 Found 1739 hits with Last Name = 'willson' and Initial = 'tm'
Found 1739 hits with Last Name = 'willson' and Initial = 'tm' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Gastrin/cholecystokinin type B receptor
(Homo sapiens (Human)) | BDBM21147

((3S)-3-[(2S)-2-[(2S)-2-{2-[(2S)-2-[(2S)-2-[(3S)-3-...)Show SMILES CSCC[C@H](NC(=O)[C@H](Cc1ccc(OS(O)(=O)=O)cc1)NC(=O)[C@@H](N)CC(O)=O)C(=O)NCC(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)N[C@@H](CCSC)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](Cc1ccccc1)C(N)=O Show InChI InChI=1S/C49H62N10O16S3/c1-76-18-16-34(55-47(69)37(58-44(66)32(50)23-41(61)62)21-28-12-14-30(15-13-28)75-78(72,73)74)45(67)53-26-40(60)54-38(22-29-25-52-33-11-7-6-10-31(29)33)48(70)56-35(17-19-77-2)46(68)59-39(24-42(63)64)49(71)57-36(43(51)65)20-27-8-4-3-5-9-27/h3-15,25,32,34-39,52H,16-24,26,50H2,1-2H3,(H2,51,65)(H,53,67)(H,54,60)(H,55,69)(H,56,70)(H,57,71)(H,58,66)(H,59,68)(H,61,62)(H,63,64)(H,72,73,74)/t32-,34-,35-,36-,37-,38-,39-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.350 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Research and Development
Curated by PDSP Ki Database
| |
J Med Chem 40: 2706-25 (1997)
Article DOI: 10.1021/jm970265x
BindingDB Entry DOI: 10.7270/Q27M06F9 |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50085044

((S)-2-(2-Benzoyl-phenylamino)-3-{4-[2-(5-methyl-2-...)Show SMILES Cc1oc(nc1CCOc1ccc(C[C@H](Nc2ccccc2C(=O)c2ccccc2)C(O)=O)cc1)-c1ccccc1 Show InChI InChI=1S/C34H30N2O5/c1-23-29(36-33(41-23)26-12-6-3-7-13-26)20-21-40-27-18-16-24(17-19-27)22-31(34(38)39)35-30-15-9-8-14-28(30)32(37)25-10-4-2-5-11-25/h2-19,31,35H,20-22H2,1H3,(H,38,39)/t31-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
PubMed
| 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Binding affinity to human Peroxisome proliferator activated receptor gamma using scintillation proximity assay |
Bioorg Med Chem Lett 11: 3111-3 (2001)
BindingDB Entry DOI: 10.7270/Q2B27TKM |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50085044

((S)-2-(2-Benzoyl-phenylamino)-3-{4-[2-(5-methyl-2-...)Show SMILES Cc1oc(nc1CCOc1ccc(C[C@H](Nc2ccccc2C(=O)c2ccccc2)C(O)=O)cc1)-c1ccccc1 Show InChI InChI=1S/C34H30N2O5/c1-23-29(36-33(41-23)26-12-6-3-7-13-26)20-21-40-27-18-16-24(17-19-27)22-31(34(38)39)35-30-15-9-8-14-28(30)32(37)25-10-4-2-5-11-25/h2-19,31,35H,20-22H2,1H3,(H,38,39)/t31-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Research and Development
Curated by ChEMBL
| Assay Description
In vitro binding to peroxisome proliferator activated receptor gamma (PPAR gamma) using [3H]-BRL 49653 as radioligand in scintillation proximity assa... |
J Med Chem 41: 5020-36 (1999)
Checked by Author
Article DOI: 10.1021/jm9804127
BindingDB Entry DOI: 10.7270/Q20K2B28 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Gastrin/cholecystokinin type B receptor
(Homo sapiens (Human)) | BDBM21147

((3S)-3-[(2S)-2-[(2S)-2-{2-[(2S)-2-[(2S)-2-[(3S)-3-...)Show SMILES CSCC[C@H](NC(=O)[C@H](Cc1ccc(OS(O)(=O)=O)cc1)NC(=O)[C@@H](N)CC(O)=O)C(=O)NCC(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)N[C@@H](CCSC)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](Cc1ccccc1)C(N)=O Show InChI InChI=1S/C49H62N10O16S3/c1-76-18-16-34(55-47(69)37(58-44(66)32(50)23-41(61)62)21-28-12-14-30(15-13-28)75-78(72,73)74)45(67)53-26-40(60)54-38(22-29-25-52-33-11-7-6-10-31(29)33)48(70)56-35(17-19-77-2)46(68)59-39(24-42(63)64)49(71)57-36(43(51)65)20-27-8-4-3-5-9-27/h3-15,25,32,34-39,52H,16-24,26,50H2,1-2H3,(H2,51,65)(H,53,67)(H,54,60)(H,55,69)(H,56,70)(H,57,71)(H,58,66)(H,59,68)(H,61,62)(H,63,64)(H,72,73,74)/t32-,34-,35-,36-,37-,38-,39-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.32 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Research and Development
Curated by PDSP Ki Database
| |
J Med Chem 40: 2706-25 (1997)
Article DOI: 10.1021/jm970265x
BindingDB Entry DOI: 10.7270/Q27M06F9 |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50418564
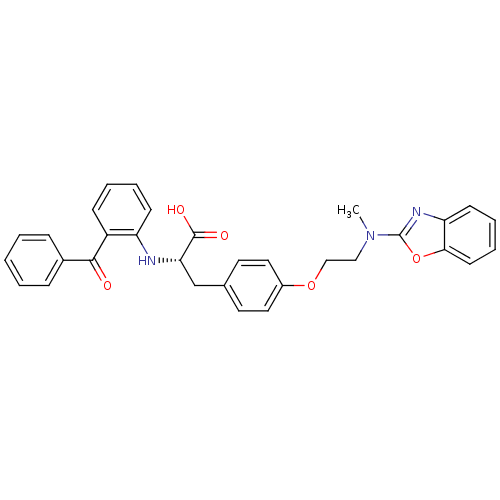
(CHEMBL423026)Show SMILES CN(CCOc1ccc(C[C@H](Nc2ccccc2C(=O)c2ccccc2)C(O)=O)cc1)c1nc2ccccc2o1 Show InChI InChI=1S/C32H29N3O5/c1-35(32-34-27-13-7-8-14-29(27)40-32)19-20-39-24-17-15-22(16-18-24)21-28(31(37)38)33-26-12-6-5-11-25(26)30(36)23-9-3-2-4-10-23/h2-18,28,33H,19-21H2,1H3,(H,37,38)/t28-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Research and Development
Curated by ChEMBL
| Assay Description
In vitro binding to peroxisome proliferator activated receptor gamma (PPAR gamma) using [3H]-BRL 49653 as radioligand in scintillation proximity assa... |
J Med Chem 41: 5020-36 (1999)
Checked by Author
Article DOI: 10.1021/jm9804127
BindingDB Entry DOI: 10.7270/Q20K2B28 |
More data for this
Ligand-Target Pair | |
Estrogen receptor
(Homo sapiens (Human)) | BDBM17292

((1S,10R,11S,14S,15S)-15-methyltetracyclo[8.7.0.0^{...)Show SMILES [H][C@@]12CC[C@H](O)[C@@]1(C)CC[C@]1([H])c3ccc(O)cc3CC[C@@]21[H] Show InChI InChI=1S/C18H24O2/c1-18-9-8-14-13-5-3-12(19)10-11(13)2-4-15(14)16(18)6-7-17(18)20/h3,5,10,14-17,19-20H,2,4,6-9H2,1H3/t14-,15-,16+,17+,18+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research & Development
Curated by ChEMBL
| Assay Description
Binding affinity for human estrogen receptor alpha |
J Med Chem 48: 2243-7 (2005)
Article DOI: 10.1021/jm040154f
BindingDB Entry DOI: 10.7270/Q24T6N42 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Estrogen receptor
(Homo sapiens (Human)) | BDBM50084948
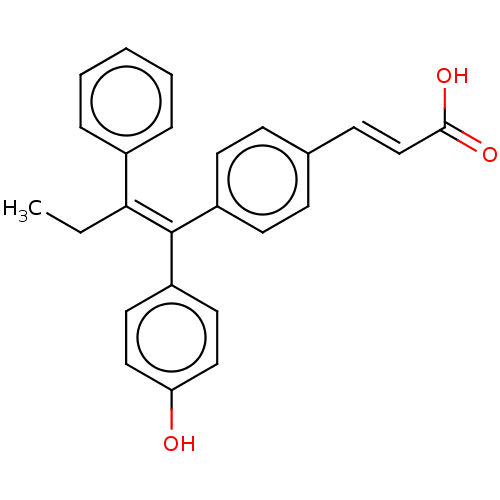
(CHEMBL195515 | GW7604)Show SMILES CC\C(=C(/c1ccc(O)cc1)c1ccc(\C=C\C(O)=O)cc1)c1ccccc1 Show InChI InChI=1S/C25H22O3/c1-2-23(19-6-4-3-5-7-19)25(21-13-15-22(26)16-14-21)20-11-8-18(9-12-20)10-17-24(27)28/h3-17,26H,2H2,1H3,(H,27,28)/b17-10+,25-23+ | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research & Development
Curated by ChEMBL
| Assay Description
Binding affinity for human estrogen receptor alpha |
J Med Chem 48: 2243-7 (2005)
Article DOI: 10.1021/jm040154f
BindingDB Entry DOI: 10.7270/Q24T6N42 |
More data for this
Ligand-Target Pair | |
Estrogen receptor
(Homo sapiens (Human)) | BDBM50276802
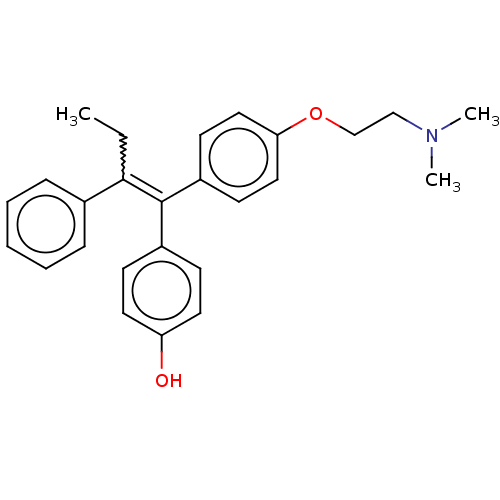
(4-OHT | Afimoxifene | TamoGel)Show SMILES CCC(=C(c1ccc(O)cc1)c1ccc(OCCN(C)C)cc1)c1ccccc1 Show InChI InChI=1S/C26H29NO2/c1-4-25(20-8-6-5-7-9-20)26(21-10-14-23(28)15-11-21)22-12-16-24(17-13-22)29-19-18-27(2)3/h5-17,28H,4,18-19H2,1-3H3/b26-25- | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 2.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research & Development
Curated by ChEMBL
| Assay Description
Binding affinity for human estrogen receptor alpha |
J Med Chem 48: 2243-7 (2005)
Article DOI: 10.1021/jm040154f
BindingDB Entry DOI: 10.7270/Q24T6N42 |
More data for this
Ligand-Target Pair | |
Cyclin-G-associated kinase
(Homo sapiens (Human)) | BDBM50524284
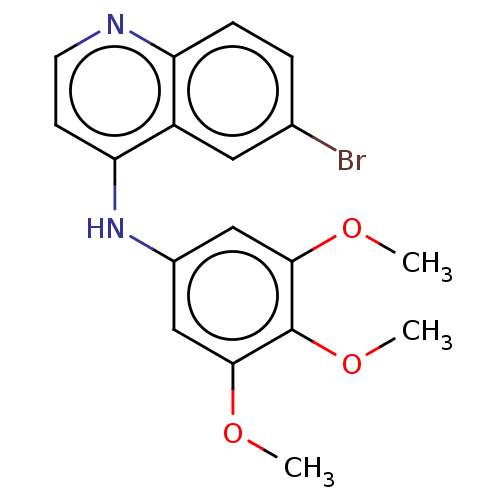
(CHEMBL4443342)Show InChI InChI=1S/C18H17BrN2O3/c1-22-16-9-12(10-17(23-2)18(16)24-3)21-15-6-7-20-14-5-4-11(19)8-13(14)15/h4-10H,1-3H3,(H,20,21) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| 3.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johann Wolfgang Goethe University
Curated by ChEMBL
| Assay Description
Binding affinity to C-terminal AVI-tagged GAK (unknown origin) (12 to 347 residues) expressed in Escherichia coli after 1.5 hrs by TR-FRET assay |
J Med Chem 62: 2830-2836 (2019)
Article DOI: 10.1021/acs.jmedchem.8b01213
BindingDB Entry DOI: 10.7270/Q28919C7 |
More data for this
Ligand-Target Pair | |
Estrogen receptor beta
(Homo sapiens (Human)) | BDBM17292

((1S,10R,11S,14S,15S)-15-methyltetracyclo[8.7.0.0^{...)Show SMILES [H][C@@]12CC[C@H](O)[C@@]1(C)CC[C@]1([H])c3ccc(O)cc3CC[C@@]21[H] Show InChI InChI=1S/C18H24O2/c1-18-9-8-14-13-5-3-12(19)10-11(13)2-4-15(14)16(18)6-7-17(18)20/h3,5,10,14-17,19-20H,2,4,6-9H2,1H3/t14-,15-,16+,17+,18+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 3.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research & Development
Curated by ChEMBL
| Assay Description
Binding affinity for human estrogen receptor beta |
J Med Chem 48: 2243-7 (2005)
Article DOI: 10.1021/jm040154f
BindingDB Entry DOI: 10.7270/Q24T6N42 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Estrogen receptor beta
(Homo sapiens (Human)) | BDBM50276802

(4-OHT | Afimoxifene | TamoGel)Show SMILES CCC(=C(c1ccc(O)cc1)c1ccc(OCCN(C)C)cc1)c1ccccc1 Show InChI InChI=1S/C26H29NO2/c1-4-25(20-8-6-5-7-9-20)26(21-10-14-23(28)15-11-21)22-12-16-24(17-13-22)29-19-18-27(2)3/h5-17,28H,4,18-19H2,1-3H3/b26-25- | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 3.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research & Development
Curated by ChEMBL
| Assay Description
Binding affinity for human estrogen receptor beta |
J Med Chem 48: 2243-7 (2005)
Article DOI: 10.1021/jm040154f
BindingDB Entry DOI: 10.7270/Q24T6N42 |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50418568
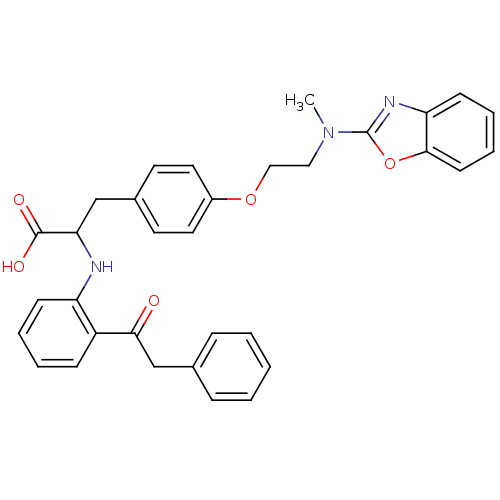
(CHEMBL146822)Show SMILES CN(CCOc1ccc(CC(Nc2ccccc2C(=O)Cc2ccccc2)C(O)=O)cc1)c1nc2ccccc2o1 Show InChI InChI=1S/C33H31N3O5/c1-36(33-35-28-13-7-8-14-31(28)41-33)19-20-40-25-17-15-24(16-18-25)21-29(32(38)39)34-27-12-6-5-11-26(27)30(37)22-23-9-3-2-4-10-23/h2-18,29,34H,19-22H2,1H3,(H,38,39) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Research and Development
Curated by ChEMBL
| Assay Description
In vitro binding to peroxisome proliferator activated receptor gamma (PPAR gamma) using [3H]-BRL 49653 as radioligand in scintillation proximity assa... |
J Med Chem 41: 5020-36 (1999)
Checked by Author
Article DOI: 10.1021/jm9804127
BindingDB Entry DOI: 10.7270/Q20K2B28 |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50000012
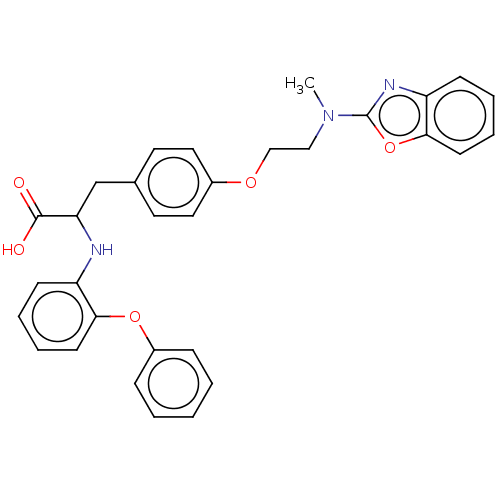
(CHEMBL147826)Show SMILES CN(CCOc1ccc(CC(Nc2ccccc2Oc2ccccc2)C(O)=O)cc1)c1nc2ccccc2o1 Show InChI InChI=1S/C31H29N3O5/c1-34(31-33-26-12-6-8-14-29(26)39-31)19-20-37-23-17-15-22(16-18-23)21-27(30(35)36)32-25-11-5-7-13-28(25)38-24-9-3-2-4-10-24/h2-18,27,32H,19-21H2,1H3,(H,35,36) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Research and Development
Curated by ChEMBL
| Assay Description
In vitro binding to peroxisome proliferator activated receptor gamma (PPAR gamma) using [3H]-BRL 49653 as radioligand in scintillation proximity assa... |
J Med Chem 41: 5020-36 (1999)
Checked by Author
Article DOI: 10.1021/jm9804127
BindingDB Entry DOI: 10.7270/Q20K2B28 |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50085046
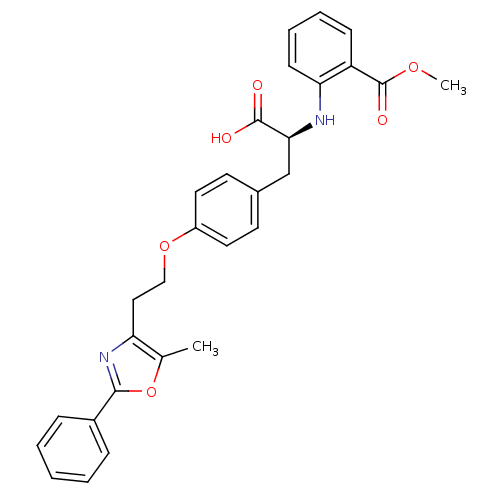
(2-((S)-1-carboxy-2-{4-[2-(5-methyl-2-phenyl-oxazol...)Show SMILES COC(=O)c1ccccc1N[C@@H](Cc1ccc(OCCc2nc(oc2C)-c2ccccc2)cc1)C(O)=O Show InChI InChI=1S/C29H28N2O6/c1-19-24(31-27(37-19)21-8-4-3-5-9-21)16-17-36-22-14-12-20(13-15-22)18-26(28(32)33)30-25-11-7-6-10-23(25)29(34)35-2/h3-15,26,30H,16-18H2,1-2H3,(H,32,33)/t26-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 3.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Binding affinity to human Peroxisome proliferator activated receptor gamma using scintillation proximity assay |
Bioorg Med Chem Lett 11: 3111-3 (2001)
BindingDB Entry DOI: 10.7270/Q2B27TKM |
More data for this
Ligand-Target Pair | |
Cyclin-G-associated kinase
(Homo sapiens (Human)) | BDBM50537138

(CHEMBL4531690)Show InChI InChI=1S/C16H15ClN4O2/c17-12-7-11-8-13(9-12)23-6-5-22-4-2-18-15-1-3-21-16(20-15)14(11)10-19-21/h1,3,7-10H,2,4-6H2,(H,18,20) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| 3.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johann Wolfgang Goethe University
Curated by ChEMBL
| Assay Description
Binding affinity to C-terminal AVI-tagged GAK (unknown origin) (12 to 347 residues) expressed in Escherichia coli after 1.5 hrs by TR-FRET assay |
J Med Chem 62: 2830-2836 (2019)
Article DOI: 10.1021/acs.jmedchem.8b01213
BindingDB Entry DOI: 10.7270/Q28919C7 |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50064451
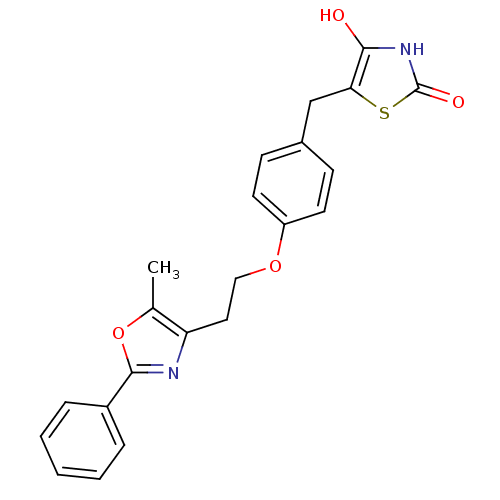
(5-{4-[2-(5-Methyl-2-phenyl-oxazol-4-yl)-ethoxy]-be...)Show SMILES Cc1oc(nc1CCOc1ccc(Cc2sc(=O)[nH]c2O)cc1)-c1ccccc1 Show InChI InChI=1S/C22H20N2O4S/c1-14-18(23-21(28-14)16-5-3-2-4-6-16)11-12-27-17-9-7-15(8-10-17)13-19-20(25)24-22(26)29-19/h2-10,25H,11-13H2,1H3,(H,24,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 4.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Research and Development
Curated by ChEMBL
| Assay Description
In vitro binding to peroxisome proliferator activated receptor gamma (PPAR gamma) using [3H]-BRL 49653 as radioligand in scintillation proximity assa... |
J Med Chem 41: 5020-36 (1999)
Checked by Author
Article DOI: 10.1021/jm9804127
BindingDB Entry DOI: 10.7270/Q20K2B28 |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50418575
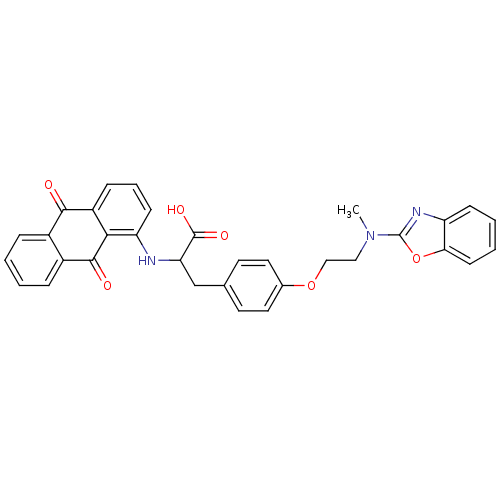
(CHEMBL358137)Show SMILES CN(CCOc1ccc(CC(Nc2cccc3C(=O)c4ccccc4C(=O)c23)C(O)=O)cc1)c1nc2ccccc2o1 Show InChI InChI=1S/C33H27N3O6/c1-36(33-35-25-10-4-5-12-28(25)42-33)17-18-41-21-15-13-20(14-16-21)19-27(32(39)40)34-26-11-6-9-24-29(26)31(38)23-8-3-2-7-22(23)30(24)37/h2-16,27,34H,17-19H2,1H3,(H,39,40) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Research and Development
Curated by ChEMBL
| Assay Description
In vitro binding to peroxisome proliferator activated receptor gamma (PPAR gamma) using [3H]-BRL 49653 as radioligand in scintillation proximity assa... |
J Med Chem 41: 5020-36 (1999)
Checked by Author
Article DOI: 10.1021/jm9804127
BindingDB Entry DOI: 10.7270/Q20K2B28 |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50418563
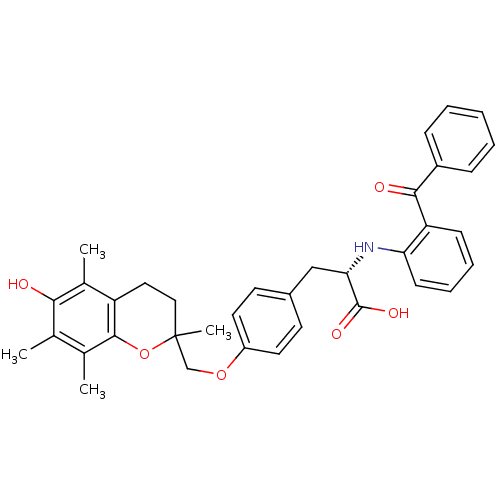
(CHEMBL148301)Show SMILES Cc1c(C)c2OC(C)(COc3ccc(C[C@H](Nc4ccccc4C(=O)c4ccccc4)C(O)=O)cc3)CCc2c(C)c1O Show InChI InChI=1S/C36H37NO6/c1-22-23(2)34-28(24(3)32(22)38)18-19-36(4,43-34)21-42-27-16-14-25(15-17-27)20-31(35(40)41)37-30-13-9-8-12-29(30)33(39)26-10-6-5-7-11-26/h5-17,31,37-38H,18-21H2,1-4H3,(H,40,41)/t31-,36?/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 5.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Research and Development
Curated by ChEMBL
| Assay Description
In vitro binding to peroxisome proliferator activated receptor gamma (PPAR gamma) using [3H]-BRL 49653 as radioligand in scintillation proximity assa... |
J Med Chem 41: 5020-36 (1999)
Checked by Author
Article DOI: 10.1021/jm9804127
BindingDB Entry DOI: 10.7270/Q20K2B28 |
More data for this
Ligand-Target Pair | |
AP2-associated protein kinase 1
(Homo sapiens (Human)) | BDBM50511373
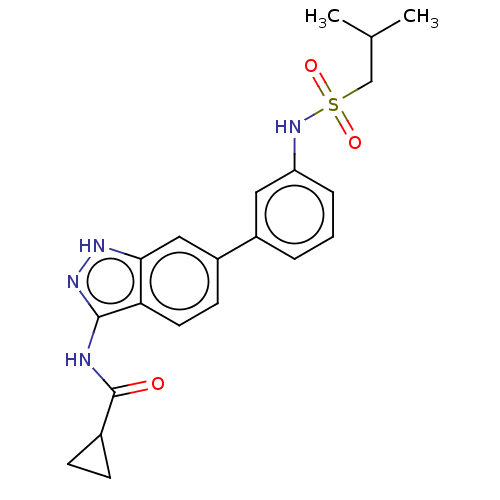
(CHEMBL4571548)Show SMILES CC(C)CS(=O)(=O)Nc1cccc(c1)-c1ccc2c(NC(=O)C3CC3)n[nH]c2c1 Show InChI InChI=1S/C21H24N4O3S/c1-13(2)12-29(27,28)25-17-5-3-4-15(10-17)16-8-9-18-19(11-16)23-24-20(18)22-21(26)14-6-7-14/h3-5,8-11,13-14,25H,6-7,12H2,1-2H3,(H2,22,23,24,26) | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 6.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of North Carolina at Chapel Hill (UNC-CH)
Curated by ChEMBL
| Assay Description
Displacement of Alexafluor labelled kinase tracer236 from biotinylated C-terminal Avi-tagged AAK1 kinase domain (31 to 396 residues) (unknown origin)... |
ACS Med Chem Lett 11: 340-345 (2020)
Article DOI: 10.1021/acsmedchemlett.9b00399
BindingDB Entry DOI: 10.7270/Q2ZC8669 |
More data for this
Ligand-Target Pair | |
AP2-associated protein kinase 1
(Homo sapiens (Human)) | BDBM50511385

(CHEMBL4534959)Show SMILES O=C(Nc1n[nH]c2cc(ccc12)-c1cccc(NS(=O)(=O)CC2CC2)c1)C1CC1 Show InChI InChI=1S/C21H22N4O3S/c26-21(14-6-7-14)22-20-18-9-8-16(11-19(18)23-24-20)15-2-1-3-17(10-15)25-29(27,28)12-13-4-5-13/h1-3,8-11,13-14,25H,4-7,12H2,(H2,22,23,24,26) | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| Article
PubMed
| 6.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of North Carolina at Chapel Hill (UNC-CH)
Curated by ChEMBL
| Assay Description
Displacement of Alexafluor labelled kinase tracer236 from biotinylated C-terminal Avi-tagged AAK1 kinase domain (31 to 396 residues) (unknown origin)... |
ACS Med Chem Lett 11: 340-345 (2020)
Article DOI: 10.1021/acsmedchemlett.9b00399
BindingDB Entry DOI: 10.7270/Q2ZC8669 |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50418571
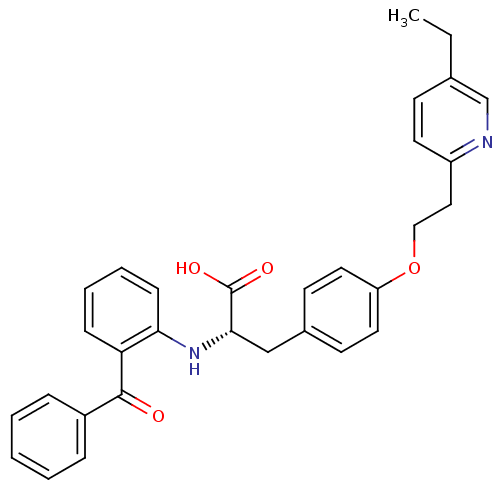
(CHEMBL346219)Show SMILES CCc1ccc(CCOc2ccc(C[C@H](Nc3ccccc3C(=O)c3ccccc3)C(O)=O)cc2)nc1 Show InChI InChI=1S/C31H30N2O4/c1-2-22-12-15-25(32-21-22)18-19-37-26-16-13-23(14-17-26)20-29(31(35)36)33-28-11-7-6-10-27(28)30(34)24-8-4-3-5-9-24/h3-17,21,29,33H,2,18-20H2,1H3,(H,35,36)/t29-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 6.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Research and Development
Curated by ChEMBL
| Assay Description
In vitro binding to peroxisome proliferator activated receptor gamma (PPAR gamma) using [3H]-BRL 49653 as radioligand in scintillation proximity assa... |
J Med Chem 41: 5020-36 (1999)
Checked by Author
Article DOI: 10.1021/jm9804127
BindingDB Entry DOI: 10.7270/Q20K2B28 |
More data for this
Ligand-Target Pair | |
Estrogen receptor
(Homo sapiens (Human)) | BDBM50475356

(CHEMBL195080)Show SMILES CCc1nc2cc(O)ccc2c(Oc2ccc(OCCN(C)C)cc2)c1-c1ccccc1 Show InChI InChI=1S/C27H28N2O3/c1-4-24-26(19-8-6-5-7-9-19)27(23-15-10-20(30)18-25(23)28-24)32-22-13-11-21(12-14-22)31-17-16-29(2)3/h5-15,18,30H,4,16-17H2,1-3H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 7.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research & Development
Curated by ChEMBL
| Assay Description
Binding affinity for human estrogen receptor alpha |
J Med Chem 48: 2243-7 (2005)
Article DOI: 10.1021/jm040154f
BindingDB Entry DOI: 10.7270/Q24T6N42 |
More data for this
Ligand-Target Pair | |
AP2-associated protein kinase 1
(Homo sapiens (Human)) | BDBM50511383
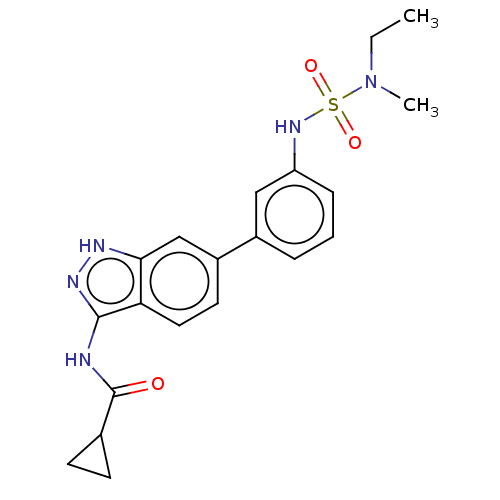
(CHEMBL4531680)Show SMILES CCN(C)S(=O)(=O)Nc1cccc(c1)-c1ccc2c(NC(=O)C3CC3)n[nH]c2c1 Show InChI InChI=1S/C20H23N5O3S/c1-3-25(2)29(27,28)24-16-6-4-5-14(11-16)15-9-10-17-18(12-15)22-23-19(17)21-20(26)13-7-8-13/h4-6,9-13,24H,3,7-8H2,1-2H3,(H2,21,22,23,26) | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
| Article
PubMed
| 8.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of North Carolina at Chapel Hill (UNC-CH)
Curated by ChEMBL
| Assay Description
Displacement of Alexafluor labelled kinase tracer236 from biotinylated C-terminal Avi-tagged AAK1 kinase domain (31 to 396 residues) (unknown origin)... |
ACS Med Chem Lett 11: 340-345 (2020)
Article DOI: 10.1021/acsmedchemlett.9b00399
BindingDB Entry DOI: 10.7270/Q2ZC8669 |
More data for this
Ligand-Target Pair | |
AP2-associated protein kinase 1
(Homo sapiens (Human)) | BDBM50511376

(CHEMBL4516665)Show SMILES O=C(Nc1n[nH]c2cc(ccc12)-c1cccc(NS(=O)(=O)C2CC2)c1)C1CC1 Show InChI InChI=1S/C20H20N4O3S/c25-20(12-4-5-12)21-19-17-9-6-14(11-18(17)22-23-19)13-2-1-3-15(10-13)24-28(26,27)16-7-8-16/h1-3,6,9-12,16,24H,4-5,7-8H2,(H2,21,22,23,25) | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| Article
PubMed
| 8.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of North Carolina at Chapel Hill (UNC-CH)
Curated by ChEMBL
| Assay Description
Displacement of Alexafluor labelled kinase tracer236 from biotinylated C-terminal Avi-tagged AAK1 kinase domain (31 to 396 residues) (unknown origin)... |
ACS Med Chem Lett 11: 340-345 (2020)
Article DOI: 10.1021/acsmedchemlett.9b00399
BindingDB Entry DOI: 10.7270/Q2ZC8669 |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(Homo sapiens (Human)) | BDBM85153
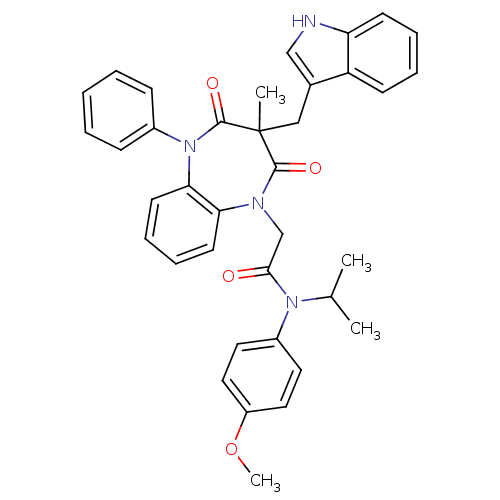
(CCK-A Agonist 20)Show SMILES COc1ccc(cc1)N(C(C)C)C(=O)CN1c2ccccc2N(c2ccccc2)C(=O)C(C)(Cc2c[nH]c3ccccc23)C1=O Show InChI InChI=1S/C37H36N4O4/c1-25(2)40(28-18-20-29(45-4)21-19-28)34(42)24-39-32-16-10-11-17-33(32)41(27-12-6-5-7-13-27)36(44)37(3,35(39)43)22-26-23-38-31-15-9-8-14-30(26)31/h5-21,23,25,38H,22,24H2,1-4H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 8.32 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Research and Development
Curated by PDSP Ki Database
| |
J Med Chem 40: 2706-25 (1997)
Article DOI: 10.1021/jm970265x
BindingDB Entry DOI: 10.7270/Q27M06F9 |
More data for this
Ligand-Target Pair | |
AP2-associated protein kinase 1
(Homo sapiens (Human)) | BDBM50511400
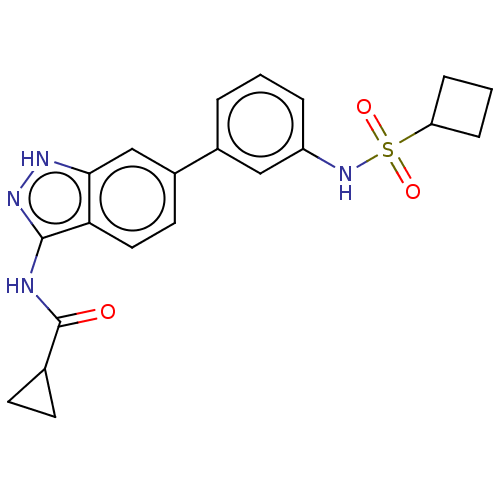
(CHEMBL4535040)Show SMILES O=C(Nc1n[nH]c2cc(ccc12)-c1cccc(NS(=O)(=O)C2CCC2)c1)C1CC1 Show InChI InChI=1S/C21H22N4O3S/c26-21(13-7-8-13)22-20-18-10-9-15(12-19(18)23-24-20)14-3-1-4-16(11-14)25-29(27,28)17-5-2-6-17/h1,3-4,9-13,17,25H,2,5-8H2,(H2,22,23,24,26) | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 8.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of North Carolina at Chapel Hill (UNC-CH)
Curated by ChEMBL
| Assay Description
Displacement of Alexafluor labelled kinase tracer236 from biotinylated C-terminal Avi-tagged AAK1 kinase domain (31 to 396 residues) (unknown origin)... |
ACS Med Chem Lett 11: 340-345 (2020)
Article DOI: 10.1021/acsmedchemlett.9b00399
BindingDB Entry DOI: 10.7270/Q2ZC8669 |
More data for this
Ligand-Target Pair | |
AP2-associated protein kinase 1
(Homo sapiens (Human)) | BDBM50511397
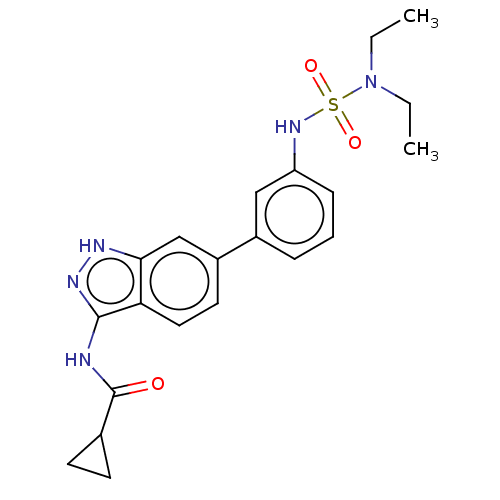
(CHEMBL4452939)Show SMILES CCN(CC)S(=O)(=O)Nc1cccc(c1)-c1ccc2c(NC(=O)C3CC3)n[nH]c2c1 Show InChI InChI=1S/C21H25N5O3S/c1-3-26(4-2)30(28,29)25-17-7-5-6-15(12-17)16-10-11-18-19(13-16)23-24-20(18)22-21(27)14-8-9-14/h5-7,10-14,25H,3-4,8-9H2,1-2H3,(H2,22,23,24,27) | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| 9.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of North Carolina at Chapel Hill (UNC-CH)
Curated by ChEMBL
| Assay Description
Displacement of Alexafluor labelled kinase tracer236 from biotinylated C-terminal Avi-tagged AAK1 kinase domain (31 to 396 residues) (unknown origin)... |
ACS Med Chem Lett 11: 340-345 (2020)
Article DOI: 10.1021/acsmedchemlett.9b00399
BindingDB Entry DOI: 10.7270/Q2ZC8669 |
More data for this
Ligand-Target Pair | |
Estrogen receptor
(Homo sapiens (Human)) | BDBM50475364
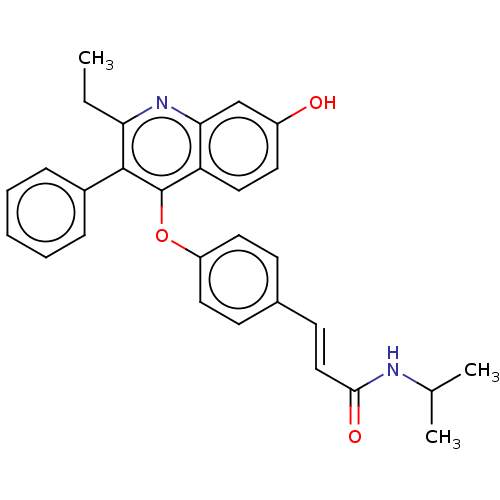
(CHEMBL370750)Show SMILES CCc1nc2cc(O)ccc2c(Oc2ccc(\C=C\C(=O)NC(C)C)cc2)c1-c1ccccc1 Show InChI InChI=1S/C29H28N2O3/c1-4-25-28(21-8-6-5-7-9-21)29(24-16-13-22(32)18-26(24)31-25)34-23-14-10-20(11-15-23)12-17-27(33)30-19(2)3/h5-19,32H,4H2,1-3H3,(H,30,33)/b17-12+ | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research & Development
Curated by ChEMBL
| Assay Description
Binding affinity for human estrogen receptor alpha |
J Med Chem 48: 2243-7 (2005)
Article DOI: 10.1021/jm040154f
BindingDB Entry DOI: 10.7270/Q24T6N42 |
More data for this
Ligand-Target Pair | |
Estrogen receptor beta
(Homo sapiens (Human)) | BDBM50475356

(CHEMBL195080)Show SMILES CCc1nc2cc(O)ccc2c(Oc2ccc(OCCN(C)C)cc2)c1-c1ccccc1 Show InChI InChI=1S/C27H28N2O3/c1-4-24-26(19-8-6-5-7-9-19)27(23-15-10-20(30)18-25(23)28-24)32-22-13-11-21(12-14-22)31-17-16-29(2)3/h5-15,18,30H,4,16-17H2,1-3H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research & Development
Curated by ChEMBL
| Assay Description
Binding affinity for human estrogen receptor beta |
J Med Chem 48: 2243-7 (2005)
Article DOI: 10.1021/jm040154f
BindingDB Entry DOI: 10.7270/Q24T6N42 |
More data for this
Ligand-Target Pair | |
Estrogen receptor beta
(Homo sapiens (Human)) | BDBM50084948

(CHEMBL195515 | GW7604)Show SMILES CC\C(=C(/c1ccc(O)cc1)c1ccc(\C=C\C(O)=O)cc1)c1ccccc1 Show InChI InChI=1S/C25H22O3/c1-2-23(19-6-4-3-5-7-19)25(21-13-15-22(26)16-14-21)20-11-8-18(9-12-20)10-17-24(27)28/h3-17,26H,2H2,1H3,(H,27,28)/b17-10+,25-23+ | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research & Development
Curated by ChEMBL
| Assay Description
Binding affinity for human estrogen receptor beta |
J Med Chem 48: 2243-7 (2005)
Article DOI: 10.1021/jm040154f
BindingDB Entry DOI: 10.7270/Q24T6N42 |
More data for this
Ligand-Target Pair | |
Estrogen receptor
(Homo sapiens (Human)) | BDBM50475363

(CHEMBL194774)Show SMILES CCc1nc2cc(O)ccc2c(Oc2ccc(\C=C\C(=O)N(C)C)cc2)c1-c1ccccc1 Show InChI InChI=1S/C28H26N2O3/c1-4-24-27(20-8-6-5-7-9-20)28(23-16-13-21(31)18-25(23)29-24)33-22-14-10-19(11-15-22)12-17-26(32)30(2)3/h5-18,31H,4H2,1-3H3/b17-12+ | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research & Development
Curated by ChEMBL
| Assay Description
Binding affinity for human estrogen receptor alpha |
J Med Chem 48: 2243-7 (2005)
Article DOI: 10.1021/jm040154f
BindingDB Entry DOI: 10.7270/Q24T6N42 |
More data for this
Ligand-Target Pair | |
AP2-associated protein kinase 1
(Homo sapiens (Human)) | BDBM50511380

(CHEMBL4452360)Show SMILES CN(C)S(=O)(=O)Nc1cccc(c1)-c1ccc2c(NC(=O)C3CC3)n[nH]c2c1 Show InChI InChI=1S/C19H21N5O3S/c1-24(2)28(26,27)23-15-5-3-4-13(10-15)14-8-9-16-17(11-14)21-22-18(16)20-19(25)12-6-7-12/h3-5,8-12,23H,6-7H2,1-2H3,(H2,20,21,22,25) | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| Article
PubMed
| 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of North Carolina at Chapel Hill (UNC-CH)
Curated by ChEMBL
| Assay Description
Displacement of Alexafluor labelled kinase tracer236 from biotinylated C-terminal Avi-tagged AAK1 kinase domain (31 to 396 residues) (unknown origin)... |
ACS Med Chem Lett 11: 340-345 (2020)
Article DOI: 10.1021/acsmedchemlett.9b00399
BindingDB Entry DOI: 10.7270/Q2ZC8669 |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(Homo sapiens (Human)) | BDBM85145
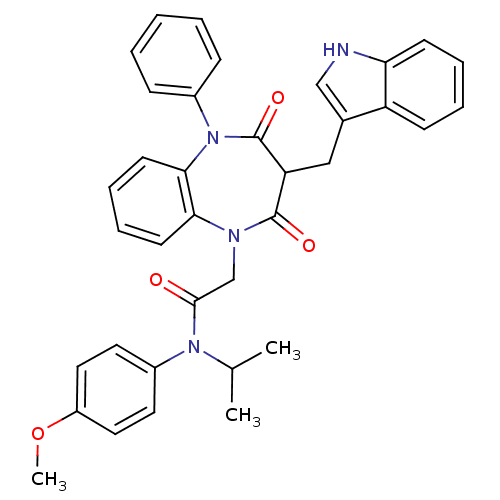
(CCK-A Agonist 15)Show SMILES COc1ccc(cc1)N(C(C)C)C(=O)CN1c2ccccc2N(c2ccccc2)C(=O)C(Cc2c[nH]c3ccccc23)C1=O Show InChI InChI=1S/C36H34N4O4/c1-24(2)39(27-17-19-28(44-3)20-18-27)34(41)23-38-32-15-9-10-16-33(32)40(26-11-5-4-6-12-26)36(43)30(35(38)42)21-25-22-37-31-14-8-7-13-29(25)31/h4-20,22,24,30,37H,21,23H2,1-3H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 11.0 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Research and Development
Curated by PDSP Ki Database
| |
J Med Chem 40: 2706-25 (1997)
Article DOI: 10.1021/jm970265x
BindingDB Entry DOI: 10.7270/Q27M06F9 |
More data for this
Ligand-Target Pair | |
AP2-associated protein kinase 1
(Homo sapiens (Human)) | BDBM50511394
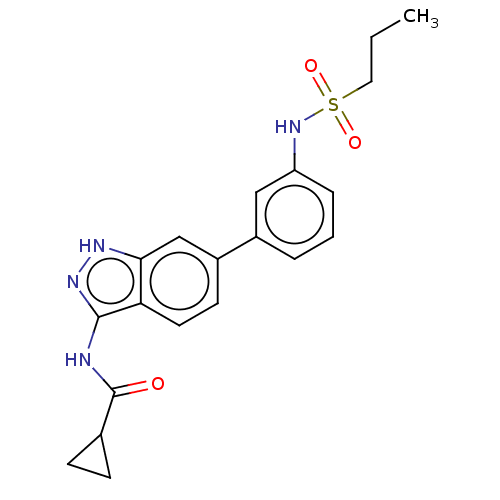
(CHEMBL4552310)Show SMILES CCCS(=O)(=O)Nc1cccc(c1)-c1ccc2c(NC(=O)C3CC3)n[nH]c2c1 Show InChI InChI=1S/C20H22N4O3S/c1-2-10-28(26,27)24-16-5-3-4-14(11-16)15-8-9-17-18(12-15)22-23-19(17)21-20(25)13-6-7-13/h3-5,8-9,11-13,24H,2,6-7,10H2,1H3,(H2,21,22,23,25) | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of North Carolina at Chapel Hill (UNC-CH)
Curated by ChEMBL
| Assay Description
Displacement of Alexafluor labelled kinase tracer236 from biotinylated C-terminal Avi-tagged AAK1 kinase domain (31 to 396 residues) (unknown origin)... |
ACS Med Chem Lett 11: 340-345 (2020)
Article DOI: 10.1021/acsmedchemlett.9b00399
BindingDB Entry DOI: 10.7270/Q2ZC8669 |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50418557
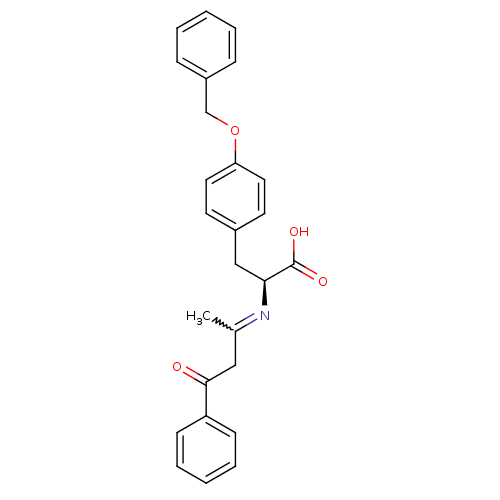
(CHEMBL1785028)Show SMILES CC(CC(=O)c1ccccc1)=N[C@@H](Cc1ccc(OCc2ccccc2)cc1)C(O)=O |r,w:1.0| Show InChI InChI=1S/C26H25NO4/c1-19(16-25(28)22-10-6-3-7-11-22)27-24(26(29)30)17-20-12-14-23(15-13-20)31-18-21-8-4-2-5-9-21/h2-15,24H,16-18H2,1H3,(H,29,30)/t24-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Research and Development
Curated by ChEMBL
| Assay Description
In vitro binding to peroxisome proliferator activated receptor gamma (PPAR gamma) using [3H]-BRL 49653 as radioligand in scintillation proximity assa... |
J Med Chem 41: 5020-36 (1999)
Checked by Author
Article DOI: 10.1021/jm9804127
BindingDB Entry DOI: 10.7270/Q20K2B28 |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50418557

(CHEMBL1785028)Show SMILES CC(CC(=O)c1ccccc1)=N[C@@H](Cc1ccc(OCc2ccccc2)cc1)C(O)=O |r,w:1.0| Show InChI InChI=1S/C26H25NO4/c1-19(16-25(28)22-10-6-3-7-11-22)27-24(26(29)30)17-20-12-14-23(15-13-20)31-18-21-8-4-2-5-9-21/h2-15,24H,16-18H2,1H3,(H,29,30)/t24-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Research and Development
Curated by ChEMBL
| Assay Description
In vitro binding to peroxisome proliferator activated receptor gamma (PPAR gamma) using [3H]-BRL 49653 as radioligand in scintillation proximity assa... |
J Med Chem 41: 5020-36 (1999)
Checked by Author
Article DOI: 10.1021/jm9804127
BindingDB Entry DOI: 10.7270/Q20K2B28 |
More data for this
Ligand-Target Pair | |
BMP-2-inducible protein kinase
(Homo sapiens (Human)) | BDBM50511376

(CHEMBL4516665)Show SMILES O=C(Nc1n[nH]c2cc(ccc12)-c1cccc(NS(=O)(=O)C2CC2)c1)C1CC1 Show InChI InChI=1S/C20H20N4O3S/c25-20(12-4-5-12)21-19-17-9-6-14(11-18(17)22-23-19)13-2-1-3-15(10-13)24-28(26,27)16-7-8-16/h1-3,6,9-12,16,24H,4-5,7-8H2,(H2,21,22,23,25) | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of North Carolina at Chapel Hill (UNC-CH)
Curated by ChEMBL
| Assay Description
Displacement of Alexafluor labelled kinase tracer236 from biotinylated C-terminal Avi-tagged BMP2K kinase domain (38 to 345 residues) (unknown origin... |
ACS Med Chem Lett 11: 340-345 (2020)
Article DOI: 10.1021/acsmedchemlett.9b00399
BindingDB Entry DOI: 10.7270/Q2ZC8669 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Gastrin/cholecystokinin type B receptor
(Homo sapiens (Human)) | BDBM85152
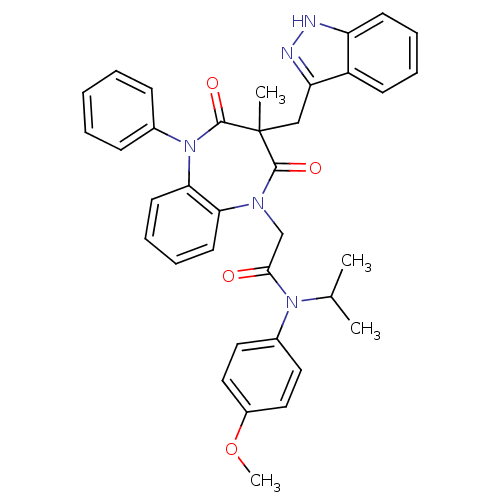
(CCK-A Agonist 23)Show SMILES COc1ccc(cc1)N(C(C)C)C(=O)CN1c2ccccc2N(c2ccccc2)C(=O)C(C)(Cc2n[nH]c3ccccc23)C1=O Show InChI InChI=1S/C36H35N5O4/c1-24(2)40(26-18-20-27(45-4)21-19-26)33(42)23-39-31-16-10-11-17-32(31)41(25-12-6-5-7-13-25)35(44)36(3,34(39)43)22-30-28-14-8-9-15-29(28)37-38-30/h5-21,24H,22-23H2,1-4H3,(H,37,38) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 13.2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Research and Development
Curated by PDSP Ki Database
| |
J Med Chem 40: 2706-25 (1997)
Article DOI: 10.1021/jm970265x
BindingDB Entry DOI: 10.7270/Q27M06F9 |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(Homo sapiens (Human)) | BDBM85148

(CCK-A Agonist 21)Show SMILES COc1ccc(cc1)N(C(C)C)C(=O)CN1c2ccccc2N(c2ccccc2)C(=O)C(Cc2c[nH]c3ccccc23)(OC)C1=O Show InChI InChI=1S/C37H36N4O5/c1-25(2)40(28-18-20-29(45-3)21-19-28)34(42)24-39-32-16-10-11-17-33(32)41(27-12-6-5-7-13-27)36(44)37(46-4,35(39)43)22-26-23-38-31-15-9-8-14-30(26)31/h5-21,23,25,38H,22,24H2,1-4H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 13.8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Research and Development
Curated by PDSP Ki Database
| |
J Med Chem 40: 2706-25 (1997)
Article DOI: 10.1021/jm970265x
BindingDB Entry DOI: 10.7270/Q27M06F9 |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(Homo sapiens (Human)) | BDBM85141
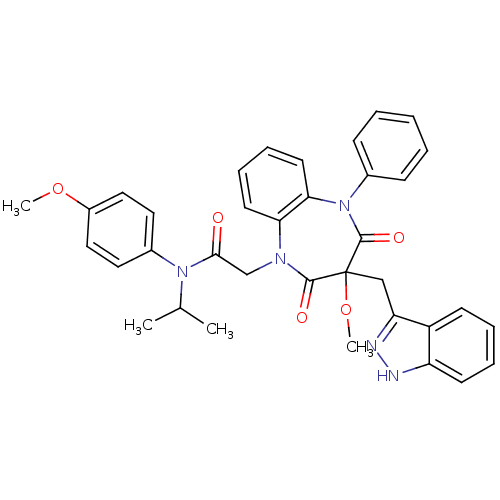
(CCK-A Agonist 24)Show SMILES COc1ccc(cc1)N(C(C)C)C(=O)CN1c2ccccc2N(c2ccccc2)C(=O)C(Cc2n[nH]c3ccccc23)(OC)C1=O Show InChI InChI=1S/C36H35N5O5/c1-24(2)40(26-18-20-27(45-3)21-19-26)33(42)23-39-31-16-10-11-17-32(31)41(25-12-6-5-7-13-25)35(44)36(46-4,34(39)43)22-30-28-14-8-9-15-29(28)37-38-30/h5-21,24H,22-23H2,1-4H3,(H,37,38) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 14.4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Research and Development
Curated by PDSP Ki Database
| |
J Med Chem 40: 2706-25 (1997)
Article DOI: 10.1021/jm970265x
BindingDB Entry DOI: 10.7270/Q27M06F9 |
More data for this
Ligand-Target Pair | |
Estrogen receptor beta
(Homo sapiens (Human)) | BDBM50475364

(CHEMBL370750)Show SMILES CCc1nc2cc(O)ccc2c(Oc2ccc(\C=C\C(=O)NC(C)C)cc2)c1-c1ccccc1 Show InChI InChI=1S/C29H28N2O3/c1-4-25-28(21-8-6-5-7-9-21)29(24-16-13-22(32)18-26(24)31-25)34-23-14-10-20(11-15-23)12-17-27(33)30-19(2)3/h5-19,32H,4H2,1-3H3,(H,30,33)/b17-12+ | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research & Development
Curated by ChEMBL
| Assay Description
Binding affinity for human estrogen receptor beta |
J Med Chem 48: 2243-7 (2005)
Article DOI: 10.1021/jm040154f
BindingDB Entry DOI: 10.7270/Q24T6N42 |
More data for this
Ligand-Target Pair | |
Estrogen receptor
(Homo sapiens (Human)) | BDBM50475361
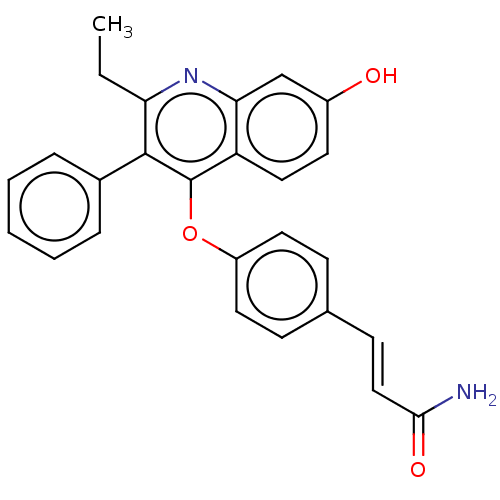
(CHEMBL363630)Show SMILES CCc1nc2cc(O)ccc2c(Oc2ccc(\C=C\C(N)=O)cc2)c1-c1ccccc1 Show InChI InChI=1S/C26H22N2O3/c1-2-22-25(18-6-4-3-5-7-18)26(21-14-11-19(29)16-23(21)28-22)31-20-12-8-17(9-13-20)10-15-24(27)30/h3-16,29H,2H2,1H3,(H2,27,30)/b15-10+ | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research & Development
Curated by ChEMBL
| Assay Description
Binding affinity for human estrogen receptor alpha |
J Med Chem 48: 2243-7 (2005)
Article DOI: 10.1021/jm040154f
BindingDB Entry DOI: 10.7270/Q24T6N42 |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(Homo sapiens (Human)) | BDBM85161
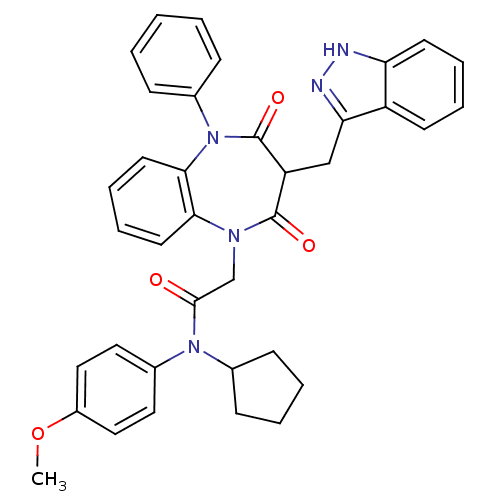
(CCK-A Agonist 43)Show SMILES COc1ccc(cc1)N(C1CCCC1)C(=O)CN1c2ccccc2N(c2ccccc2)C(=O)C(Cc2n[nH]c3ccccc23)C1=O Show InChI InChI=1S/C37H35N5O4/c1-46-28-21-19-27(20-22-28)41(25-13-5-6-14-25)35(43)24-40-33-17-9-10-18-34(33)42(26-11-3-2-4-12-26)37(45)30(36(40)44)23-32-29-15-7-8-16-31(29)38-39-32/h2-4,7-12,15-22,25,30H,5-6,13-14,23-24H2,1H3,(H,38,39) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 17.0 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Research and Development
Curated by PDSP Ki Database
| |
J Med Chem 40: 2706-25 (1997)
Article DOI: 10.1021/jm970265x
BindingDB Entry DOI: 10.7270/Q27M06F9 |
More data for this
Ligand-Target Pair | |
BMP-2-inducible protein kinase
(Homo sapiens (Human)) | BDBM50511400

(CHEMBL4535040)Show SMILES O=C(Nc1n[nH]c2cc(ccc12)-c1cccc(NS(=O)(=O)C2CCC2)c1)C1CC1 Show InChI InChI=1S/C21H22N4O3S/c26-21(13-7-8-13)22-20-18-10-9-15(12-19(18)23-24-20)14-3-1-4-16(11-14)25-29(27,28)17-5-2-6-17/h1,3-4,9-13,17,25H,2,5-8H2,(H2,22,23,24,26) | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 17 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of North Carolina at Chapel Hill (UNC-CH)
Curated by ChEMBL
| Assay Description
Displacement of Alexafluor labelled kinase tracer236 from biotinylated C-terminal Avi-tagged BMP2K kinase domain (38 to 345 residues) (unknown origin... |
ACS Med Chem Lett 11: 340-345 (2020)
Article DOI: 10.1021/acsmedchemlett.9b00399
BindingDB Entry DOI: 10.7270/Q2ZC8669 |
More data for this
Ligand-Target Pair | |
BMP-2-inducible protein kinase
(Homo sapiens (Human)) | BDBM50511385

(CHEMBL4534959)Show SMILES O=C(Nc1n[nH]c2cc(ccc12)-c1cccc(NS(=O)(=O)CC2CC2)c1)C1CC1 Show InChI InChI=1S/C21H22N4O3S/c26-21(14-6-7-14)22-20-18-9-8-16(11-19(18)23-24-20)15-2-1-3-17(10-15)25-29(27,28)12-13-4-5-13/h1-3,8-11,13-14,25H,4-7,12H2,(H2,22,23,24,26) | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| 17 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of North Carolina at Chapel Hill (UNC-CH)
Curated by ChEMBL
| Assay Description
Displacement of Alexafluor labelled kinase tracer236 from biotinylated C-terminal Avi-tagged BMP2K kinase domain (38 to 345 residues) (unknown origin... |
ACS Med Chem Lett 11: 340-345 (2020)
Article DOI: 10.1021/acsmedchemlett.9b00399
BindingDB Entry DOI: 10.7270/Q2ZC8669 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
BMP-2-inducible protein kinase
(Homo sapiens (Human)) | BDBM50511373

(CHEMBL4571548)Show SMILES CC(C)CS(=O)(=O)Nc1cccc(c1)-c1ccc2c(NC(=O)C3CC3)n[nH]c2c1 Show InChI InChI=1S/C21H24N4O3S/c1-13(2)12-29(27,28)25-17-5-3-4-15(10-17)16-8-9-18-19(11-16)23-24-20(18)22-21(26)14-6-7-14/h3-5,8-11,13-14,25H,6-7,12H2,1-2H3,(H2,22,23,24,26) | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 17 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of North Carolina at Chapel Hill (UNC-CH)
Curated by ChEMBL
| Assay Description
Displacement of Alexafluor labelled kinase tracer236 from biotinylated C-terminal Avi-tagged BMP2K kinase domain (38 to 345 residues) (unknown origin... |
ACS Med Chem Lett 11: 340-345 (2020)
Article DOI: 10.1021/acsmedchemlett.9b00399
BindingDB Entry DOI: 10.7270/Q2ZC8669 |
More data for this
Ligand-Target Pair | |
BMP-2-inducible protein kinase
(Homo sapiens (Human)) | BDBM50511397

(CHEMBL4452939)Show SMILES CCN(CC)S(=O)(=O)Nc1cccc(c1)-c1ccc2c(NC(=O)C3CC3)n[nH]c2c1 Show InChI InChI=1S/C21H25N5O3S/c1-3-26(4-2)30(28,29)25-17-7-5-6-15(12-17)16-10-11-18-19(13-16)23-24-20(18)22-21(27)14-8-9-14/h5-7,10-14,25H,3-4,8-9H2,1-2H3,(H2,22,23,24,27) | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| 17 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of North Carolina at Chapel Hill (UNC-CH)
Curated by ChEMBL
| Assay Description
Displacement of Alexafluor labelled kinase tracer236 from biotinylated C-terminal Avi-tagged BMP2K kinase domain (38 to 345 residues) (unknown origin... |
ACS Med Chem Lett 11: 340-345 (2020)
Article DOI: 10.1021/acsmedchemlett.9b00399
BindingDB Entry DOI: 10.7270/Q2ZC8669 |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(Homo sapiens (Human)) | BDBM85155
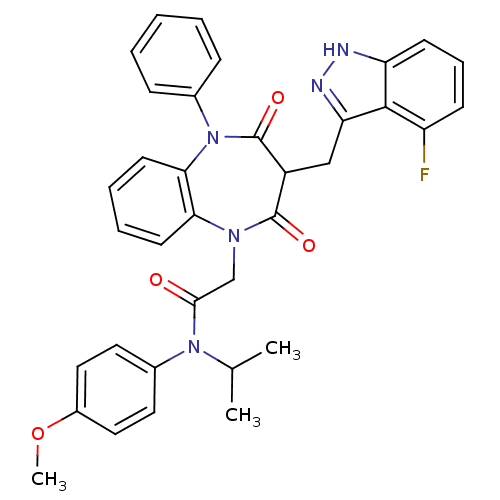
(CCK-A Agonist 31)Show SMILES COc1ccc(cc1)N(C(C)C)C(=O)CN1c2ccccc2N(c2ccccc2)C(=O)C(Cc2n[nH]c3cccc(F)c23)C1=O Show InChI InChI=1S/C35H32FN5O4/c1-22(2)40(24-16-18-25(45-3)19-17-24)32(42)21-39-30-14-7-8-15-31(30)41(23-10-5-4-6-11-23)35(44)26(34(39)43)20-29-33-27(36)12-9-13-28(33)37-38-29/h4-19,22,26H,20-21H2,1-3H3,(H,37,38) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 17.4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Research and Development
Curated by PDSP Ki Database
| |
J Med Chem 40: 2706-25 (1997)
Article DOI: 10.1021/jm970265x
BindingDB Entry DOI: 10.7270/Q27M06F9 |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(Homo sapiens (Human)) | BDBM85147
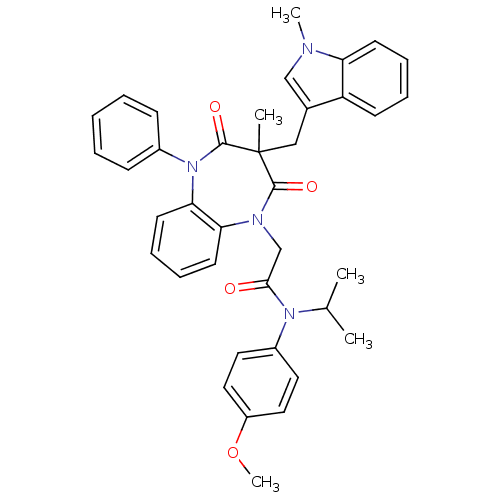
(CCK-A Agonist 22)Show SMILES COc1ccc(cc1)N(C(C)C)C(=O)CN1c2ccccc2N(c2ccccc2)C(=O)C(C)(Cc2cn(C)c3ccccc23)C1=O Show InChI InChI=1S/C38H38N4O4/c1-26(2)41(29-19-21-30(46-5)22-20-29)35(43)25-40-33-17-11-12-18-34(33)42(28-13-7-6-8-14-28)37(45)38(3,36(40)44)23-27-24-39(4)32-16-10-9-15-31(27)32/h6-22,24,26H,23,25H2,1-5H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 17.8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Research and Development
Curated by PDSP Ki Database
| |
J Med Chem 40: 2706-25 (1997)
Article DOI: 10.1021/jm970265x
BindingDB Entry DOI: 10.7270/Q27M06F9 |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50290271

(CHEMBL432747 | Nonanoic acid {2-[4-(2,4-dioxo-thia...)Show SMILES CCCCCCCCC(=O)N(C)CCOc1ccc(Cc2sc(=O)[nH]c2O)cc1 Show InChI InChI=1S/C22H32N2O4S/c1-3-4-5-6-7-8-9-20(25)24(2)14-15-28-18-12-10-17(11-13-18)16-19-21(26)23-22(27)29-19/h10-13,26H,3-9,14-16H2,1-2H3,(H,23,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 18 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity was determined by displacement of 20 nM [3H]- thiazolidinedione from 4 nM biotinylated human peroxisome proliferator-activated recep... |
Bioorg Med Chem Lett 7: 2491-2496 (1997)
Article DOI: 10.1016/S0960-894X(97)10017-8
BindingDB Entry DOI: 10.7270/Q2DB81VF |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data