

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Histone deacetylase 6 (Homo sapiens (Human)) | BDBM50583624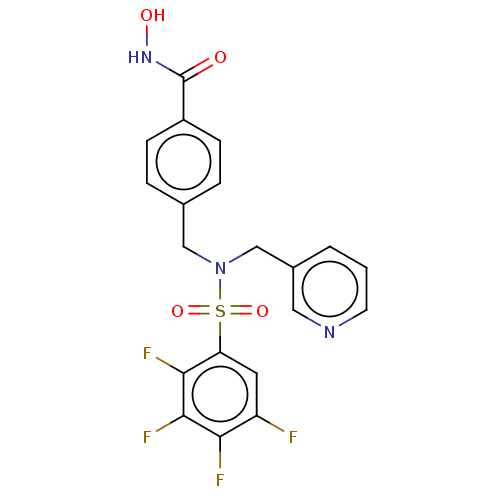 (CHEMBL5075135) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | Article PubMed | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Binding affinity to human HDAC6 by jump dilution assay | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01922 BindingDB Entry DOI: 10.7270/Q2NC6530 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1ab (2019-nCoV) | BDBM535166 (WO2022013684, Example 18) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | WIPO WO2022013684 | 1.55 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The proteolytic activity of the main protease, 3CLpro, of SARS-CoV-2 was monitored using a continuous fluorescence resonance energy transfer (FRET) a... | Citation and Details BindingDB Entry DOI: 10.7270/Q2WD43SW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM24621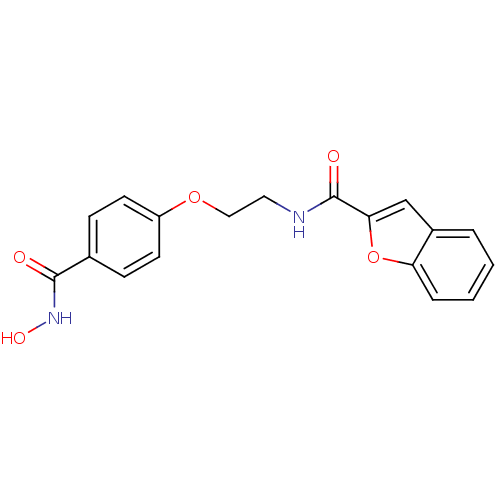 (CG-003 | N-{2-[4-(hydroxycarbamoyl)phenoxy]ethyl}-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents | Article PubMed | 4 | -47.5 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
Celera Genomics | Assay Description HDAC activity was measured using a continuous trypsin-coupled assay. For inhibitor characterization, measurements were done using 96-well assay plat... | Mol Cancer Ther 5: 1309-17 (2006) Article DOI: 10.1158/1535-7163.MCT-05-0442 BindingDB Entry DOI: 10.7270/Q2DF6PJJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 6 (Homo sapiens (Human)) | BDBM50603512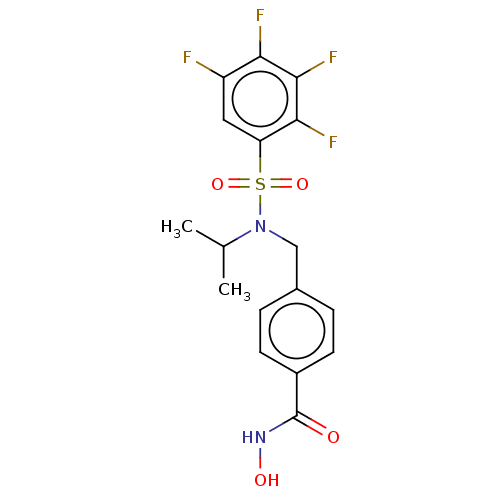 (CHEMBL5177475) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | Article PubMed | 4.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01585 BindingDB Entry DOI: 10.7270/Q2QV3RKM | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein [1424-1463]/[1502-1685] (West Nile virus (WNV)) | BDBM24732 ((2S)-6-amino-N-[(1S)-5-amino-1-{[(2S)-5-carbamimid...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 6 | -48.8 | 32 | n/a | n/a | n/a | n/a | 9.5 | 37 |
University of Queensland | Assay Description Inhibition of WNV protease activity was measured using recombinant WNV protease, which first incubated with inhibitor in 96-well plates. Catalysis wa... | J Med Chem 51: 5714-21 (2008) Article DOI: 10.1021/jm800503y BindingDB Entry DOI: 10.7270/Q27P8WPF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM24620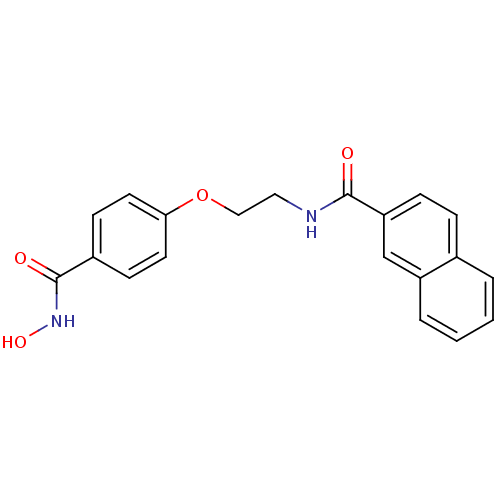 (CG-002 | N-{2-[4-(hydroxycarbamoyl)phenoxy]ethyl}n...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 6 | -46.5 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
Celera Genomics | Assay Description HDAC activity was measured using a continuous trypsin-coupled assay. For inhibitor characterization, measurements were done using 96-well assay plat... | Mol Cancer Ther 5: 1309-17 (2006) Article DOI: 10.1158/1535-7163.MCT-05-0442 BindingDB Entry DOI: 10.7270/Q2DF6PJJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1ab (2019-nCoV) | BDBM535159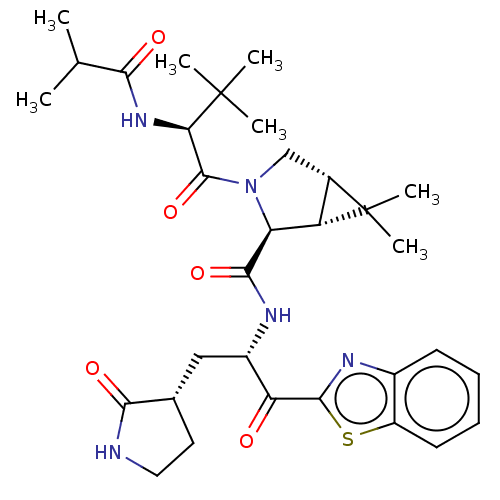 (WO2022013684, Example 14) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | WIPO WO2022013684 | 6.02 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The proteolytic activity of the main protease, 3CLpro, of SARS-CoV-2 was monitored using a continuous fluorescence resonance energy transfer (FRET) a... | Citation and Details BindingDB Entry DOI: 10.7270/Q2WD43SW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1ab (2019-nCoV) | BDBM535160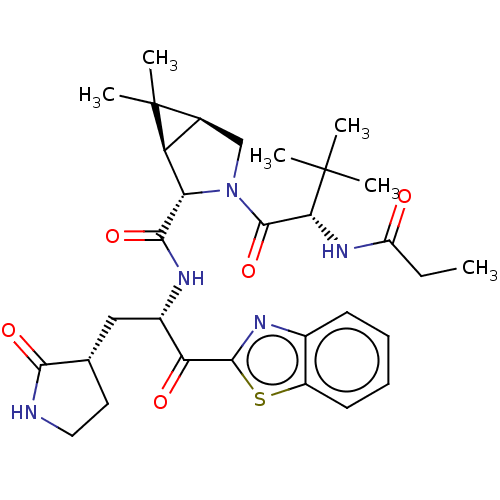 (WO2022013684, Example 15) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | WIPO WO2022013684 | 6.14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The proteolytic activity of the main protease, 3CLpro, of SARS-CoV-2 was monitored using a continuous fluorescence resonance energy transfer (FRET) a... | Citation and Details BindingDB Entry DOI: 10.7270/Q2WD43SW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM24622 (3-[(dimethylamino)methyl]-N-{2-[4-(hydroxycarbamoy...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 7 | -46.1 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
Celera Genomics | Assay Description HDAC activity was measured using a continuous trypsin-coupled assay. For inhibitor characterization, measurements were done using 96-well assay plat... | Mol Cancer Ther 5: 1309-17 (2006) Article DOI: 10.1158/1535-7163.MCT-05-0442 BindingDB Entry DOI: 10.7270/Q2DF6PJJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1ab (2019-nCoV) | BDBM535126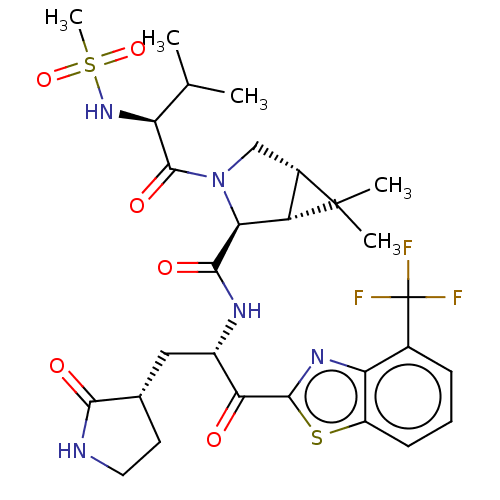 ((1R,2S,5S)-6,6-Dimethyl-3-[N-(methylsulfonyl)-L-va...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | WIPO WO2022013684 | 7.74 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The proteolytic activity of the main protease, 3CLpro, of SARS-CoV-2 was monitored using a continuous fluorescence resonance energy transfer (FRET) a... | Citation and Details BindingDB Entry DOI: 10.7270/Q2WD43SW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 3 (Homo sapiens (Human)) | BDBM24622 (3-[(dimethylamino)methyl]-N-{2-[4-(hydroxycarbamoy...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 8.20 | -45.7 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
Celera Genomics | Assay Description HDAC activity was measured using a continuous trypsin-coupled assay. For inhibitor characterization, measurements were done using 96-well assay plat... | Mol Cancer Ther 5: 1309-17 (2006) Article DOI: 10.1158/1535-7163.MCT-05-0442 BindingDB Entry DOI: 10.7270/Q2DF6PJJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein [1424-1463]/[1502-1685] (West Nile virus (WNV)) | BDBM24731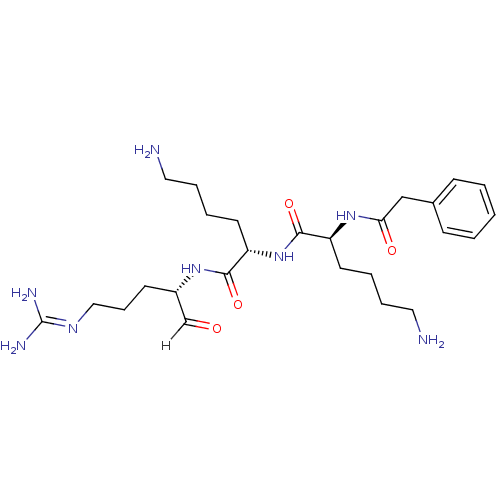 ((2S)-6-amino-N-[(1S)-5-amino-1-{[(2S)-5-carbamimid...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 9 | -47.8 | 51 | n/a | n/a | n/a | n/a | 9.5 | 37 |
University of Queensland | Assay Description Inhibition of WNV protease activity was measured using recombinant WNV protease, which first incubated with inhibitor in 96-well plates. Catalysis wa... | J Med Chem 51: 5714-21 (2008) Article DOI: 10.1021/jm800503y BindingDB Entry DOI: 10.7270/Q27P8WPF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1ab (2019-nCoV) | BDBM535168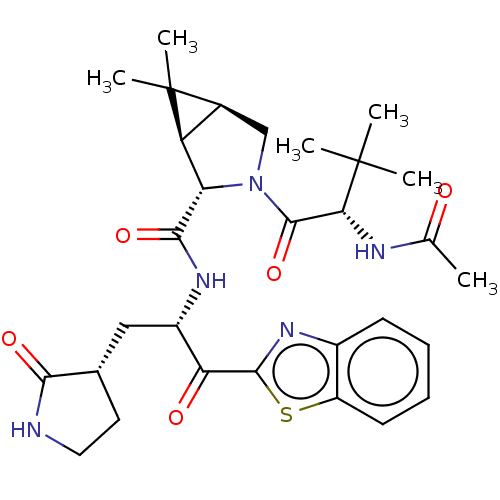 (WO2022013684, Example 19) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | WIPO WO2022013684 | 9.77 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The proteolytic activity of the main protease, 3CLpro, of SARS-CoV-2 was monitored using a continuous fluorescence resonance energy transfer (FRET) a... | Citation and Details BindingDB Entry DOI: 10.7270/Q2WD43SW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1ab (2019-nCoV) | BDBM535164 (WO2022013684, Example 16) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | WIPO WO2022013684 | 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The proteolytic activity of the main protease, 3CLpro, of SARS-CoV-2 was monitored using a continuous fluorescence resonance energy transfer (FRET) a... | Citation and Details BindingDB Entry DOI: 10.7270/Q2WD43SW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein [1424-1463]/[1502-1685] (West Nile virus (WNV)) | BDBM24734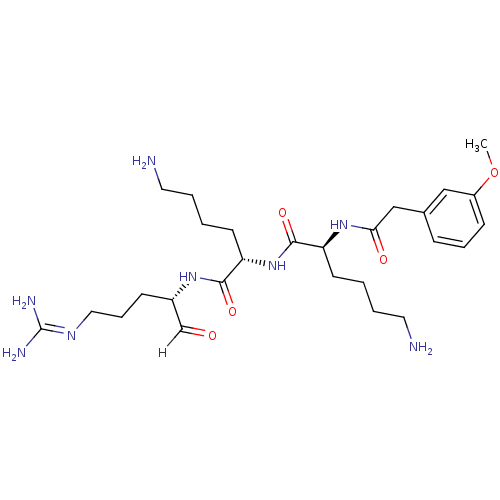 ((2S)-6-amino-N-[(1S)-5-amino-1-{[(2S)-5-carbamimid...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 11 | -47.3 | 60 | n/a | n/a | n/a | n/a | 9.5 | 37 |
University of Queensland | Assay Description Inhibition of WNV protease activity was measured using recombinant WNV protease, which first incubated with inhibitor in 96-well plates. Catalysis wa... | J Med Chem 51: 5714-21 (2008) Article DOI: 10.1021/jm800503y BindingDB Entry DOI: 10.7270/Q27P8WPF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1ab (2019-nCoV) | BDBM535123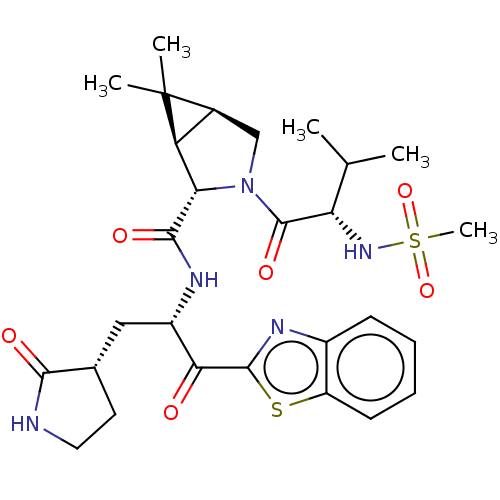 ((1R,2S,5S)-N-{(2S)-1-(1,3-Benzothiazol-2-yl)-1-oxo...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | WIPO WO2022013684 | 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The proteolytic activity of the main protease, 3CLpro, of SARS-CoV-2 was monitored using a continuous fluorescence resonance energy transfer (FRET) a... | Citation and Details BindingDB Entry DOI: 10.7270/Q2WD43SW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein [1424-1463]/[1502-1685] (West Nile virus (WNV)) | BDBM24746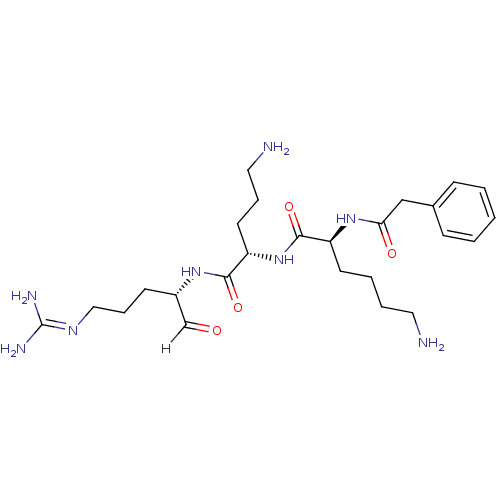 ((2S)-6-amino-N-[(1S)-4-amino-1-{[(2S)-5-carbamimid...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 13 | -46.8 | 73 | n/a | n/a | n/a | n/a | 9.5 | 37 |
University of Queensland | Assay Description Inhibition of WNV protease activity was measured using recombinant WNV protease, which first incubated with inhibitor in 96-well plates. Catalysis wa... | J Med Chem 51: 5714-21 (2008) Article DOI: 10.1021/jm800503y BindingDB Entry DOI: 10.7270/Q27P8WPF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1ab (2019-nCoV) | BDBM535143 (WO2022013684, Example 11) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | WIPO WO2022013684 | 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The proteolytic activity of the main protease, 3CLpro, of SARS-CoV-2 was monitored using a continuous fluorescence resonance energy transfer (FRET) a... | Citation and Details BindingDB Entry DOI: 10.7270/Q2WD43SW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1ab (2019-nCoV) | BDBM535129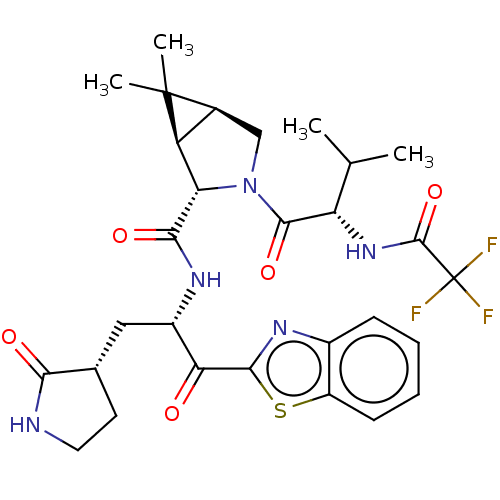 ((1R,2S,5S)-N-{(2S)-1-(1,3-Benzothiazol-2-yl)-1-oxo...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | WIPO WO2022013684 | 15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The proteolytic activity of the main protease, 3CLpro, of SARS-CoV-2 was monitored using a continuous fluorescence resonance energy transfer (FRET) a... | Citation and Details BindingDB Entry DOI: 10.7270/Q2WD43SW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 6 (Homo sapiens (Human)) | BDBM50595960 (CHEMBL5192865) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | MCE PC cid PC sid UniChem | Article PubMed | 17 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00420 BindingDB Entry DOI: 10.7270/Q2MP579X | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 6 (Homo sapiens (Human)) | BDBM50595960 (CHEMBL5192865) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | MCE PC cid PC sid UniChem | Article PubMed | 17 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01585 BindingDB Entry DOI: 10.7270/Q2QV3RKM | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 6 (Homo sapiens (Human)) | BDBM24622 (3-[(dimethylamino)methyl]-N-{2-[4-(hydroxycarbamoy...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 17 | -43.9 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
Celera Genomics | Assay Description HDAC activity was measured using a continuous trypsin-coupled assay. For inhibitor characterization, measurements were done using 96-well assay plat... | Mol Cancer Ther 5: 1309-17 (2006) Article DOI: 10.1158/1535-7163.MCT-05-0442 BindingDB Entry DOI: 10.7270/Q2DF6PJJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein [1424-1463]/[1502-1685] (West Nile virus (WNV)) | BDBM24739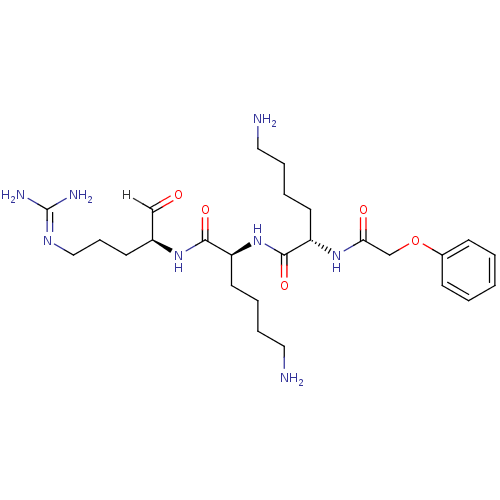 ((2S)-6-amino-N-[(1S)-5-amino-1-{[(2S)-5-carbamimid...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 19 | -45.8 | 107 | n/a | n/a | n/a | n/a | 9.5 | 37 |
University of Queensland | Assay Description Inhibition of WNV protease activity was measured using recombinant WNV protease, which first incubated with inhibitor in 96-well plates. Catalysis wa... | J Med Chem 51: 5714-21 (2008) Article DOI: 10.1021/jm800503y BindingDB Entry DOI: 10.7270/Q27P8WPF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 2 (Homo sapiens (Human)) | BDBM24622 (3-[(dimethylamino)methyl]-N-{2-[4-(hydroxycarbamoy...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 19 | -43.6 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
Celera Genomics | Assay Description HDAC activity was measured using a continuous trypsin-coupled assay. For inhibitor characterization, measurements were done using 96-well assay plat... | Mol Cancer Ther 5: 1309-17 (2006) Article DOI: 10.1158/1535-7163.MCT-05-0442 BindingDB Entry DOI: 10.7270/Q2DF6PJJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein [1424-1463]/[1502-1685] (West Nile virus (WNV)) | BDBM24733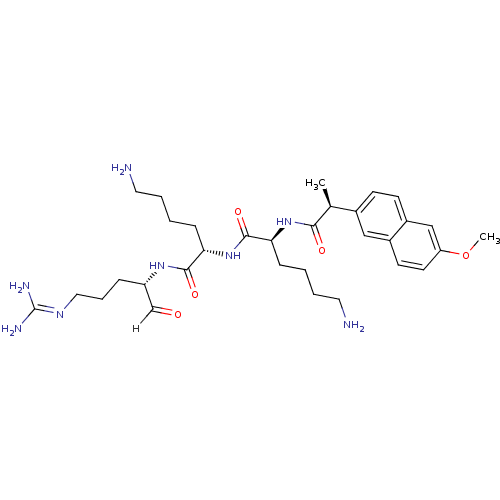 ((2S)-6-amino-N-[(1S)-5-amino-1-{[(2S)-5-carbamimid...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 20 | -45.7 | 112 | n/a | n/a | n/a | n/a | 9.5 | 37 |
University of Queensland | Assay Description Inhibition of WNV protease activity was measured using recombinant WNV protease, which first incubated with inhibitor in 96-well plates. Catalysis wa... | J Med Chem 51: 5714-21 (2008) Article DOI: 10.1021/jm800503y BindingDB Entry DOI: 10.7270/Q27P8WPF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1ab (2019-nCoV) | BDBM535141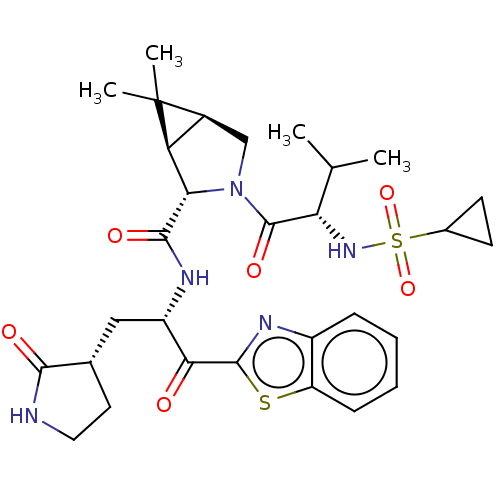 (WO2022013684, Example 10) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | WIPO WO2022013684 | 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The proteolytic activity of the main protease, 3CLpro, of SARS-CoV-2 was monitored using a continuous fluorescence resonance energy transfer (FRET) a... | Citation and Details BindingDB Entry DOI: 10.7270/Q2WD43SW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1ab (2019-nCoV) | BDBM535075 ((6S)-N-{(2S)-1-(1,3-Benzothiazol-2-yl)-1-oxo-3-[(3...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | WIPO WO2022013684 | 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The proteolytic activity of the main protease, 3CLpro, of SARS-CoV-2 was monitored using a continuous fluorescence resonance energy transfer (FRET) a... | Citation and Details BindingDB Entry DOI: 10.7270/Q2WD43SW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyamine deacetylase HDAC10 (Homo sapiens (Human)) | BDBM24622 (3-[(dimethylamino)methyl]-N-{2-[4-(hydroxycarbamoy...) | NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 24 | -43.1 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
Celera Genomics | Assay Description HDAC activity was measured using a continuous trypsin-coupled assay. For inhibitor characterization, measurements were done using 96-well assay plat... | Mol Cancer Ther 5: 1309-17 (2006) Article DOI: 10.1158/1535-7163.MCT-05-0442 BindingDB Entry DOI: 10.7270/Q2DF6PJJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein [1424-1463]/[1502-1685] (West Nile virus (WNV)) | BDBM24735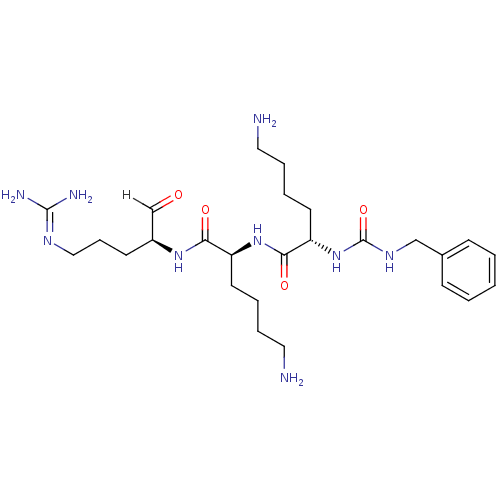 ((2S)-6-amino-N-[(1S)-5-amino-1-{[(2S)-5-carbamimid...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 26 | -45.0 | 146 | n/a | n/a | n/a | n/a | 9.5 | 37 |
University of Queensland | Assay Description Inhibition of WNV protease activity was measured using recombinant WNV protease, which first incubated with inhibitor in 96-well plates. Catalysis wa... | J Med Chem 51: 5714-21 (2008) Article DOI: 10.1021/jm800503y BindingDB Entry DOI: 10.7270/Q27P8WPF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein [1424-1463]/[1502-1685] (West Nile virus (WNV)) | BDBM24741 ((2S)-6-amino-N-[(2S)-5-carbamimidamido-1-oxopentan...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 28 | -44.8 | 154 | n/a | n/a | n/a | n/a | 9.5 | 37 |
University of Queensland | Assay Description Inhibition of WNV protease activity was measured using recombinant WNV protease, which first incubated with inhibitor in 96-well plates. Catalysis wa... | J Med Chem 51: 5714-21 (2008) Article DOI: 10.1021/jm800503y BindingDB Entry DOI: 10.7270/Q27P8WPF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1ab (2019-nCoV) | BDBM535145 (WO2022013684, Example 12) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | WIPO WO2022013684 | 34 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The proteolytic activity of the main protease, 3CLpro, of SARS-CoV-2 was monitored using a continuous fluorescence resonance energy transfer (FRET) a... | Citation and Details BindingDB Entry DOI: 10.7270/Q2WD43SW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 6 (Homo sapiens (Human)) | BDBM19149 (CHEMBL98 | N-hydroxy-N'-phenyloctanediamide | SAHA...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank Article PubMed | <35 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Toronto Mississauga Curated by ChEMBL | Assay Description Inhibition of recombinant full length human HDAC6 expressed in baculovirus infected Sf9 cells assessed as inhibitory constant using FAM-RHKK-Ac as su... | ACS Med Chem Lett 11: 56-64 (2020) Article DOI: 10.1021/acsmedchemlett.9b00471 BindingDB Entry DOI: 10.7270/Q26976Z8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 6 (Homo sapiens (Human)) | BDBM50519457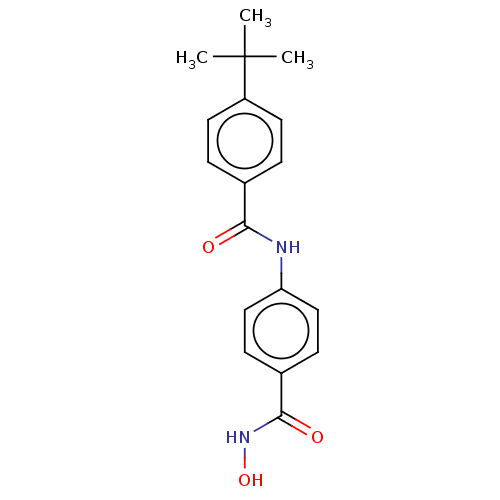 (CHEMBL4452620) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | 35 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Toronto Mississauga Curated by ChEMBL | Assay Description Inhibition of recombinant full length human HDAC6 expressed in baculovirus infected Sf9 cells assessed as inhibitory constant using FAM-RHKK-Ac as su... | ACS Med Chem Lett 11: 56-64 (2020) Article DOI: 10.1021/acsmedchemlett.9b00471 BindingDB Entry DOI: 10.7270/Q26976Z8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein [1424-1463]/[1502-1685] (West Nile virus (WNV)) | BDBM24736 (Capped tripeptide aldehyde inhibitor, 24 | benzyl ...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 40 | -43.9 | 222 | n/a | n/a | n/a | n/a | 9.5 | 37 |
University of Queensland | Assay Description Inhibition of WNV protease activity was measured using recombinant WNV protease, which first incubated with inhibitor in 96-well plates. Catalysis wa... | J Med Chem 51: 5714-21 (2008) Article DOI: 10.1021/jm800503y BindingDB Entry DOI: 10.7270/Q27P8WPF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein [1424-1463]/[1502-1685] (West Nile virus (WNV)) | BDBM24728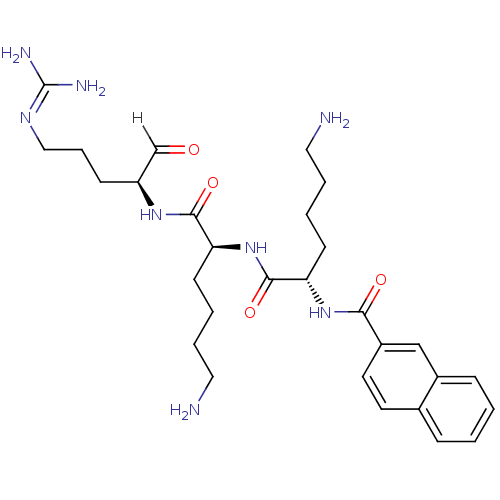 ((2S)-6-amino-N-[(1S)-5-amino-1-{[(2S)-5-carbamimid...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 41 | -43.9 | 231 | n/a | n/a | n/a | n/a | 9.5 | 37 |
University of Queensland | Assay Description Inhibition of WNV protease activity was measured using recombinant WNV protease, which first incubated with inhibitor in 96-well plates. Catalysis wa... | J Med Chem 51: 5714-21 (2008) Article DOI: 10.1021/jm800503y BindingDB Entry DOI: 10.7270/Q27P8WPF | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM24618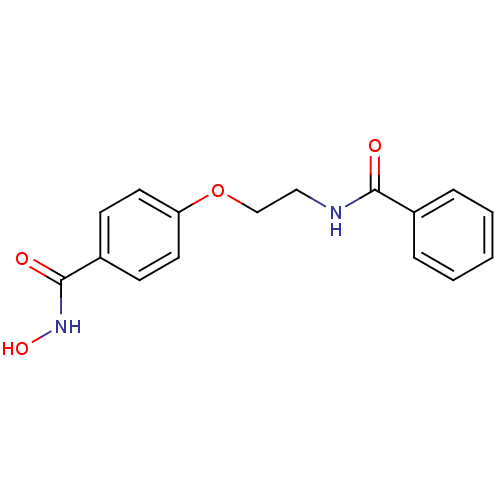 (CG-001 | N-hydroxy-4-[2-(phenylformamido)ethoxy]be...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 43 | -41.6 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
Celera Genomics | Assay Description HDAC activity was measured using a continuous trypsin-coupled assay. For inhibitor characterization, measurements were done using 96-well assay plat... | Mol Cancer Ther 5: 1309-17 (2006) Article DOI: 10.1158/1535-7163.MCT-05-0442 BindingDB Entry DOI: 10.7270/Q2DF6PJJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein [1424-1463]/[1502-1685] (West Nile virus (WNV)) | BDBM24744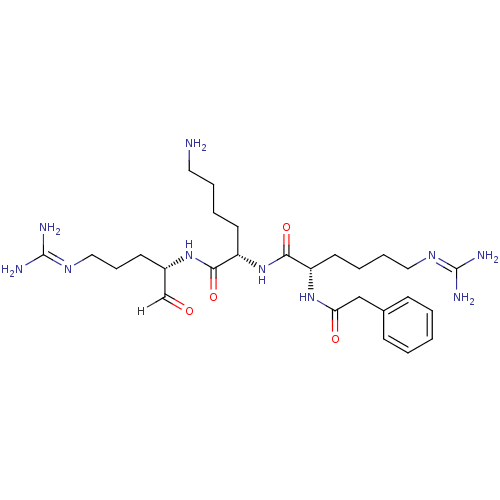 ((2S)-N-[(1S)-5-amino-1-{[(2S)-5-carbamimidamido-1-...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 44 | -43.7 | 245 | n/a | n/a | n/a | n/a | 9.5 | 37 |
University of Queensland | Assay Description Inhibition of WNV protease activity was measured using recombinant WNV protease, which first incubated with inhibitor in 96-well plates. Catalysis wa... | J Med Chem 51: 5714-21 (2008) Article DOI: 10.1021/jm800503y BindingDB Entry DOI: 10.7270/Q27P8WPF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein [1424-1463]/[1502-1685] (West Nile virus (WNV)) | BDBM24742 ((2S)-6-amino-2-[(2S)-5-amino-2-(1-phenylacetamido)...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 46 | -43.6 | 255 | n/a | n/a | n/a | n/a | 9.5 | 37 |
University of Queensland | Assay Description Inhibition of WNV protease activity was measured using recombinant WNV protease, which first incubated with inhibitor in 96-well plates. Catalysis wa... | J Med Chem 51: 5714-21 (2008) Article DOI: 10.1021/jm800503y BindingDB Entry DOI: 10.7270/Q27P8WPF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein [1424-1463]/[1502-1685] (West Nile virus (WNV)) | BDBM24737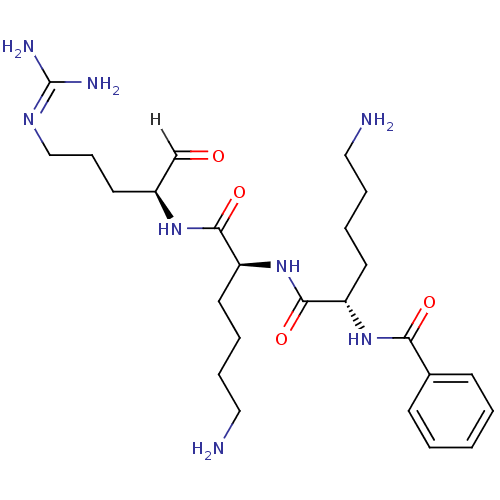 ((2S)-6-amino-N-[(1S)-5-amino-1-{[(2S)-5-carbamimid...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 49 | -43.4 | 271 | n/a | n/a | n/a | n/a | 9.5 | 37 |
University of Queensland | Assay Description Inhibition of WNV protease activity was measured using recombinant WNV protease, which first incubated with inhibitor in 96-well plates. Catalysis wa... | J Med Chem 51: 5714-21 (2008) Article DOI: 10.1021/jm800503y BindingDB Entry DOI: 10.7270/Q27P8WPF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein [1424-1463]/[1502-1685] (West Nile virus (WNV)) | BDBM24748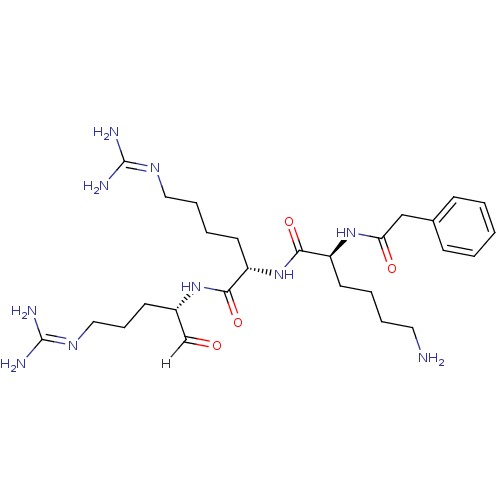 ((2S)-2-[(2S)-6-amino-2-(1-phenylacetamido)hexanami...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 53 | -43.2 | 297 | n/a | n/a | n/a | n/a | 9.5 | 37 |
University of Queensland | Assay Description Inhibition of WNV protease activity was measured using recombinant WNV protease, which first incubated with inhibitor in 96-well plates. Catalysis wa... | J Med Chem 51: 5714-21 (2008) Article DOI: 10.1021/jm800503y BindingDB Entry DOI: 10.7270/Q27P8WPF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein [1424-1463]/[1502-1685] (West Nile virus (WNV)) | BDBM24745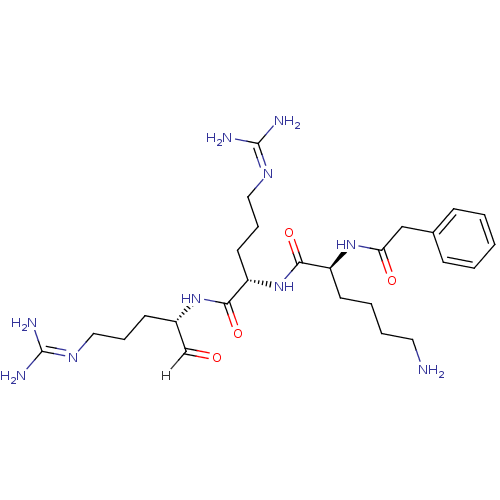 ((2S)-6-amino-N-[(1S)-4-carbamimidamido-1-{[(2S)-5-...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 58 | -43.0 | 325 | n/a | n/a | n/a | n/a | 9.5 | 37 |
University of Queensland | Assay Description Inhibition of WNV protease activity was measured using recombinant WNV protease, which first incubated with inhibitor in 96-well plates. Catalysis wa... | J Med Chem 51: 5714-21 (2008) Article DOI: 10.1021/jm800503y BindingDB Entry DOI: 10.7270/Q27P8WPF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein [1424-1463]/[1502-1685] (West Nile virus (WNV)) | BDBM24738 ((2S)-6-amino-N-[(1S)-5-amino-1-{[(2S)-5-carbamimid...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 81 | -42.1 | 454 | n/a | n/a | n/a | n/a | 9.5 | 37 |
University of Queensland | Assay Description Inhibition of WNV protease activity was measured using recombinant WNV protease, which first incubated with inhibitor in 96-well plates. Catalysis wa... | J Med Chem 51: 5714-21 (2008) Article DOI: 10.1021/jm800503y BindingDB Entry DOI: 10.7270/Q27P8WPF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1ab (2019-nCoV) | BDBM535165 (WO2022013684, Example 17) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | WIPO WO2022013684 | 99 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The proteolytic activity of the main protease, 3CLpro, of SARS-CoV-2 was monitored using a continuous fluorescence resonance energy transfer (FRET) a... | Citation and Details BindingDB Entry DOI: 10.7270/Q2WD43SW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein [1424-1463]/[1502-1685] (West Nile virus (WNV)) | BDBM24730 ((2S)-6-amino-2-[(2S)-6-amino-2-[(2E)-3-phenylprop-...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 104 | -41.5 | 580 | n/a | n/a | n/a | n/a | 9.5 | 37 |
University of Queensland | Assay Description Inhibition of WNV protease activity was measured using recombinant WNV protease, which first incubated with inhibitor in 96-well plates. Catalysis wa... | J Med Chem 51: 5714-21 (2008) Article DOI: 10.1021/jm800503y BindingDB Entry DOI: 10.7270/Q27P8WPF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein [1424-1463]/[1502-1685] (West Nile virus (WNV)) | BDBM24743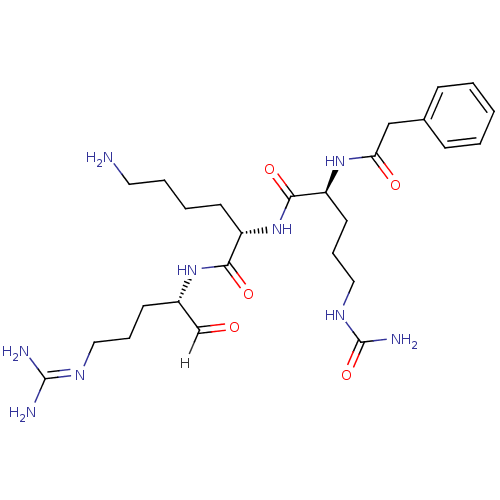 ((2S)-6-amino-N-[(2S)-5-carbamimidamido-1-oxopentan...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Similars | Article PubMed | 111 | -41.3 | 619 | n/a | n/a | n/a | n/a | 9.5 | 37 |
University of Queensland | Assay Description Inhibition of WNV protease activity was measured using recombinant WNV protease, which first incubated with inhibitor in 96-well plates. Catalysis wa... | J Med Chem 51: 5714-21 (2008) Article DOI: 10.1021/jm800503y BindingDB Entry DOI: 10.7270/Q27P8WPF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1ab (2019-nCoV) | BDBM535130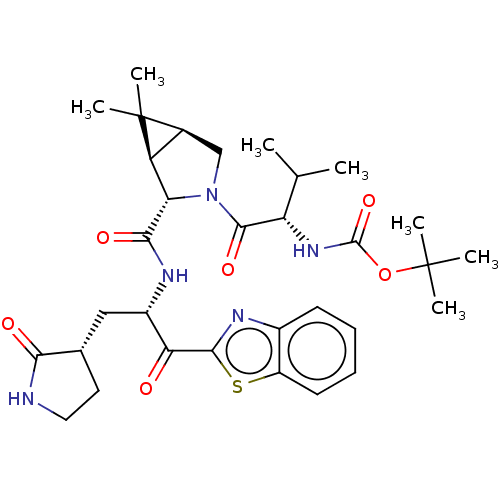 (WO2022013684, Example 6 | tert-Butyl {(2S)-1-[(1R,...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | WIPO WO2022013684 | 137 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The proteolytic activity of the main protease, 3CLpro, of SARS-CoV-2 was monitored using a continuous fluorescence resonance energy transfer (FRET) a... | Citation and Details BindingDB Entry DOI: 10.7270/Q2WD43SW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1ab (2019-nCoV) | BDBM535146 (WO2022013684, Example 13) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | WIPO WO2022013684 | 139 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The proteolytic activity of the main protease, 3CLpro, of SARS-CoV-2 was monitored using a continuous fluorescence resonance energy transfer (FRET) a... | Citation and Details BindingDB Entry DOI: 10.7270/Q2WD43SW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein [1424-1463]/[1502-1685] (West Nile virus (WNV)) | BDBM24740 ((2S)-6-amino-N-[(1S)-5-amino-1-{[(2S)-5-carbamimid...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 160 | -40.3 | 891 | n/a | n/a | n/a | n/a | 9.5 | 37 |
University of Queensland | Assay Description Inhibition of WNV protease activity was measured using recombinant WNV protease, which first incubated with inhibitor in 96-well plates. Catalysis wa... | J Med Chem 51: 5714-21 (2008) Article DOI: 10.1021/jm800503y BindingDB Entry DOI: 10.7270/Q27P8WPF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 6 (Homo sapiens (Human)) | BDBM50519456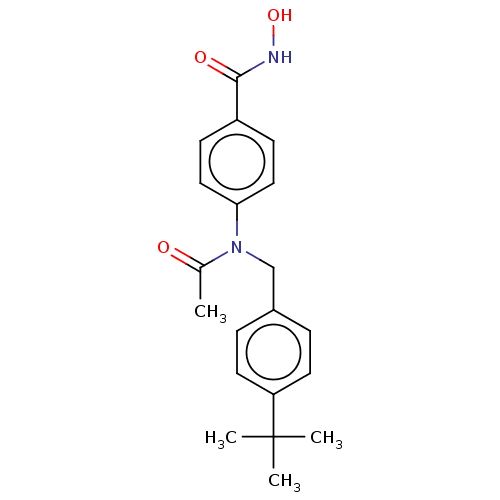 (CHEMBL4565296) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 199 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Toronto Mississauga Curated by ChEMBL | Assay Description Inhibition of recombinant full length human HDAC6 expressed in baculovirus infected Sf9 cells assessed as inhibitory constant using FAM-RHKK-Ac as su... | ACS Med Chem Lett 11: 56-64 (2020) Article DOI: 10.1021/acsmedchemlett.9b00471 BindingDB Entry DOI: 10.7270/Q26976Z8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 6 (Homo sapiens (Human)) | BDBM50519455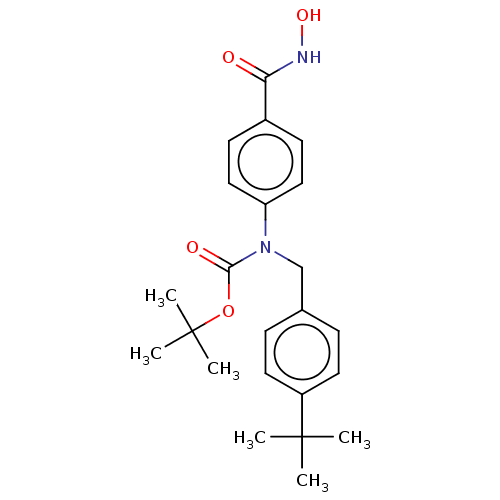 (CHEMBL4559559) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 201 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Toronto Mississauga Curated by ChEMBL | Assay Description Inhibition of recombinant full length human HDAC6 expressed in baculovirus infected Sf9 cells assessed as inhibitory constant using FAM-RHKK-Ac as su... | ACS Med Chem Lett 11: 56-64 (2020) Article DOI: 10.1021/acsmedchemlett.9b00471 BindingDB Entry DOI: 10.7270/Q26976Z8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 1440 total ) | Next | Last >> |