

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Replicase polyprotein 1ab (Human SARS coronavirus (SARS-CoV) (Severe acute re...) | BDBM59242 (Benzotriazole ester, 8 | acs.jmedchem.1c00409_ST.6...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid PDB UniChem | Article PubMed | 7.5 | n/a | n/a | n/a | n/a | n/a | n/a | 7.5 | n/a |
Academia Sinica | Assay Description Inhibition assay against SARS-CoV 3CL protease, a fluorometric assay was utilized to determine the inhibition constants of the samples. | Chem Biol 13: 261-8 (2006) Article DOI: 10.1016/j.chembiol.2005.12.008 BindingDB Entry DOI: 10.7270/Q21C1V9G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1ab (Human SARS coronavirus (SARS-CoV) (Severe acute re...) | BDBM59240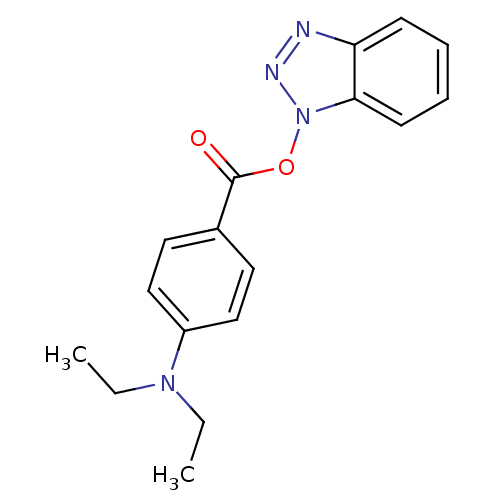 (Benzotriazole ester, 6 | acs.jmedchem.1c00409_ST.8) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 11.1 | n/a | n/a | n/a | n/a | n/a | n/a | 7.5 | n/a |
Academia Sinica | Assay Description Inhibition assay against SARS-CoV 3CL protease, a fluorometric assay was utilized to determine the inhibition constants of the samples. | Chem Biol 13: 261-8 (2006) Article DOI: 10.1016/j.chembiol.2005.12.008 BindingDB Entry DOI: 10.7270/Q21C1V9G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1ab (Human SARS coronavirus (SARS-CoV) (Severe acute re...) | BDBM59239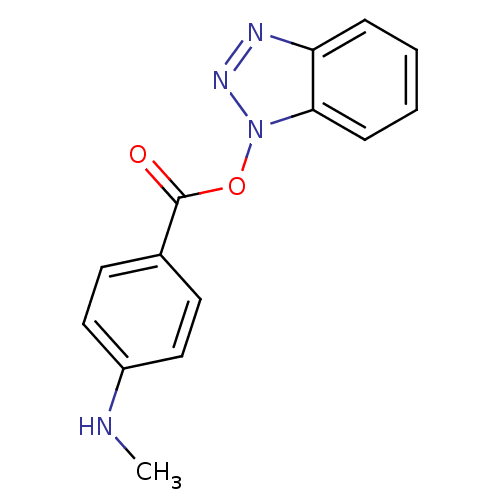 (Benzotriazole ester, 5 | acs.jmedchem.1c00409_ST.9) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 12.1 | n/a | n/a | n/a | n/a | n/a | n/a | 7.5 | n/a |
Academia Sinica | Assay Description Inhibition assay against SARS-CoV 3CL protease, a fluorometric assay was utilized to determine the inhibition constants of the samples. | Chem Biol 13: 261-8 (2006) Article DOI: 10.1016/j.chembiol.2005.12.008 BindingDB Entry DOI: 10.7270/Q21C1V9G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1ab (Human SARS coronavirus (SARS-CoV) (Severe acute re...) | BDBM59243 (Benzotriazole ester, 9 | acs.jmedchem.1c00409_ST.1...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 12.3 | n/a | n/a | n/a | n/a | n/a | n/a | 7.5 | n/a |
Academia Sinica | Assay Description Inhibition assay against SARS-CoV 3CL protease, a fluorometric assay was utilized to determine the inhibition constants of the samples. | Chem Biol 13: 261-8 (2006) Article DOI: 10.1016/j.chembiol.2005.12.008 BindingDB Entry DOI: 10.7270/Q21C1V9G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1ab (Human SARS coronavirus (SARS-CoV) (Severe acute re...) | BDBM59244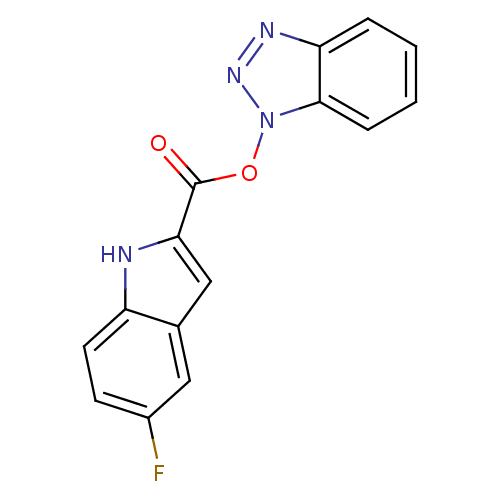 (Benzotriazole ester, 10 | acs.jmedchem.1c00409_ST....) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 13.8 | n/a | n/a | n/a | n/a | n/a | n/a | 7.5 | n/a |
Academia Sinica | Assay Description Inhibition assay against SARS-CoV 3CL protease, a fluorometric assay was utilized to determine the inhibition constants of the samples. | Chem Biol 13: 261-8 (2006) Article DOI: 10.1016/j.chembiol.2005.12.008 BindingDB Entry DOI: 10.7270/Q21C1V9G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1ab (Human SARS coronavirus (SARS-CoV) (Severe acute re...) | BDBM59238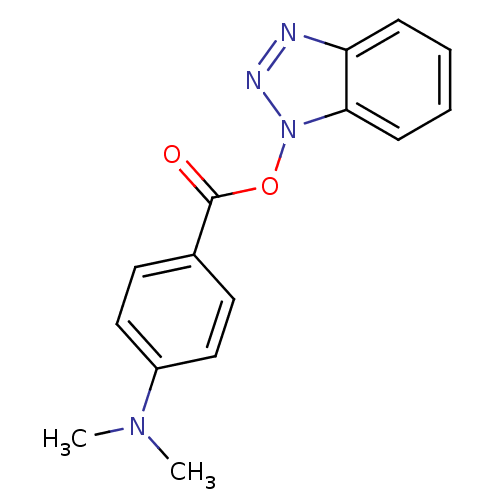 (Benzotriazole ester, 4 | acs.jmedchem.1c00409_ST.1...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | 17.4 | n/a | n/a | n/a | n/a | n/a | n/a | 7.5 | n/a |
Academia Sinica | Assay Description Inhibition assay against SARS-CoV 3CL protease, a fluorometric assay was utilized to determine the inhibition constants of the samples. | Chem Biol 13: 261-8 (2006) Article DOI: 10.1016/j.chembiol.2005.12.008 BindingDB Entry DOI: 10.7270/Q21C1V9G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1ab (Human SARS coronavirus (SARS-CoV) (Severe acute re...) | BDBM59237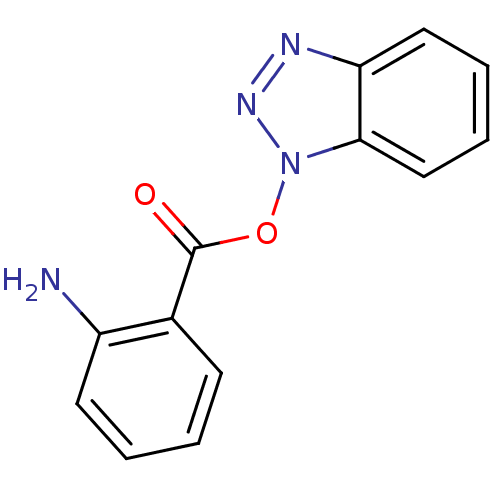 (Benzotriazole ester, 3 | acs.jmedchem.1c00409_ST.1...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 19.5 | n/a | n/a | n/a | n/a | n/a | n/a | 7.5 | n/a |
Academia Sinica | Assay Description Inhibition assay against SARS-CoV 3CL protease, a fluorometric assay was utilized to determine the inhibition constants of the samples. | Chem Biol 13: 261-8 (2006) Article DOI: 10.1016/j.chembiol.2005.12.008 BindingDB Entry DOI: 10.7270/Q21C1V9G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1ab (Human SARS coronavirus (SARS-CoV) (Severe acute re...) | BDBM59241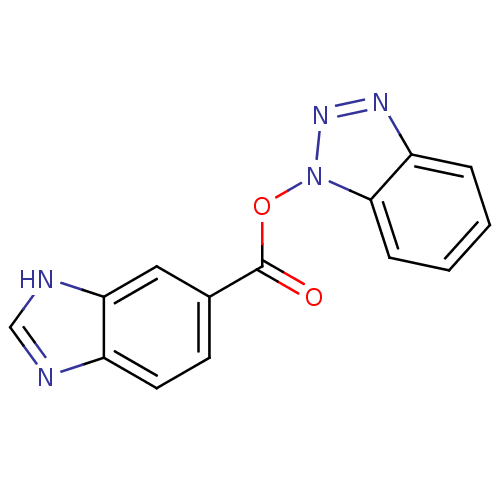 (Benzotriazole ester, 7) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 22.9 | n/a | n/a | n/a | n/a | n/a | n/a | 7.5 | n/a |
Academia Sinica | Assay Description Inhibition assay against SARS-CoV 3CL protease, a fluorometric assay was utilized to determine the inhibition constants of the samples. | Chem Biol 13: 261-8 (2006) Article DOI: 10.1016/j.chembiol.2005.12.008 BindingDB Entry DOI: 10.7270/Q21C1V9G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1ab (Human SARS coronavirus (SARS-CoV) (Severe acute re...) | BDBM11234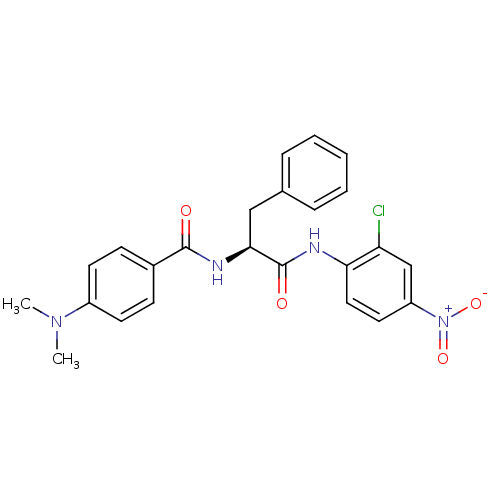 ((2S)-N-(2-chloro-4-nitrophenyl)-2-{[4-(dimethylami...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 30 | -42.9 | 60 | n/a | n/a | n/a | n/a | 7.0 | 25 |
National Taiwan University | Assay Description The effects of compound on enzyme activity were measured by using a fluorogenic peptide cleavage assay. Enhanced fluorescence caused by cleavage of t... | J Med Chem 48: 4469-73 (2005) Article DOI: 10.1021/jm050184y BindingDB Entry DOI: 10.7270/Q20K26S1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrolipoyl dehydrogenase (Mycobacterium tuberculosis) | BDBM119876 (SL932 | US9073941, 503) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 37 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Weill Cornell Medical College | Assay Description IC50 values were determined with serial dilutions (from 100 to 0.1 uM) of inhibitors by a spectrophotometric assay with DTNB, lipoamide, and NADH or ... | Biochemistry 52: 9375-84 (2013) Article DOI: 10.1021/bi401077f BindingDB Entry DOI: 10.7270/Q29K48X6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prephenate dehydrogenase (Mycobacterium tuberculosis H37Rv) | BDBM119876 (SL932 | US9073941, 503) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 78 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Weill Cornell Medical College | Assay Description IC50 values were determined with serial dilutions (from 100 to 0.1 uM) of inhibitors by a spectrophotometric assay with DTNB, lipoamide, and NADH or ... | Biochemistry 52: 9375-84 (2013) Article DOI: 10.1021/bi401077f BindingDB Entry DOI: 10.7270/Q29K48X6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrolipoyl dehydrogenase (Mycobacterium tuberculosis) | BDBM119877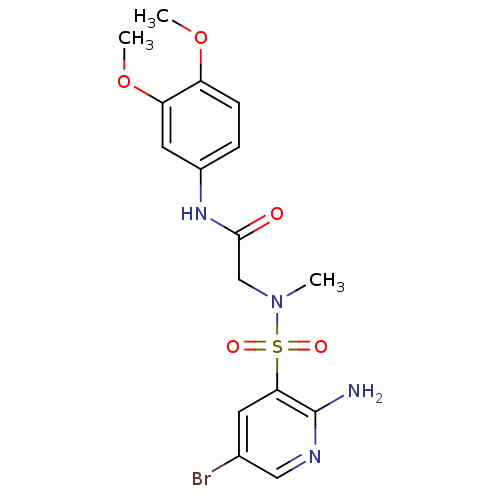 (SL809 | US9073941, 502) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | 93 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Weill Cornell Medical College | Assay Description IC50 values were determined with serial dilutions (from 100 to 0.1 uM) of inhibitors by a spectrophotometric assay with DTNB, lipoamide, and NADH or ... | Biochemistry 52: 9375-84 (2013) Article DOI: 10.1021/bi401077f BindingDB Entry DOI: 10.7270/Q29K48X6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrolipoyl dehydrogenase (Mycobacterium tuberculosis) | BDBM119878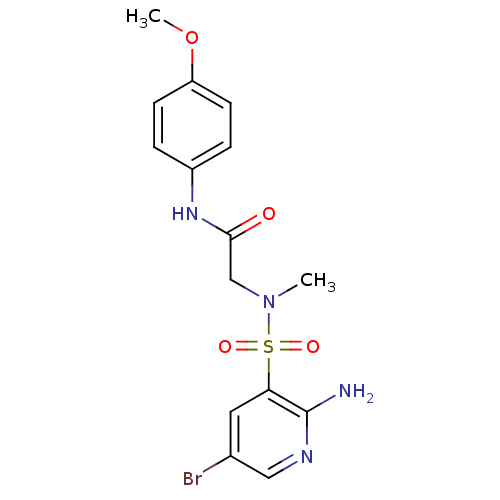 (SL827 | US9073941, 500) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Weill Cornell Medical College | Assay Description IC50 values were determined with serial dilutions (from 100 to 0.1 uM) of inhibitors by a spectrophotometric assay with DTNB, lipoamide, and NADH or ... | Biochemistry 52: 9375-84 (2013) Article DOI: 10.1021/bi401077f BindingDB Entry DOI: 10.7270/Q29K48X6 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Dihydrolipoyl dehydrogenase (Mycobacterium tuberculosis) | BDBM119880 (SL418) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 143 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Weill Cornell Medical College | Assay Description IC50 values were determined with serial dilutions (from 100 to 0.1 uM) of inhibitors by a spectrophotometric assay with DTNB, lipoamide, and NADH or ... | Biochemistry 52: 9375-84 (2013) Article DOI: 10.1021/bi401077f BindingDB Entry DOI: 10.7270/Q29K48X6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prephenate dehydrogenase (Mycobacterium tuberculosis H37Rv) | BDBM119878 (SL827 | US9073941, 500) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid PDB UniChem Similars | Article PubMed | 155 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Weill Cornell Medical College | Assay Description IC50 values were determined with serial dilutions (from 100 to 0.1 uM) of inhibitors by a spectrophotometric assay with DTNB, lipoamide, and NADH or ... | Biochemistry 52: 9375-84 (2013) Article DOI: 10.1021/bi401077f BindingDB Entry DOI: 10.7270/Q29K48X6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prephenate dehydrogenase (Mycobacterium tuberculosis H37Rv) | BDBM119877 (SL809 | US9073941, 502) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | 155 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Weill Cornell Medical College | Assay Description IC50 values were determined with serial dilutions (from 100 to 0.1 uM) of inhibitors by a spectrophotometric assay with DTNB, lipoamide, and NADH or ... | Biochemistry 52: 9375-84 (2013) Article DOI: 10.1021/bi401077f BindingDB Entry DOI: 10.7270/Q29K48X6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrolipoyl dehydrogenase (Mycobacterium tuberculosis) | BDBM119879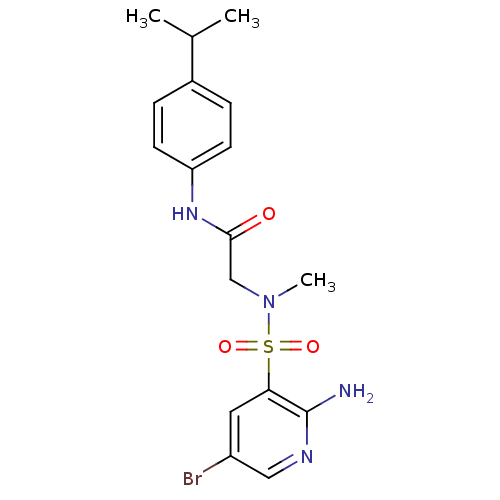 (SL917 | US9073941, 505) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 233 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Weill Cornell Medical College | Assay Description IC50 values were determined with serial dilutions (from 100 to 0.1 uM) of inhibitors by a spectrophotometric assay with DTNB, lipoamide, and NADH or ... | Biochemistry 52: 9375-84 (2013) Article DOI: 10.1021/bi401077f BindingDB Entry DOI: 10.7270/Q29K48X6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prephenate dehydrogenase (Mycobacterium tuberculosis H37Rv) | BDBM119879 (SL917 | US9073941, 505) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 289 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Weill Cornell Medical College | Assay Description IC50 values were determined with serial dilutions (from 100 to 0.1 uM) of inhibitors by a spectrophotometric assay with DTNB, lipoamide, and NADH or ... | Biochemistry 52: 9375-84 (2013) Article DOI: 10.1021/bi401077f BindingDB Entry DOI: 10.7270/Q29K48X6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prephenate dehydrogenase (Mycobacterium tuberculosis H37Rv) | BDBM119880 (SL418) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 333 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Weill Cornell Medical College | Assay Description IC50 values were determined with serial dilutions (from 100 to 0.1 uM) of inhibitors by a spectrophotometric assay with DTNB, lipoamide, and NADH or ... | Biochemistry 52: 9375-84 (2013) Article DOI: 10.1021/bi401077f BindingDB Entry DOI: 10.7270/Q29K48X6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1ab (Human SARS coronavirus (SARS-CoV) (Severe acute re...) | BDBM11261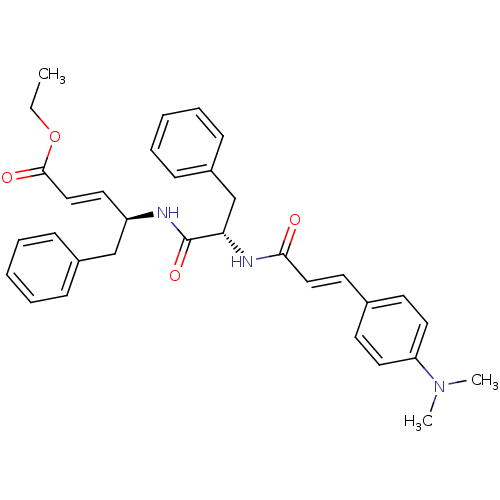 (dipeptidomimetic unsaturated ester 18c | ethyl (2E...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 520 | -35.9 | 1.00E+3 | n/a | n/a | n/a | n/a | 7.0 | 25 |
National Taiwan University | Assay Description The effects of compound on enzyme activity were measured by using a fluorogenic peptide cleavage assay. Enhanced fluorescence caused by cleavage of t... | Bioorg Med Chem 13: 5240-52 (2005) Article DOI: 10.1016/j.bmc.2005.05.065 BindingDB Entry DOI: 10.7270/Q2VT1Q9R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1ab (Human SARS coronavirus (SARS-CoV) (Severe acute re...) | BDBM59246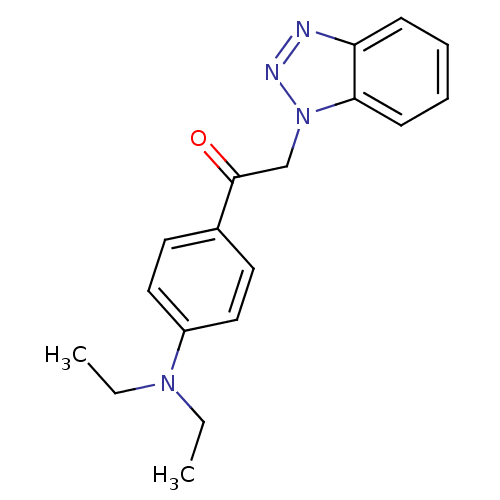 (Benzotriazole ester, 14) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | 7.5 | n/a |
Academia Sinica | Assay Description Inhibition assay against SARS-CoV 3CL protease, a fluorometric assay was utilized to determine the inhibition constants of the samples. | Chem Biol 13: 261-8 (2006) Article DOI: 10.1016/j.chembiol.2005.12.008 BindingDB Entry DOI: 10.7270/Q21C1V9G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1ab (Human SARS coronavirus (SARS-CoV) (Severe acute re...) | BDBM11240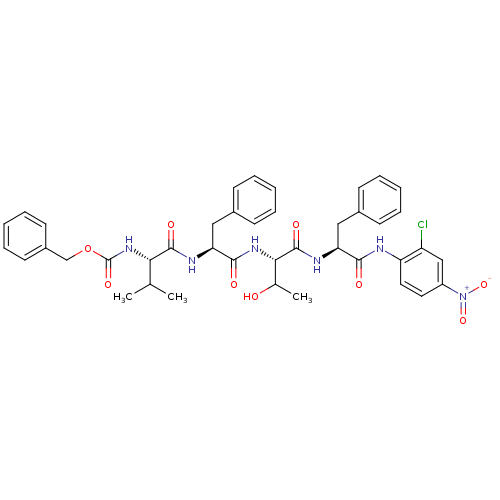 (Anilide Inhibitor 4k | N-[(benzyloxy)carbonyl]-L-v...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 1.51E+3 | -33.2 | 6.00E+3 | n/a | n/a | n/a | n/a | 7.0 | 25 |
National Taiwan University | Assay Description The effects of compound on enzyme activity were measured by using a fluorogenic peptide cleavage assay. Enhanced fluorescence caused by cleavage of t... | J Med Chem 48: 4469-73 (2005) Article DOI: 10.1021/jm050184y BindingDB Entry DOI: 10.7270/Q20K26S1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1ab (Human SARS coronavirus (SARS-CoV) (Severe acute re...) | BDBM11239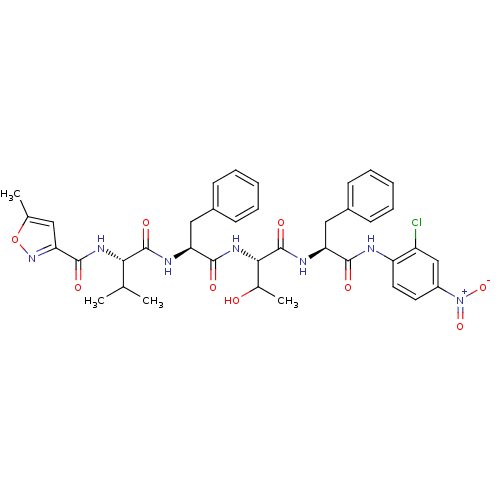 ((2S)-N-[(1S)-1-{[(1S,2S)-1-{[(1S)-1-[(2-chloro-4-n...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 1.61E+3 | -33.1 | 5.00E+3 | n/a | n/a | n/a | n/a | 7.0 | 25 |
National Taiwan University | Assay Description The effects of compound on enzyme activity were measured by using a fluorogenic peptide cleavage assay. Enhanced fluorescence caused by cleavage of t... | J Med Chem 48: 4469-73 (2005) Article DOI: 10.1021/jm050184y BindingDB Entry DOI: 10.7270/Q20K26S1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1ab (Human SARS coronavirus (SARS-CoV) (Severe acute re...) | BDBM11236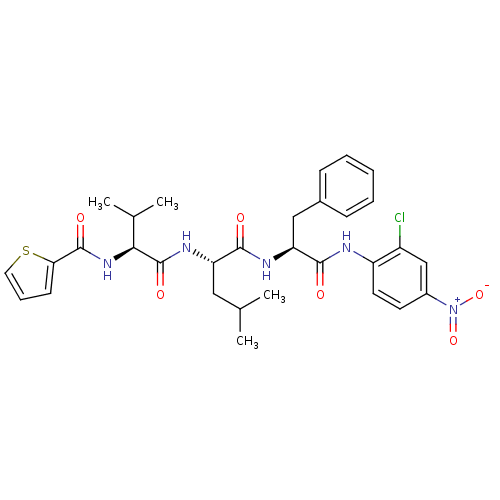 ((2S)-N-[(1S)-1-[(2-chloro-4-nitrophenyl)carbamoyl]...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 2.29E+3 | -32.2 | 5.00E+3 | n/a | n/a | n/a | n/a | 7.0 | 25 |
National Taiwan University | Assay Description The effects of compound on enzyme activity were measured by using a fluorogenic peptide cleavage assay. Enhanced fluorescence caused by cleavage of t... | J Med Chem 48: 4469-73 (2005) Article DOI: 10.1021/jm050184y BindingDB Entry DOI: 10.7270/Q20K26S1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1ab (Human SARS coronavirus (SARS-CoV) (Severe acute re...) | BDBM11260 (dipeptidomimetic unsaturated ester 18b | ethyl (2E...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Similars | Article PubMed | 2.48E+3 | -32.0 | 5.00E+3 | n/a | n/a | n/a | n/a | 7.0 | 25 |
National Taiwan University | Assay Description The effects of compound on enzyme activity were measured by using a fluorogenic peptide cleavage assay. Enhanced fluorescence caused by cleavage of t... | Bioorg Med Chem 13: 5240-52 (2005) Article DOI: 10.1016/j.bmc.2005.05.065 BindingDB Entry DOI: 10.7270/Q2VT1Q9R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1ab (Human SARS coronavirus (SARS-CoV) (Severe acute re...) | BDBM11237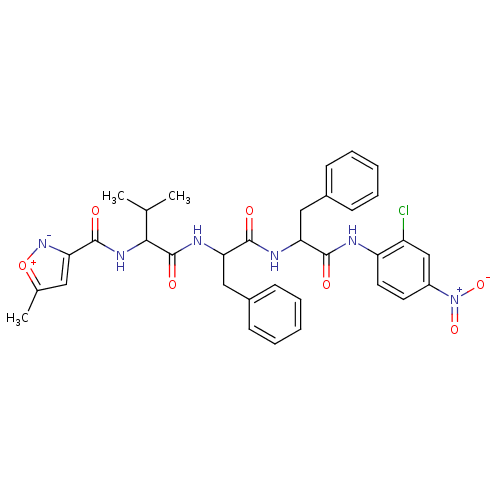 ((2S)-N-[(1S)-1-{[(1S)-1-[(2-chloro-4-nitrophenyl)c...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 2.90E+3 | -31.6 | 7.00E+3 | n/a | n/a | n/a | n/a | 7.0 | 25 |
National Taiwan University | Assay Description The effects of compound on enzyme activity were measured by using a fluorogenic peptide cleavage assay. Enhanced fluorescence caused by cleavage of t... | J Med Chem 48: 4469-73 (2005) Article DOI: 10.1021/jm050184y BindingDB Entry DOI: 10.7270/Q20K26S1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1ab (Human SARS coronavirus (SARS-CoV) (Severe acute re...) | BDBM11263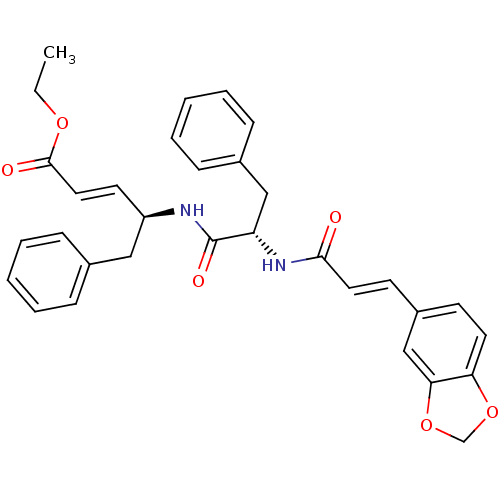 (dipeptidomimetic unsaturated ester 18e | ethyl (2E...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 3.05E+3 | -31.5 | 7.00E+3 | n/a | n/a | n/a | n/a | 7.0 | 25 |
National Taiwan University | Assay Description The effects of compound on enzyme activity were measured by using a fluorogenic peptide cleavage assay. Enhanced fluorescence caused by cleavage of t... | Bioorg Med Chem 13: 5240-52 (2005) Article DOI: 10.1016/j.bmc.2005.05.065 BindingDB Entry DOI: 10.7270/Q2VT1Q9R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1ab (Human SARS coronavirus (SARS-CoV) (Severe acute re...) | BDBM11241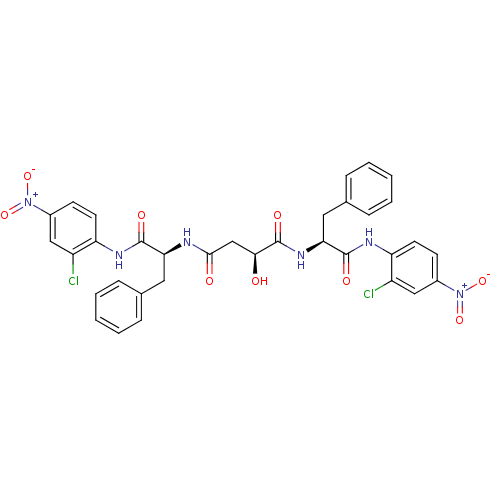 ((2S)-N,N'-bis[(1S)-1-[(2-chloro-4-nitrophenyl)carb...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 3.10E+3 | -31.4 | 4.00E+3 | n/a | n/a | n/a | n/a | 7.0 | 25 |
National Taiwan University | Assay Description The effects of compound on enzyme activity were measured by using a fluorogenic peptide cleavage assay. Enhanced fluorescence caused by cleavage of t... | J Med Chem 48: 4469-73 (2005) Article DOI: 10.1021/jm050184y BindingDB Entry DOI: 10.7270/Q20K26S1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1ab (Human SARS coronavirus (SARS-CoV) (Severe acute re...) | BDBM11238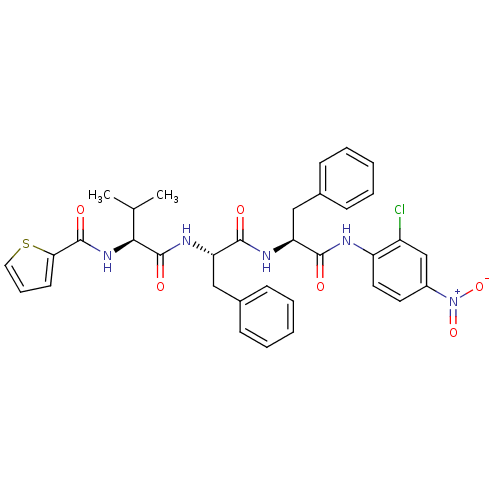 ((2S)-N-[(1S)-1-{[(1S)-1-[(2-chloro-4-nitrophenyl)c...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 4.30E+3 | -30.6 | 5.00E+3 | n/a | n/a | n/a | n/a | 7.0 | 25 |
National Taiwan University | Assay Description The effects of compound on enzyme activity were measured by using a fluorogenic peptide cleavage assay. Enhanced fluorescence caused by cleavage of t... | J Med Chem 48: 4469-73 (2005) Article DOI: 10.1021/jm050184y BindingDB Entry DOI: 10.7270/Q20K26S1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1ab (Human SARS coronavirus (SARS-CoV) (Severe acute re...) | BDBM59249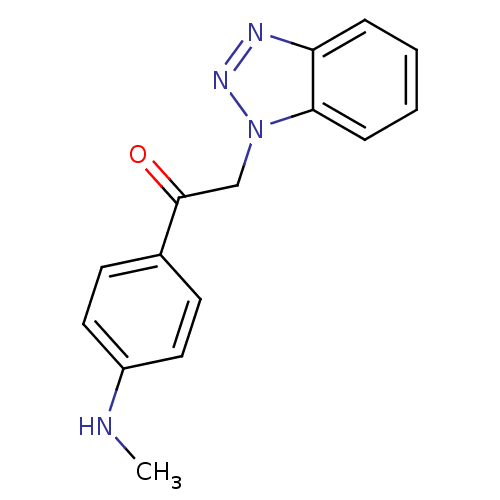 (Benzotriazole ester, 17 | acs.jmedchem.1c00409_ST....) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 4.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | 7.5 | n/a |
Academia Sinica | Assay Description Inhibition assay against SARS-CoV 3CL protease, a fluorometric assay was utilized to determine the inhibition constants of the samples. | Chem Biol 13: 261-8 (2006) Article DOI: 10.1016/j.chembiol.2005.12.008 BindingDB Entry DOI: 10.7270/Q21C1V9G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1ab (Human SARS coronavirus (SARS-CoV) (Severe acute re...) | BDBM11259 (dipeptidomimetic unsaturated ester 18a | ethyl (2E...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 6.44E+3 | -29.6 | 1.00E+4 | n/a | n/a | n/a | n/a | 7.0 | 25 |
National Taiwan University | Assay Description The effects of compound on enzyme activity were measured by using a fluorogenic peptide cleavage assay. Enhanced fluorescence caused by cleavage of t... | Bioorg Med Chem 13: 5240-52 (2005) Article DOI: 10.1016/j.bmc.2005.05.065 BindingDB Entry DOI: 10.7270/Q2VT1Q9R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1ab (Human SARS coronavirus (SARS-CoV) (Severe acute re...) | BDBM59250 (Benzotriazole ester, 18 | acs.jmedchem.1c00409_ST....) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 6.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a | 7.5 | n/a |
Academia Sinica | Assay Description Inhibition assay against SARS-CoV 3CL protease, a fluorometric assay was utilized to determine the inhibition constants of the samples. | Chem Biol 13: 261-8 (2006) Article DOI: 10.1016/j.chembiol.2005.12.008 BindingDB Entry DOI: 10.7270/Q21C1V9G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1ab (Human SARS coronavirus (SARS-CoV) (Severe acute re...) | BDBM11262 (dipeptidomimetic unsaturated ester 18d | ethyl (2E...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 9.05E+3 | -28.8 | 1.00E+4 | n/a | n/a | n/a | n/a | 7.0 | 25 |
National Taiwan University | Assay Description The effects of compound on enzyme activity were measured by using a fluorogenic peptide cleavage assay. Enhanced fluorescence caused by cleavage of t... | Bioorg Med Chem 13: 5240-52 (2005) Article DOI: 10.1016/j.bmc.2005.05.065 BindingDB Entry DOI: 10.7270/Q2VT1Q9R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1ab (Human SARS coronavirus (SARS-CoV) (Severe acute re...) | BDBM59251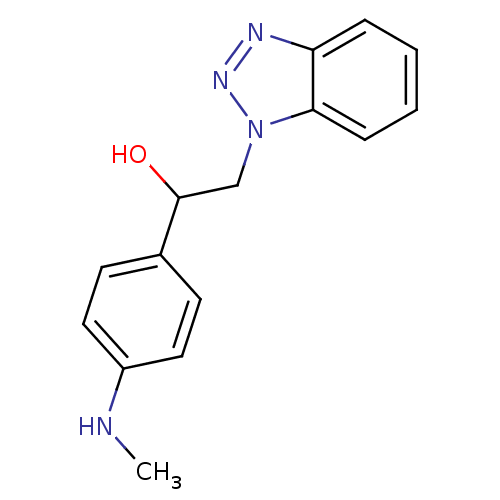 (Benzotriazole ester, 19) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | 7.5 | n/a |
Academia Sinica | Assay Description Inhibition assay against SARS-CoV 3CL protease, a fluorometric assay was utilized to determine the inhibition constants of the samples. | Chem Biol 13: 261-8 (2006) Article DOI: 10.1016/j.chembiol.2005.12.008 BindingDB Entry DOI: 10.7270/Q21C1V9G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1ab (Human SARS coronavirus (SARS-CoV) (Severe acute re...) | BDBM59252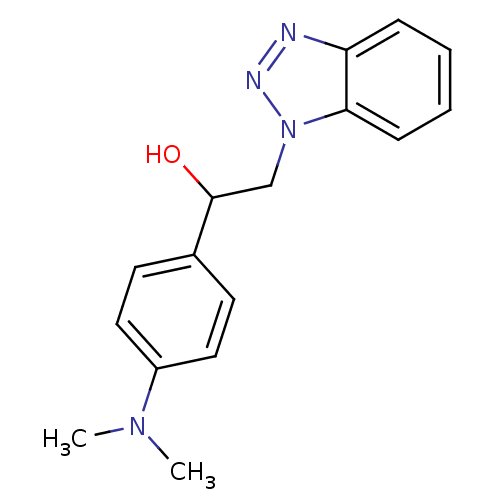 (Benzotriazole ester, 20) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | 7.5 | n/a |
Academia Sinica | Assay Description Inhibition assay against SARS-CoV 3CL protease, a fluorometric assay was utilized to determine the inhibition constants of the samples. | Chem Biol 13: 261-8 (2006) Article DOI: 10.1016/j.chembiol.2005.12.008 BindingDB Entry DOI: 10.7270/Q21C1V9G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM50223090 (3-(4-(3-chloro-4-fluorophenylamino)quinazolin-6-yl...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Development Center for Biotechnology Curated by ChEMBL | Assay Description Inhibition of EGFR | Bioorg Med Chem Lett 17: 6373-7 (2007) Article DOI: 10.1016/j.bmcl.2007.08.061 BindingDB Entry DOI: 10.7270/Q25Q4VT1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM5446 (CHEMBL553 | ERLOTINIB HYDROCHLORIDE | Erlotinib | ...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank MMDB PDB Article PubMed | n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
Development Center for Biotechnology Curated by ChEMBL | Assay Description Inhibition of EGFR | Bioorg Med Chem Lett 17: 6373-7 (2007) Article DOI: 10.1016/j.bmcl.2007.08.061 BindingDB Entry DOI: 10.7270/Q25Q4VT1 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM50095259 ((6,7-Dimethoxy-quinazolin-4-yl)-(3-ethynyl-phenyl)...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 29 | n/a | n/a | n/a | n/a | n/a | n/a |
Development Center for Biotechnology Curated by ChEMBL | Assay Description Inhibition of EGFR | Bioorg Med Chem Lett 17: 6373-7 (2007) Article DOI: 10.1016/j.bmcl.2007.08.061 BindingDB Entry DOI: 10.7270/Q25Q4VT1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM5447 (CHEMBL939 | GEFITINIB | Iressa | N-(3-Chloro-4-flu...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank MMDB PDB Article PubMed | n/a | n/a | 39 | n/a | n/a | n/a | n/a | n/a | n/a |
Development Center for Biotechnology Curated by ChEMBL | Assay Description Inhibition of EGFR | Bioorg Med Chem Lett 17: 6373-7 (2007) Article DOI: 10.1016/j.bmcl.2007.08.061 BindingDB Entry DOI: 10.7270/Q25Q4VT1 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM50223084 (CHEMBL251498 | N-(3-chloro-4-fluorophenyl)-6-(3-mo...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
Development Center for Biotechnology Curated by ChEMBL | Assay Description Inhibition of EGFR | Bioorg Med Chem Lett 17: 6373-7 (2007) Article DOI: 10.1016/j.bmcl.2007.08.061 BindingDB Entry DOI: 10.7270/Q25Q4VT1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM50223082 (CHEMBL251700 | N-(3-chloro-4-fluorophenyl)-6-(3-(p...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 89 | n/a | n/a | n/a | n/a | n/a | n/a |
Development Center for Biotechnology Curated by ChEMBL | Assay Description Inhibition of EGFR | Bioorg Med Chem Lett 17: 6373-7 (2007) Article DOI: 10.1016/j.bmcl.2007.08.061 BindingDB Entry DOI: 10.7270/Q25Q4VT1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM50223095 (4-(4-(3-chloro-4-fluorophenylamino)quinazolin-6-yl...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 95 | n/a | n/a | n/a | n/a | n/a | n/a |
Development Center for Biotechnology Curated by ChEMBL | Assay Description Inhibition of EGFR | Bioorg Med Chem Lett 17: 6373-7 (2007) Article DOI: 10.1016/j.bmcl.2007.08.061 BindingDB Entry DOI: 10.7270/Q25Q4VT1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrolipoyl dehydrogenase (Mycobacterium tuberculosis) | BDBM119881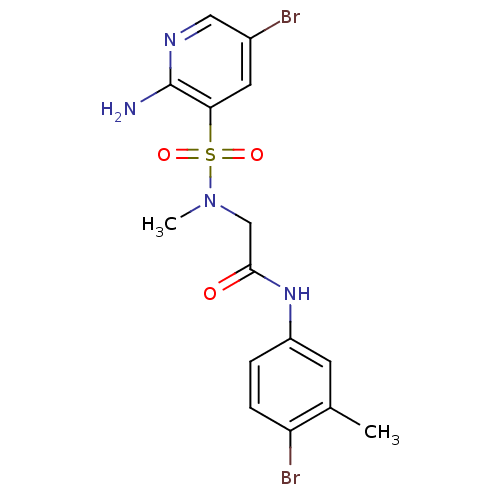 (K906-3584) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | <100 | n/a | n/a | n/a | n/a | n/a | n/a |
Weill Cornell Medical College | Assay Description IC50 values were determined with serial dilutions (from 100 to 0.1 uM) of inhibitors by a spectrophotometric assay with DTNB, lipoamide, and NADH or ... | Biochemistry 52: 9375-84 (2013) Article DOI: 10.1021/bi401077f BindingDB Entry DOI: 10.7270/Q29K48X6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM50223092 (5-(4-(3-chloro-4-fluorophenylamino)quinazolin-6-yl...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 114 | n/a | n/a | n/a | n/a | n/a | n/a |
Development Center for Biotechnology Curated by ChEMBL | Assay Description Inhibition of EGFR | Bioorg Med Chem Lett 17: 6373-7 (2007) Article DOI: 10.1016/j.bmcl.2007.08.061 BindingDB Entry DOI: 10.7270/Q25Q4VT1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM50223085 (CHEMBL249509 | N-(3-chloro-4-fluorophenyl)-6-ethyn...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 127 | n/a | n/a | n/a | n/a | n/a | n/a |
Development Center for Biotechnology Curated by ChEMBL | Assay Description Inhibition of EGFR | Bioorg Med Chem Lett 17: 6373-7 (2007) Article DOI: 10.1016/j.bmcl.2007.08.061 BindingDB Entry DOI: 10.7270/Q25Q4VT1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM50223093 (4-(4-(3-chloro-4-fluorophenylamino)quinazolin-6-yl...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 152 | n/a | n/a | n/a | n/a | n/a | n/a |
Development Center for Biotechnology Curated by ChEMBL | Assay Description Inhibition of EGFR | Bioorg Med Chem Lett 17: 6373-7 (2007) Article DOI: 10.1016/j.bmcl.2007.08.061 BindingDB Entry DOI: 10.7270/Q25Q4VT1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM50223088 (4-(4-(3-chloro-4-fluorophenylamino)quinazolin-6-yl...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 186 | n/a | n/a | n/a | n/a | n/a | n/a |
Development Center for Biotechnology Curated by ChEMBL | Assay Description Inhibition of EGFR | Bioorg Med Chem Lett 17: 6373-7 (2007) Article DOI: 10.1016/j.bmcl.2007.08.061 BindingDB Entry DOI: 10.7270/Q25Q4VT1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM50223086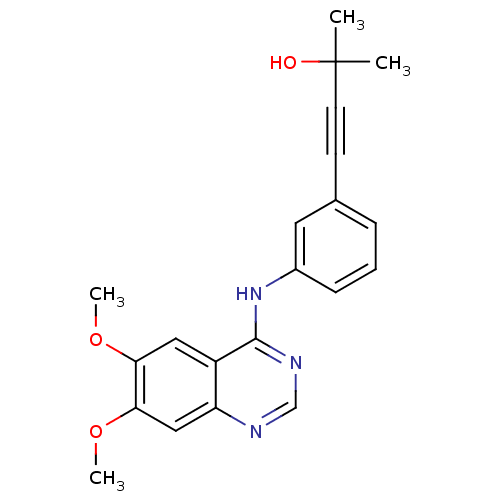 (4-(3-(6,7-dimethoxyquinazolin-4-ylamino)phenyl)-2-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 206 | n/a | n/a | n/a | n/a | n/a | n/a |
Development Center for Biotechnology Curated by ChEMBL | Assay Description Inhibition of EGFR | Bioorg Med Chem Lett 17: 6373-7 (2007) Article DOI: 10.1016/j.bmcl.2007.08.061 BindingDB Entry DOI: 10.7270/Q25Q4VT1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM50223094 (CHEMBL249511 | N-(3-chloro-4-fluorophenyl)-6-(2-ph...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 331 | n/a | n/a | n/a | n/a | n/a | n/a |
Development Center for Biotechnology Curated by ChEMBL | Assay Description Inhibition of EGFR | Bioorg Med Chem Lett 17: 6373-7 (2007) Article DOI: 10.1016/j.bmcl.2007.08.061 BindingDB Entry DOI: 10.7270/Q25Q4VT1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM50223091 (4-(3-(6,7-dimethoxyquinazolin-4-ylamino)phenyl)but...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 402 | n/a | n/a | n/a | n/a | n/a | n/a |
Development Center for Biotechnology Curated by ChEMBL | Assay Description Inhibition of EGFR | Bioorg Med Chem Lett 17: 6373-7 (2007) Article DOI: 10.1016/j.bmcl.2007.08.061 BindingDB Entry DOI: 10.7270/Q25Q4VT1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 108 total ) | Next | Last >> |