

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Promotilin (Homo sapiens (Human)) | BDBM50143037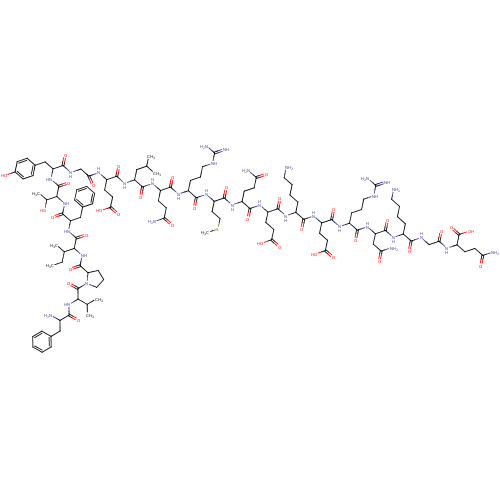 (CHEMBL411576 | MOTILIN) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by PDSP Ki Database | Br J Pharmacol 140: 948-54 (2003) Article DOI: 10.1038/sj.bjp.0705505 BindingDB Entry DOI: 10.7270/Q2N58JZF | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Promotilin (Homo sapiens (Human)) | BDBM86314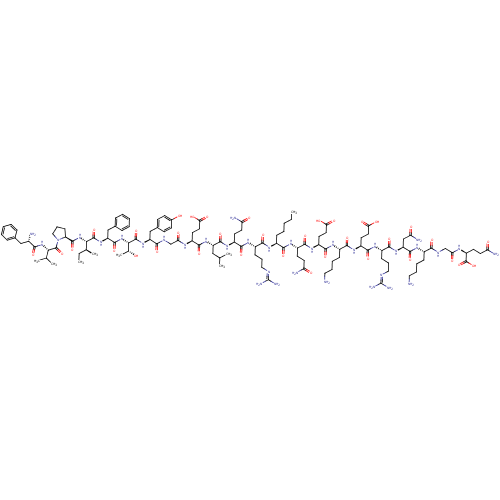 (CAS_0 | NSC_0 | [Nle13]-Motilin) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Similars | Article PubMed | 0.447 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by PDSP Ki Database | Br J Pharmacol 140: 948-54 (2003) Article DOI: 10.1038/sj.bjp.0705505 BindingDB Entry DOI: 10.7270/Q2N58JZF | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Promotilin (RABBIT) | BDBM86314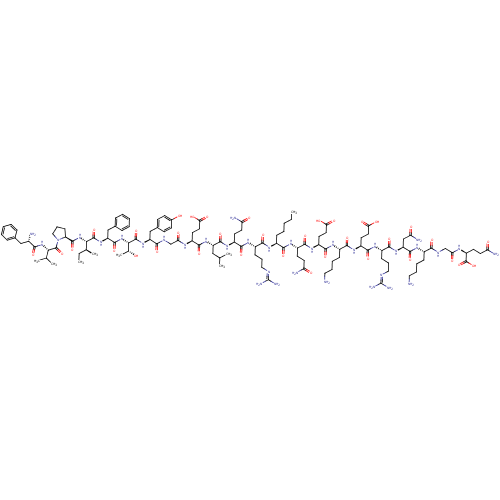 (CAS_0 | NSC_0 | [Nle13]-Motilin) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Similars | Article PubMed | 0.850 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by PDSP Ki Database | Br J Pharmacol 140: 948-54 (2003) Article DOI: 10.1038/sj.bjp.0705505 BindingDB Entry DOI: 10.7270/Q2N58JZF | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Promotilin (RABBIT) | BDBM50143037 (CHEMBL411576 | MOTILIN) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.980 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by PDSP Ki Database | Br J Pharmacol 140: 948-54 (2003) Article DOI: 10.1038/sj.bjp.0705505 BindingDB Entry DOI: 10.7270/Q2N58JZF | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Promotilin (Homo sapiens (Human)) | BDBM50143037 (CHEMBL411576 | MOTILIN) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.29 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by PDSP Ki Database | Br J Pharmacol 140: 948-54 (2003) Article DOI: 10.1038/sj.bjp.0705505 BindingDB Entry DOI: 10.7270/Q2N58JZF | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Promotilin (RABBIT) | BDBM50143037 (CHEMBL411576 | MOTILIN) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.35 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by PDSP Ki Database | Br J Pharmacol 140: 948-54 (2003) Article DOI: 10.1038/sj.bjp.0705505 BindingDB Entry DOI: 10.7270/Q2N58JZF | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Promotilin (RABBIT) | BDBM86314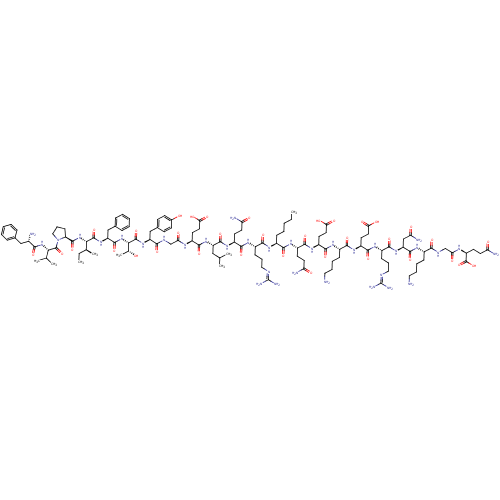 (CAS_0 | NSC_0 | [Nle13]-Motilin) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Similars | Article PubMed | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by PDSP Ki Database | Br J Pharmacol 140: 948-54 (2003) Article DOI: 10.1038/sj.bjp.0705505 BindingDB Entry DOI: 10.7270/Q2N58JZF | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Promotilin (Homo sapiens (Human)) | BDBM86314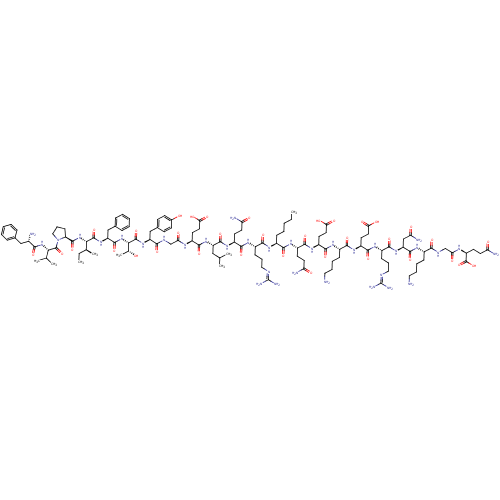 (CAS_0 | NSC_0 | [Nle13]-Motilin) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Similars | Article PubMed | 1.95 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by PDSP Ki Database | Br J Pharmacol 140: 948-54 (2003) Article DOI: 10.1038/sj.bjp.0705505 BindingDB Entry DOI: 10.7270/Q2N58JZF | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1B (Homo sapiens (Human)) | BDBM50477760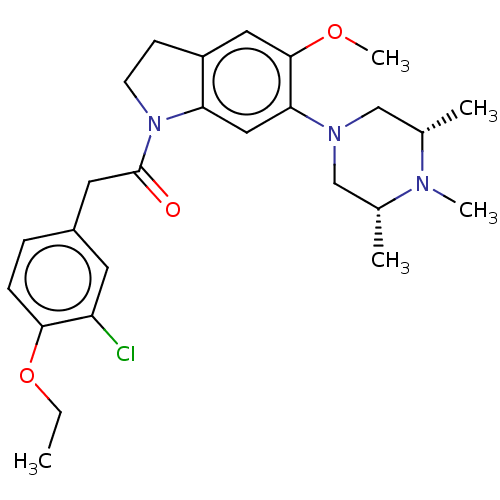 (CHEMBL250973) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Binding affinity at 5HT1B receptor | Bioorg Med Chem Lett 17: 6584-7 (2007) Article DOI: 10.1016/j.bmcl.2007.09.067 BindingDB Entry DOI: 10.7270/Q2MW2KX2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Promotilin (RABBIT) | BDBM86313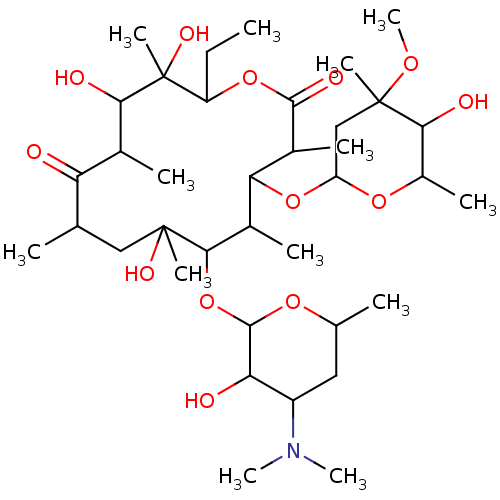 (CAS_114-07-8 | NSC_12560 | erythromycin-A) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 191 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by PDSP Ki Database | Br J Pharmacol 140: 948-54 (2003) Article DOI: 10.1038/sj.bjp.0705505 BindingDB Entry DOI: 10.7270/Q2N58JZF | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Promotilin (Homo sapiens (Human)) | BDBM86313 (CAS_114-07-8 | NSC_12560 | erythromycin-A) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 513 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by PDSP Ki Database | Br J Pharmacol 140: 948-54 (2003) Article DOI: 10.1038/sj.bjp.0705505 BindingDB Entry DOI: 10.7270/Q2N58JZF | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1B (Homo sapiens (Human)) | BDBM50477753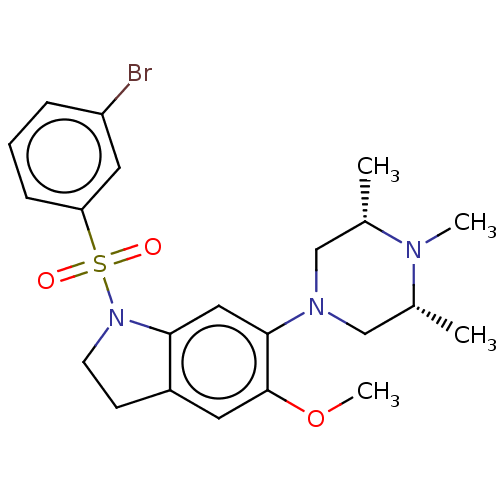 (CHEMBL399704) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Binding affinity at 5HT1B receptor | Bioorg Med Chem Lett 17: 6584-7 (2007) Article DOI: 10.1016/j.bmcl.2007.09.067 BindingDB Entry DOI: 10.7270/Q2MW2KX2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional dihydrofolate reductase-thymidylate synthase (Toxoplasma gondii) | BDBM18268 (5-methyl-6-{[(3,4,5-trimethoxyphenyl)amino]methyl}...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Vyera Pharmaceuticals, LLC Curated by ChEMBL | Assay Description Inhibition of Toxoplasma gondii DHFR-TS expressed in Escherichia coli BL21 competent cells using DHF as substrate preincubated for 15 mins followed b... | J Med Chem 62: 1562-1576 (2019) Article DOI: 10.1021/acs.jmedchem.8b01754 BindingDB Entry DOI: 10.7270/Q2T43XJX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional dihydrofolate reductase-thymidylate synthase (Toxoplasma gondii) | BDBM50531784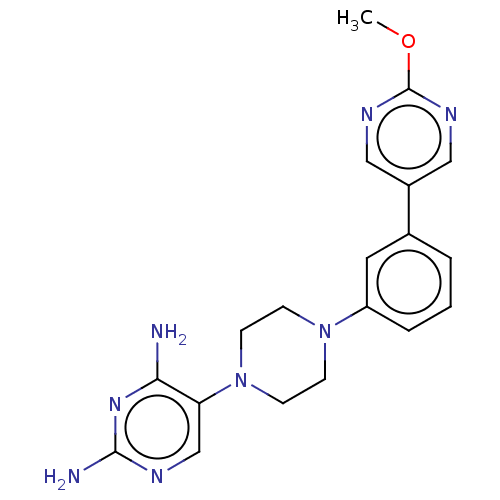 (Fanotaprim | TRC-2533 | TRC-2533-NX | US11530198, ...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | PDB Article PubMed | n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Vyera Pharmaceuticals, LLC Curated by ChEMBL | Assay Description Inhibition of Toxoplasma gondii DHFR-TS expressed in Escherichia coli BL21 competent cells using DHF as substrate preincubated for 15 mins followed b... | J Med Chem 62: 1562-1576 (2019) Article DOI: 10.1021/acs.jmedchem.8b01754 BindingDB Entry DOI: 10.7270/Q2T43XJX | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Bifunctional dihydrofolate reductase-thymidylate synthase (Toxoplasma gondii) | BDBM50531801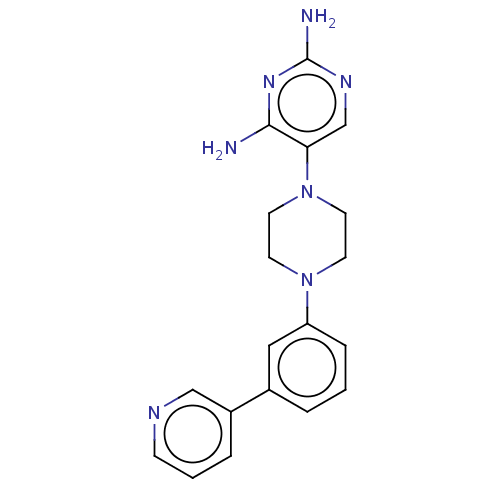 (CHEMBL4461133) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Vyera Pharmaceuticals, LLC Curated by ChEMBL | Assay Description Inhibition of Toxoplasma gondii DHFR-TS expressed in Escherichia coli BL21 competent cells using DHF as substrate preincubated for 15 mins followed b... | J Med Chem 62: 1562-1576 (2019) Article DOI: 10.1021/acs.jmedchem.8b01754 BindingDB Entry DOI: 10.7270/Q2T43XJX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional dihydrofolate reductase-thymidylate synthase (Toxoplasma gondii) | BDBM50531789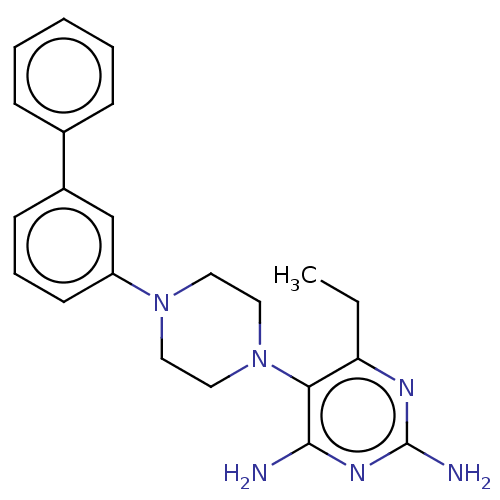 (CHEMBL4582435) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Vyera Pharmaceuticals, LLC Curated by ChEMBL | Assay Description Inhibition of Toxoplasma gondii DHFR-TS expressed in Escherichia coli BL21 competent cells using DHF as substrate preincubated for 15 mins followed b... | J Med Chem 62: 1562-1576 (2019) Article DOI: 10.1021/acs.jmedchem.8b01754 BindingDB Entry DOI: 10.7270/Q2T43XJX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional dihydrofolate reductase-thymidylate synthase (Toxoplasma gondii) | BDBM50531785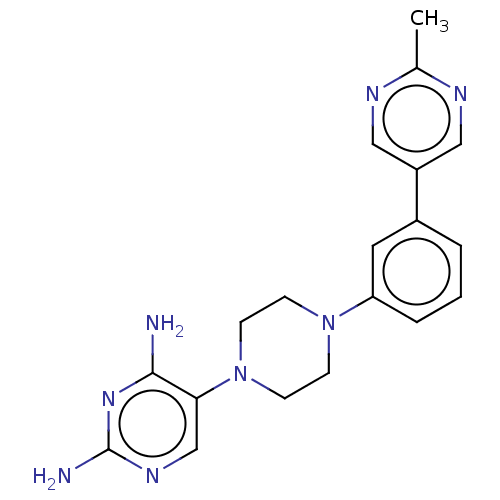 (CHEMBL4543226 | US11530198, Example 69) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid PDB UniChem | PDB Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Vyera Pharmaceuticals, LLC Curated by ChEMBL | Assay Description Inhibition of Toxoplasma gondii DHFR-TS expressed in Escherichia coli BL21 competent cells using DHF as substrate preincubated for 15 mins followed b... | J Med Chem 62: 1562-1576 (2019) Article DOI: 10.1021/acs.jmedchem.8b01754 BindingDB Entry DOI: 10.7270/Q2T43XJX | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Dihydrofolate reductase (Homo sapiens (Human)) | BDBM18268 (5-methyl-6-{[(3,4,5-trimethoxyphenyl)amino]methyl}...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank Article PubMed | n/a | n/a | 4.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Vyera Pharmaceuticals, LLC Curated by ChEMBL | Assay Description Inhibition of human DHFR expressed in Escherichia coli BL21 competent cells using DHF as substrate preincubated for 15 mins followed by substrate and... | J Med Chem 62: 1562-1576 (2019) Article DOI: 10.1021/acs.jmedchem.8b01754 BindingDB Entry DOI: 10.7270/Q2T43XJX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional dihydrofolate reductase-thymidylate synthase (Toxoplasma gondii) | BDBM50531777 (CHEMBL4591654) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Vyera Pharmaceuticals, LLC Curated by ChEMBL | Assay Description Inhibition of Toxoplasma gondii DHFR-TS expressed in Escherichia coli BL21 competent cells using DHF as substrate preincubated for 15 mins followed b... | J Med Chem 62: 1562-1576 (2019) Article DOI: 10.1021/acs.jmedchem.8b01754 BindingDB Entry DOI: 10.7270/Q2T43XJX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Homo sapiens (Human)) | BDBM66082 ((2S)-2-[[4-[(2,4-diaminopteridin-6-yl)methyl-methy...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE KEGG PC cid PC sid PDB UniChem Similars | DrugBank PDB Article PubMed | n/a | n/a | 4.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Vyera Pharmaceuticals, LLC Curated by ChEMBL | Assay Description Inhibition of human DHFR expressed in Escherichia coli BL21 competent cells using DHF as substrate preincubated for 15 mins followed by substrate and... | J Med Chem 62: 1562-1576 (2019) Article DOI: 10.1021/acs.jmedchem.8b01754 BindingDB Entry DOI: 10.7270/Q2T43XJX | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Bifunctional dihydrofolate reductase-thymidylate synthase (Toxoplasma gondii) | BDBM50531786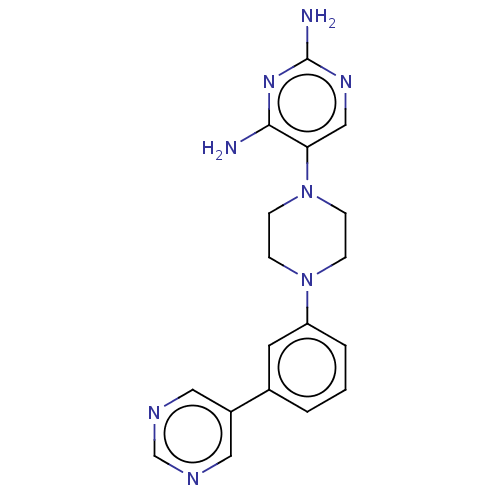 (CHEMBL4573445 | US11530198, Example 74) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Vyera Pharmaceuticals, LLC Curated by ChEMBL | Assay Description Inhibition of Toxoplasma gondii DHFR-TS expressed in Escherichia coli BL21 competent cells using DHF as substrate preincubated for 15 mins followed b... | J Med Chem 62: 1562-1576 (2019) Article DOI: 10.1021/acs.jmedchem.8b01754 BindingDB Entry DOI: 10.7270/Q2T43XJX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional dihydrofolate reductase-thymidylate synthase (Toxoplasma gondii) | BDBM50531780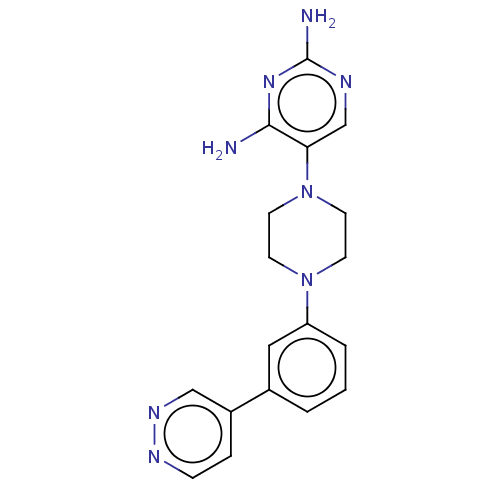 (CHEMBL4464707 | US11530198, Example 73) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Vyera Pharmaceuticals, LLC Curated by ChEMBL | Assay Description Inhibition of Toxoplasma gondii DHFR-TS expressed in Escherichia coli BL21 competent cells using DHF as substrate preincubated for 15 mins followed b... | J Med Chem 62: 1562-1576 (2019) Article DOI: 10.1021/acs.jmedchem.8b01754 BindingDB Entry DOI: 10.7270/Q2T43XJX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional dihydrofolate reductase-thymidylate synthase (Toxoplasma gondii) | BDBM50203246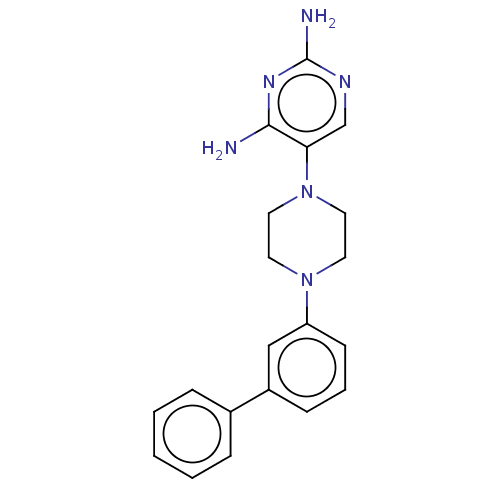 (CHEMBL3953119 | US11530198, Example 2) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Vyera Pharmaceuticals, LLC Curated by ChEMBL | Assay Description Inhibition of Toxoplasma gondii DHFR-TS expressed in Escherichia coli BL21 competent cells using DHF as substrate preincubated for 15 mins followed b... | J Med Chem 62: 1562-1576 (2019) Article DOI: 10.1021/acs.jmedchem.8b01754 BindingDB Entry DOI: 10.7270/Q2T43XJX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional dihydrofolate reductase-thymidylate synthase (Toxoplasma gondii) | BDBM50531781 (CHEMBL4459877) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 9.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Vyera Pharmaceuticals, LLC Curated by ChEMBL | Assay Description Inhibition of Toxoplasma gondii DHFR-TS expressed in Escherichia coli BL21 competent cells using DHF as substrate preincubated for 15 mins followed b... | J Med Chem 62: 1562-1576 (2019) Article DOI: 10.1021/acs.jmedchem.8b01754 BindingDB Entry DOI: 10.7270/Q2T43XJX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional dihydrofolate reductase-thymidylate synthase (Toxoplasma gondii) | BDBM50531797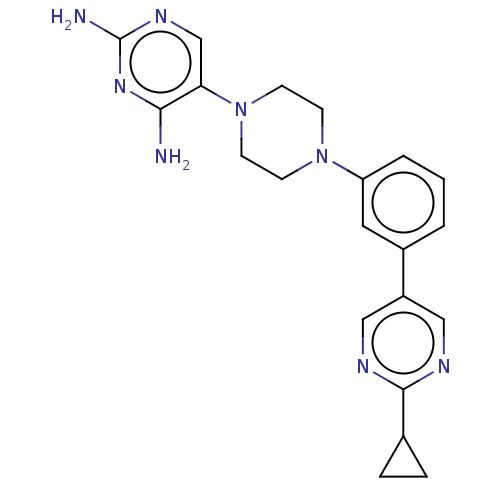 (CHEMBL4550110 | US11530198, Example 86) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 9.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Vyera Pharmaceuticals, LLC Curated by ChEMBL | Assay Description Inhibition of Toxoplasma gondii DHFR-TS expressed in Escherichia coli BL21 competent cells using DHF as substrate preincubated for 15 mins followed b... | J Med Chem 62: 1562-1576 (2019) Article DOI: 10.1021/acs.jmedchem.8b01754 BindingDB Entry DOI: 10.7270/Q2T43XJX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional dihydrofolate reductase-thymidylate synthase (Toxoplasma gondii) | BDBM50531788 (CHEMBL4519642) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Vyera Pharmaceuticals, LLC Curated by ChEMBL | Assay Description Inhibition of Toxoplasma gondii DHFR-TS expressed in Escherichia coli BL21 competent cells using DHF as substrate preincubated for 15 mins followed b... | J Med Chem 62: 1562-1576 (2019) Article DOI: 10.1021/acs.jmedchem.8b01754 BindingDB Entry DOI: 10.7270/Q2T43XJX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional dihydrofolate reductase-thymidylate synthase (Toxoplasma gondii) | BDBM50531798 (CHEMBL4466302 | US11530198, Example 104) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Vyera Pharmaceuticals, LLC Curated by ChEMBL | Assay Description Inhibition of Toxoplasma gondii DHFR-TS expressed in Escherichia coli BL21 competent cells using DHF as substrate preincubated for 15 mins followed b... | J Med Chem 62: 1562-1576 (2019) Article DOI: 10.1021/acs.jmedchem.8b01754 BindingDB Entry DOI: 10.7270/Q2T43XJX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional dihydrofolate reductase-thymidylate synthase (Toxoplasma gondii) | BDBM50531790 (CHEMBL4439025) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Vyera Pharmaceuticals, LLC Curated by ChEMBL | Assay Description Inhibition of Toxoplasma gondii DHFR-TS expressed in Escherichia coli BL21 competent cells using DHF as substrate preincubated for 15 mins followed b... | J Med Chem 62: 1562-1576 (2019) Article DOI: 10.1021/acs.jmedchem.8b01754 BindingDB Entry DOI: 10.7270/Q2T43XJX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional dihydrofolate reductase-thymidylate synthase (Toxoplasma gondii) | BDBM50531787 (CHEMBL4584470 | US11530198, Example 103) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 27 | n/a | n/a | n/a | n/a | n/a | n/a |
Vyera Pharmaceuticals, LLC Curated by ChEMBL | Assay Description Inhibition of Toxoplasma gondii DHFR-TS expressed in Escherichia coli BL21 competent cells using DHF as substrate preincubated for 15 mins followed b... | J Med Chem 62: 1562-1576 (2019) Article DOI: 10.1021/acs.jmedchem.8b01754 BindingDB Entry DOI: 10.7270/Q2T43XJX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional dihydrofolate reductase-thymidylate synthase (Toxoplasma gondii) | BDBM50531799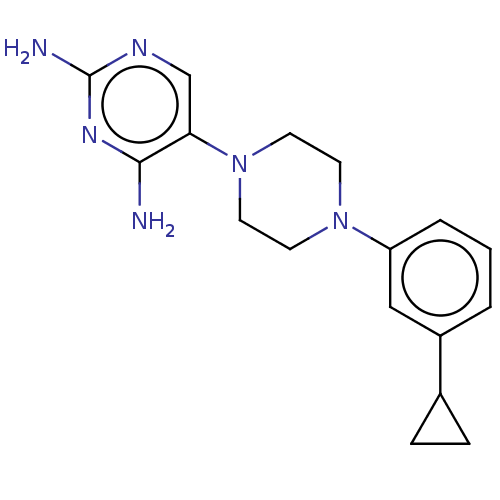 (CHEMBL4435247 | US11530198, Example 66) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 29 | n/a | n/a | n/a | n/a | n/a | n/a |
Vyera Pharmaceuticals, LLC Curated by ChEMBL | Assay Description Inhibition of Toxoplasma gondii DHFR-TS expressed in Escherichia coli BL21 competent cells using DHF as substrate preincubated for 15 mins followed b... | J Med Chem 62: 1562-1576 (2019) Article DOI: 10.1021/acs.jmedchem.8b01754 BindingDB Entry DOI: 10.7270/Q2T43XJX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM514512 (8-(7- (Trifluoromethyl)imidazo[1,5- a]pyridin-5-yl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 29.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Recombinant human IDO or TDO was incubated in 50 mM KPO4 (pH 7.0), 0.5 mM EGTA, 0.5 mM EDTA, 0.05% Triton™ X100, 20 mM ascorbate, 10 μM methylen... | Citation and Details BindingDB Entry DOI: 10.7270/Q2MK6H2F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional dihydrofolate reductase-thymidylate synthase (Toxoplasma gondii) | BDBM50531796 (CHEMBL4544467 | US11530198, Example 12) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 31 | n/a | n/a | n/a | n/a | n/a | n/a |
Vyera Pharmaceuticals, LLC Curated by ChEMBL | Assay Description Inhibition of Toxoplasma gondii DHFR-TS expressed in Escherichia coli BL21 competent cells using DHF as substrate preincubated for 15 mins followed b... | J Med Chem 62: 1562-1576 (2019) Article DOI: 10.1021/acs.jmedchem.8b01754 BindingDB Entry DOI: 10.7270/Q2T43XJX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional dihydrofolate reductase-thymidylate synthase (Toxoplasma gondii) | BDBM50531795 (CHEMBL4552468) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 32 | n/a | n/a | n/a | n/a | n/a | n/a |
Vyera Pharmaceuticals, LLC Curated by ChEMBL | Assay Description Inhibition of Toxoplasma gondii DHFR-TS expressed in Escherichia coli BL21 competent cells using DHF as substrate preincubated for 15 mins followed b... | J Med Chem 62: 1562-1576 (2019) Article DOI: 10.1021/acs.jmedchem.8b01754 BindingDB Entry DOI: 10.7270/Q2T43XJX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional dihydrofolate reductase-thymidylate synthase (Toxoplasma gondii) | BDBM50531783 (CHEMBL4591892 | US11530198, Example 87) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 33 | n/a | n/a | n/a | n/a | n/a | n/a |
Vyera Pharmaceuticals, LLC Curated by ChEMBL | Assay Description Inhibition of Toxoplasma gondii DHFR-TS expressed in Escherichia coli BL21 competent cells using DHF as substrate preincubated for 15 mins followed b... | J Med Chem 62: 1562-1576 (2019) Article DOI: 10.1021/acs.jmedchem.8b01754 BindingDB Entry DOI: 10.7270/Q2T43XJX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional dihydrofolate reductase-thymidylate synthase (Toxoplasma gondii) | BDBM50531792 (CHEMBL4544894) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
Vyera Pharmaceuticals, LLC Curated by ChEMBL | Assay Description Inhibition of Toxoplasma gondii DHFR-TS expressed in Escherichia coli BL21 competent cells using DHF as substrate preincubated for 15 mins followed b... | J Med Chem 62: 1562-1576 (2019) Article DOI: 10.1021/acs.jmedchem.8b01754 BindingDB Entry DOI: 10.7270/Q2T43XJX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional dihydrofolate reductase-thymidylate synthase (Toxoplasma gondii) | BDBM50531791 (CHEMBL4456834 | US11530198, Example 6) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 46 | n/a | n/a | n/a | n/a | n/a | n/a |
Vyera Pharmaceuticals, LLC Curated by ChEMBL | Assay Description Inhibition of Toxoplasma gondii DHFR-TS expressed in Escherichia coli BL21 competent cells using DHF as substrate preincubated for 15 mins followed b... | J Med Chem 62: 1562-1576 (2019) Article DOI: 10.1021/acs.jmedchem.8b01754 BindingDB Entry DOI: 10.7270/Q2T43XJX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional dihydrofolate reductase-thymidylate synthase (Toxoplasma gondii) | BDBM50531778 (CHEMBL4546142 | US11530198, Example 9) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 58 | n/a | n/a | n/a | n/a | n/a | n/a |
Vyera Pharmaceuticals, LLC Curated by ChEMBL | Assay Description Inhibition of Toxoplasma gondii DHFR-TS expressed in Escherichia coli BL21 competent cells using DHF as substrate preincubated for 15 mins followed b... | J Med Chem 62: 1562-1576 (2019) Article DOI: 10.1021/acs.jmedchem.8b01754 BindingDB Entry DOI: 10.7270/Q2T43XJX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional dihydrofolate reductase-thymidylate synthase (Toxoplasma gondii) | BDBM50531800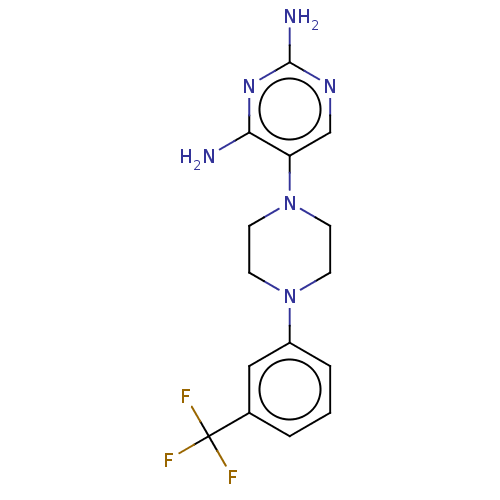 (CHEMBL4535103 | US11530198, Example 3) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 62 | n/a | n/a | n/a | n/a | n/a | n/a |
Vyera Pharmaceuticals, LLC Curated by ChEMBL | Assay Description Inhibition of Toxoplasma gondii DHFR-TS expressed in Escherichia coli BL21 competent cells using DHF as substrate preincubated for 15 mins followed b... | J Med Chem 62: 1562-1576 (2019) Article DOI: 10.1021/acs.jmedchem.8b01754 BindingDB Entry DOI: 10.7270/Q2T43XJX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM514513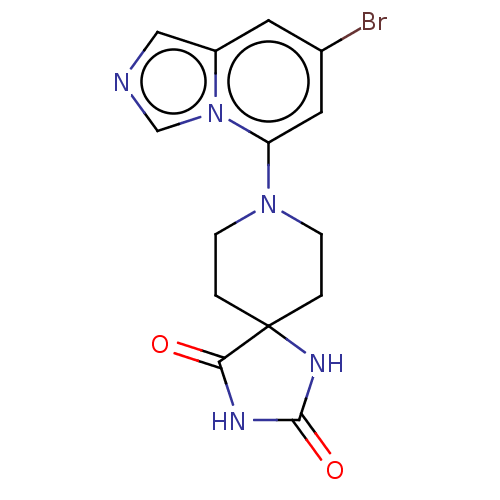 (8-(7-bromoimidazo[1,5- a]pyridin-5-yl)-1,3,8- tria...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 64.6 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Recombinant human IDO or TDO was incubated in 50 mM KPO4 (pH 7.0), 0.5 mM EGTA, 0.5 mM EDTA, 0.05% Triton™ X100, 20 mM ascorbate, 10 μM methylen... | Citation and Details BindingDB Entry DOI: 10.7270/Q2MK6H2F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional dihydrofolate reductase-thymidylate synthase (Toxoplasma gondii) | BDBM50531794 (CHEMBL4549054 | US11530198, Example 7) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 75 | n/a | n/a | n/a | n/a | n/a | n/a |
Vyera Pharmaceuticals, LLC Curated by ChEMBL | Assay Description Inhibition of Toxoplasma gondii DHFR-TS expressed in Escherichia coli BL21 competent cells using DHF as substrate preincubated for 15 mins followed b... | J Med Chem 62: 1562-1576 (2019) Article DOI: 10.1021/acs.jmedchem.8b01754 BindingDB Entry DOI: 10.7270/Q2T43XJX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional dihydrofolate reductase-thymidylate synthase (Toxoplasma gondii) | BDBM66082 ((2S)-2-[[4-[(2,4-diaminopteridin-6-yl)methyl-methy...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase DrugBank MCE KEGG PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 78 | n/a | n/a | n/a | n/a | n/a | n/a |
Vyera Pharmaceuticals, LLC Curated by ChEMBL | Assay Description Inhibition of Toxoplasma gondii DHFR-TS expressed in Escherichia coli BL21 competent cells using DHF as substrate preincubated for 15 mins followed b... | J Med Chem 62: 1562-1576 (2019) Article DOI: 10.1021/acs.jmedchem.8b01754 BindingDB Entry DOI: 10.7270/Q2T43XJX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM514559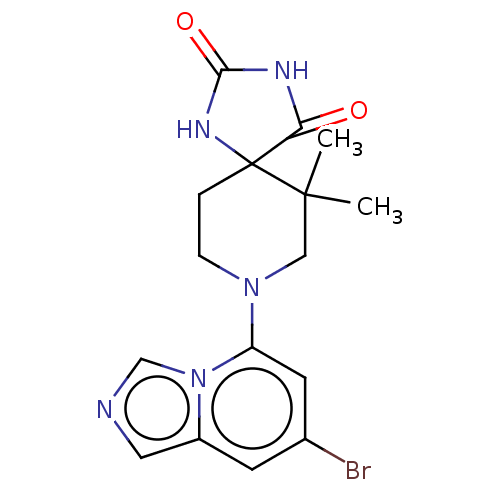 (8-(7-bromoimidazo[1,5- a]pyridin-5-yl)-6,6-dimethy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 87.1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Recombinant human IDO or TDO was incubated in 50 mM KPO4 (pH 7.0), 0.5 mM EGTA, 0.5 mM EDTA, 0.05% Triton™ X100, 20 mM ascorbate, 10 μM methylen... | Citation and Details BindingDB Entry DOI: 10.7270/Q2MK6H2F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM514527 (8-(7-chloroimidazo[1,5- a]pyridin-5-yl)-6,6-dimeth...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 102 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Recombinant human IDO or TDO was incubated in 50 mM KPO4 (pH 7.0), 0.5 mM EGTA, 0.5 mM EDTA, 0.05% Triton™ X100, 20 mM ascorbate, 10 μM methylen... | Citation and Details BindingDB Entry DOI: 10.7270/Q2MK6H2F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional dihydrofolate reductase-thymidylate synthase (Toxoplasma gondii) | BDBM18512 (5-(4-chlorophenyl)-6-ethylpyrimidine-2,4-diamine |...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 139 | n/a | n/a | n/a | n/a | n/a | n/a |
Vyera Pharmaceuticals, LLC Curated by ChEMBL | Assay Description Inhibition of Toxoplasma gondii DHFR-TS expressed in Escherichia coli BL21 competent cells using DHF as substrate preincubated for 15 mins followed b... | J Med Chem 62: 1562-1576 (2019) Article DOI: 10.1021/acs.jmedchem.8b01754 BindingDB Entry DOI: 10.7270/Q2T43XJX | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM514527 (8-(7-chloroimidazo[1,5- a]pyridin-5-yl)-6,6-dimeth...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 148 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Recombinant human IDO or TDO was incubated in 50 mM KPO4 (pH 7.0), 0.5 mM EGTA, 0.5 mM EDTA, 0.05% Triton™ X100, 20 mM ascorbate, 10 μM methylen... | Citation and Details BindingDB Entry DOI: 10.7270/Q2MK6H2F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM423348 (US10501458, Compound 1021) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 158 | n/a | n/a | n/a | n/a | n/a | n/a |
Iomet Pharma Ltd.; Merck Sharp & Dohme Corp. US Patent | Assay Description Compounds to be tested were serially diluted in ten 3-fold steps in DMSO starting from 10 mM DMSO stocks. Compound dilutions or DMSO alone were then ... | US Patent US10501458 (2019) BindingDB Entry DOI: 10.7270/Q26T0Q1W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional dihydrofolate reductase-thymidylate synthase (Toxoplasma gondii) | BDBM50531793 (CHEMBL4525462 | US11530198, Example 8) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 181 | n/a | n/a | n/a | n/a | n/a | n/a |
Vyera Pharmaceuticals, LLC Curated by ChEMBL | Assay Description Inhibition of Toxoplasma gondii DHFR-TS expressed in Escherichia coli BL21 competent cells using DHF as substrate preincubated for 15 mins followed b... | J Med Chem 62: 1562-1576 (2019) Article DOI: 10.1021/acs.jmedchem.8b01754 BindingDB Entry DOI: 10.7270/Q2T43XJX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Homo sapiens (Human)) | BDBM50531789 (CHEMBL4582435) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
Vyera Pharmaceuticals, LLC Curated by ChEMBL | Assay Description Inhibition of human DHFR expressed in Escherichia coli BL21 competent cells using DHF as substrate preincubated for 15 mins followed by substrate and... | J Med Chem 62: 1562-1576 (2019) Article DOI: 10.1021/acs.jmedchem.8b01754 BindingDB Entry DOI: 10.7270/Q2T43XJX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional dihydrofolate reductase-thymidylate synthase (Toxoplasma gondii) | BDBM50531779 (CHEMBL4553229 | US11530198, Example 4) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 209 | n/a | n/a | n/a | n/a | n/a | n/a |
Vyera Pharmaceuticals, LLC Curated by ChEMBL | Assay Description Inhibition of Toxoplasma gondii DHFR-TS expressed in Escherichia coli BL21 competent cells using DHF as substrate preincubated for 15 mins followed b... | J Med Chem 62: 1562-1576 (2019) Article DOI: 10.1021/acs.jmedchem.8b01754 BindingDB Entry DOI: 10.7270/Q2T43XJX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Homo sapiens (Human)) | BDBM50531781 (CHEMBL4459877) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 237 | n/a | n/a | n/a | n/a | n/a | n/a |
Vyera Pharmaceuticals, LLC Curated by ChEMBL | Assay Description Inhibition of human DHFR expressed in Escherichia coli BL21 competent cells using DHF as substrate preincubated for 15 mins followed by substrate and... | J Med Chem 62: 1562-1576 (2019) Article DOI: 10.1021/acs.jmedchem.8b01754 BindingDB Entry DOI: 10.7270/Q2T43XJX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 231 total ) | Next | Last >> |