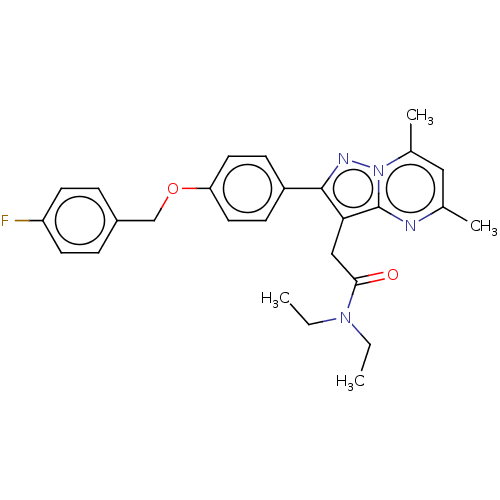
(CHEMBL3408969)Show SMILES CCN(CC)C(=O)Cc1c(nn2c(C)cc(C)nc12)-c1ccc(OCc2ccc(F)cc2)cc1 Show InChI InChI=1S/C27H29FN4O2/c1-5-31(6-2)25(33)16-24-26(30-32-19(4)15-18(3)29-27(24)32)21-9-13-23(14-10-21)34-17-20-7-11-22(28)12-8-20/h7-15H,5-6,16-17H2,1-4H3 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.770 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Stanford University School of Medicine
Curated by ChEMBL
| Assay Description
Displacement of [3H]PK11195 from TSPO in Wistar rat kidney mitochondrial fractions incubated for 90 mins by liquid scintillation counting method |
Eur J Med Chem 93: 392-400 (2015)
Article DOI: 10.1016/j.ejmech.2015.02.004
BindingDB Entry DOI: 10.7270/Q2G73GF4 |
More data for this
Ligand-Target Pair | |
Translocator protein
(Rattus norvegicus (rat)) | BDBM50073591
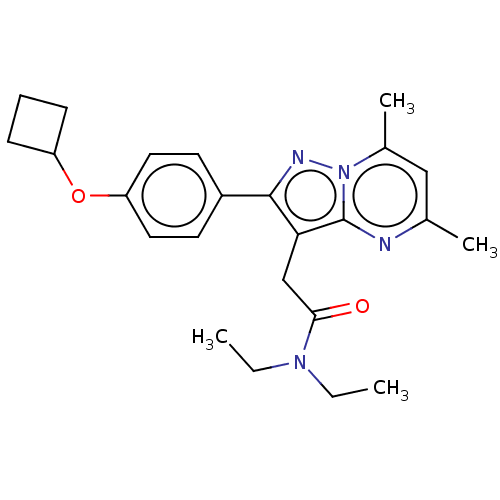
(CHEMBL3408960)Show SMILES CCN(CC)C(=O)Cc1c(nn2c(C)cc(C)nc12)-c1ccc(OC2CCC2)cc1 Show InChI InChI=1S/C24H30N4O2/c1-5-27(6-2)22(29)15-21-23(26-28-17(4)14-16(3)25-24(21)28)18-10-12-20(13-11-18)30-19-8-7-9-19/h10-14,19H,5-9,15H2,1-4H3 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.860 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Stanford University School of Medicine
Curated by ChEMBL
| Assay Description
Displacement of [3H]PK11195 from TSPO in Wistar rat kidney mitochondrial fractions incubated for 90 mins by liquid scintillation counting method |
Eur J Med Chem 93: 392-400 (2015)
Article DOI: 10.1016/j.ejmech.2015.02.004
BindingDB Entry DOI: 10.7270/Q2G73GF4 |
More data for this
Ligand-Target Pair | |
Translocator protein
(Homo sapiens (Human)) | BDBM50073588
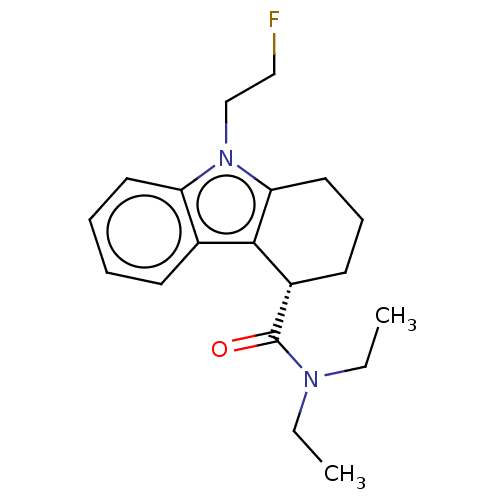
(CHEMBL3408974)Show InChI InChI=1S/C19H25FN2O/c1-3-21(4-2)19(23)15-9-7-11-17-18(15)14-8-5-6-10-16(14)22(17)13-12-20/h5-6,8,10,15H,3-4,7,9,11-13H2,1-2H3/t15-/m0/s1 | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.870 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Stanford University School of Medicine
Curated by ChEMBL
| Assay Description
Inhibition of TSPO (unknown origin) |
Eur J Med Chem 93: 392-400 (2015)
Article DOI: 10.1016/j.ejmech.2015.02.004
BindingDB Entry DOI: 10.7270/Q2G73GF4 |
More data for this
Ligand-Target Pair | |
Translocator protein
(Rattus norvegicus (rat)) | BDBM50073595
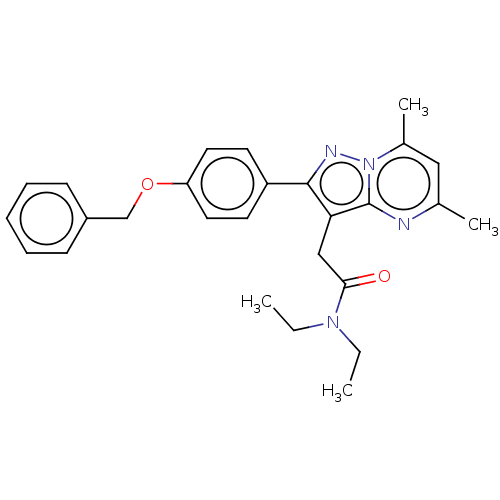
(CHEMBL3408966)Show SMILES CCN(CC)C(=O)Cc1c(nn2c(C)cc(C)nc12)-c1ccc(OCc2ccccc2)cc1 Show InChI InChI=1S/C27H30N4O2/c1-5-30(6-2)25(32)17-24-26(29-31-20(4)16-19(3)28-27(24)31)22-12-14-23(15-13-22)33-18-21-10-8-7-9-11-21/h7-16H,5-6,17-18H2,1-4H3 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.990 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Stanford University School of Medicine
Curated by ChEMBL
| Assay Description
Displacement of [3H]PK11195 from TSPO in Wistar rat kidney mitochondrial fractions incubated for 90 mins by liquid scintillation counting method |
Eur J Med Chem 93: 392-400 (2015)
Article DOI: 10.1016/j.ejmech.2015.02.004
BindingDB Entry DOI: 10.7270/Q2G73GF4 |
More data for this
Ligand-Target Pair | |
Translocator protein
(Rattus norvegicus (rat)) | BDBM50073603

(CHEMBL3408963)Show SMILES CCN(CC)C(=O)Cc1c(nn2c(C)cc(C)nc12)-c1ccc(OCC2CCC2)cc1 Show InChI InChI=1S/C25H32N4O2/c1-5-28(6-2)23(30)15-22-24(27-29-18(4)14-17(3)26-25(22)29)20-10-12-21(13-11-20)31-16-19-8-7-9-19/h10-14,19H,5-9,15-16H2,1-4H3 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Stanford University School of Medicine
Curated by ChEMBL
| Assay Description
Displacement of [3H]PK11195 from TSPO in Wistar rat kidney mitochondrial fractions incubated for 90 mins by liquid scintillation counting method |
Eur J Med Chem 93: 392-400 (2015)
Article DOI: 10.1016/j.ejmech.2015.02.004
BindingDB Entry DOI: 10.7270/Q2G73GF4 |
More data for this
Ligand-Target Pair | |
Translocator protein
(Rattus norvegicus (rat)) | BDBM50073599
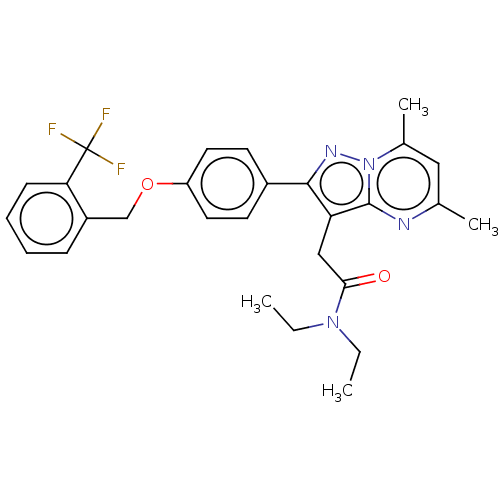
(CHEMBL3408970)Show SMILES CCN(CC)C(=O)Cc1c(nn2c(C)cc(C)nc12)-c1ccc(OCc2ccccc2C(F)(F)F)cc1 Show InChI InChI=1S/C28H29F3N4O2/c1-5-34(6-2)25(36)16-23-26(33-35-19(4)15-18(3)32-27(23)35)20-11-13-22(14-12-20)37-17-21-9-7-8-10-24(21)28(29,30)31/h7-15H,5-6,16-17H2,1-4H3 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Stanford University School of Medicine
Curated by ChEMBL
| Assay Description
Displacement of [3H]PK11195 from TSPO in Wistar rat kidney mitochondrial fractions incubated for 90 mins by liquid scintillation counting method |
Eur J Med Chem 93: 392-400 (2015)
Article DOI: 10.1016/j.ejmech.2015.02.004
BindingDB Entry DOI: 10.7270/Q2G73GF4 |
More data for this
Ligand-Target Pair | |
Translocator protein
(Rattus norvegicus (rat)) | BDBM50073592
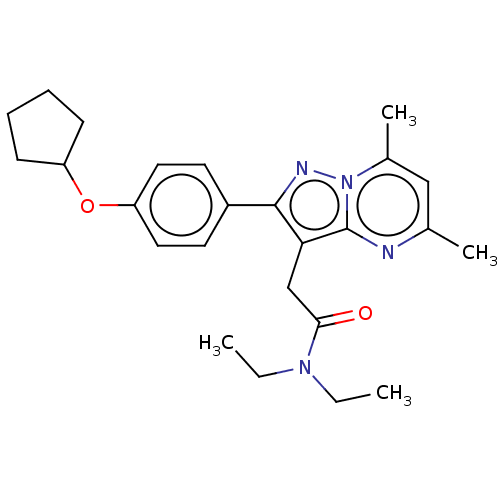
(CHEMBL3408961)Show SMILES CCN(CC)C(=O)Cc1c(nn2c(C)cc(C)nc12)-c1ccc(OC2CCCC2)cc1 Show InChI InChI=1S/C25H32N4O2/c1-5-28(6-2)23(30)16-22-24(27-29-18(4)15-17(3)26-25(22)29)19-11-13-21(14-12-19)31-20-9-7-8-10-20/h11-15,20H,5-10,16H2,1-4H3 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Stanford University School of Medicine
Curated by ChEMBL
| Assay Description
Displacement of [3H]PK11195 from TSPO in Wistar rat kidney mitochondrial fractions incubated for 90 mins by liquid scintillation counting method |
Eur J Med Chem 93: 392-400 (2015)
Article DOI: 10.1016/j.ejmech.2015.02.004
BindingDB Entry DOI: 10.7270/Q2G73GF4 |
More data for this
Ligand-Target Pair | |
Translocator protein
(Rattus norvegicus (rat)) | BDBM50073601

(CHEMBL3408972)Show SMILES CCN(CC)C(=O)Cc1c(nn2c(C)cc(C)nc12)-c1ccc(OCc2ccc(cc2)C(F)(F)F)cc1 Show InChI InChI=1S/C28H29F3N4O2/c1-5-34(6-2)25(36)16-24-26(33-35-19(4)15-18(3)32-27(24)35)21-9-13-23(14-10-21)37-17-20-7-11-22(12-8-20)28(29,30)31/h7-15H,5-6,16-17H2,1-4H3 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Stanford University School of Medicine
Curated by ChEMBL
| Assay Description
Displacement of [3H]PK11195 from TSPO in Wistar rat kidney mitochondrial fractions incubated for 90 mins by liquid scintillation counting method |
Eur J Med Chem 93: 392-400 (2015)
Article DOI: 10.1016/j.ejmech.2015.02.004
BindingDB Entry DOI: 10.7270/Q2G73GF4 |
More data for this
Ligand-Target Pair | |
Translocator protein
(Rattus norvegicus (rat)) | BDBM50073604

(CHEMBL3408964)Show SMILES CCN(CC)C(=O)Cc1c(nn2c(C)cc(C)nc12)-c1ccc(OCC2CCCC2)cc1 Show InChI InChI=1S/C26H34N4O2/c1-5-29(6-2)24(31)16-23-25(28-30-19(4)15-18(3)27-26(23)30)21-11-13-22(14-12-21)32-17-20-9-7-8-10-20/h11-15,20H,5-10,16-17H2,1-4H3 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Stanford University School of Medicine
Curated by ChEMBL
| Assay Description
Displacement of [3H]PK11195 from TSPO in Wistar rat kidney mitochondrial fractions incubated for 90 mins by liquid scintillation counting method |
Eur J Med Chem 93: 392-400 (2015)
Article DOI: 10.1016/j.ejmech.2015.02.004
BindingDB Entry DOI: 10.7270/Q2G73GF4 |
More data for this
Ligand-Target Pair | |
Translocator protein
(Rattus norvegicus (rat)) | BDBM50073589
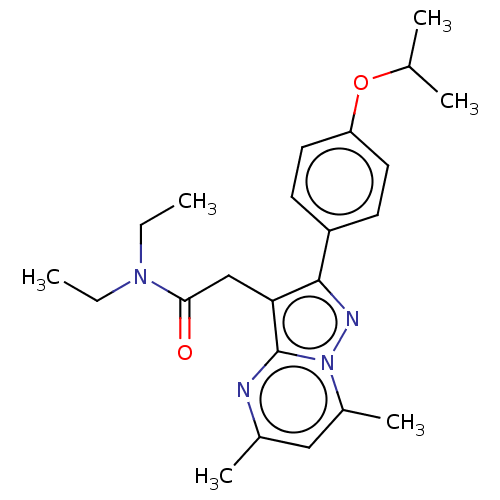
(CHEMBL3408958)Show SMILES CCN(CC)C(=O)Cc1c(nn2c(C)cc(C)nc12)-c1ccc(OC(C)C)cc1 Show InChI InChI=1S/C23H30N4O2/c1-7-26(8-2)21(28)14-20-22(18-9-11-19(12-10-18)29-15(3)4)25-27-17(6)13-16(5)24-23(20)27/h9-13,15H,7-8,14H2,1-6H3 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Stanford University School of Medicine
Curated by ChEMBL
| Assay Description
Displacement of [3H]PK11195 from TSPO in Wistar rat kidney mitochondrial fractions incubated for 90 mins by liquid scintillation counting method |
Eur J Med Chem 93: 392-400 (2015)
Article DOI: 10.1016/j.ejmech.2015.02.004
BindingDB Entry DOI: 10.7270/Q2G73GF4 |
More data for this
Ligand-Target Pair | |
Translocator protein
(Rattus norvegicus (rat)) | BDBM50073590

(CHEMBL3408959)Show SMILES CCN(CC)C(=O)Cc1c(nn2c(C)cc(C)nc12)-c1ccc(OCC(C)C)cc1 Show InChI InChI=1S/C24H32N4O2/c1-7-27(8-2)22(29)14-21-23(26-28-18(6)13-17(5)25-24(21)28)19-9-11-20(12-10-19)30-15-16(3)4/h9-13,16H,7-8,14-15H2,1-6H3 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 2.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Stanford University School of Medicine
Curated by ChEMBL
| Assay Description
Displacement of [3H]PK11195 from TSPO in Wistar rat kidney mitochondrial fractions incubated for 90 mins by liquid scintillation counting method |
Eur J Med Chem 93: 392-400 (2015)
Article DOI: 10.1016/j.ejmech.2015.02.004
BindingDB Entry DOI: 10.7270/Q2G73GF4 |
More data for this
Ligand-Target Pair | |
Translocator protein
(Homo sapiens (Human)) | BDBM50229594

(CHEMBL253597 | N-(2-methoxybenzyl)-N-(4-phenoxypyr...)Show InChI InChI=1S/C21H20N2O3/c1-16(24)23(15-17-8-6-7-11-20(17)25-2)19-14-22-13-12-21(19)26-18-9-4-3-5-10-18/h3-14H,15H2,1-2H3 | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 2.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Stanford University School of Medicine
Curated by ChEMBL
| Assay Description
Inhibition of human TSPO |
Eur J Med Chem 93: 392-400 (2015)
Article DOI: 10.1016/j.ejmech.2015.02.004
BindingDB Entry DOI: 10.7270/Q2G73GF4 |
More data for this
Ligand-Target Pair | |
Translocator protein
(Rattus norvegicus (rat)) | BDBM22032
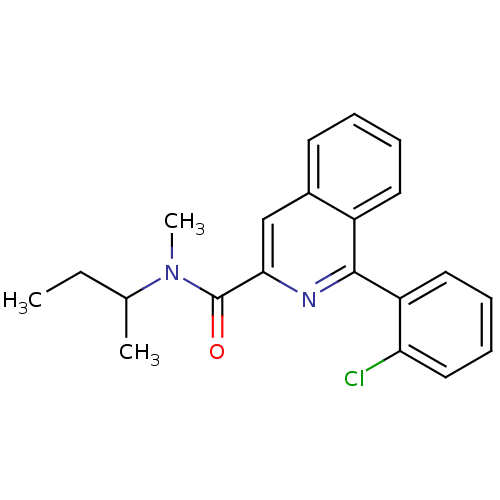
(1-(2-chlorophenyl)-N-methyl-N-(1-methylpropyl)isoq...)Show InChI InChI=1S/C21H21ClN2O/c1-4-14(2)24(3)21(25)19-13-15-9-5-6-10-16(15)20(23-19)17-11-7-8-12-18(17)22/h5-14H,4H2,1-3H3 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Stanford University School of Medicine
Curated by ChEMBL
| Assay Description
Inhibition of rat TSPO |
Eur J Med Chem 93: 392-400 (2015)
Article DOI: 10.1016/j.ejmech.2015.02.004
BindingDB Entry DOI: 10.7270/Q2G73GF4 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Translocator protein
(Rattus norvegicus (rat)) | BDBM50073593

(CHEMBL3408962)Show SMILES CCN(CC)C(=O)Cc1c(nn2c(C)cc(C)nc12)-c1ccc(OCC2CC2)cc1 Show InChI InChI=1S/C24H30N4O2/c1-5-27(6-2)22(29)14-21-23(26-28-17(4)13-16(3)25-24(21)28)19-9-11-20(12-10-19)30-15-18-7-8-18/h9-13,18H,5-8,14-15H2,1-4H3 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Stanford University School of Medicine
Curated by ChEMBL
| Assay Description
Displacement of [3H]PK11195 from TSPO in Wistar rat kidney mitochondrial fractions incubated for 90 mins by liquid scintillation counting method |
Eur J Med Chem 93: 392-400 (2015)
Article DOI: 10.1016/j.ejmech.2015.02.004
BindingDB Entry DOI: 10.7270/Q2G73GF4 |
More data for this
Ligand-Target Pair | |
Translocator protein
(Rattus norvegicus (rat)) | BDBM50073594
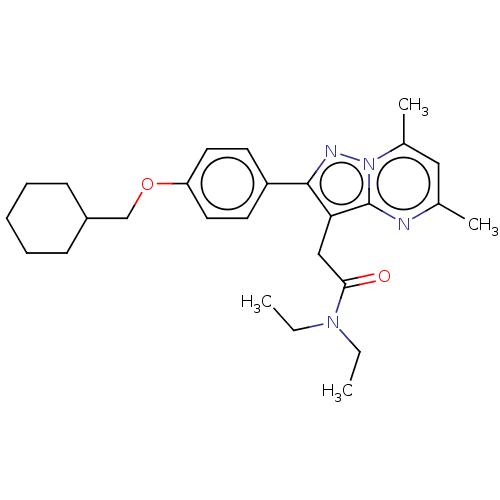
(CHEMBL3408965)Show SMILES CCN(CC)C(=O)Cc1c(nn2c(C)cc(C)nc12)-c1ccc(OCC2CCCCC2)cc1 Show InChI InChI=1S/C27H36N4O2/c1-5-30(6-2)25(32)17-24-26(29-31-20(4)16-19(3)28-27(24)31)22-12-14-23(15-13-22)33-18-21-10-8-7-9-11-21/h12-16,21H,5-11,17-18H2,1-4H3 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Stanford University School of Medicine
Curated by ChEMBL
| Assay Description
Displacement of [3H]PK11195 from TSPO in Wistar rat kidney mitochondrial fractions incubated for 90 mins by liquid scintillation counting method |
Eur J Med Chem 93: 392-400 (2015)
Article DOI: 10.1016/j.ejmech.2015.02.004
BindingDB Entry DOI: 10.7270/Q2G73GF4 |
More data for this
Ligand-Target Pair | |
Translocator protein
(Homo sapiens (Human)) | BDBM22032

(1-(2-chlorophenyl)-N-methyl-N-(1-methylpropyl)isoq...)Show InChI InChI=1S/C21H21ClN2O/c1-4-14(2)24(3)21(25)19-13-15-9-5-6-10-16(15)20(23-19)17-11-7-8-12-18(17)22/h5-14H,4H2,1-3H3 | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 4.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Stanford University School of Medicine
Curated by ChEMBL
| Assay Description
Inhibition of human TSPO |
Eur J Med Chem 93: 392-400 (2015)
Article DOI: 10.1016/j.ejmech.2015.02.004
BindingDB Entry DOI: 10.7270/Q2G73GF4 |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor/Kappa-type opioid receptor/Mu-type opioid receptor/Sigma non-opioid intracellular receptor 1
(Rattus norvegicus (rat)-RAT) | BDBM50222203

(CHEMBL159427)Show InChI InChI=1S/C22H35NO2/c1-5-7-8-13-23-14-11-22(12-15-23)19-16-17(24)9-10-18(19)21(3,4)25-20(22)6-2/h9-10,16,20,24H,5-8,11-15H2,1-4H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Tested for Opioid receptors affinity determined by the capacity to displace bound, radiolabeled dihydromorphine from rat brain homogenates. |
J Med Chem 23: 688-90 (1980)
BindingDB Entry DOI: 10.7270/Q2J968KW |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor/Kappa-type opioid receptor/Mu-type opioid receptor/Sigma non-opioid intracellular receptor 1
(Rattus norvegicus (rat)-RAT) | BDBM50222204

(CHEMBL161463)Show InChI InChI=1S/C23H37NO2/c1-5-8-9-14-24-15-12-23(13-16-24)20-17-18(25)10-11-19(20)22(4,7-3)26-21(23)6-2/h10-11,17,21,25H,5-9,12-16H2,1-4H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Tested for Opioid receptors affinity determined by the capacity to displace bound, radiolabeled dihydromorphine from rat brain homogenates. |
J Med Chem 23: 688-90 (1980)
BindingDB Entry DOI: 10.7270/Q2J968KW |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor/Kappa-type opioid receptor/Mu-type opioid receptor/Sigma non-opioid intracellular receptor 1
(Rattus norvegicus (rat)-RAT) | BDBM50222206

(CHEMBL161330)Show InChI InChI=1S/C20H29NO2/c1-5-11-21-12-9-20(10-13-21)17-14-15(22)7-8-16(17)19(3,4)23-18(20)6-2/h5,7-8,14,18,22H,1,6,9-13H2,2-4H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Tested for Opioid receptors affinity determined by the capacity to displace bound, radiolabeled dihydromorphine from rat brain homogenates. |
J Med Chem 23: 688-90 (1980)
BindingDB Entry DOI: 10.7270/Q2J968KW |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor/Kappa-type opioid receptor/Mu-type opioid receptor/Sigma non-opioid intracellular receptor 1
(Rattus norvegicus (rat)-RAT) | BDBM50222207

(CHEMBL159934)Show InChI InChI=1S/C18H27NO2/c1-5-16-18(8-10-19(4)11-9-18)15-12-13(20)6-7-14(15)17(2,3)21-16/h6-7,12,16,20H,5,8-11H2,1-4H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Tested for Opioid receptors affinity determined by the capacity to displace bound, radiolabeled dihydromorphine from rat brain homogenates. |
J Med Chem 23: 688-90 (1980)
BindingDB Entry DOI: 10.7270/Q2J968KW |
More data for this
Ligand-Target Pair | |
Interleukin-1 receptor-associated kinase 4
(Homo sapiens (Human)) | BDBM50612201
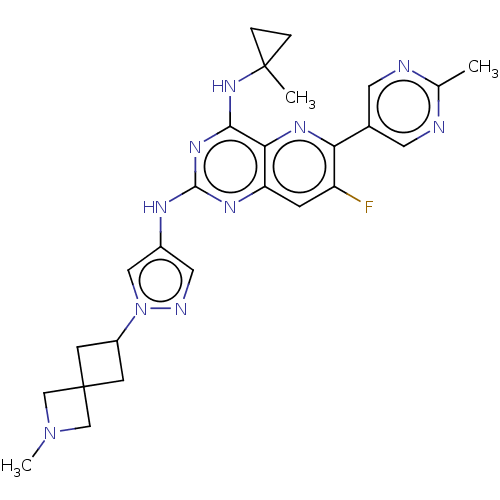
(CHEMBL5270030) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Interleukin-1 receptor-associated kinase 4
(Homo sapiens (Human)) | BDBM50612188

(CHEMBL5283628) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Interleukin-1 receptor-associated kinase 4
(Homo sapiens (Human)) | BDBM50612189

(CHEMBL5281595) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Interleukin-1 receptor-associated kinase 4
(Homo sapiens (Human)) | BDBM50612187
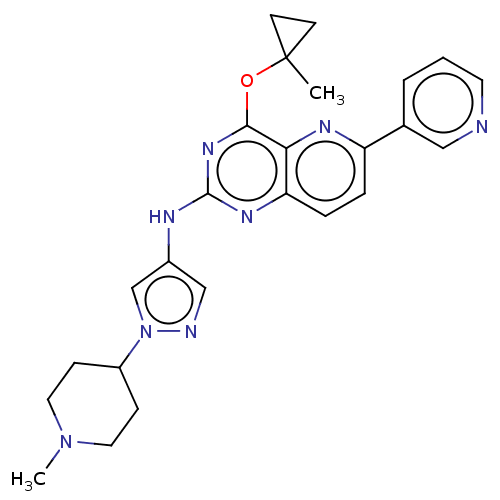
(CHEMBL5288696) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Interleukin-1 receptor-associated kinase 4
(Homo sapiens (Human)) | BDBM50612193

(CHEMBL5290090) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Mer
(Homo sapiens (Human)) | BDBM50573136

(CHEMBL4875486)Show SMILES CN(Cc1ccc2nccn2c1)c1nnc(o1)-c1ccc(cc1C)-c1c(C)nn(C)c1C#N | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of MER (unknown origin) by LC-MS analysis |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00920
BindingDB Entry DOI: 10.7270/Q2S18686 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Interleukin-1 receptor-associated kinase 4
(Homo sapiens (Human)) | BDBM50612191

(CHEMBL5273100) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| | n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Interleukin-1 receptor-associated kinase 4
(Homo sapiens (Human)) | BDBM50612202

(CHEMBL5274954) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Interleukin-1 receptor-associated kinase 4
(Homo sapiens (Human)) | BDBM50612181

(CHEMBL5281524) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Interleukin-1 receptor-associated kinase 4
(Homo sapiens (Human)) | BDBM50593685
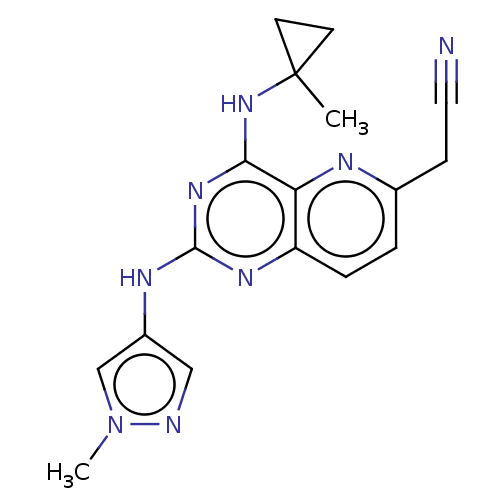
(CHEMBL5179237) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Tyrosine-protein kinase Mer
(Homo sapiens (Human)) | BDBM50573135

(CHEMBL4873217)Show SMILES CN(Cc1ccc2nccn2c1)c1nnc(o1)-c1ccc(cc1C)-c1cnn(C)c1C | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of MER (unknown origin) by LC-MS analysis |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00920
BindingDB Entry DOI: 10.7270/Q2S18686 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Interleukin-1 receptor-associated kinase 1
(Homo sapiens (Human)) | BDBM50612188

(CHEMBL5283628) | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
P2X purinoceptor 7
(Homo sapiens (Human)) | BDBM50237730
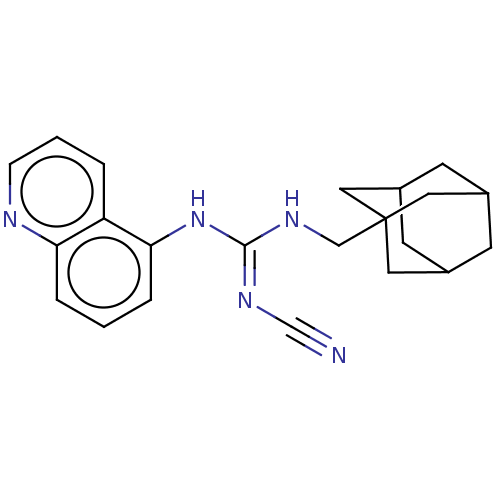
(CHEMBL4074449)Show SMILES N#C\N=C(/NCC12CC3CC(CC(C3)C1)C2)Nc1cccc2ncccc12 |TLB:5:6:9.8.13:11,THB:7:8:11:15.6.14,7:6:9.8.13:11,14:6:9:13.12.11,14:12:9:15.7.6| Show InChI InChI=1S/C22H25N5/c23-14-26-21(27-20-5-1-4-19-18(20)3-2-6-24-19)25-13-22-10-15-7-16(11-22)9-17(8-15)12-22/h1-6,15-17H,7-13H2,(H2,25,26,27) | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Sydney
Curated by ChEMBL
| Assay Description
Inhibition of [3H]-Ro- 15-1788 binding to human GABA-A receptor alpha-5-beta-3-gamma-2 subunits |
Eur J Med Chem 130: 433-439 (2017)
Article DOI: 10.1016/j.ejmech.2017.02.060
BindingDB Entry DOI: 10.7270/Q2SJ1NW5 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Mer
(Homo sapiens (Human)) | BDBM50573128
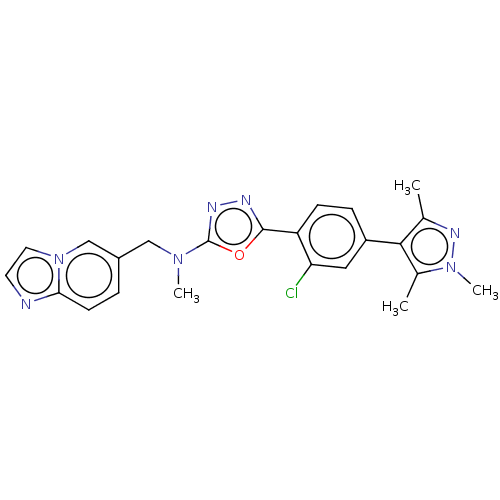
(CHEMBL4865752)Show SMILES CN(Cc1ccc2nccn2c1)c1nnc(o1)-c1ccc(cc1Cl)-c1c(C)nn(C)c1C | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of MER (unknown origin) by LC-MS analysis |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00920
BindingDB Entry DOI: 10.7270/Q2S18686 |
More data for this
Ligand-Target Pair | |
Protein kinase C alpha type
(Rattus norvegicus (Rat)) | BDBM50282632
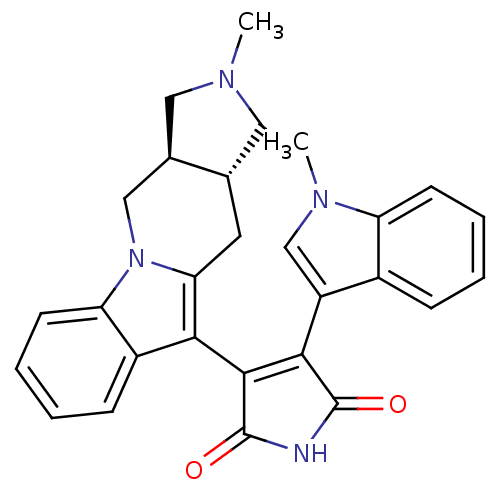
(3-((3aR,10aR)-2-Methyl-2,3,3a,4,10,10a-hexahydro-1...)Show SMILES CN1C[C@@H]2Cc3c(C4=C(C(=O)NC4=O)c4cn(C)c5ccccc45)c4ccccc4n3C[C@H]2C1 |r,t:7| Show InChI InChI=1S/C28H26N4O2/c1-30-12-16-11-23-24(19-8-4-6-10-22(19)32(23)14-17(16)13-30)26-25(27(33)29-28(26)34)20-15-31(2)21-9-5-3-7-18(20)21/h3-10,15-17H,11-14H2,1-2H3,(H,29,33,34)/t16-,17+/m0/s1 | PDB
MMDB
Reactome pathway
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 2.85 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of Protein kinase C alpha |
Bioorg Med Chem Lett 4: 1303-1308 (1994)
Article DOI: 10.1016/S0960-894X(01)80349-8
BindingDB Entry DOI: 10.7270/Q27H1JJ4 |
More data for this
Ligand-Target Pair | |
Interleukin-1 receptor-associated kinase 4
(Homo sapiens (Human)) | BDBM50505743

(CHEMBL4582624)Show SMILES COC(=O)N1CCN(CC1)[C@H]1CC[C@@H](CC1)Nc1nc(Nc2cnn(C)c2)nc2ccc(CC#N)nc12 |r,wU:13.17,wD:10.10,(21.97,-21.58,;21.98,-20.04,;20.66,-19.26,;20.68,-17.72,;19.32,-20.02,;19.31,-21.56,;17.97,-22.32,;16.64,-21.54,;16.65,-19.99,;17.99,-19.23,;15.3,-22.29,;13.96,-21.52,;12.62,-22.28,;12.62,-23.82,;13.95,-24.6,;15.29,-23.83,;11.28,-24.59,;11.28,-26.13,;12.62,-26.91,;12.62,-28.45,;13.95,-29.22,;15.28,-28.46,;15.43,-26.92,;16.94,-26.61,;17.7,-27.94,;19.24,-27.93,;16.67,-29.08,;11.28,-29.22,;9.95,-28.45,;8.63,-29.24,;7.29,-28.48,;7.27,-26.93,;5.93,-26.19,;5.91,-24.65,;5.88,-23.11,;8.6,-26.15,;9.94,-26.91,)| Show InChI InChI=1S/C25H32N10O2/c1-33-16-19(15-27-33)30-24-31-21-8-5-18(9-10-26)28-22(21)23(32-24)29-17-3-6-20(7-4-17)34-11-13-35(14-12-34)25(36)37-2/h5,8,15-17,20H,3-4,6-7,9,11-14H2,1-2H3,(H2,29,30,31,32)/t17-,20- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| PDB
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
GTPase KRas
(Homo sapiens (Human)) | BDBM50605537

(CHEMBL5183988)Show SMILES [H][C@]12CN([C@H](C)CN1c1ncnc3c(F)c(c(Cl)c(OCC2)c13)-c1c(O)cccc1F)C(=O)C=C |r,wD:4.4,1.0,(2.77,3.02,;2.46,1.44,;3.89,1.99,;5.09,1.02,;4.85,-.5,;6.05,-1.47,;3.42,-1.05,;2.22,-.09,;1.01,-1.04,;1.67,-2.43,;.79,-3.65,;-.74,-3.57,;-1.4,-2.18,;-2.94,-2.1,;-3.81,-3.32,;-3.6,-.67,;-2.72,.6,;-3.38,1.99,;-1.19,.52,;-1.24,2.3,;.1,3.07,;1.6,2.71,;-.53,-.96,;-5.14,-.59,;-6.01,-1.81,;-5.35,-3.2,;-7.55,-1.73,;-8.2,-.29,;-7.33,.93,;-5.79,.85,;-4.92,2.12,;6.53,1.58,;7.73,.61,;6.77,3.1,;8.2,3.65,)| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00369
BindingDB Entry DOI: 10.7270/Q2W09B1P |
More data for this
Ligand-Target Pair | |
Interleukin-1 receptor-associated kinase 4
(Homo sapiens (Human)) | BDBM50612180

(CHEMBL5274369) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Interleukin-1 receptor-associated kinase 4
(Homo sapiens (Human)) | BDBM50612200
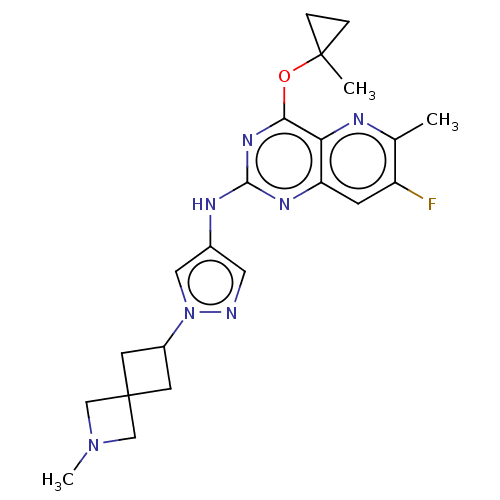
(CHEMBL5286869) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Interleukin-1 receptor-associated kinase 4
(Homo sapiens (Human)) | BDBM50612199

(CHEMBL5285779) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Interleukin-1 receptor-associated kinase 4
(Homo sapiens (Human)) | BDBM50612198
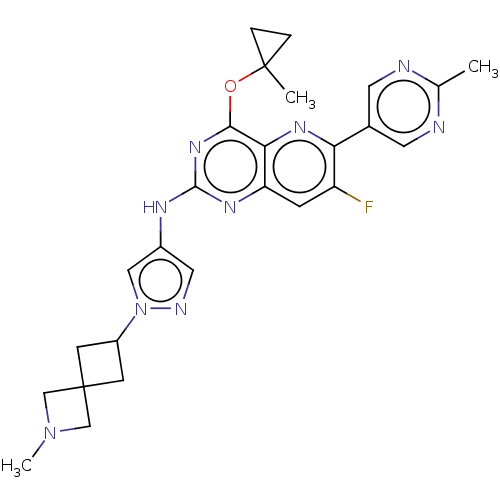
(CHEMBL5270903) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PDB
UniChem
| PDB
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Interleukin-1 receptor-associated kinase 4
(Homo sapiens (Human)) | BDBM50612195

(CHEMBL5288931) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PDB
UniChem
| PDB
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Interleukin-1 receptor-associated kinase 4
(Homo sapiens (Human)) | BDBM50612183
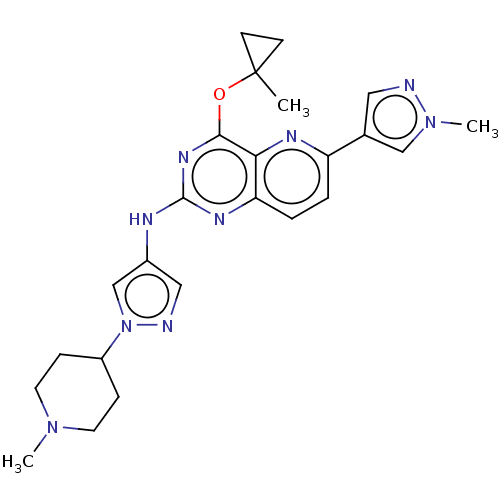
(CHEMBL5266181) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PDB
UniChem
| PDB
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Interleukin-1 receptor-associated kinase 4
(Homo sapiens (Human)) | BDBM50612179

(CHEMBL5272567) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data