

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Aromatase (Homo sapiens (Human)) | BDBM19461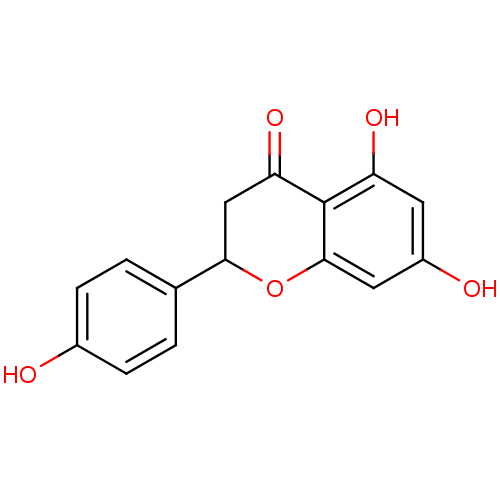 (α-CA inhibitor, 5 | 5,7-dihydroxy-2-(4-hydrox...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 0.00110 | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Central Institute of Medicinal and Aromatic Plants | Assay Description Inhibition assay using aromatase enzyme. | Chem Biol Drug Des 80: 616-624 (2012) Article DOI: 10.1111/j.1747-0285.2012.01439.x BindingDB Entry DOI: 10.7270/Q24X56CG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM92556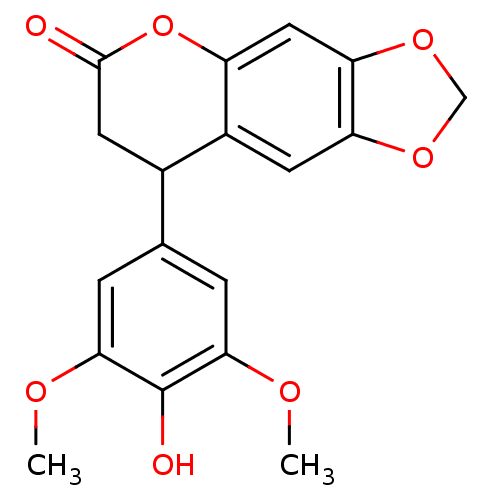 (Neoflavonoid, 8) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | 0.00182 | n/a | 1.21E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Central Institute of Medicinal and Aromatic Plants | Assay Description Inhibition assay using aromatase enzyme. | Chem Biol Drug Des 80: 616-624 (2012) Article DOI: 10.1111/j.1747-0285.2012.01439.x BindingDB Entry DOI: 10.7270/Q24X56CG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM92555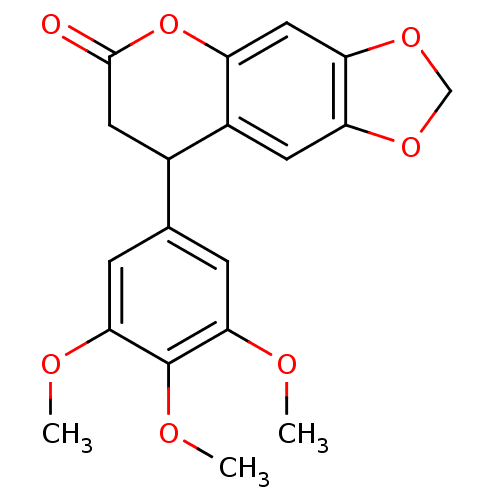 (Neoflavonoid, 7) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | 0.00216 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Central Institute of Medicinal and Aromatic Plants | Assay Description Inhibition assay using aromatase enzyme. | Chem Biol Drug Des 80: 616-624 (2012) Article DOI: 10.1111/j.1747-0285.2012.01439.x BindingDB Entry DOI: 10.7270/Q24X56CG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM92557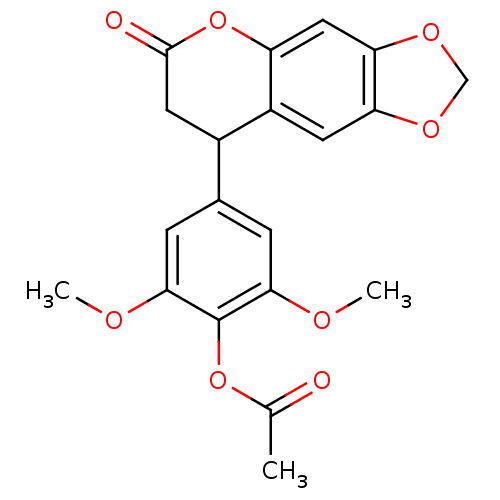 (Neoflavonoid, 9) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.00256 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Central Institute of Medicinal and Aromatic Plants | Assay Description Inhibition assay using aromatase enzyme. | Chem Biol Drug Des 80: 616-624 (2012) Article DOI: 10.1111/j.1747-0285.2012.01439.x BindingDB Entry DOI: 10.7270/Q24X56CG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM92559 (Neoflavonoid, 11) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.00425 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Central Institute of Medicinal and Aromatic Plants | Assay Description Inhibition assay using aromatase enzyme. | Chem Biol Drug Des 80: 616-624 (2012) Article DOI: 10.1111/j.1747-0285.2012.01439.x BindingDB Entry DOI: 10.7270/Q24X56CG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM92560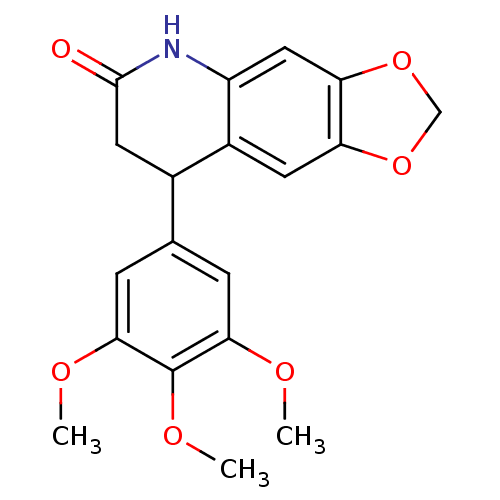 (Neoflavonoid, 19) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | 0.00597 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Central Institute of Medicinal and Aromatic Plants | Assay Description Inhibition assay using aromatase enzyme. | Chem Biol Drug Des 80: 616-624 (2012) Article DOI: 10.1111/j.1747-0285.2012.01439.x BindingDB Entry DOI: 10.7270/Q24X56CG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM92558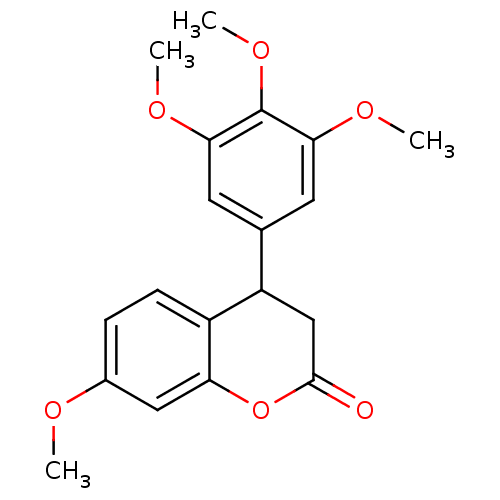 (Neoflavonoid, 10) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.00838 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Central Institute of Medicinal and Aromatic Plants | Assay Description Inhibition assay using aromatase enzyme. | Chem Biol Drug Des 80: 616-624 (2012) Article DOI: 10.1111/j.1747-0285.2012.01439.x BindingDB Entry DOI: 10.7270/Q24X56CG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM92561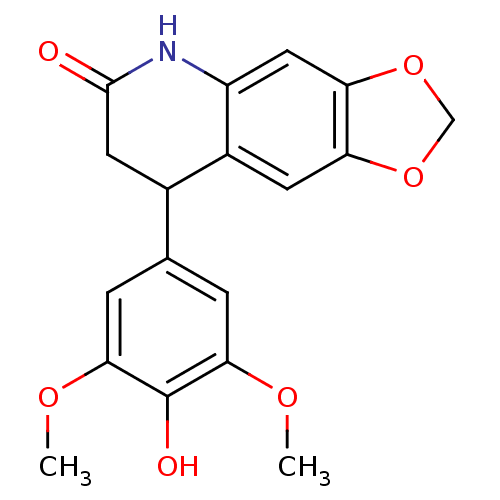 (Neoflavonoid, 20) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | 0.0118 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Central Institute of Medicinal and Aromatic Plants | Assay Description Inhibition assay using aromatase enzyme. | Chem Biol Drug Des 80: 616-624 (2012) Article DOI: 10.1111/j.1747-0285.2012.01439.x BindingDB Entry DOI: 10.7270/Q24X56CG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM13061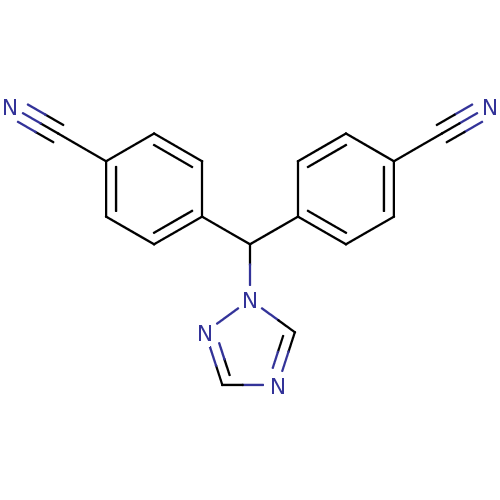 (4,4 -(1H-1,2,4-triazol-1-ylmethanediyl)dibenzonitr...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid UniChem Patents Similars | DrugBank Article PubMed | 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Competitive inhibition of human aromatase using dibenzylfluorescein substrate after 10 mins preincubation measured every 10 sec for 5 mins by Michael... | Bioorg Med Chem 20: 2427-34 (2012) Article DOI: 10.1016/j.bmc.2012.01.047 BindingDB Entry DOI: 10.7270/Q2DJ5G3W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sigma non-opioid intracellular receptor 1 (Cavia porcellus (Guinea pig)) | BDBM50179280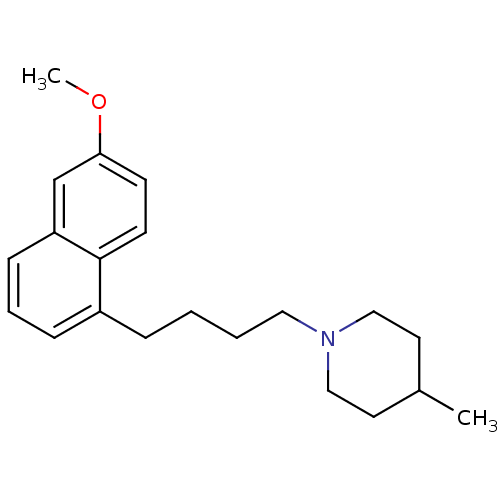 (1-(4-(6-methoxynaphthalen-1-yl)butyl)-4-methylpipe...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ Degli Studi di Bari ALDO MORO Curated by ChEMBL | Assay Description Displacement of [3H]-pentazocine from sigma 1 receptor in guinea pig brain membrane after 120 mins | Eur J Med Chem 108: 577-85 (2016) Article DOI: 10.1016/j.ejmech.2015.12.014 BindingDB Entry DOI: 10.7270/Q22Z17C3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM8611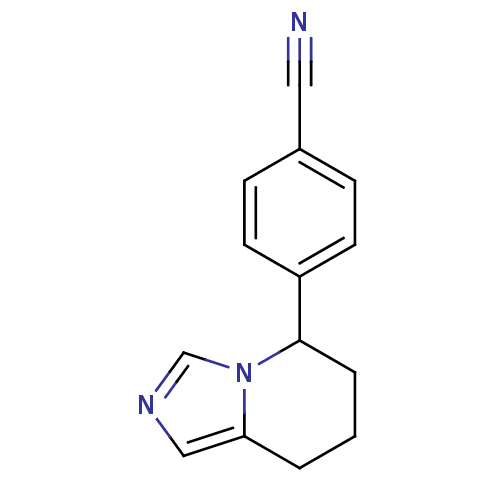 (4-{5H,6H,7H,8H-imidazo[1,5-a]pyridin-5-yl}benzonit...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Competitive inhibition of human aromatase using dibenzylfluorescein substrate after 10 mins preincubation measured every 10 sec for 5 mins by Michael... | Bioorg Med Chem 20: 2427-34 (2012) Article DOI: 10.1016/j.bmc.2012.01.047 BindingDB Entry DOI: 10.7270/Q2DJ5G3W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50366125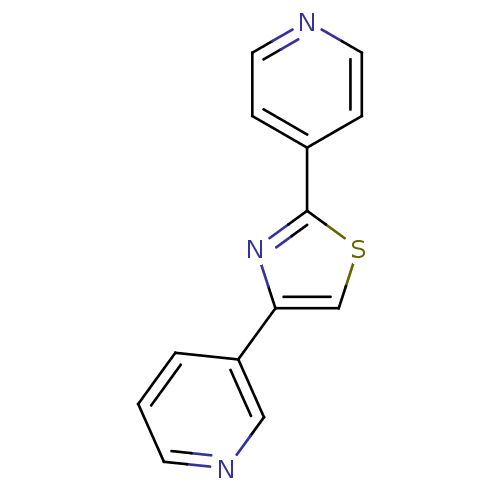 (CHEMBL1957214) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Competitive inhibition of human aromatase using dibenzylfluorescein substrate after 10 mins preincubation measured every 10 sec for 5 mins by Michael... | Bioorg Med Chem 20: 2427-34 (2012) Article DOI: 10.1016/j.bmc.2012.01.047 BindingDB Entry DOI: 10.7270/Q2DJ5G3W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sigma non-opioid intracellular receptor 1 (Cavia porcellus (Guinea pig)) | BDBM50299711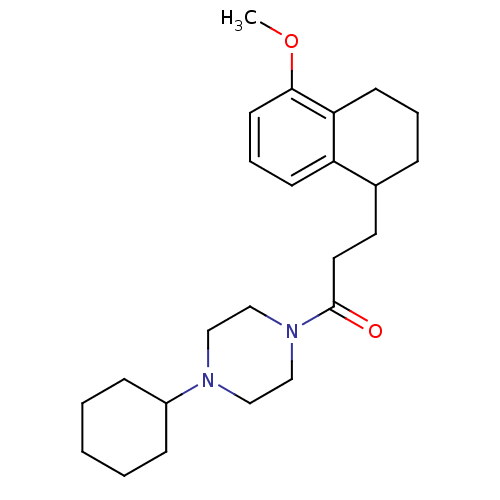 (4-Cyclohexyl-1-[3-(5-methoxy-1,2,3,4-tetrahydronap...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ Degli Studi di Bari ALDO MORO Curated by ChEMBL | Assay Description Displacement of [3H]-pentazocine from sigma 1 receptor in guinea pig brain membrane after 120 mins | Eur J Med Chem 108: 577-85 (2016) Article DOI: 10.1016/j.ejmech.2015.12.014 BindingDB Entry DOI: 10.7270/Q22Z17C3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM10015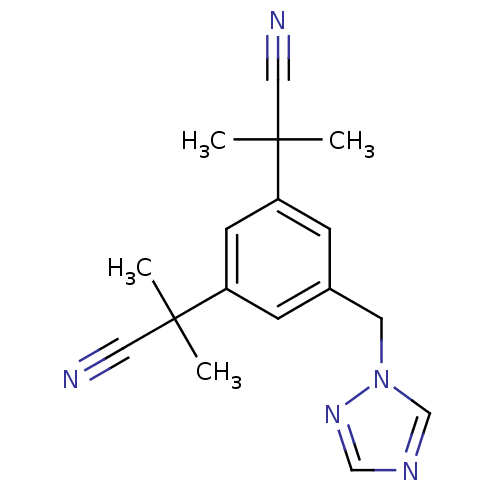 (2-[3-(1-cyano-1-methylethyl)-5-(1H-1,2,4-triazol-1...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid UniChem Patents Similars | DrugBank Article PubMed | 0.130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Competitive inhibition of human aromatase using dibenzylfluorescein substrate after 10 mins preincubation measured every 10 sec for 5 mins by Michael... | Bioorg Med Chem 20: 2427-34 (2012) Article DOI: 10.1016/j.bmc.2012.01.047 BindingDB Entry DOI: 10.7270/Q2DJ5G3W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM50139773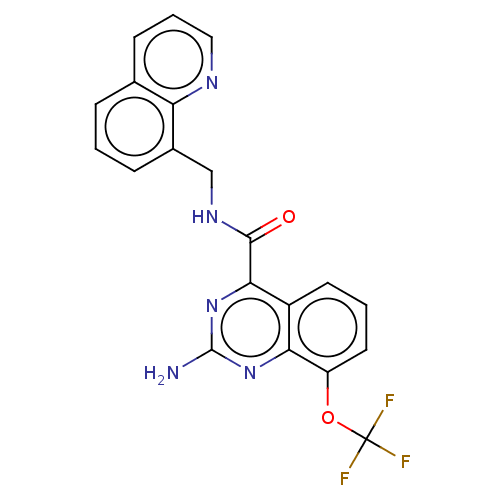 (CHEMBL3765379 | US10138212, Example 101) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]SCH-58261 from human adenosine A2A receptor expressed in HEK cell membranes after 60 mins by microplate scintillation counting an... | Bioorg Med Chem Lett 26: 1348-54 (2016) Article DOI: 10.1016/j.bmcl.2015.11.048 BindingDB Entry DOI: 10.7270/Q2S46TT3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM50200981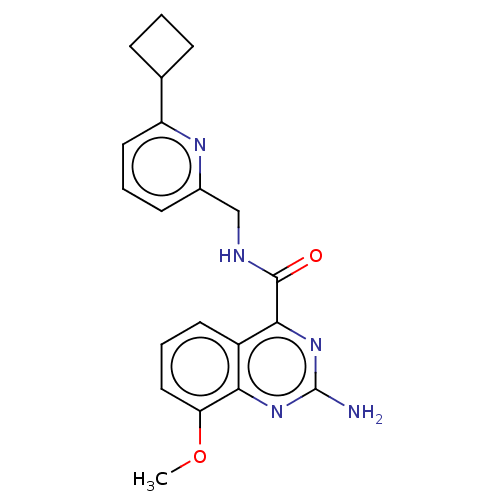 (CHEMBL3960148 | US10138212, Example 6) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description Binding affinities of compounds of the invention for the human A2a receptor were determined in a competition binding assay using Scintillation Proxim... | US Patent US10138212 (2018) BindingDB Entry DOI: 10.7270/Q2WM1GGB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM50139773 (CHEMBL3765379 | US10138212, Example 101) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description Binding affinities of compounds of the invention for the human A2a receptor were determined in a competition binding assay using Scintillation Proxim... | US Patent US10138212 (2018) BindingDB Entry DOI: 10.7270/Q2WM1GGB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM50139771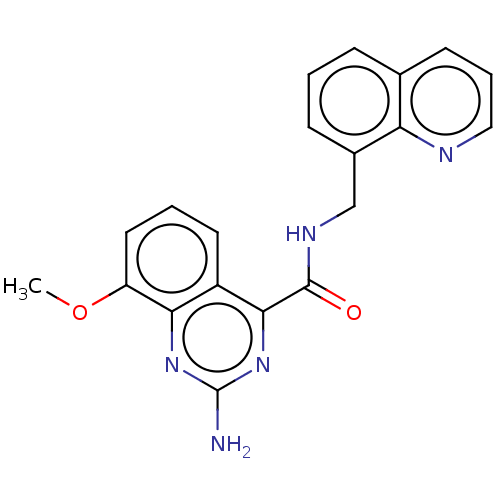 (CHEMBL3765580 | US10138212, Example 12) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]SCH-58261 from human adenosine A2A receptor expressed in HEK cell membranes after 60 mins by microplate scintillation counting an... | Bioorg Med Chem Lett 26: 1348-54 (2016) Article DOI: 10.1016/j.bmcl.2015.11.048 BindingDB Entry DOI: 10.7270/Q2S46TT3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM50201019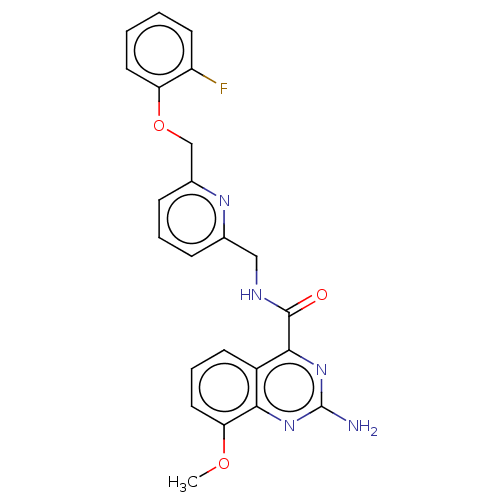 (CHEMBL3973920 | US10138212, Example 44) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | US Patent | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description Binding affinities of compounds of the invention for the human A2a receptor were determined in a competition binding assay using Scintillation Proxim... | US Patent US10138212 (2018) BindingDB Entry DOI: 10.7270/Q2WM1GGB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Heat shock protein HSP 90-alpha (Homo sapiens (Human)) | BDBM50401333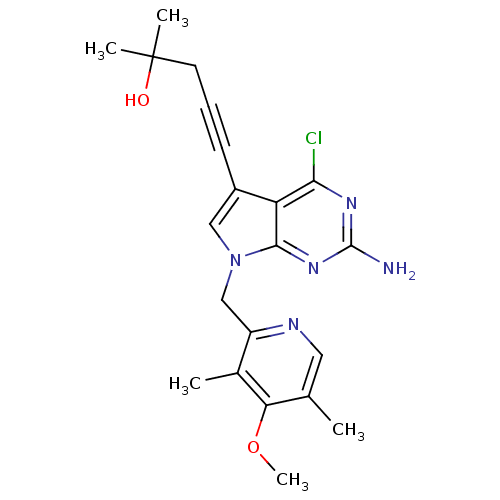 (CHEMBL1230584) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Similars | MMDB Article PubMed | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Biogen Idec Inc. Curated by ChEMBL | Assay Description Binding affinity at recombinant Hsp90alpha incubated for 16 hrs by fluorescence polarization competition assay | J Med Chem 55: 7786-95 (2012) Article DOI: 10.1021/jm300810x BindingDB Entry DOI: 10.7270/Q2V125Z3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM50201006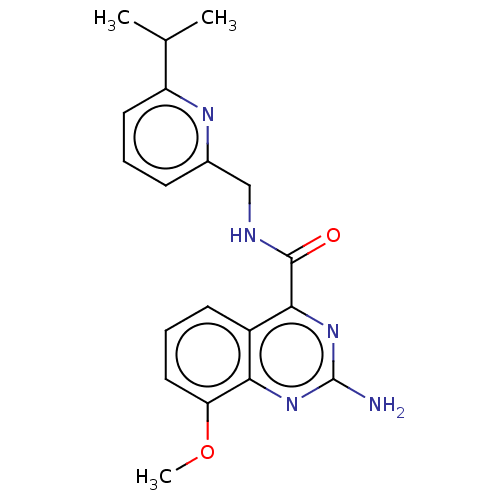 (CHEMBL3923709 | US10138212, Example 5) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description Binding affinities of compounds of the invention for the human A2a receptor were determined in a competition binding assay using Scintillation Proxim... | US Patent US10138212 (2018) BindingDB Entry DOI: 10.7270/Q2WM1GGB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM303248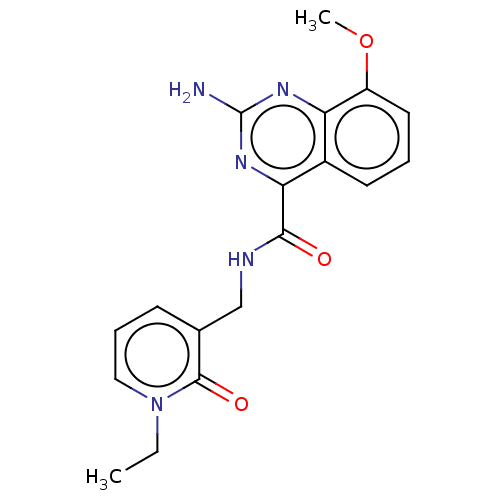 (2-amino-N-[(1-ethyl-2- oxo-3-pyridyl)methyl]-8- me...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description Binding affinities of compounds of the invention for the human A2a receptor were determined in a competition binding assay using Scintillation Proxim... | US Patent US10138212 (2018) BindingDB Entry DOI: 10.7270/Q2WM1GGB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM50139771 (CHEMBL3765580 | US10138212, Example 12) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description Binding affinities of compounds of the invention for the human A2a receptor were determined in a competition binding assay using Scintillation Proxim... | US Patent US10138212 (2018) BindingDB Entry DOI: 10.7270/Q2WM1GGB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM303246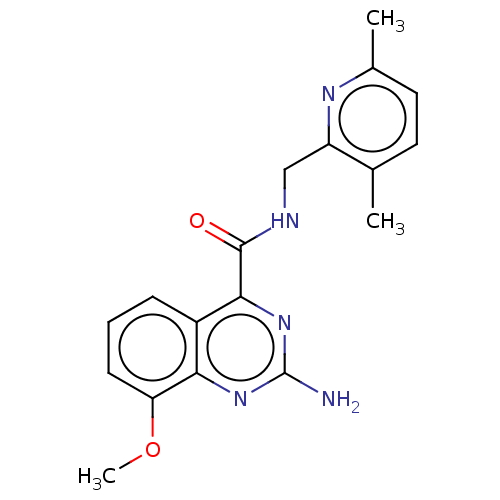 (2-amino-N-[(3,6- dimethyl-2- pyridyl)methyl]-8- me...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description Binding affinities of compounds of the invention for the human A2a receptor were determined in a competition binding assay using Scintillation Proxim... | US Patent US10138212 (2018) BindingDB Entry DOI: 10.7270/Q2WM1GGB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM303280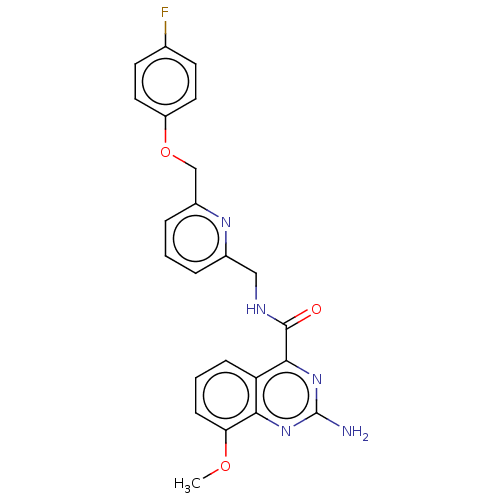 (2-amino-N-[[6-[(4- fluorophenoxy)methyl]- 2-pyridy...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description Binding affinities of compounds of the invention for the human A2a receptor were determined in a competition binding assay using Scintillation Proxim... | US Patent US10138212 (2018) BindingDB Entry DOI: 10.7270/Q2WM1GGB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM50139748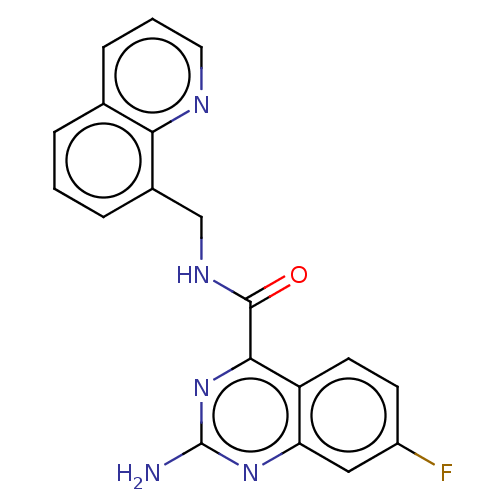 (CHEMBL3763717) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]SCH-58261 from human adenosine A2A receptor expressed in HEK cell membranes after 60 mins by microplate scintillation counting an... | Bioorg Med Chem Lett 26: 1348-54 (2016) Article DOI: 10.1016/j.bmcl.2015.11.048 BindingDB Entry DOI: 10.7270/Q2S46TT3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM303181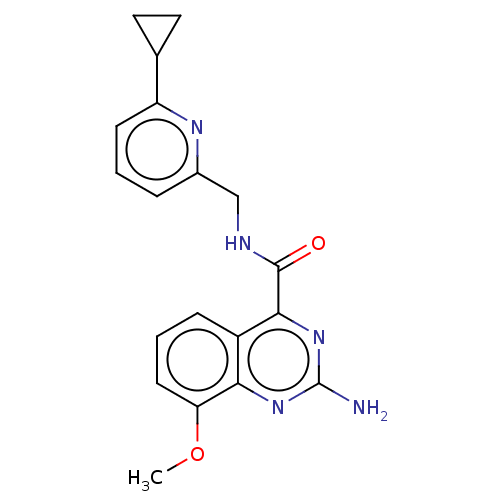 (2-amino-N-[(6- cyclopropyl-2- pyridyl)methyl]-8- m...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description Binding affinities of compounds of the invention for the human A2a receptor were determined in a competition binding assay using Scintillation Proxim... | US Patent US10138212 (2018) BindingDB Entry DOI: 10.7270/Q2WM1GGB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polycomb protein EED (Homo sapiens (Human)) | BDBM50594944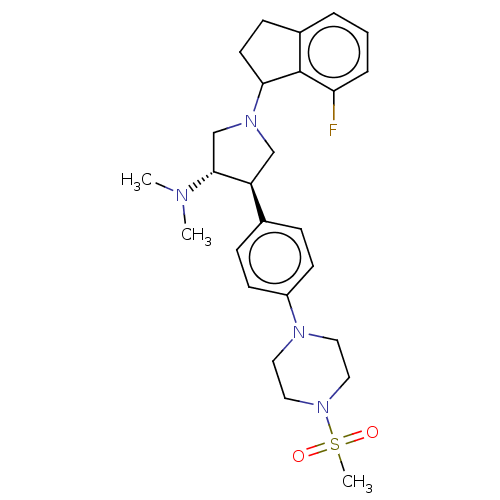 (CHEMBL5181703) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1016/j.ejmech.2022.114144 BindingDB Entry DOI: 10.7270/Q2DN4921 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM303251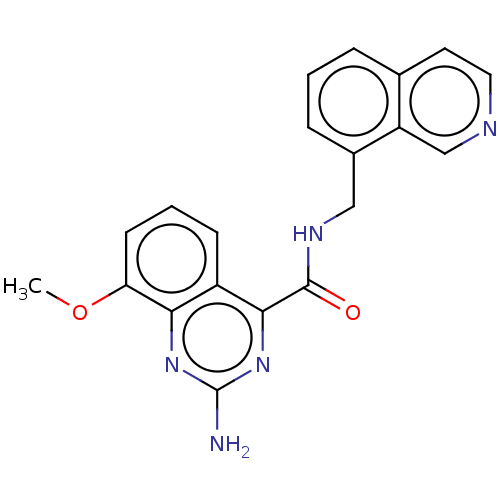 (2-amino-N-(8- isoquinolylmethyl)-8- methoxy-quinaz...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description Binding affinities of compounds of the invention for the human A2a receptor were determined in a competition binding assay using Scintillation Proxim... | US Patent US10138212 (2018) BindingDB Entry DOI: 10.7270/Q2WM1GGB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM50200984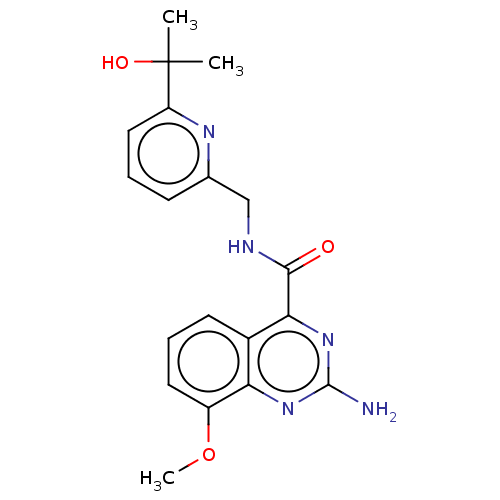 (CHEMBL3932655 | US10138212, Example 9) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description Binding affinities of compounds of the invention for the human A2a receptor were determined in a competition binding assay using Scintillation Proxim... | US Patent US10138212 (2018) BindingDB Entry DOI: 10.7270/Q2WM1GGB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM50200989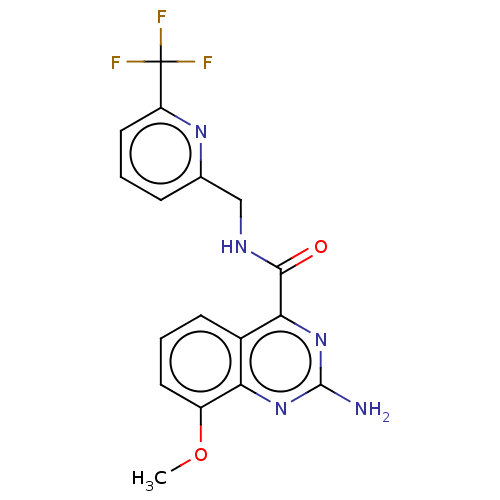 (CHEMBL3906827 | US10138212, Example 18) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | US Patent | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description Binding affinities of compounds of the invention for the human A2a receptor were determined in a competition binding assay using Scintillation Proxim... | US Patent US10138212 (2018) BindingDB Entry DOI: 10.7270/Q2WM1GGB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM303252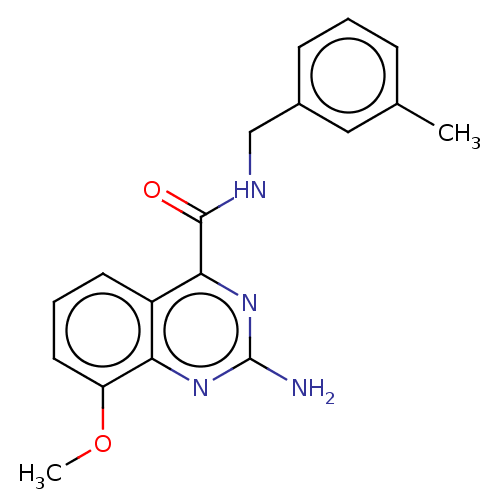 (2-amino-8-methoxy-N- (m- tolylmethyl)quinazoline- ...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description Binding affinities of compounds of the invention for the human A2a receptor were determined in a competition binding assay using Scintillation Proxim... | US Patent US10138212 (2018) BindingDB Entry DOI: 10.7270/Q2WM1GGB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cysteine protease (Trypanosoma brucei rhodesiense) | BDBM50574836 (CHEMBL4871105) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.450 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of Trypanosoma brucei rhodesain assessed as fluorescence using Cbz-Phe-Arg-AMC as substrate measured at second inhibition step by fluorome... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01002 BindingDB Entry DOI: 10.7270/Q2CC14G4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM303298 (2-amino-8-fluoro-N-[(2- pyrazol-1- ylphenyl)methyl...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description Binding affinities of compounds of the invention for the human A2a receptor were determined in a competition binding assay using Scintillation Proxim... | US Patent US10138212 (2018) BindingDB Entry DOI: 10.7270/Q2WM1GGB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM303284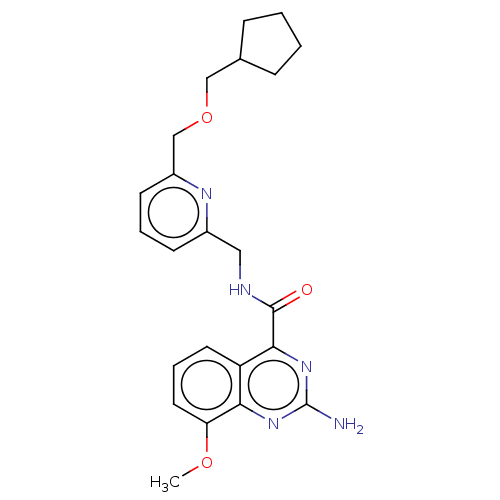 (2-amino-N-[[6- (cyclopentylmethoxy- methyl)-2-pyri...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description Binding affinities of compounds of the invention for the human A2a receptor were determined in a competition binding assay using Scintillation Proxim... | US Patent US10138212 (2018) BindingDB Entry DOI: 10.7270/Q2WM1GGB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM303255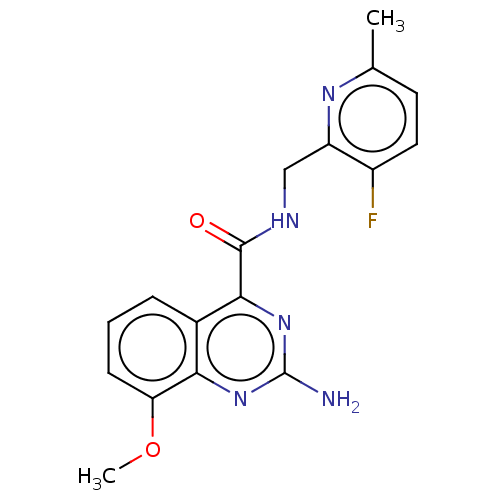 (2-amino-N-[(3-fluoro-6- methyl-2- pyridyl)methyl]-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description Binding affinities of compounds of the invention for the human A2a receptor were determined in a competition binding assay using Scintillation Proxim... | US Patent US10138212 (2018) BindingDB Entry DOI: 10.7270/Q2WM1GGB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM50201017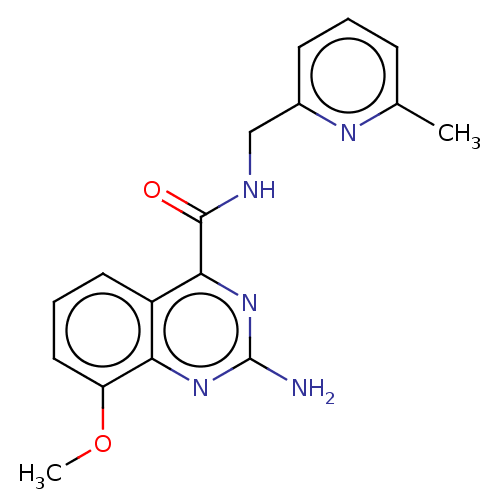 (CHEMBL3941632 | US10138212, Example 15) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description Binding affinities of compounds of the invention for the human A2a receptor were determined in a competition binding assay using Scintillation Proxim... | US Patent US10138212 (2018) BindingDB Entry DOI: 10.7270/Q2WM1GGB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM303313 (2-amino-8-fluoro-N-[(3- fluoro-6-methyl-2- pyridyl...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description Binding affinities of compounds of the invention for the human A2a receptor were determined in a competition binding assay using Scintillation Proxim... | US Patent US10138212 (2018) BindingDB Entry DOI: 10.7270/Q2WM1GGB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM50200986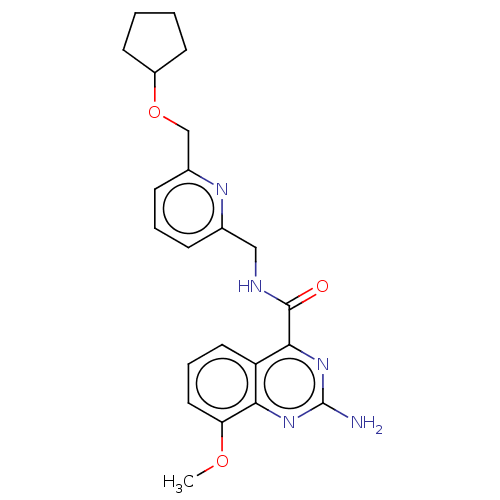 (CHEMBL3902955 | US10138212, Example 48) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description Binding affinities of compounds of the invention for the human A2a receptor were determined in a competition binding assay using Scintillation Proxim... | US Patent US10138212 (2018) BindingDB Entry DOI: 10.7270/Q2WM1GGB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM50139655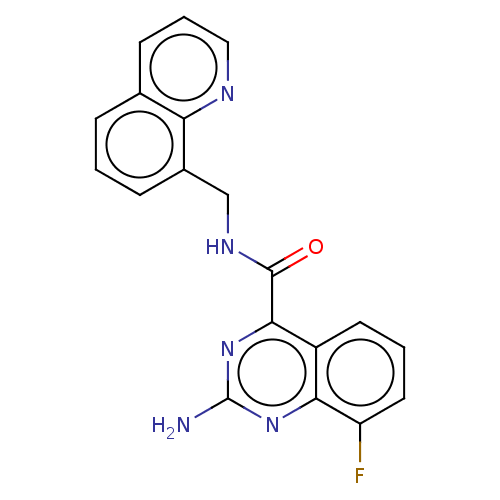 (CHEMBL3764083) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]SCH-58261 from human adenosine A2A receptor expressed in HEK cell membranes after 60 mins by microplate scintillation counting an... | Bioorg Med Chem Lett 26: 1348-54 (2016) Article DOI: 10.1016/j.bmcl.2015.11.048 BindingDB Entry DOI: 10.7270/Q2S46TT3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM303145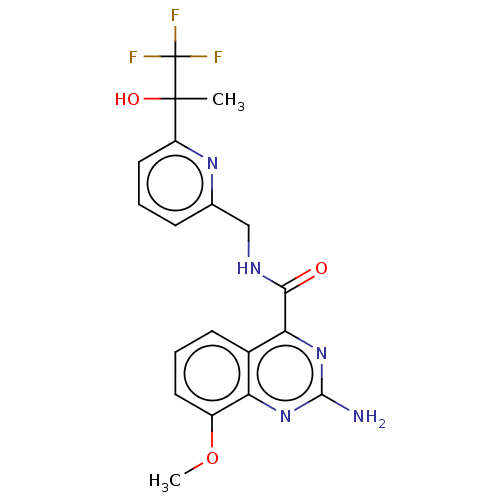 (2-amino-8-methoxy-N- [[6-(2,2,2-trifluoro-1- hydro...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description Binding affinities of compounds of the invention for the human A2a receptor were determined in a competition binding assay using Scintillation Proxim... | US Patent US10138212 (2018) BindingDB Entry DOI: 10.7270/Q2WM1GGB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxytocin receptor (Homo sapiens (Human)) | BDBM50523555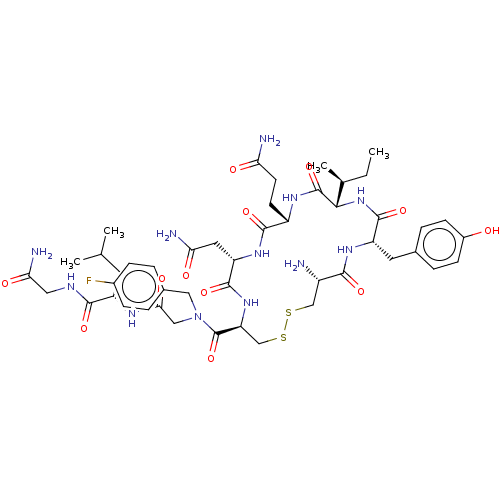 (CHEMBL4474284) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.510 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tohoku University and Department of Pharmaceutical Sciences Curated by ChEMBL | Assay Description Displacement of [3H]OT from recombinant human OTR expressed in HEK293 cell membranes measured after 1 hr | J Med Chem 62: 3297-3310 (2019) Article DOI: 10.1021/acs.jmedchem.8b01691 BindingDB Entry DOI: 10.7270/Q2GF0XXQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxytocin receptor (Homo sapiens (Human)) | BDBM50205990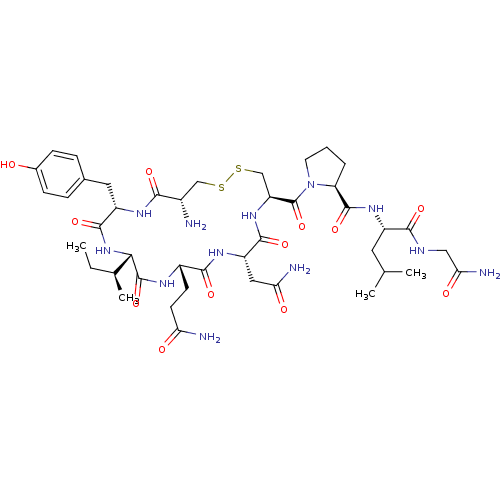 (CHEMBL395429 | OXYTOCIN) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | 0.580 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tohoku University and Department of Pharmaceutical Sciences Curated by ChEMBL | Assay Description Displacement of [3H]OT from recombinant human OTR expressed in HEK293 cell membranes measured after 1 hr | J Med Chem 62: 3297-3310 (2019) Article DOI: 10.1021/acs.jmedchem.8b01691 BindingDB Entry DOI: 10.7270/Q2GF0XXQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM303319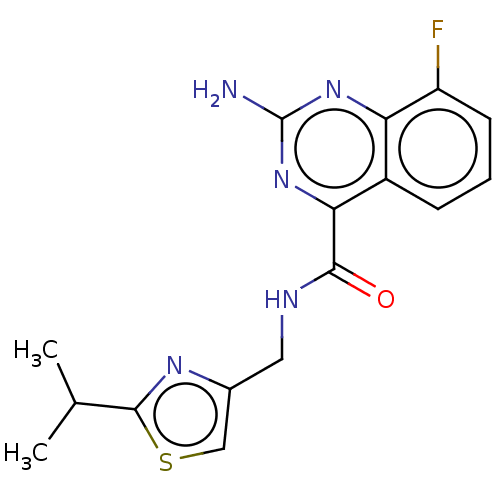 (2-amino-8-fluoro-N-[(2- isopropylthiazol-4- yl)met...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description Binding affinities of compounds of the invention for the human A2a receptor were determined in a competition binding assay using Scintillation Proxim... | US Patent US10138212 (2018) BindingDB Entry DOI: 10.7270/Q2WM1GGB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM50201020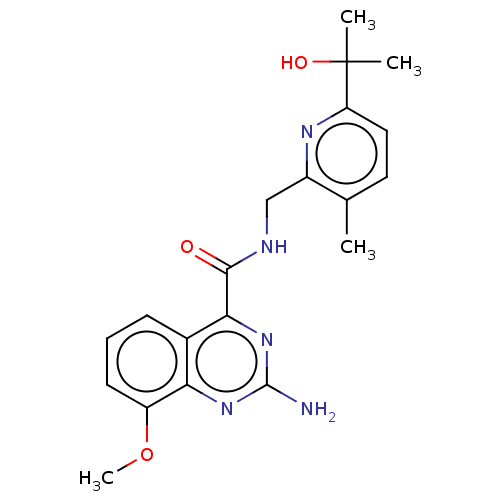 (CHEMBL3951425 | US10138212, Example 2) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description Binding affinities of compounds of the invention for the human A2a receptor were determined in a competition binding assay using Scintillation Proxim... | US Patent US10138212 (2018) BindingDB Entry DOI: 10.7270/Q2WM1GGB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM303150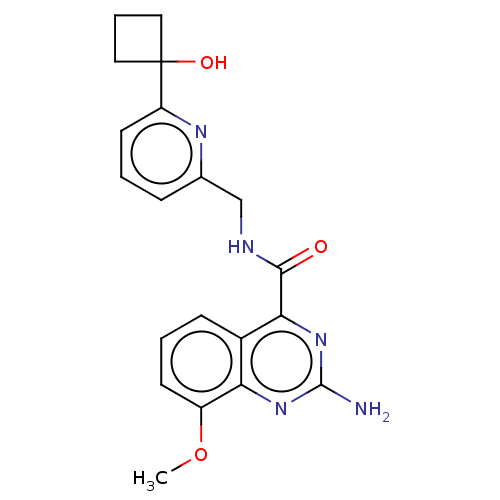 (2-amino-N-[[6-(1- hydroxycyclobutyl)-2- pyridyl]me...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description Binding affinities of compounds of the invention for the human A2a receptor were determined in a competition binding assay using Scintillation Proxim... | US Patent US10138212 (2018) BindingDB Entry DOI: 10.7270/Q2WM1GGB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Rattus norvegicus (rat)) | BDBM50139771 (CHEMBL3765580 | US10138212, Example 12) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Binding affinity to rat A2A adenosine receptor | Bioorg Med Chem Lett 26: 1348-54 (2016) Article DOI: 10.1016/j.bmcl.2015.11.048 BindingDB Entry DOI: 10.7270/Q2S46TT3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50366128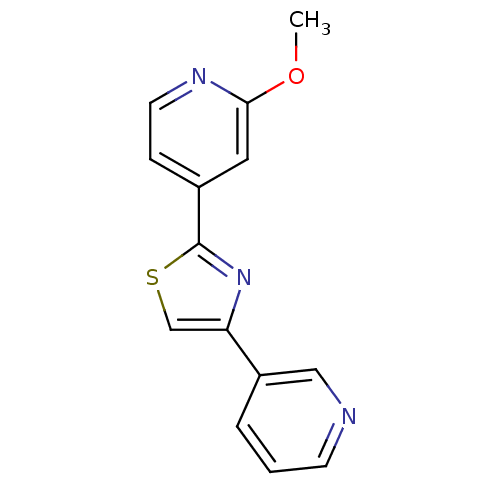 (CHEMBL1957217) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.650 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Competitive inhibition of human aromatase using dibenzylfluorescein substrate after 10 mins preincubation measured every 10 sec for 5 mins by Michael... | Bioorg Med Chem 20: 2427-34 (2012) Article DOI: 10.1016/j.bmc.2012.01.047 BindingDB Entry DOI: 10.7270/Q2DJ5G3W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM50139765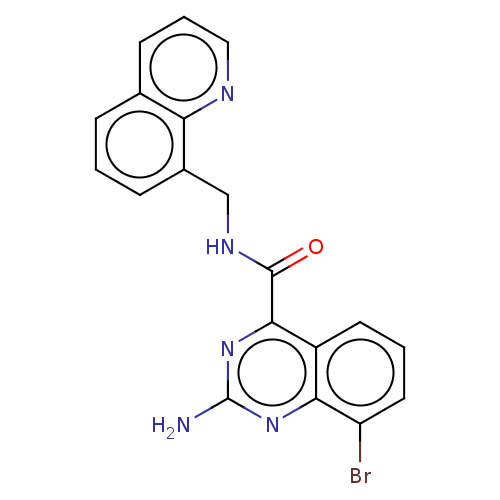 (CHEMBL3763830 | US10138212, Example 96) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description Binding affinities of compounds of the invention for the human A2a receptor were determined in a competition binding assay using Scintillation Proxim... | US Patent US10138212 (2018) BindingDB Entry DOI: 10.7270/Q2WM1GGB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM50139765 (CHEMBL3763830 | US10138212, Example 96) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]SCH-58261 from human adenosine A2A receptor expressed in HEK cell membranes after 60 mins by microplate scintillation counting an... | Bioorg Med Chem Lett 26: 1348-54 (2016) Article DOI: 10.1016/j.bmcl.2015.11.048 BindingDB Entry DOI: 10.7270/Q2S46TT3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 3771 total ) | Next | Last >> |