 Found 391 hits with Last Name = 'wittlin' and Initial = 's'
Found 391 hits with Last Name = 'wittlin' and Initial = 's' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Dihydrofolate reductase
(Homo sapiens (Human)) | BDBM18793
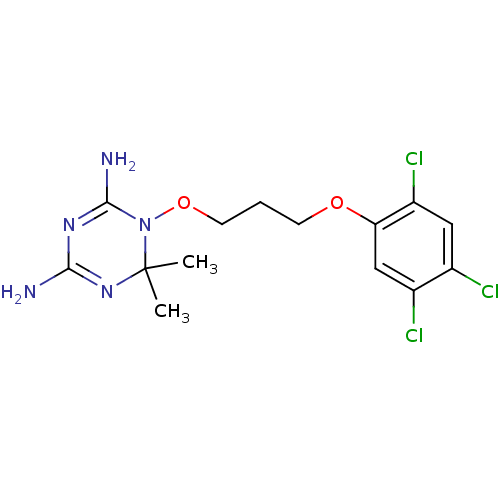
(6,6-dimethyl-1-[3-(2,4,5-trichlorophenoxy)propoxy]...)Show SMILES CC1(C)N=C(N)N=C(N)N1OCCCOc1cc(Cl)c(Cl)cc1Cl |t:3,6| Show InChI InChI=1S/C14H18Cl3N5O2/c1-14(2)21-12(18)20-13(19)22(14)24-5-3-4-23-11-7-9(16)8(15)6-10(11)17/h6-7H,3-5H2,1-2H3,(H4,18,19,20,21) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
| Article
PubMed
| 7.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kenya Medical Research Institute/Wellcome Trust Collaborative Research Program
Curated by ChEMBL
| Assay Description
Binding affinity to human recombinant DHFR expressed in Escherichia coli BL21(DE3) by competitive binding assay |
Antimicrob Agents Chemother 54: 2603-10 (2010)
Article DOI: 10.1128/AAC.01526-09
BindingDB Entry DOI: 10.7270/Q2VX0GQW |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Homo sapiens (Human)) | BDBM50035483
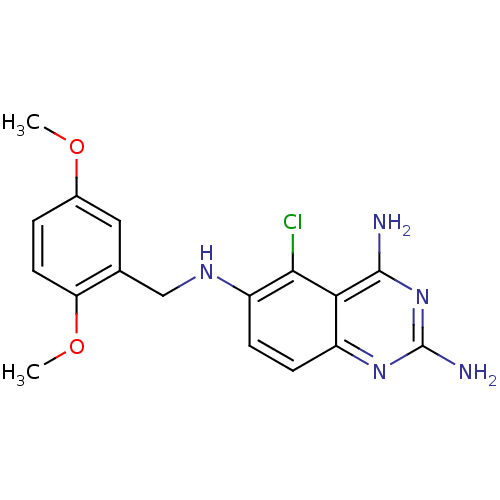
(5-Chloro-N*6*-(2,5-dimethoxy-benzyl)-quinazoline-2...)Show InChI InChI=1S/C17H18ClN5O2/c1-24-10-3-6-13(25-2)9(7-10)8-21-12-5-4-11-14(15(12)18)16(19)23-17(20)22-11/h3-7,21H,8H2,1-2H3,(H4,19,20,22,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 10.2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kenya Medical Research Institute/Wellcome Trust Collaborative Research Program
Curated by ChEMBL
| Assay Description
Binding affinity to human recombinant DHFR expressed in Escherichia coli BL21(DE3) by competitive binding assay |
Antimicrob Agents Chemother 54: 2603-10 (2010)
Article DOI: 10.1128/AAC.01526-09
BindingDB Entry DOI: 10.7270/Q2VX0GQW |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Homo sapiens (Human)) | BDBM18512

(5-(4-chlorophenyl)-6-ethylpyrimidine-2,4-diamine |...)Show InChI InChI=1S/C12H13ClN4/c1-2-9-10(11(14)17-12(15)16-9)7-3-5-8(13)6-4-7/h3-6H,2H2,1H3,(H4,14,15,16,17) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| 30.8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kenya Medical Research Institute/Wellcome Trust Collaborative Research Program
Curated by ChEMBL
| Assay Description
Binding affinity to human recombinant DHFR expressed in Escherichia coli BL21(DE3) by competitive binding assay |
Antimicrob Agents Chemother 54: 2603-10 (2010)
Article DOI: 10.1128/AAC.01526-09
BindingDB Entry DOI: 10.7270/Q2VX0GQW |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Homo sapiens (Human)) | BDBM18792
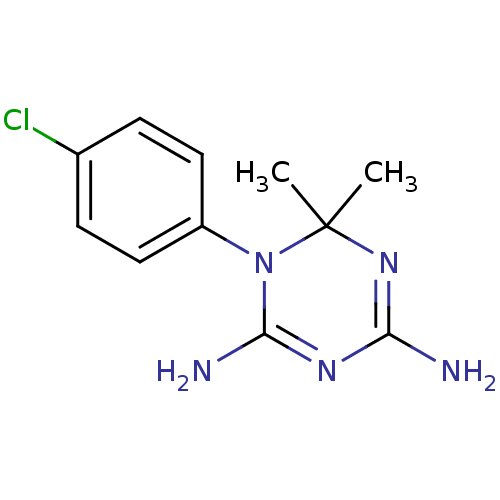
(1-(4-chlorophenyl)-6,6-dimethyl-1,6-dihydro-1,3,5-...)Show InChI InChI=1S/C11H14ClN5/c1-11(2)16-9(13)15-10(14)17(11)8-5-3-7(12)4-6-8/h3-6H,1-2H3,(H4,13,14,15,16) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 55.6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kenya Medical Research Institute/Wellcome Trust Collaborative Research Program
Curated by ChEMBL
| Assay Description
Binding affinity to human recombinant DHFR expressed in Escherichia coli BL21(DE3) by competitive binding assay |
Antimicrob Agents Chemother 54: 2603-10 (2010)
Article DOI: 10.1128/AAC.01526-09
BindingDB Entry DOI: 10.7270/Q2VX0GQW |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM24226

(1-[(4-fluorophenyl)methyl]-N-{1-[2-(4-methoxypheny...)Show SMILES COc1ccc(CCN2CCC(CC2)Nc2nc3ccccc3n2Cc2ccc(F)cc2)cc1 Show InChI InChI=1S/C28H31FN4O/c1-34-25-12-8-21(9-13-25)14-17-32-18-15-24(16-19-32)30-28-31-26-4-2-3-5-27(26)33(28)20-22-6-10-23(29)11-7-22/h2-13,24H,14-20H2,1H3,(H,30,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
| n/a | n/a | 4.20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Plasmepsin X
(Plasmodium falciparum (isolate 3D7)) | BDBM50591320

(CHEMBL5190700)Show SMILES C[C@H]1C[C@H](CCO1)N1C(=O)C[C@](C)(NC1=N)c1cccc(NC(=O)c2cccc(c2)C#N)c1Cl |r| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01336
BindingDB Entry DOI: 10.7270/Q2988C16 |
More data for this
Ligand-Target Pair | |
Plasmepsin X
(Plasmodium falciparum (isolate 3D7)) | BDBM50591320

(CHEMBL5190700)Show SMILES C[C@H]1C[C@H](CCO1)N1C(=O)C[C@](C)(NC1=N)c1cccc(NC(=O)c2cccc(c2)C#N)c1Cl |r| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01336
BindingDB Entry DOI: 10.7270/Q2988C16 |
More data for this
Ligand-Target Pair | |
Plasmepsin X
(Plasmodium falciparum (isolate 3D7)) | BDBM50591317
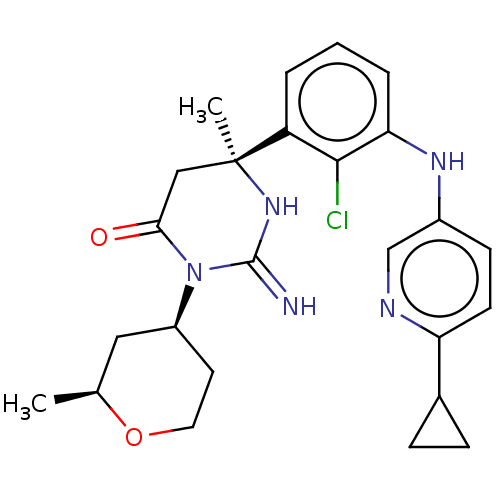
(CHEMBL5191783)Show SMILES C[C@H]1C[C@H](CCO1)N1C(=O)C[C@](C)(NC1=N)c1cccc(Nc2ccc(nc2)C2CC2)c1Cl |r| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01336
BindingDB Entry DOI: 10.7270/Q2988C16 |
More data for this
Ligand-Target Pair | |
Plasmepsin X
(Plasmodium falciparum (isolate 3D7)) | BDBM50591318

(CHEMBL5204196)Show SMILES C[C@H]1C[C@H](CCO1)N1C(=O)C[C@](C)(NC1=N)c1cccc(Nc2cnc(nc2)C2CC2)c1Cl |r| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01336
BindingDB Entry DOI: 10.7270/Q2988C16 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Plasmepsin X
(Plasmodium falciparum (isolate 3D7)) | BDBM50591315
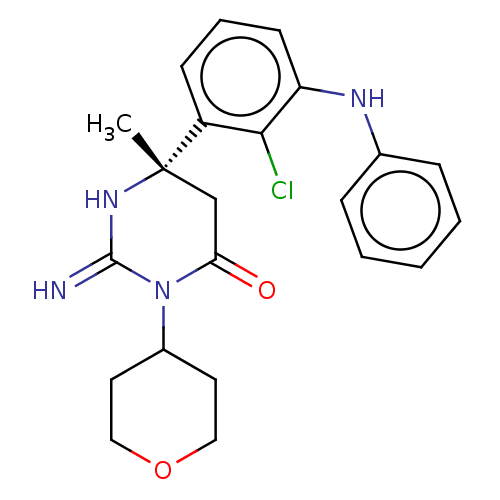
(CHEMBL5172999)Show SMILES C[C@]1(CC(=O)N(C2CCOCC2)C(=N)N1)c1cccc(Nc2ccccc2)c1Cl |r| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01336
BindingDB Entry DOI: 10.7270/Q2988C16 |
More data for this
Ligand-Target Pair | |
Dihydroorotate dehydrogenase (quinone), mitochondrial
(Rattus norvegicus (rat)) | BDBM50018011
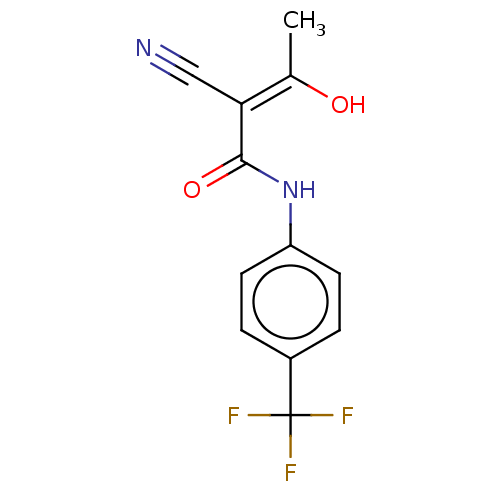
(Aubagio | CHEBI:68540 | HMR-1726 | TERIFLUNOMIDE)Show InChI InChI=1S/C12H9F3N2O2/c1-7(18)10(6-16)11(19)17-9-4-2-8(3-5-9)12(13,14)15/h2-5,18H,1H3,(H,17,19)/b10-7- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Washington
Curated by ChEMBL
| Assay Description
Inhibition of C-terminal His6-tagged rat DHODH expressed in Escherichia coli BL21(DE3) cells using L-DHO as substrate by DCIP dye based assay |
J Med Chem 63: 4929-4956 (2020)
Article DOI: 10.1021/acs.jmedchem.0c00311
BindingDB Entry DOI: 10.7270/Q2M90D6Z |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Plasmepsin X
(Plasmodium falciparum (isolate 3D7)) | BDBM50591319

(CHEMBL5204856)Show SMILES C[C@]1(CC(=O)N(C2CCOCC2)C(=N)N1)c1cccc(NC(=O)c2ccccc2)c1Cl |r| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 32 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01336
BindingDB Entry DOI: 10.7270/Q2988C16 |
More data for this
Ligand-Target Pair | |
Plasmepsin X
(Plasmodium falciparum (isolate 3D7)) | BDBM50591316

(CHEMBL5208335)Show SMILES C[C@]1(CC(=O)N(C2CCOCC2)C(=N)N1)c1cccc(Nc2ccc(nc2)C2CC2)c1Cl |r| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 37 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01336
BindingDB Entry DOI: 10.7270/Q2988C16 |
More data for this
Ligand-Target Pair | |
Cathepsin D
(Homo sapiens (Human)) | BDBM50591315

(CHEMBL5172999)Show SMILES C[C@]1(CC(=O)N(C2CCOCC2)C(=N)N1)c1cccc(Nc2ccccc2)c1Cl |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 58 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01336
BindingDB Entry DOI: 10.7270/Q2988C16 |
More data for this
Ligand-Target Pair | |
Cathepsin D
(Homo sapiens (Human)) | BDBM50591317

(CHEMBL5191783)Show SMILES C[C@H]1C[C@H](CCO1)N1C(=O)C[C@](C)(NC1=N)c1cccc(Nc2ccc(nc2)C2CC2)c1Cl |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 64 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01336
BindingDB Entry DOI: 10.7270/Q2988C16 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50238633
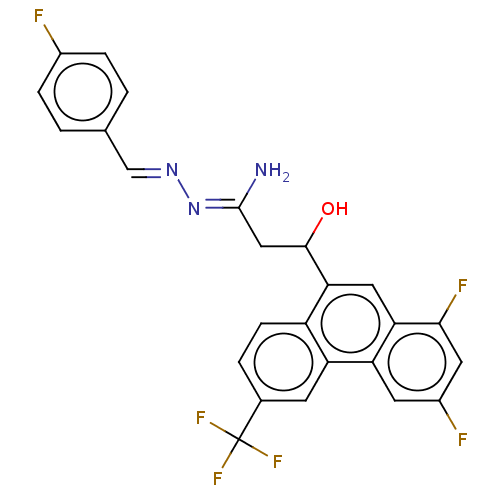
(CHEMBL4081080)Show SMILES N\C(CC(O)c1cc2c(F)cc(F)cc2c2cc(ccc12)C(F)(F)F)=N/N=C/c1ccc(F)cc1 Show InChI InChI=1S/C25H17F6N3O/c26-15-4-1-13(2-5-15)12-33-34-24(32)11-23(35)21-10-20-19(8-16(27)9-22(20)28)18-7-14(25(29,30)31)3-6-17(18)21/h1-10,12,23,35H,11H2,(H2,32,34)/b33-12+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 67 | n/a | n/a | n/a | n/a | n/a | n/a |
Heinrich-Heine-Universit£t D£sseldorf
Curated by ChEMBL
| Assay Description
Inhibition of human ERG by fluorescence polarization assay |
J Med Chem 60: 6036-6044 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00140
BindingDB Entry DOI: 10.7270/Q24M96TQ |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50238633

(CHEMBL4081080)Show SMILES N\C(CC(O)c1cc2c(F)cc(F)cc2c2cc(ccc12)C(F)(F)F)=N/N=C/c1ccc(F)cc1 Show InChI InChI=1S/C25H17F6N3O/c26-15-4-1-13(2-5-15)12-33-34-24(32)11-23(35)21-10-20-19(8-16(27)9-22(20)28)18-7-14(25(29,30)31)3-6-17(18)21/h1-10,12,23,35H,11H2,(H2,32,34)/b33-12+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 70 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01744
BindingDB Entry DOI: 10.7270/Q2WQ07WG |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50536194

(CHEMBL4569641)Show SMILES C(C1CCN(CC1)c1cc(nc(n1)-c1ccncc1)-c1cccnc1)N1CCOCC1 Show InChI InChI=1S/C24H28N6O/c1-2-21(17-26-7-1)22-16-23(28-24(27-22)20-3-8-25-9-4-20)30-10-5-19(6-11-30)18-29-12-14-31-15-13-29/h1-4,7-9,16-17,19H,5-6,10-15,18H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Dundee
Curated by ChEMBL
| Assay Description
Inhibition of human CYP3A4 expressed in Escherichia coli pre-incubated for 5 mins before regenerating cofactor solution addition using Diethoxyfluore... |
J Med Chem 59: 6101-20 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00028
BindingDB Entry DOI: 10.7270/Q2H41VZX |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50536193
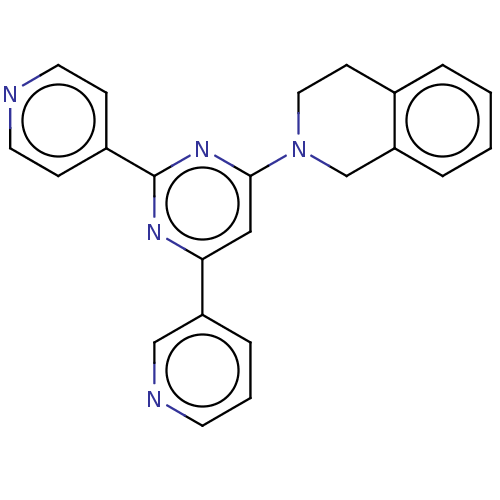
(CHEMBL548646 | GNF-Pf-1447 | TCMDC-125419)Show SMILES C1Cc2ccccc2CN1c1cc(nc(n1)-c1ccncc1)-c1cccnc1 Show InChI InChI=1S/C23H19N5/c1-2-5-20-16-28(13-9-17(20)4-1)22-14-21(19-6-3-10-25-15-19)26-23(27-22)18-7-11-24-12-8-18/h1-8,10-12,14-15H,9,13,16H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Dundee
Curated by ChEMBL
| Assay Description
Inhibition of human CYP3A4 expressed in Escherichia coli pre-incubated for 5 mins before regenerating cofactor solution addition using Diethoxyfluore... |
J Med Chem 59: 6101-20 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00028
BindingDB Entry DOI: 10.7270/Q2H41VZX |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50536191

(CHEMBL4584780)Show SMILES C1CN(CCO1)C1CCN(CC1)c1cc(nc(n1)-c1ccncc1)-c1cccnc1 Show InChI InChI=1S/C23H26N6O/c1-2-19(17-25-7-1)21-16-22(27-23(26-21)18-3-8-24-9-4-18)29-10-5-20(6-11-29)28-12-14-30-15-13-28/h1-4,7-9,16-17,20H,5-6,10-15H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Dundee
Curated by ChEMBL
| Assay Description
Inhibition of human CYP3A4 expressed in Escherichia coli pre-incubated for 5 mins before regenerating cofactor solution addition using Diethoxyfluore... |
J Med Chem 59: 6101-20 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00028
BindingDB Entry DOI: 10.7270/Q2H41VZX |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50609885

(CHEMBL5273046) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| | n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50379157
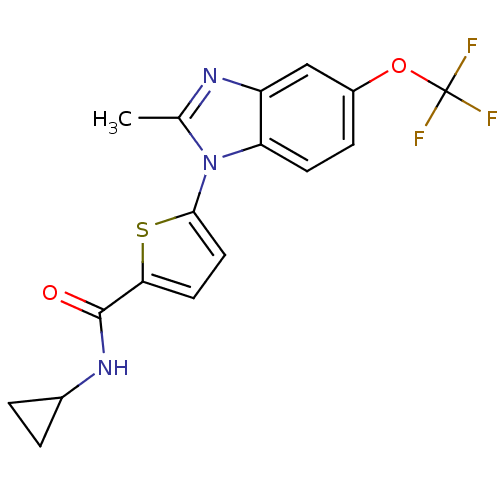
(CHEMBL1234899 | US8703811, 57)Show SMILES Cc1nc2cc(OC(F)(F)F)ccc2n1-c1ccc(s1)C(=O)NC1CC1 Show InChI InChI=1S/C17H14F3N3O2S/c1-9-21-12-8-11(25-17(18,19)20)4-5-13(12)23(9)15-7-6-14(26-15)16(24)22-10-2-3-10/h4-8,10H,2-3H2,1H3,(H,22,24) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of human CYP2D6 using bufuralol as substrate after 10 mins by LC-MS/MS analysis |
ACS Med Chem Lett 2: 708-713 (2011)
Article DOI: 10.1021/ml200143c
BindingDB Entry DOI: 10.7270/Q2C24XDS |
More data for this
Ligand-Target Pair | |
Dihydroorotate dehydrogenase (quinone), mitochondrial
(Mus musculus) | BDBM50018011

(Aubagio | CHEBI:68540 | HMR-1726 | TERIFLUNOMIDE)Show InChI InChI=1S/C12H9F3N2O2/c1-7(18)10(6-16)11(19)17-9-4-2-8(3-5-9)12(13,14)15/h2-5,18H,1H3,(H,17,19)/b10-7- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Washington
Curated by ChEMBL
| Assay Description
Inhibition of C-terminal His6-tagged mouse DHODH expressed in Escherichia coli BL21(DE3) cells using L-DHO as substrate by DCIP dye based assay |
J Med Chem 63: 4929-4956 (2020)
Article DOI: 10.1021/acs.jmedchem.0c00311
BindingDB Entry DOI: 10.7270/Q2M90D6Z |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Cytochrome P450 2C19
(Homo sapiens (Human)) | BDBM50305505

(CHEMBL227855 | OZ-209 | dispiro[adamantane-2,2'-[1...)Show SMILES NC[C@H]1CC[C@]2(CC1)OO[C@@]1(O2)C2CC3CC(C2)CC1C3 |r,wU:10.10,2.1,wD:5.12,TLB:20:19:15.14.13:17,11:10:16.17.18:14.13.20,9:10:15.14.13:17,THB:20:14:17:10.18.19,11:10:15.14.13:17,9:10:16.17.18:14.13.20,15:14:10:16.17.18,15:16:10:14.13.20,(42.83,-6.44,;42.06,-5.09,;40.52,-5.09,;39.74,-6.41,;38.21,-6.41,;37.44,-5.07,;38.21,-3.74,;39.75,-3.75,;36.96,-3.61,;35.42,-3.62,;34.95,-5.09,;36.2,-5.99,;33.79,-6.1,;32.6,-5.28,;32.09,-3.96,;30.33,-3.92,;31.64,-4.76,;32.15,-6.19,;32.83,-3.69,;34.45,-3.65,;33.19,-2.96,)| Show InChI InChI=1S/C17H27NO3/c18-10-11-1-3-16(4-2-11)19-17(21-20-16)14-6-12-5-13(8-14)9-15(17)7-12/h11-15H,1-10,18H2/t11-,12?,13?,14?,15?,16+,17- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Nebraska Medical Center
Curated by ChEMBL
| Assay Description
Inhibition of CYP2C19 |
Bioorg Med Chem Lett 20: 563-6 (2010)
Article DOI: 10.1016/j.bmcl.2009.11.088
BindingDB Entry DOI: 10.7270/Q2RJ4JKQ |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50609892
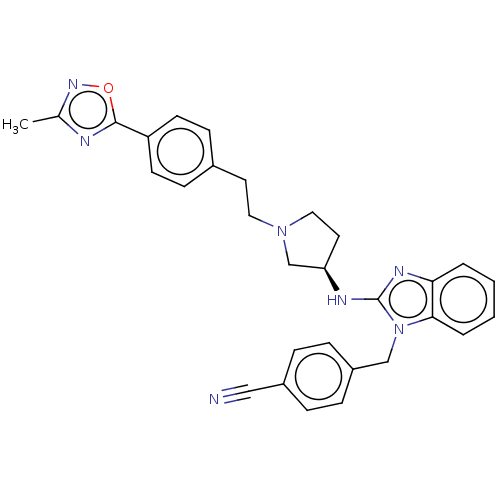
(CHEMBL5291291) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| | n/a | n/a | 130 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Plasmepsin IX
(Plasmodium falciparum (isolate 3D7)) | BDBM50591320

(CHEMBL5190700)Show SMILES C[C@H]1C[C@H](CCO1)N1C(=O)C[C@](C)(NC1=N)c1cccc(NC(=O)c2cccc(c2)C#N)c1Cl |r| | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 142 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01336
BindingDB Entry DOI: 10.7270/Q2988C16 |
More data for this
Ligand-Target Pair | |
Plasmepsin IX
(Plasmodium falciparum (isolate 3D7)) | BDBM50591320

(CHEMBL5190700)Show SMILES C[C@H]1C[C@H](CCO1)N1C(=O)C[C@](C)(NC1=N)c1cccc(NC(=O)c2cccc(c2)C#N)c1Cl |r| | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 142 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01336
BindingDB Entry DOI: 10.7270/Q2988C16 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM79214
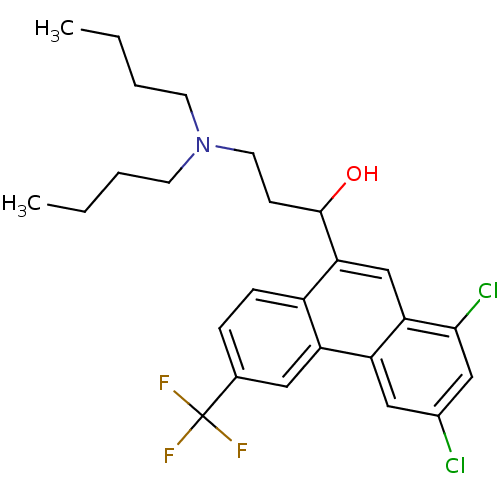
(1-[1,3-bis(chloranyl)-6-(trifluoromethyl)phenanthr...)Show SMILES CCCCN(CCCC)CCC(O)c1cc2c(Cl)cc(Cl)cc2c2cc(ccc12)C(F)(F)F Show InChI InChI=1S/C26H30Cl2F3NO/c1-3-5-10-32(11-6-4-2)12-9-25(33)23-16-22-21(14-18(27)15-24(22)28)20-13-17(26(29,30)31)7-8-19(20)23/h7-8,13-16,25,33H,3-6,9-12H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
PC cid
PC sid
UniChem
Similars
| DrugBank
Article
PubMed
| n/a | n/a | 156 | n/a | n/a | n/a | n/a | n/a | n/a |
Heinrich-Heine-Universit£t D£sseldorf
Curated by ChEMBL
| Assay Description
Inhibition of 5-HT uptake in rat synaptosomal fraction |
J Med Chem 60: 6036-6044 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00140
BindingDB Entry DOI: 10.7270/Q24M96TQ |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM79214

(1-[1,3-bis(chloranyl)-6-(trifluoromethyl)phenanthr...)Show SMILES CCCCN(CCCC)CCC(O)c1cc2c(Cl)cc(Cl)cc2c2cc(ccc12)C(F)(F)F Show InChI InChI=1S/C26H30Cl2F3NO/c1-3-5-10-32(11-6-4-2)12-9-25(33)23-16-22-21(14-18(27)15-24(22)28)20-13-17(26(29,30)31)7-8-19(20)23/h7-8,13-16,25,33H,3-6,9-12H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
PC cid
PC sid
UniChem
Similars
| DrugBank
Article
PubMed
| n/a | n/a | 160 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01744
BindingDB Entry DOI: 10.7270/Q2WQ07WG |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50591317

(CHEMBL5191783)Show SMILES C[C@H]1C[C@H](CCO1)N1C(=O)C[C@](C)(NC1=N)c1cccc(Nc2ccc(nc2)C2CC2)c1Cl |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 180 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01336
BindingDB Entry DOI: 10.7270/Q2988C16 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50606526

(CHEMBL5219819)Show SMILES Cl.COc1ccc(cc1)\N=C(/N)CC(O)c1cc2c(F)cc(F)cc2c2cc(ccc12)C(F)(F)F | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 190 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01744
BindingDB Entry DOI: 10.7270/Q2WQ07WG |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM81939
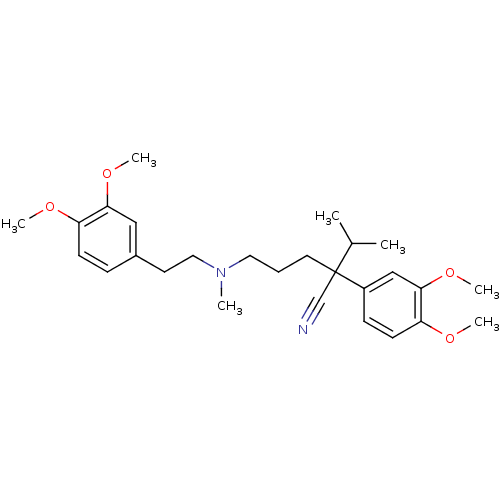
(CAS_52-53-9 | NSC_62969 | VERAPAMIL)Show SMILES COc1ccc(CCN(C)CCCC(C#N)(C(C)C)c2ccc(OC)c(OC)c2)cc1OC Show InChI InChI=1S/C27H38N2O4/c1-20(2)27(19-28,22-10-12-24(31-5)26(18-22)33-7)14-8-15-29(3)16-13-21-9-11-23(30-4)25(17-21)32-6/h9-12,17-18,20H,8,13-16H2,1-7H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Similars
| DrugBank
Article
PubMed
| n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Cape Town
Curated by ChEMBL
| Assay Description
Inhibition of human ERG expressed in CHO cells assessed as reduction in peak channel current by IonWork patch-clamp electrophysiology method |
J Med Chem 62: 1022-1035 (2019)
Article DOI: 10.1021/acs.jmedchem.8b01769
BindingDB Entry DOI: 10.7270/Q2HX1H22 |
More data for this
Ligand-Target Pair | |
Cathepsin D
(Homo sapiens (Human)) | BDBM50591318

(CHEMBL5204196)Show SMILES C[C@H]1C[C@H](CCO1)N1C(=O)C[C@](C)(NC1=N)c1cccc(Nc2cnc(nc2)C2CC2)c1Cl |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | 215 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01336
BindingDB Entry DOI: 10.7270/Q2988C16 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50567995
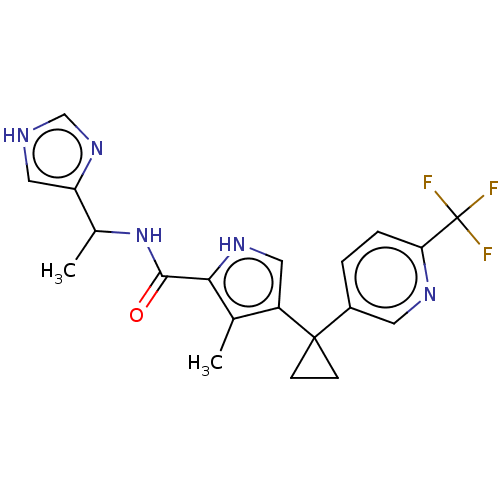
(CHEMBL4851471 | US11903936, Compound 20)Show SMILES CC(NC(=O)c1[nH]cc(c1C)C1(CC1)c1ccc(nc1)C(F)(F)F)c1c[nH]cn1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | <250 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of CYP2D6 (unknown origin) by UPLC-MS analysis |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00173
BindingDB Entry DOI: 10.7270/Q2NZ8CD4 |
More data for this
Ligand-Target Pair | |
Plasmepsin IX
(Plasmodium falciparum (isolate 3D7)) | BDBM50591317

(CHEMBL5191783)Show SMILES C[C@H]1C[C@H](CCO1)N1C(=O)C[C@](C)(NC1=N)c1cccc(Nc2ccc(nc2)C2CC2)c1Cl |r| | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 250 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01336
BindingDB Entry DOI: 10.7270/Q2988C16 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 1A2
(Homo sapiens (Human)) | BDBM50567995

(CHEMBL4851471 | US11903936, Compound 20)Show SMILES CC(NC(=O)c1[nH]cc(c1C)C1(CC1)c1ccc(nc1)C(F)(F)F)c1c[nH]cn1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | <250 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of CYP1A2 (unknown origin) using phenacetin O-deethylation by UPLC-MS analysis |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00173
BindingDB Entry DOI: 10.7270/Q2NZ8CD4 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C19
(Homo sapiens (Human)) | BDBM50567995

(CHEMBL4851471 | US11903936, Compound 20)Show SMILES CC(NC(=O)c1[nH]cc(c1C)C1(CC1)c1ccc(nc1)C(F)(F)F)c1c[nH]cn1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | <250 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of CYP2C19 (unknown origin) by UPLC-MS analysis |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00173
BindingDB Entry DOI: 10.7270/Q2NZ8CD4 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM50567995

(CHEMBL4851471 | US11903936, Compound 20)Show SMILES CC(NC(=O)c1[nH]cc(c1C)C1(CC1)c1ccc(nc1)C(F)(F)F)c1c[nH]cn1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | <250 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of CYP2C9 (unknown origin) by UPLC-MS analysis |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00173
BindingDB Entry DOI: 10.7270/Q2NZ8CD4 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50609889

(CHEMBL5275583) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| | n/a | n/a | 250 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Dihydroorotate dehydrogenase (quinone), mitochondrial
(Homo sapiens (Human)) | BDBM50018011

(Aubagio | CHEBI:68540 | HMR-1726 | TERIFLUNOMIDE)Show InChI InChI=1S/C12H9F3N2O2/c1-7(18)10(6-16)11(19)17-9-4-2-8(3-5-9)12(13,14)15/h2-5,18H,1H3,(H,17,19)/b10-7- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Washington
Curated by ChEMBL
| Assay Description
Inhibition of C-terminal His6-tagged human DHODH expressed in Escherichia coli BL21(DE3) cells using L-DHO as substrate by DCIP dye based assay |
J Med Chem 63: 4929-4956 (2020)
Article DOI: 10.1021/acs.jmedchem.0c00311
BindingDB Entry DOI: 10.7270/Q2M90D6Z |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50609887
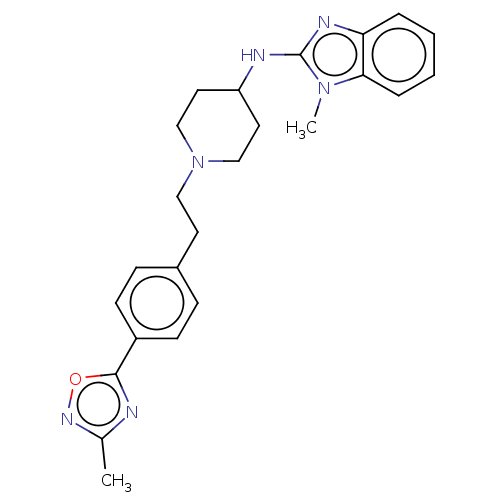
(CHEMBL5280818) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| | n/a | n/a | 350 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50238630

(CHEBI:156095 | Lumefantrine)Show SMILES CCCCN(CCCC)CC(O)c1cc(Cl)cc2\C(=C/c3ccc(Cl)cc3)c3cc(Cl)ccc3-c12 Show InChI InChI=1S/C30H32Cl3NO/c1-3-5-13-34(14-6-4-2)19-29(35)28-18-23(33)17-27-25(15-20-7-9-21(31)10-8-20)26-16-22(32)11-12-24(26)30(27)28/h7-12,15-18,29,35H,3-6,13-14,19H2,1-2H3/b25-15- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 377 | n/a | n/a | n/a | n/a | n/a | n/a |
Heinrich-Heine-Universit£t D£sseldorf
Curated by ChEMBL
| Assay Description
Inhibition of noradrenaline uptake in rat synaptosomal fraction |
J Med Chem 60: 6036-6044 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00140
BindingDB Entry DOI: 10.7270/Q24M96TQ |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50109750
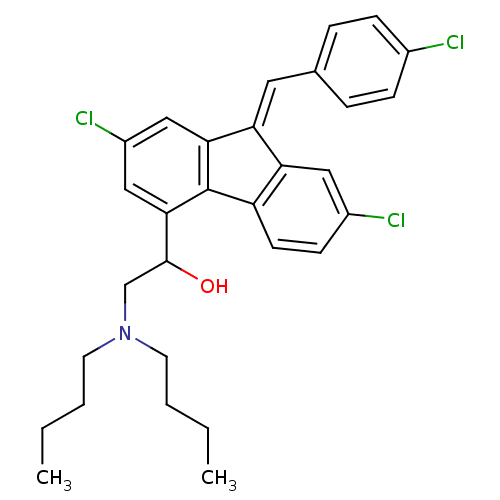
(2-Dibutylamino-1-{2,7-dichloro-9-[1-(4-chloro-phen...)Show SMILES CCCCN(CCCC)CC(O)c1cc(Cl)cc2\C(=C\c3ccc(Cl)cc3)c3cc(Cl)ccc3-c12 Show InChI InChI=1S/C30H32Cl3NO/c1-3-5-13-34(14-6-4-2)19-29(35)28-18-23(33)17-27-25(15-20-7-9-21(31)10-8-20)26-16-22(32)11-12-24(26)30(27)28/h7-12,15-18,29,35H,3-6,13-14,19H2,1-2H3/b25-15+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 380 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01744
BindingDB Entry DOI: 10.7270/Q2WQ07WG |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50238629
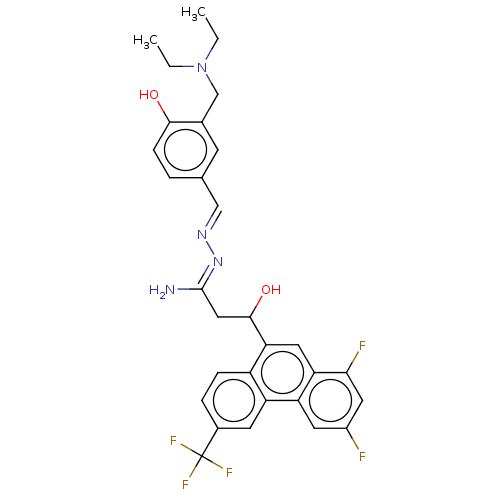
(CHEMBL4105332)Show SMILES CCN(CC)Cc1cc(\C=N\N=C(/N)CC(O)c2cc3c(F)cc(F)cc3c3cc(ccc23)C(F)(F)F)ccc1O Show InChI InChI=1S/C30H29F5N4O2/c1-3-39(4-2)16-18-9-17(5-8-27(18)40)15-37-38-29(36)14-28(41)25-13-24-23(11-20(31)12-26(24)32)22-10-19(30(33,34)35)6-7-21(22)25/h5-13,15,28,40-41H,3-4,14,16H2,1-2H3,(H2,36,38)/b37-15+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 390 | n/a | n/a | n/a | n/a | n/a | n/a |
Heinrich-Heine-Universit£t D£sseldorf
Curated by ChEMBL
| Assay Description
Inhibition of human ERG by fluorescence polarization assay |
J Med Chem 60: 6036-6044 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00140
BindingDB Entry DOI: 10.7270/Q24M96TQ |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50554407
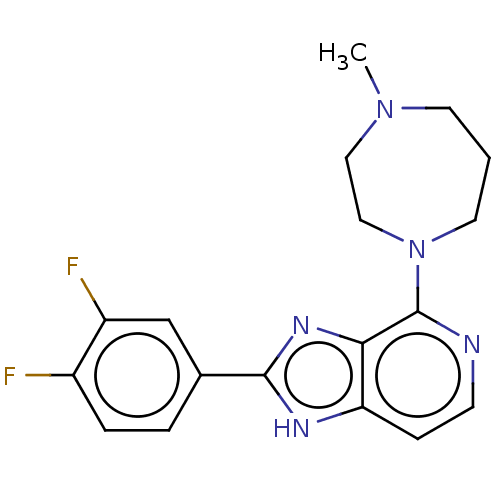
(CHEMBL4746185)Show SMILES CN1CCCN(CC1)c1nccc2[nH]c(nc12)-c1ccc(F)c(F)c1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 400 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human ERG by patch clamp method |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01411
BindingDB Entry DOI: 10.7270/Q2TB1BKN |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50554417

(CHEMBL4758157)Show SMILES CN1CCCN(CC1)c1nccc2[nH]c(nc12)-c1cc(F)cc(F)c1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 400 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human ERG by patch clamp method |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01411
BindingDB Entry DOI: 10.7270/Q2TB1BKN |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50357427
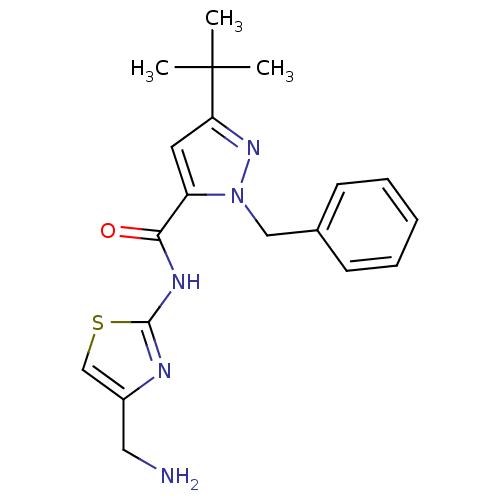
(CHEMBL1917511)Show SMILES CC(C)(C)c1cc(C(=O)Nc2nc(CN)cs2)n(Cc2ccccc2)n1 Show InChI InChI=1S/C19H23N5OS/c1-19(2,3)16-9-15(17(25)22-18-21-14(10-20)12-26-18)24(23-16)11-13-7-5-4-6-8-13/h4-9,12H,10-11,20H2,1-3H3,(H,21,22,25) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 400 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Cape Town
Curated by ChEMBL
| Assay Description
Inhibition of CYP2D6-mediated dextromethorphan O-demethylation in human liver microsomes by LCMS analysis |
J Med Chem 54: 7713-9 (2011)
Article DOI: 10.1021/jm201108k
BindingDB Entry DOI: 10.7270/Q20865Q4 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50030835
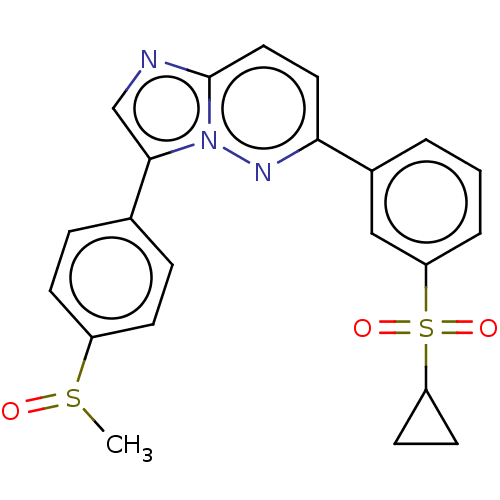
(CHEMBL3355638)Show SMILES CS(=O)c1ccc(cc1)-c1cnc2ccc(nn12)-c1cccc(c1)S(=O)(=O)C1CC1 Show InChI InChI=1S/C22H19N3O3S2/c1-29(26)17-7-5-15(6-8-17)21-14-23-22-12-11-20(24-25(21)22)16-3-2-4-19(13-16)30(27,28)18-9-10-18/h2-8,11-14,18H,9-10H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 400 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Cape Town
Curated by ChEMBL
| Assay Description
Inhibition of human ERG channel by patch clamp technique |
J Med Chem 57: 8839-48 (2014)
Article DOI: 10.1021/jm500887k
BindingDB Entry DOI: 10.7270/Q2ZP47Q1 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50030836
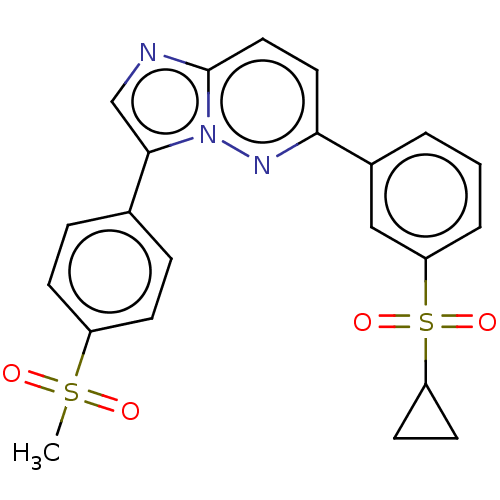
(CHEMBL3355639)Show SMILES CS(=O)(=O)c1ccc(cc1)-c1cnc2ccc(nn12)-c1cccc(c1)S(=O)(=O)C1CC1 Show InChI InChI=1S/C22H19N3O4S2/c1-30(26,27)17-7-5-15(6-8-17)21-14-23-22-12-11-20(24-25(21)22)16-3-2-4-19(13-16)31(28,29)18-9-10-18/h2-8,11-14,18H,9-10H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 400 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Cape Town
Curated by ChEMBL
| Assay Description
Inhibition of human ERG channel by patch clamp technique |
J Med Chem 57: 8839-48 (2014)
Article DOI: 10.1021/jm500887k
BindingDB Entry DOI: 10.7270/Q2ZP47Q1 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50030836

(CHEMBL3355639)Show SMILES CS(=O)(=O)c1ccc(cc1)-c1cnc2ccc(nn12)-c1cccc(c1)S(=O)(=O)C1CC1 Show InChI InChI=1S/C22H19N3O4S2/c1-30(26,27)17-7-5-15(6-8-17)21-14-23-22-12-11-20(24-25(21)22)16-3-2-4-19(13-16)31(28,29)18-9-10-18/h2-8,11-14,18H,9-10H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 400 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Cape Town
Curated by ChEMBL
| Assay Description
Inhibition of human ERG potassium channel by ionworks patch-clamp electrophysiology assay |
J Med Chem 58: 8713-22 (2015)
Article DOI: 10.1021/acs.jmedchem.5b01605
BindingDB Entry DOI: 10.7270/Q24F1SJD |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data