 Found 40 hits with Last Name = 'wisastra' and Initial = 'r'
Found 40 hits with Last Name = 'wisastra' and Initial = 'r' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Seed linoleate 13S-lipoxygenase-1
(Glycine max (soybean)) | BDBM50401193
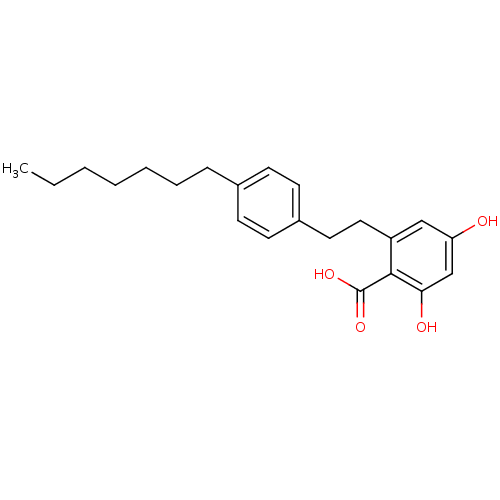
(CHEMBL1934604)Show InChI InChI=1S/C22H28O4/c1-2-3-4-5-6-7-16-8-10-17(11-9-16)12-13-18-14-19(23)15-20(24)21(18)22(25)26/h8-11,14-15,23-24H,2-7,12-13H2,1H3,(H,25,26) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 9.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Groningen Research Institute of Pharmacy
Curated by ChEMBL
| Assay Description
Inhibition of soyabean lipoxygenase-1 assessed as inhibition constant for enzyme-inhibitor complex by Lineweaver-Burk plot |
Bioorg Med Chem 20: 5027-32 (2012)
Article DOI: 10.1016/j.bmc.2012.06.019
BindingDB Entry DOI: 10.7270/Q20R9QJG |
More data for this
Ligand-Target Pair | |
Seed linoleate 13S-lipoxygenase-1
(Glycine max (soybean)) | BDBM50401193

(CHEMBL1934604)Show InChI InChI=1S/C22H28O4/c1-2-3-4-5-6-7-16-8-10-17(11-9-16)12-13-18-14-19(23)15-20(24)21(18)22(25)26/h8-11,14-15,23-24H,2-7,12-13H2,1H3,(H,25,26) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.57E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Groningen Research Institute of Pharmacy
Curated by ChEMBL
| Assay Description
Inhibition of soyabean lipoxygenase-1 assessed as inhibition constant for enzyme-substrate-inhibitor complex by Lineweaver-Burk plot |
Bioorg Med Chem 20: 5027-32 (2012)
Article DOI: 10.1016/j.bmc.2012.06.019
BindingDB Entry DOI: 10.7270/Q20R9QJG |
More data for this
Ligand-Target Pair | |
Polyunsaturated fatty acid 5-lipoxygenase
(Homo sapiens (Human)) | BDBM50444319
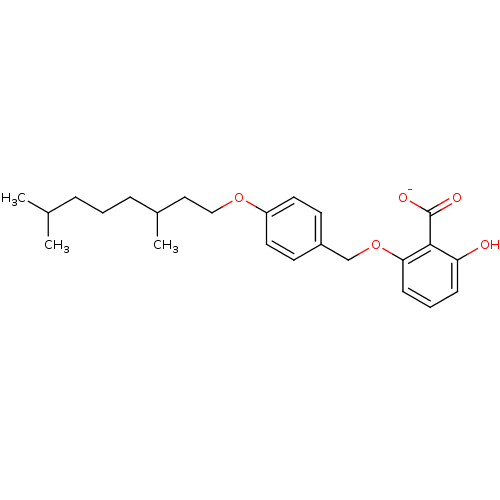
(CHEMBL3094153)Show SMILES CC(C)CCCC(C)CCOc1ccc(COc2cccc(O)c2C([O-])=O)cc1 Show InChI InChI=1S/C24H32O5/c1-17(2)6-4-7-18(3)14-15-28-20-12-10-19(11-13-20)16-29-22-9-5-8-21(25)23(22)24(26)27/h5,8-13,17-18,25H,4,6-7,14-16H2,1-3H3,(H,26,27)/p-1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 7.03E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Groningen
Curated by ChEMBL
| Assay Description
Binding affinity to human 5-LOX using linoleic acid as substrate |
Bioorg Med Chem 21: 7763-78 (2013)
Article DOI: 10.1016/j.bmc.2013.10.015
BindingDB Entry DOI: 10.7270/Q2HD7X3K |
More data for this
Ligand-Target Pair | |
Polyunsaturated fatty acid 5-lipoxygenase
(Homo sapiens (Human)) | BDBM50444319

(CHEMBL3094153)Show SMILES CC(C)CCCC(C)CCOc1ccc(COc2cccc(O)c2C([O-])=O)cc1 Show InChI InChI=1S/C24H32O5/c1-17(2)6-4-7-18(3)14-15-28-20-12-10-19(11-13-20)16-29-22-9-5-8-21(25)23(22)24(26)27/h5,8-13,17-18,25H,4,6-7,14-16H2,1-3H3,(H,26,27)/p-1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 1.40E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Groningen
Curated by ChEMBL
| Assay Description
Binding affinity to human 5-LOX-linoleic acid complex |
Bioorg Med Chem 21: 7763-78 (2013)
Article DOI: 10.1016/j.bmc.2013.10.015
BindingDB Entry DOI: 10.7270/Q2HD7X3K |
More data for this
Ligand-Target Pair | |
Macrophage metalloelastase
(Homo sapiens (Human)) | BDBM50035507

(CHEMBL3355722)Show SMILES CC(C)C[C@H](CC(=O)NO)C(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@@H](CCCCN)C(N)=O |r| Show InChI InChI=1S/C23H37N5O5/c1-15(2)12-17(14-20(29)28-33)22(31)27-19(13-16-8-4-3-5-9-16)23(32)26-18(21(25)30)10-6-7-11-24/h3-5,8-9,15,17-19,33H,6-7,10-14,24H2,1-2H3,(H2,25,30)(H,26,32)(H,27,31)(H,28,29)/t17-,18+,19+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
University Medical Center Groningen
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant MMP12 catalytic domain Mca-Pro-Leu-Gly-Leu-Dpa-Ala-Arg-NH2 fluorogenic substrate |
Bioorg Med Chem 23: 192-202 (2014)
Article DOI: 10.1016/j.bmc.2014.11.013
BindingDB Entry DOI: 10.7270/Q26Q1ZVW |
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase-17
(Homo sapiens (Human)) | BDBM50035507

(CHEMBL3355722)Show SMILES CC(C)C[C@H](CC(=O)NO)C(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@@H](CCCCN)C(N)=O |r| Show InChI InChI=1S/C23H37N5O5/c1-15(2)12-17(14-20(29)28-33)22(31)27-19(13-16-8-4-3-5-9-16)23(32)26-18(21(25)30)10-6-7-11-24/h3-5,8-9,15,17-19,33H,6-7,10-14,24H2,1-2H3,(H2,25,30)(H,26,32)(H,27,31)(H,28,29)/t17-,18+,19+/m1/s1 | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.70 | n/a | n/a | n/a | n/a | n/a | n/a |
University Medical Center Groningen
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant ADAM17 using Mca-PLAQAV-Dpa-RSSSR-NH2 |
Bioorg Med Chem 23: 192-202 (2014)
Article DOI: 10.1016/j.bmc.2014.11.013
BindingDB Entry DOI: 10.7270/Q26Q1ZVW |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50035507

(CHEMBL3355722)Show SMILES CC(C)C[C@H](CC(=O)NO)C(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@@H](CCCCN)C(N)=O |r| Show InChI InChI=1S/C23H37N5O5/c1-15(2)12-17(14-20(29)28-33)22(31)27-19(13-16-8-4-3-5-9-16)23(32)26-18(21(25)30)10-6-7-11-24/h3-5,8-9,15,17-19,33H,6-7,10-14,24H2,1-2H3,(H2,25,30)(H,26,32)(H,27,31)(H,28,29)/t17-,18+,19+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7.40 | n/a | n/a | n/a | n/a | n/a | n/a |
University Medical Center Groningen
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant MMP2 catalytic domain using Mca-Pro-Leu-Gly-Leu-Dpa-Ala-Arg-NH2 fluorogenic substrate |
Bioorg Med Chem 23: 192-202 (2014)
Article DOI: 10.1016/j.bmc.2014.11.013
BindingDB Entry DOI: 10.7270/Q26Q1ZVW |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50035475

(CHEMBL3355723)Show SMILES CC(C)C[C@H](CC(=O)NO)C(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@@H](CCCCNC(=O)c1ccc(F)cc1)C(N)=O |r| Show InChI InChI=1S/C30H40FN5O6/c1-19(2)16-22(18-26(37)36-42)29(40)35-25(17-20-8-4-3-5-9-20)30(41)34-24(27(32)38)10-6-7-15-33-28(39)21-11-13-23(31)14-12-21/h3-5,8-9,11-14,19,22,24-25,42H,6-7,10,15-18H2,1-2H3,(H2,32,38)(H,33,39)(H,34,41)(H,35,40)(H,36,37)/t22-,24+,25+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
University Medical Center Groningen
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant MMP2 catalytic domain using Mca-Pro-Leu-Gly-Leu-Dpa-Ala-Arg-NH2 fluorogenic substrate |
Bioorg Med Chem 23: 192-202 (2014)
Article DOI: 10.1016/j.bmc.2014.11.013
BindingDB Entry DOI: 10.7270/Q26Q1ZVW |
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase-9
(Homo sapiens (Human)) | BDBM50035507

(CHEMBL3355722)Show SMILES CC(C)C[C@H](CC(=O)NO)C(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@@H](CCCCN)C(N)=O |r| Show InChI InChI=1S/C23H37N5O5/c1-15(2)12-17(14-20(29)28-33)22(31)27-19(13-16-8-4-3-5-9-16)23(32)26-18(21(25)30)10-6-7-11-24/h3-5,8-9,15,17-19,33H,6-7,10-14,24H2,1-2H3,(H2,25,30)(H,26,32)(H,27,31)(H,28,29)/t17-,18+,19+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
University Medical Center Groningen
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant MMP9 catalytic domain using Mca-Pro-Leu-Gly-Leu-Dpa-Ala-Arg-NH2 fluorogenic substrate |
Bioorg Med Chem 23: 192-202 (2014)
Article DOI: 10.1016/j.bmc.2014.11.013
BindingDB Entry DOI: 10.7270/Q26Q1ZVW |
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase-17
(Homo sapiens (Human)) | BDBM50035475

(CHEMBL3355723)Show SMILES CC(C)C[C@H](CC(=O)NO)C(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@@H](CCCCNC(=O)c1ccc(F)cc1)C(N)=O |r| Show InChI InChI=1S/C30H40FN5O6/c1-19(2)16-22(18-26(37)36-42)29(40)35-25(17-20-8-4-3-5-9-20)30(41)34-24(27(32)38)10-6-7-15-33-28(39)21-11-13-23(31)14-12-21/h3-5,8-9,11-14,19,22,24-25,42H,6-7,10,15-18H2,1-2H3,(H2,32,38)(H,33,39)(H,34,41)(H,35,40)(H,36,37)/t22-,24+,25+/m1/s1 | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
University Medical Center Groningen
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant ADAM17 using Mca-PLAQAV-Dpa-RSSSR-NH2 |
Bioorg Med Chem 23: 192-202 (2014)
Article DOI: 10.1016/j.bmc.2014.11.013
BindingDB Entry DOI: 10.7270/Q26Q1ZVW |
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase-9
(Homo sapiens (Human)) | BDBM50035475

(CHEMBL3355723)Show SMILES CC(C)C[C@H](CC(=O)NO)C(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@@H](CCCCNC(=O)c1ccc(F)cc1)C(N)=O |r| Show InChI InChI=1S/C30H40FN5O6/c1-19(2)16-22(18-26(37)36-42)29(40)35-25(17-20-8-4-3-5-9-20)30(41)34-24(27(32)38)10-6-7-15-33-28(39)21-11-13-23(31)14-12-21/h3-5,8-9,11-14,19,22,24-25,42H,6-7,10,15-18H2,1-2H3,(H2,32,38)(H,33,39)(H,34,41)(H,35,40)(H,36,37)/t22-,24+,25+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 32 | n/a | n/a | n/a | n/a | n/a | n/a |
University Medical Center Groningen
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant MMP9 catalytic domain using Mca-Pro-Leu-Gly-Leu-Dpa-Ala-Arg-NH2 fluorogenic substrate |
Bioorg Med Chem 23: 192-202 (2014)
Article DOI: 10.1016/j.bmc.2014.11.013
BindingDB Entry DOI: 10.7270/Q26Q1ZVW |
More data for this
Ligand-Target Pair | |
Macrophage metalloelastase
(Homo sapiens (Human)) | BDBM50035475

(CHEMBL3355723)Show SMILES CC(C)C[C@H](CC(=O)NO)C(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@@H](CCCCNC(=O)c1ccc(F)cc1)C(N)=O |r| Show InChI InChI=1S/C30H40FN5O6/c1-19(2)16-22(18-26(37)36-42)29(40)35-25(17-20-8-4-3-5-9-20)30(41)34-24(27(32)38)10-6-7-15-33-28(39)21-11-13-23(31)14-12-21/h3-5,8-9,11-14,19,22,24-25,42H,6-7,10,15-18H2,1-2H3,(H2,32,38)(H,33,39)(H,34,41)(H,35,40)(H,36,37)/t22-,24+,25+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 138 | n/a | n/a | n/a | n/a | n/a | n/a |
University Medical Center Groningen
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant MMP12 catalytic domain Mca-Pro-Leu-Gly-Leu-Dpa-Ala-Arg-NH2 fluorogenic substrate |
Bioorg Med Chem 23: 192-202 (2014)
Article DOI: 10.1016/j.bmc.2014.11.013
BindingDB Entry DOI: 10.7270/Q26Q1ZVW |
More data for this
Ligand-Target Pair | |
Polyunsaturated fatty acid 5-lipoxygenase
(Homo sapiens (Human)) | BDBM50000541

((+-)-1-(1-Benzo[b]thien-2-ylethyl)-1-hydroxyurea |...)Show InChI InChI=1S/C11H12N2O2S/c1-7(13(15)11(12)14)10-6-8-4-2-3-5-9(8)16-10/h2-7,15H,1H3,(H2,12,14) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| n/a | n/a | 500 | n/a | n/a | n/a | n/a | n/a | n/a |
Groningen Research Institute of Pharmacy
Curated by ChEMBL
| Assay Description
Inhibition of 5-LOX |
Bioorg Med Chem 20: 5027-32 (2012)
Article DOI: 10.1016/j.bmc.2012.06.019
BindingDB Entry DOI: 10.7270/Q20R9QJG |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50035472
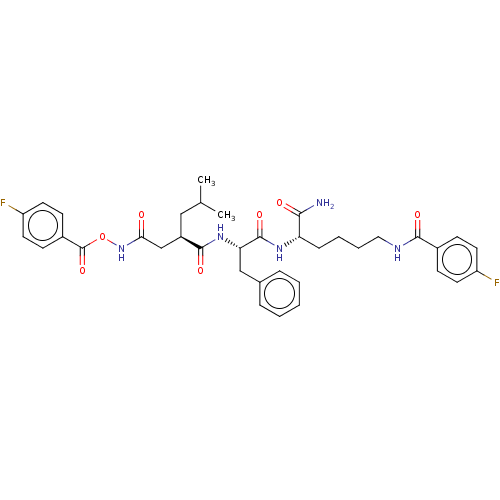
(CHEMBL3355724)Show SMILES CC(C)C[C@H](CC(=O)NOC(=O)c1ccc(F)cc1)C(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@@H](CCCCNC(=O)c1ccc(F)cc1)C(N)=O |r| Show InChI InChI=1S/C37H43F2N5O7/c1-23(2)20-27(22-32(45)44-51-37(50)26-13-17-29(39)18-14-26)35(48)43-31(21-24-8-4-3-5-9-24)36(49)42-30(33(40)46)10-6-7-19-41-34(47)25-11-15-28(38)16-12-25/h3-5,8-9,11-18,23,27,30-31H,6-7,10,19-22H2,1-2H3,(H2,40,46)(H,41,47)(H,42,49)(H,43,48)(H,44,45)/t27-,30+,31+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.45E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University Medical Center Groningen
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant MMP2 catalytic domain using Mca-Pro-Leu-Gly-Leu-Dpa-Ala-Arg-NH2 fluorogenic substrate |
Bioorg Med Chem 23: 192-202 (2014)
Article DOI: 10.1016/j.bmc.2014.11.013
BindingDB Entry DOI: 10.7270/Q26Q1ZVW |
More data for this
Ligand-Target Pair | |
Histone acetyltransferase KAT2B
(Homo sapiens (Human)) | BDBM50247839
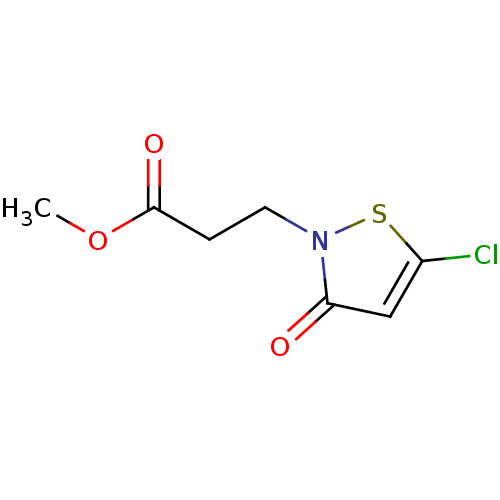
(CHEMBL447612 | methyl 3-(5-chloro-3-oxoisothiazol-...)Show InChI InChI=1S/C7H8ClNO3S/c1-12-7(11)2-3-9-6(10)4-5(8)13-9/h4H,2-3H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Groningen
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant HAT PCAF |
Bioorg Med Chem 17: 460-6 (2009)
Article DOI: 10.1016/j.bmc.2008.12.008
BindingDB Entry DOI: 10.7270/Q2BC3ZDM |
More data for this
Ligand-Target Pair | |
Histone acetyltransferase KAT2B
(Homo sapiens (Human)) | BDBM50247843
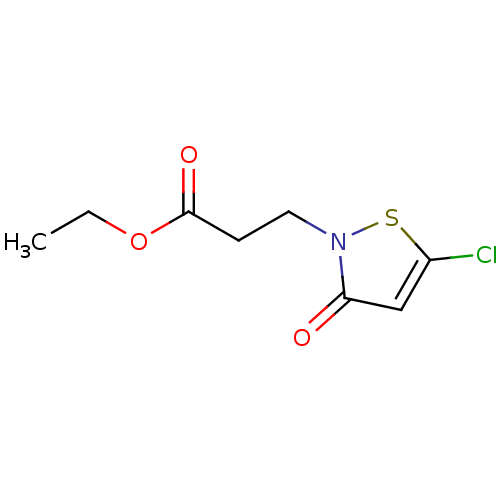
(CHEMBL510872 | Ethyl 3-(5-chloro-3-oxoisothiazol-2...)Show InChI InChI=1S/C8H10ClNO3S/c1-2-13-8(12)3-4-10-7(11)5-6(9)14-10/h5H,2-4H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Groningen
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant HAT PCAF |
Bioorg Med Chem 17: 460-6 (2009)
Article DOI: 10.1016/j.bmc.2008.12.008
BindingDB Entry DOI: 10.7270/Q2BC3ZDM |
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase-9
(Homo sapiens (Human)) | BDBM50035472

(CHEMBL3355724)Show SMILES CC(C)C[C@H](CC(=O)NOC(=O)c1ccc(F)cc1)C(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@@H](CCCCNC(=O)c1ccc(F)cc1)C(N)=O |r| Show InChI InChI=1S/C37H43F2N5O7/c1-23(2)20-27(22-32(45)44-51-37(50)26-13-17-29(39)18-14-26)35(48)43-31(21-24-8-4-3-5-9-24)36(49)42-30(33(40)46)10-6-7-19-41-34(47)25-11-15-28(38)16-12-25/h3-5,8-9,11-18,23,27,30-31H,6-7,10,19-22H2,1-2H3,(H2,40,46)(H,41,47)(H,42,49)(H,43,48)(H,44,45)/t27-,30+,31+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.42E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University Medical Center Groningen
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant MMP9 catalytic domain using Mca-Pro-Leu-Gly-Leu-Dpa-Ala-Arg-NH2 fluorogenic substrate |
Bioorg Med Chem 23: 192-202 (2014)
Article DOI: 10.1016/j.bmc.2014.11.013
BindingDB Entry DOI: 10.7270/Q26Q1ZVW |
More data for this
Ligand-Target Pair | |
Histone acetyltransferase KAT2B
(Homo sapiens (Human)) | BDBM50247840
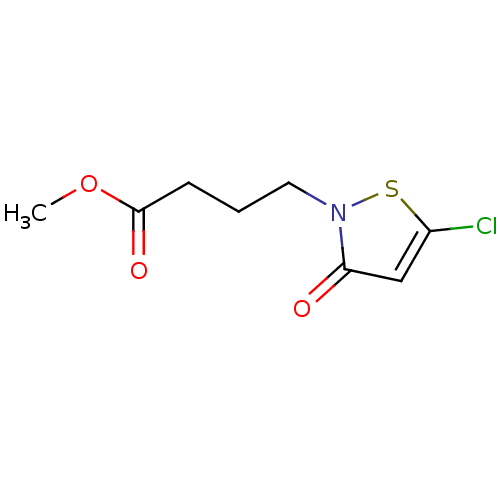
(CHEMBL489116 | Methyl 4-(5-chloro-3-oxoisothiazol-...)Show InChI InChI=1S/C8H10ClNO3S/c1-13-8(12)3-2-4-10-7(11)5-6(9)14-10/h5H,2-4H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Groningen
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant HAT PCAF |
Bioorg Med Chem 17: 460-6 (2009)
Article DOI: 10.1016/j.bmc.2008.12.008
BindingDB Entry DOI: 10.7270/Q2BC3ZDM |
More data for this
Ligand-Target Pair | |
Histone acetyltransferase KAT2B
(Homo sapiens (Human)) | BDBM50247844
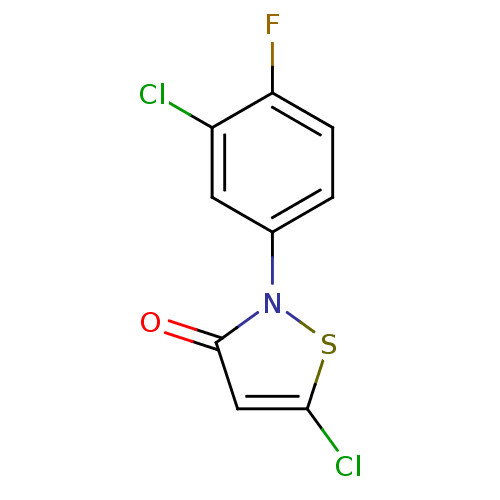
(5-Chloro-2-(3-chloro-4-fluorophenyl)isothiazol-3(2...)Show InChI InChI=1S/C9H4Cl2FNOS/c10-6-3-5(1-2-7(6)12)13-9(14)4-8(11)15-13/h1-4H | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Groningen
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant HAT PCAF |
Bioorg Med Chem 17: 460-6 (2009)
Article DOI: 10.1016/j.bmc.2008.12.008
BindingDB Entry DOI: 10.7270/Q2BC3ZDM |
More data for this
Ligand-Target Pair | |
Histone acetyltransferase KAT2B
(Homo sapiens (Human)) | BDBM50247845
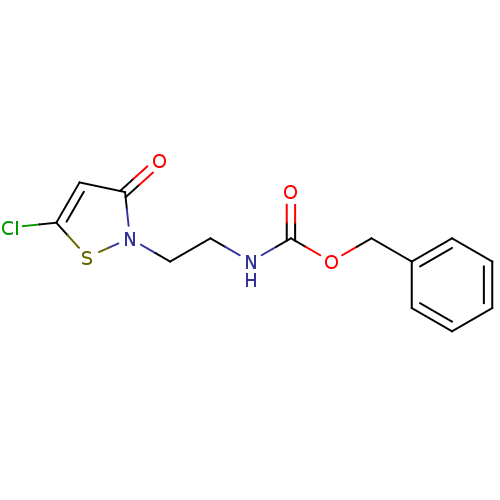
(Benzyl [2-(5-chloro-3-oxoisothiazol-2(3H)-yl)ethyl...)Show InChI InChI=1S/C13H13ClN2O3S/c14-11-8-12(17)16(20-11)7-6-15-13(18)19-9-10-4-2-1-3-5-10/h1-5,8H,6-7,9H2,(H,15,18) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Groningen
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant HAT PCAF |
Bioorg Med Chem 17: 460-6 (2009)
Article DOI: 10.1016/j.bmc.2008.12.008
BindingDB Entry DOI: 10.7270/Q2BC3ZDM |
More data for this
Ligand-Target Pair | |
Histone acetyltransferase KAT2B
(Homo sapiens (Human)) | BDBM50247841
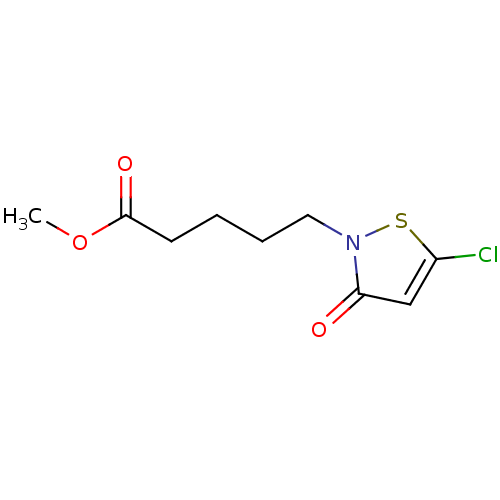
(CHEMBL443133 | Methyl 4-(5-chloro-3-oxoisothiazol-...)Show InChI InChI=1S/C9H12ClNO3S/c1-14-9(13)4-2-3-5-11-8(12)6-7(10)15-11/h6H,2-5H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Groningen
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant HAT PCAF |
Bioorg Med Chem 17: 460-6 (2009)
Article DOI: 10.1016/j.bmc.2008.12.008
BindingDB Entry DOI: 10.7270/Q2BC3ZDM |
More data for this
Ligand-Target Pair | |
Histone acetyltransferase KAT2B
(Homo sapiens (Human)) | BDBM50247837
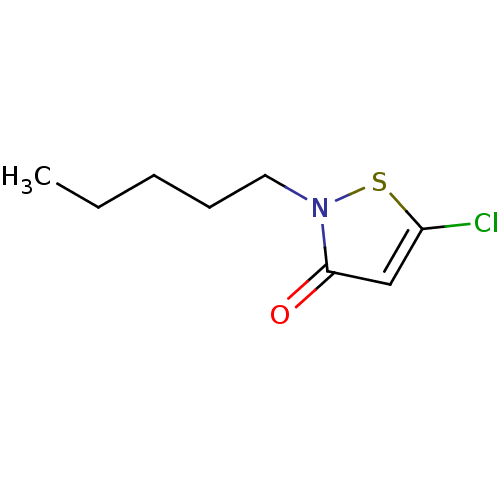
(5-Chloro-2-pentylisothiazol-3(2H)-one | CHEMBL4891...)Show InChI InChI=1S/C8H12ClNOS/c1-2-3-4-5-10-8(11)6-7(9)12-10/h6H,2-5H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Groningen
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant HAT PCAF |
Bioorg Med Chem 17: 460-6 (2009)
Article DOI: 10.1016/j.bmc.2008.12.008
BindingDB Entry DOI: 10.7270/Q2BC3ZDM |
More data for this
Ligand-Target Pair | |
Histone acetyltransferase KAT2B
(Homo sapiens (Human)) | BDBM50247838
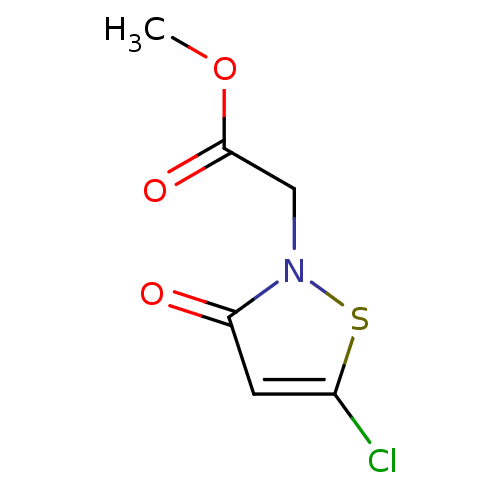
(CHEMBL466866 | Methyl 2-(5-chloro-3-oxoisothiazol-...)Show InChI InChI=1S/C6H6ClNO3S/c1-11-6(10)3-8-5(9)2-4(7)12-8/h2H,3H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Groningen
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant HAT PCAF |
Bioorg Med Chem 17: 460-6 (2009)
Article DOI: 10.1016/j.bmc.2008.12.008
BindingDB Entry DOI: 10.7270/Q2BC3ZDM |
More data for this
Ligand-Target Pair | |
Histone acetyltransferase KAT2B
(Homo sapiens (Human)) | BDBM50247820
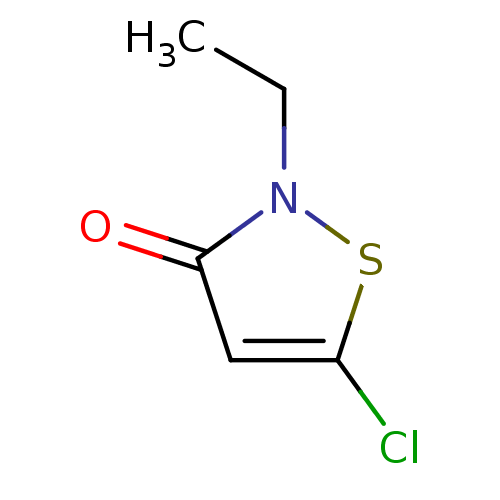
(5-Chloro-2-ethylisothiazol-3(2H)-one | CHEMBL46147...)Show InChI InChI=1S/C5H6ClNOS/c1-2-7-5(8)3-4(6)9-7/h3H,2H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Groningen
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant HAT PCAF |
Bioorg Med Chem 17: 460-6 (2009)
Article DOI: 10.1016/j.bmc.2008.12.008
BindingDB Entry DOI: 10.7270/Q2BC3ZDM |
More data for this
Ligand-Target Pair | |
Histone acetyltransferase KAT2B
(Homo sapiens (Human)) | BDBM50247842
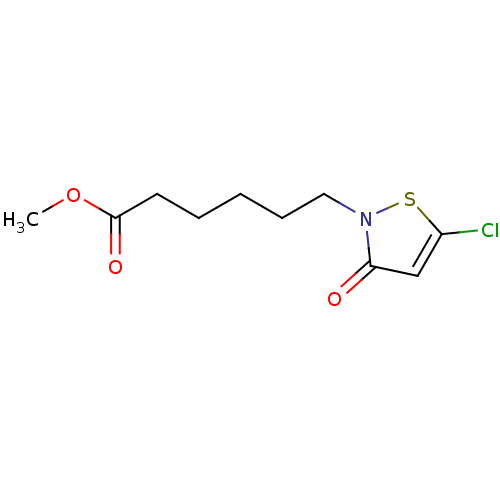
(CHEMBL442599 | Methyl 6-(5-chloro-3-oxoisothiazol-...)Show InChI InChI=1S/C10H14ClNO3S/c1-15-10(14)5-3-2-4-6-12-9(13)7-8(11)16-12/h7H,2-6H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Groningen
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant HAT PCAF |
Bioorg Med Chem 17: 460-6 (2009)
Article DOI: 10.1016/j.bmc.2008.12.008
BindingDB Entry DOI: 10.7270/Q2BC3ZDM |
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase-17
(Homo sapiens (Human)) | BDBM50035472

(CHEMBL3355724)Show SMILES CC(C)C[C@H](CC(=O)NOC(=O)c1ccc(F)cc1)C(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@@H](CCCCNC(=O)c1ccc(F)cc1)C(N)=O |r| Show InChI InChI=1S/C37H43F2N5O7/c1-23(2)20-27(22-32(45)44-51-37(50)26-13-17-29(39)18-14-26)35(48)43-31(21-24-8-4-3-5-9-24)36(49)42-30(33(40)46)10-6-7-19-41-34(47)25-11-15-28(38)16-12-25/h3-5,8-9,11-18,23,27,30-31H,6-7,10,19-22H2,1-2H3,(H2,40,46)(H,41,47)(H,42,49)(H,43,48)(H,44,45)/t27-,30+,31+/m1/s1 | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.26E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University Medical Center Groningen
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant ADAM17 using Mca-PLAQAV-Dpa-RSSSR-NH2 |
Bioorg Med Chem 23: 192-202 (2014)
Article DOI: 10.1016/j.bmc.2014.11.013
BindingDB Entry DOI: 10.7270/Q26Q1ZVW |
More data for this
Ligand-Target Pair | |
Histone acetyltransferase KAT2B
(Homo sapiens (Human)) | BDBM50247868
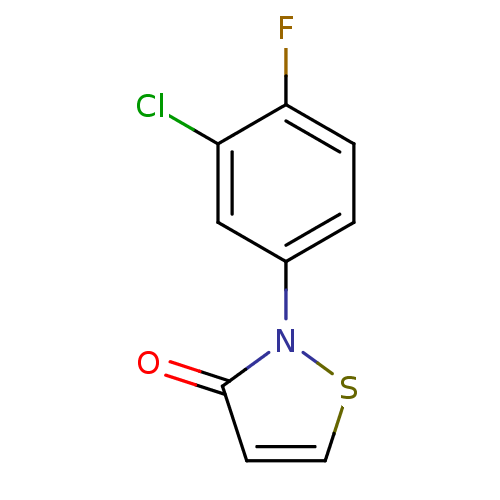
(2-(3-Chloro-4-fluorophenyl)isothiazol-3(2H)-one | ...)Show InChI InChI=1S/C9H5ClFNOS/c10-7-5-6(1-2-8(7)11)12-9(13)3-4-14-12/h1-5H | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 4.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Groningen
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant HAT PCAF |
Bioorg Med Chem 17: 460-6 (2009)
Article DOI: 10.1016/j.bmc.2008.12.008
BindingDB Entry DOI: 10.7270/Q2BC3ZDM |
More data for this
Ligand-Target Pair | |
Histone acetyltransferase KAT2B
(Homo sapiens (Human)) | BDBM50247871
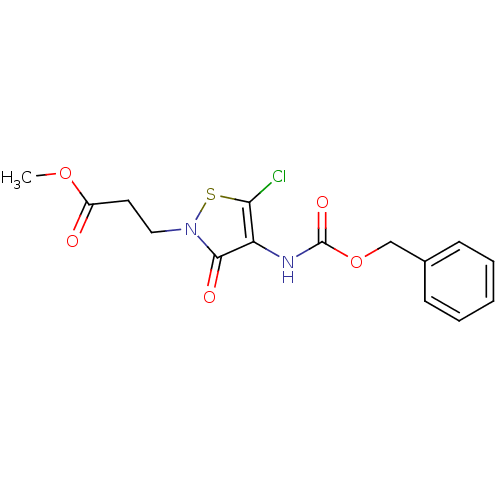
(CHEMBL521965 | Methyl 3-[4-{[(benzyloxy)carbonyl]a...)Show InChI InChI=1S/C15H15ClN2O5S/c1-22-11(19)7-8-18-14(20)12(13(16)24-18)17-15(21)23-9-10-5-3-2-4-6-10/h2-6H,7-9H2,1H3,(H,17,21) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Groningen
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant HAT PCAF |
Bioorg Med Chem 17: 460-6 (2009)
Article DOI: 10.1016/j.bmc.2008.12.008
BindingDB Entry DOI: 10.7270/Q2BC3ZDM |
More data for this
Ligand-Target Pair | |
Histone acetyltransferase KAT2B
(Homo sapiens (Human)) | BDBM50247869
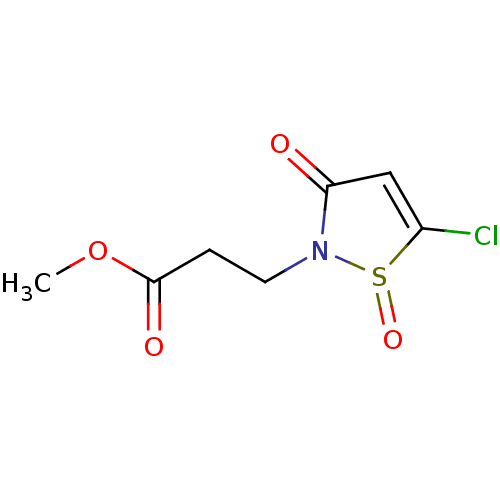
(CHEMBL509992 | methyl 3-(5-chloro-1-oxido-3-oxoiso...)Show InChI InChI=1S/C7H8ClNO4S/c1-13-7(11)2-3-9-6(10)4-5(8)14(9)12/h4H,2-3H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Groningen
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant HAT PCAF |
Bioorg Med Chem 17: 460-6 (2009)
Article DOI: 10.1016/j.bmc.2008.12.008
BindingDB Entry DOI: 10.7270/Q2BC3ZDM |
More data for this
Ligand-Target Pair | |
Histone acetyltransferase KAT2B
(Homo sapiens (Human)) | BDBM50247867
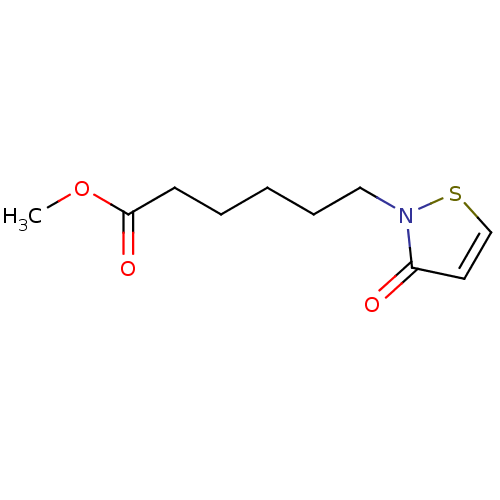
(CHEMBL500338 | methyl 6-(3-oxoisothiazol-2(3H)-yl)...)Show InChI InChI=1S/C10H15NO3S/c1-14-10(13)5-3-2-4-7-11-9(12)6-8-15-11/h6,8H,2-5,7H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Groningen
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant HAT PCAF |
Bioorg Med Chem 17: 460-6 (2009)
Article DOI: 10.1016/j.bmc.2008.12.008
BindingDB Entry DOI: 10.7270/Q2BC3ZDM |
More data for this
Ligand-Target Pair | |
Histone acetyltransferase KAT2B
(Homo sapiens (Human)) | BDBM50247866

(CHEMBL491669 | Methyl 4-(3-oxoisothiazol-2(3H)-yl)...)Show InChI InChI=1S/C8H11NO3S/c1-12-8(11)3-2-5-9-7(10)4-6-13-9/h4,6H,2-3,5H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Groningen
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant HAT PCAF |
Bioorg Med Chem 17: 460-6 (2009)
Article DOI: 10.1016/j.bmc.2008.12.008
BindingDB Entry DOI: 10.7270/Q2BC3ZDM |
More data for this
Ligand-Target Pair | |
Histone acetyltransferase KAT2B
(Homo sapiens (Human)) | BDBM50247865
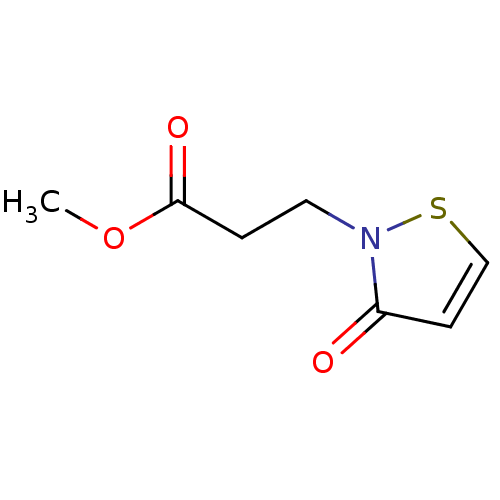
(CHEMBL450644 | Methyl 3-(3-oxoisothiazol-2(3H)-yl)...)Show InChI InChI=1S/C7H9NO3S/c1-11-7(10)2-4-8-6(9)3-5-12-8/h3,5H,2,4H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Groningen
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant HAT PCAF |
Bioorg Med Chem 17: 460-6 (2009)
Article DOI: 10.1016/j.bmc.2008.12.008
BindingDB Entry DOI: 10.7270/Q2BC3ZDM |
More data for this
Ligand-Target Pair | |
Histone acetyltransferase KAT2B
(Homo sapiens (Human)) | BDBM50247864

(2-pentylisothiazol-3(2H)-one | CHEMBL491668)Show InChI InChI=1S/C8H13NOS/c1-2-3-4-6-9-8(10)5-7-11-9/h5,7H,2-4,6H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Groningen
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant HAT PCAF |
Bioorg Med Chem 17: 460-6 (2009)
Article DOI: 10.1016/j.bmc.2008.12.008
BindingDB Entry DOI: 10.7270/Q2BC3ZDM |
More data for this
Ligand-Target Pair | |
Histone acetyltransferase KAT2B
(Homo sapiens (Human)) | BDBM50247870

(CHEMBL491670 | Methyl 3-[(5-chloroisothiazol-3-yl)...)Show InChI InChI=1S/C7H9ClN2O2S/c1-12-7(11)2-3-9-6-4-5(8)13-10-6/h4H,2-3H2,1H3,(H,9,10) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Groningen
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant HAT PCAF |
Bioorg Med Chem 17: 460-6 (2009)
Article DOI: 10.1016/j.bmc.2008.12.008
BindingDB Entry DOI: 10.7270/Q2BC3ZDM |
More data for this
Ligand-Target Pair | |
Histone acetyltransferase KAT2B
(Homo sapiens (Human)) | BDBM50247846
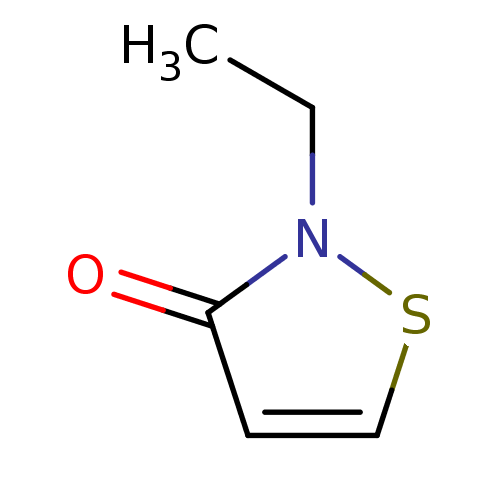
(2-Ethylisothiazol-3(2H)-one | CHEMBL490685)Show InChI InChI=1S/C5H7NOS/c1-2-6-5(7)3-4-8-6/h3-4H,2H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Groningen
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant HAT PCAF |
Bioorg Med Chem 17: 460-6 (2009)
Article DOI: 10.1016/j.bmc.2008.12.008
BindingDB Entry DOI: 10.7270/Q2BC3ZDM |
More data for this
Ligand-Target Pair | |
Seed linoleate 13S-lipoxygenase-1
(Glycine max (soybean)) | BDBM50401193

(CHEMBL1934604)Show InChI InChI=1S/C22H28O4/c1-2-3-4-5-6-7-16-8-10-17(11-9-16)12-13-18-14-19(23)15-20(24)21(18)22(25)26/h8-11,14-15,23-24H,2-7,12-13H2,1H3,(H,25,26) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.11E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Groningen Research Institute of Pharmacy
Curated by ChEMBL
| Assay Description
Inhibition of soyabean lipoxygenase-1 assessed as formation of 13-HPOD from linolenic acid preincubated for 10 mins measured by real-time spectrophot... |
Bioorg Med Chem 20: 5027-32 (2012)
Article DOI: 10.1016/j.bmc.2012.06.019
BindingDB Entry DOI: 10.7270/Q20R9QJG |
More data for this
Ligand-Target Pair | |
Probable linoleate 9S-lipoxygenase 5
(Solanum tuberosum) | BDBM50240436
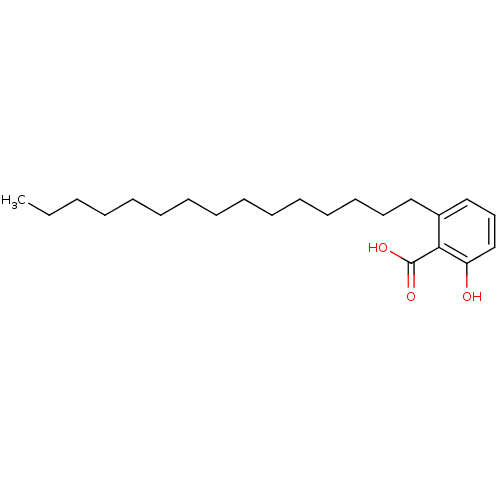
(2-Hydroxy-6-pentadecyl-benzoic acid | 2-hydroxy-6-...)Show InChI InChI=1S/C22H36O3/c1-2-3-4-5-6-7-8-9-10-11-12-13-14-16-19-17-15-18-20(23)21(19)22(24)25/h15,17-18,23H,2-14,16H2,1H3,(H,24,25) | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 4.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Groningen Research Institute of Pharmacy
Curated by ChEMBL
| Assay Description
Inhibition of potato 5-lipoxygenase assessed as residual activity preincubated for 10 mins measured by real-time spectrophotometric analysis |
Bioorg Med Chem 20: 5027-32 (2012)
Article DOI: 10.1016/j.bmc.2012.06.019
BindingDB Entry DOI: 10.7270/Q20R9QJG |
More data for this
Ligand-Target Pair | |
Seed linoleate 13S-lipoxygenase-1
(Glycine max (soybean)) | BDBM50240436

(2-Hydroxy-6-pentadecyl-benzoic acid | 2-hydroxy-6-...)Show InChI InChI=1S/C22H36O3/c1-2-3-4-5-6-7-8-9-10-11-12-13-14-16-19-17-15-18-20(23)21(19)22(24)25/h15,17-18,23H,2-14,16H2,1H3,(H,24,25) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 5.19E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Groningen Research Institute of Pharmacy
Curated by ChEMBL
| Assay Description
Inhibition of soyabean lipoxygenase-1 assessed as formation of 13-HPOD from linolenic acid preincubated for 10 mins measured by real-time spectrophot... |
Bioorg Med Chem 20: 5027-32 (2012)
Article DOI: 10.1016/j.bmc.2012.06.019
BindingDB Entry DOI: 10.7270/Q20R9QJG |
More data for this
Ligand-Target Pair | |
Seed linoleate 13S-lipoxygenase-1
(Glycine max (soybean)) | BDBM50401192

(CHEMBL418973)Show InChI InChI=1S/C21H34O4/c1-2-3-4-5-6-7-8-9-10-11-12-13-17-25-19-16-14-15-18(22)20(19)21(23)24/h14-16,22H,2-13,17H2,1H3,(H,23,24) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.54E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Groningen Research Institute of Pharmacy
Curated by ChEMBL
| Assay Description
Inhibition of soyabean lipoxygenase-1 assessed as formation of 13-HPOD from linolenic acid preincubated for 10 mins measured by real-time spectrophot... |
Bioorg Med Chem 20: 5027-32 (2012)
Article DOI: 10.1016/j.bmc.2012.06.019
BindingDB Entry DOI: 10.7270/Q20R9QJG |
More data for this
Ligand-Target Pair | |
Seed linoleate 13S-lipoxygenase-1
(Glycine max (soybean)) | BDBM50401191

(CHEMBL2206368)Show SMILES OC(=O)c1c(O)cc(O)cc1CCc1ccc(Cc2nc3ccccc3o2)cc1 Show InChI InChI=1S/C23H19NO5/c25-17-12-16(22(23(27)28)19(26)13-17)10-9-14-5-7-15(8-6-14)11-21-24-18-3-1-2-4-20(18)29-21/h1-8,12-13,25-26H,9-11H2,(H,27,28) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5.85E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Groningen Research Institute of Pharmacy
Curated by ChEMBL
| Assay Description
Inhibition of soyabean lipoxygenase-1 assessed as formation of 13-HPOD from linolenic acid preincubated for 10 mins measured by real-time spectrophot... |
Bioorg Med Chem 20: 5027-32 (2012)
Article DOI: 10.1016/j.bmc.2012.06.019
BindingDB Entry DOI: 10.7270/Q20R9QJG |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data