

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mu-type opioid receptor (MOUSE) | BDBM50408679 (CHEMBL5287792) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | 0.00260 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Assay Description Antagonist activity at cloned muscarinic M2 receptor-Gqi5 chimeric protein expressed in CHO cells assessed as acetylcholine-induced change in cytosol... | Citation and Details | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (MOUSE) | BDBM50408509 (CHEMBL5277326) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | 0.00340 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Assay Description Antagonist activity at AT1 receptor in rat aortic rings | Citation and Details | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (MOUSE) | BDBM50408675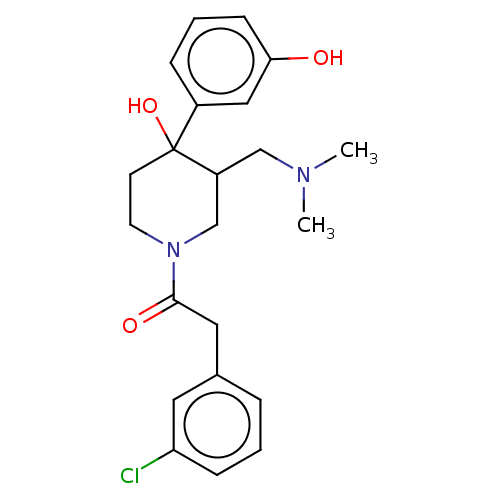 (CHEMBL5270915) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Assay Description Antagonist activity at cloned muscarinic M1 receptor expressed in CHO cells assessed as acetylcholine-induced change in cytosolic calcium concentrati... | Citation and Details | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urotensin-2 receptor (Homo sapiens (Human)) | BDBM50244020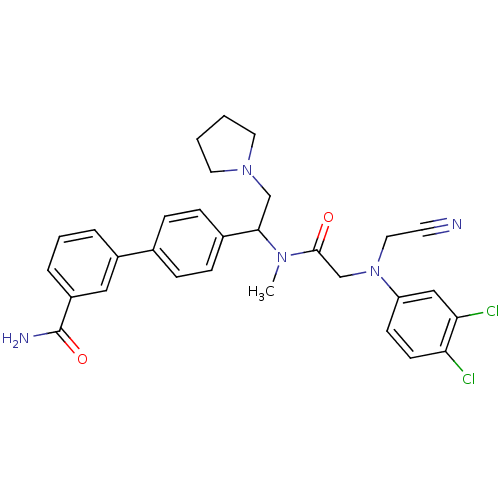 (4'-[1-({2-[Cyanomethyl-(3,4-dichloro-phenyl)-amino...) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals Curated by ChEMBL | Assay Description Binding affinity to human urotensin2 receptor | Bioorg Med Chem Lett 18: 3716-9 (2008) Article DOI: 10.1016/j.bmcl.2008.05.058 BindingDB Entry DOI: 10.7270/Q2RN37NJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urotensin-2 receptor (Homo sapiens (Human)) | BDBM50377220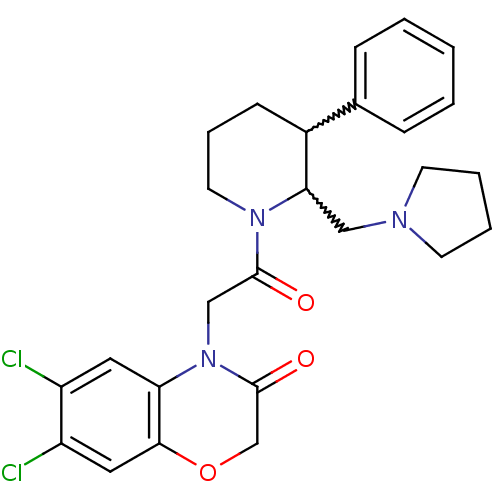 (CHEMBL255509) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals Curated by ChEMBL | Assay Description Displacement of [125I]hU-2 from human recombinant Urotensin 2 receptor expressed in HEK293 cells | Bioorg Med Chem Lett 18: 3500-3 (2008) Article DOI: 10.1016/j.bmcl.2008.05.027 BindingDB Entry DOI: 10.7270/Q29024Q7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50463297 (CHEMBL4246433) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Fudan University Curated by ChEMBL | Assay Description Displacement of [3H]DAMGO from human MOR expressed in CHO cell membranes after 30 mins by liquid scintillation counting analysis | Bioorg Med Chem 26: 4254-4263 (2018) Article DOI: 10.1016/j.bmc.2018.07.020 BindingDB Entry DOI: 10.7270/Q28W3GZJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50463294 (CHEMBL4249256) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Fudan University Curated by ChEMBL | Assay Description Displacement of [3H]U69,593 from human KOR expressed in CHO cell membranes after 30 mins by liquid scintillation counting analysis | Bioorg Med Chem 26: 4254-4263 (2018) Article DOI: 10.1016/j.bmc.2018.07.020 BindingDB Entry DOI: 10.7270/Q28W3GZJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urotensin-2 receptor (Homo sapiens (Human)) | BDBM50377218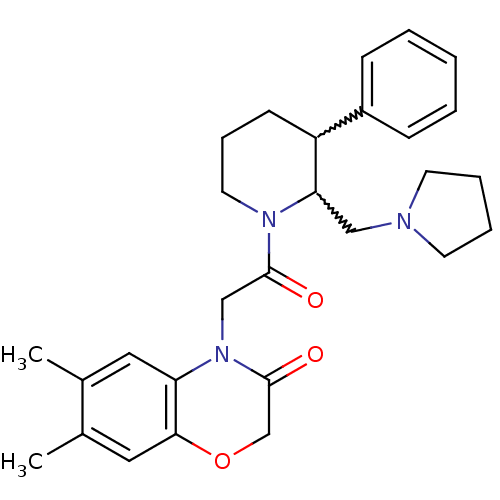 (CHEMBL257171) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals Curated by ChEMBL | Assay Description Displacement of [125I]hU-2 from human recombinant Urotensin 2 receptor expressed in HEK293 cells | Bioorg Med Chem Lett 18: 3500-3 (2008) Article DOI: 10.1016/j.bmcl.2008.05.027 BindingDB Entry DOI: 10.7270/Q29024Q7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| B1 bradykinin receptor (Homo sapiens (Human)) | BDBM50209744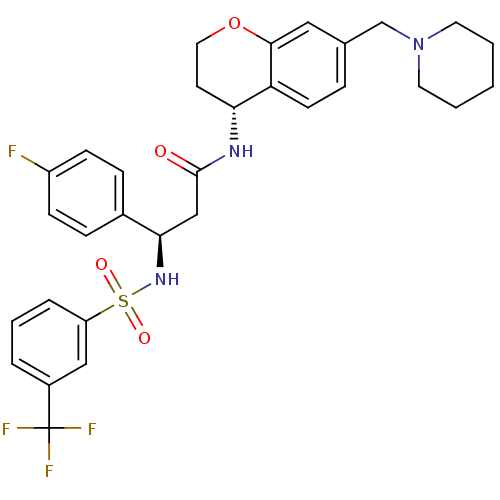 ((R)-3-(4-fluorophenyl)-N-((R)-7-(piperidin-1-ylmet...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc. Curated by ChEMBL | Assay Description Displacement of [3H]DAK from human bradykinin B1 receptor expressed in CHOD cells | J Med Chem 50: 2200-12 (2007) Article DOI: 10.1021/jm070055c BindingDB Entry DOI: 10.7270/Q2MS3SG2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50506108 (CHEMBL4449252) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.470 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Fudan University Curated by ChEMBL | Assay Description Displacement of [3H]U69593 from human KOR expressed in CHO cell membranes incubated for 30 mins by liquid scintillation counting | J Med Chem 62: 11054-11070 (2019) Article DOI: 10.1021/acs.jmedchem.9b00857 BindingDB Entry DOI: 10.7270/Q2VD72RV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM21397 (8-[4-(4-fluorophenyl)-4-keto-butyl]-1-phenyl-1,3,8...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 0.480 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Fudan University | Assay Description The transfection of plasmid and membrane preparations was conducted as described in our previous report, with 0.7 nm [3H] SCH23390 (D1R) or [3H]Spipe... | Chem Biol Drug Des 88: 599-607 (2016) Article DOI: 10.1111/cbdd.12796 BindingDB Entry DOI: 10.7270/Q2QF8RQ5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50463297 (CHEMBL4246433) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Fudan University Curated by ChEMBL | Assay Description Displacement of [3H]U69,593 from human KOR expressed in CHO cell membranes after 30 mins by liquid scintillation counting analysis | Bioorg Med Chem 26: 4254-4263 (2018) Article DOI: 10.1016/j.bmc.2018.07.020 BindingDB Entry DOI: 10.7270/Q28W3GZJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urotensin-2 receptor (Homo sapiens (Human)) | BDBM50377217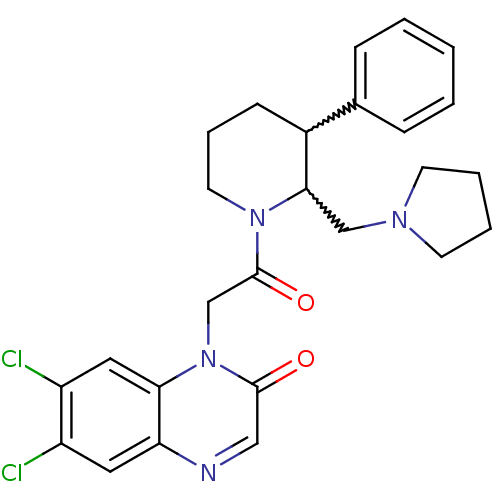 (CHEMBL256989) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals Curated by ChEMBL | Assay Description Displacement of [125I]hU-2 from human recombinant Urotensin 2 receptor expressed in HEK293 cells | Bioorg Med Chem Lett 18: 3500-3 (2008) Article DOI: 10.1016/j.bmcl.2008.05.027 BindingDB Entry DOI: 10.7270/Q29024Q7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| B1 bradykinin receptor (Homo sapiens (Human)) | BDBM50322858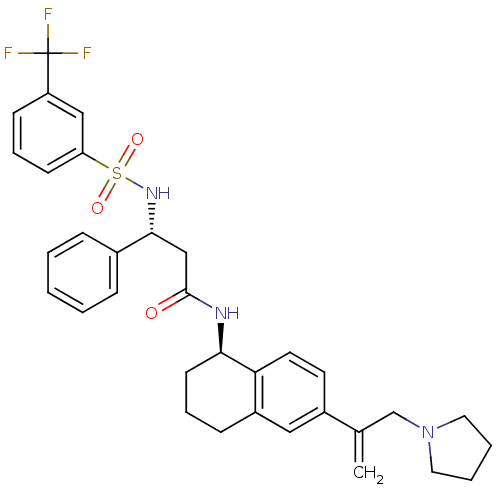 ((R)-3-phenyl-N-((R)-6-(3-(pyrrolidin-1-yl)prop-1-e...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc. Curated by ChEMBL | Assay Description Displacement of [3H]Lys-desArg9-BK from human bradykinin B1 receptor | Bioorg Med Chem Lett 20: 4593-7 (2010) Article DOI: 10.1016/j.bmcl.2010.06.010 BindingDB Entry DOI: 10.7270/Q25Q4W88 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| B1 bradykinin receptor (Homo sapiens (Human)) | BDBM50203200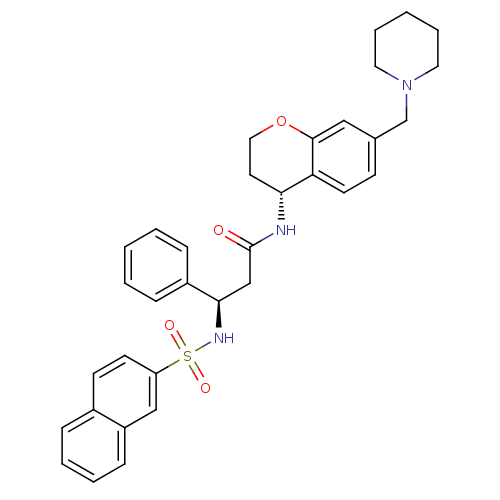 ((R)-3-(naphthalene-7-sulfonamido)-3-phenyl-N-((R)-...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc. Curated by ChEMBL | Assay Description Displacement of [3H]DAK from human bradykinin B1 receptor expressed in CHOD cells | J Med Chem 50: 2200-12 (2007) Article DOI: 10.1021/jm070055c BindingDB Entry DOI: 10.7270/Q2MS3SG2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (MOUSE) | BDBM50408677 (CHEMBL5274117) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Assay Description Antagonist activity at cloned muscarinic M2 receptor-Gqi5 chimeric protein expressed in CHO cells assessed as acetylcholine-induced change in cytosol... | Citation and Details | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM21130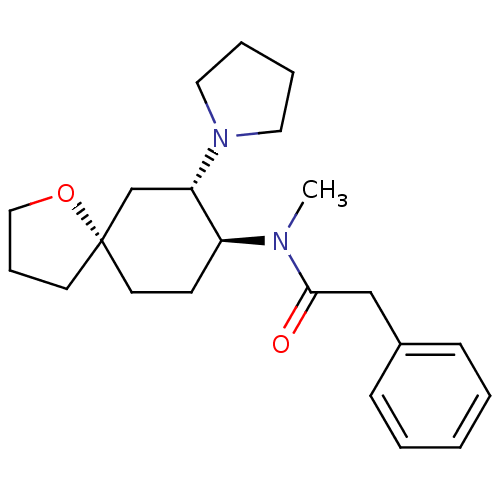 (N-methyl-2-phenyl-N-[(5R,7S,8S)-7-(pyrrolidin-1-yl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Patents Similars | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Assay Description Antagonist activity at oxytocin receptor in Wistar rat uterus assessed as inhibition of oxytocin-induced uterotonic activity treated 1 min prior to o... | Citation and Details | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrin alpha-V/beta-3 (Homo sapiens (Human)) | BDBM50083761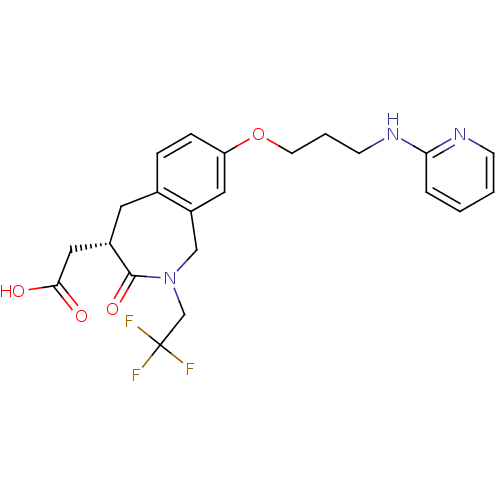 (CHEMBL87429 | [3-Oxo-8-[3-(pyridin-2-ylamino)-prop...) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals Curated by ChEMBL | Assay Description Binding affinity for Vitronectin receptor (alpha V beta 3) | J Med Chem 43: 22-6 (2000) BindingDB Entry DOI: 10.7270/Q290230D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urotensin-2 receptor (Homo sapiens (Human)) | BDBM50377215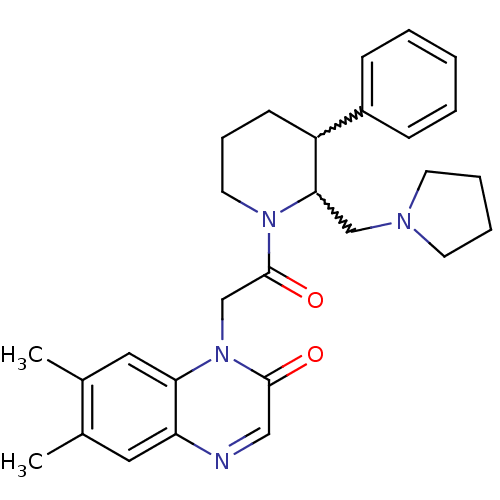 (CHEMBL257415) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals Curated by ChEMBL | Assay Description Displacement of [125I]hU-2 from human recombinant Urotensin 2 receptor expressed in HEK293 cells | Bioorg Med Chem Lett 18: 3500-3 (2008) Article DOI: 10.1016/j.bmcl.2008.05.027 BindingDB Entry DOI: 10.7270/Q29024Q7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM21397 (8-[4-(4-fluorophenyl)-4-keto-butyl]-1-phenyl-1,3,8...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 1.08 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Fudan University | Assay Description The transfection of plasmid and membrane preparations was conducted as described in our previous report, with 0.7 nm [3H] SCH23390 (D1R) or [3H]Spipe... | Chem Biol Drug Des 88: 599-607 (2016) Article DOI: 10.1111/cbdd.12796 BindingDB Entry DOI: 10.7270/Q2QF8RQ5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urotensin-2 receptor (Homo sapiens (Human)) | BDBM50377227 (CHEMBL255462) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals Curated by ChEMBL | Assay Description Displacement of [125I]hU-2 from human recombinant Urotensin 2 receptor expressed in HEK293 cells | Bioorg Med Chem Lett 18: 3500-3 (2008) Article DOI: 10.1016/j.bmcl.2008.05.027 BindingDB Entry DOI: 10.7270/Q29024Q7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrin alpha-V/beta-3 (Homo sapiens (Human)) | BDBM50083763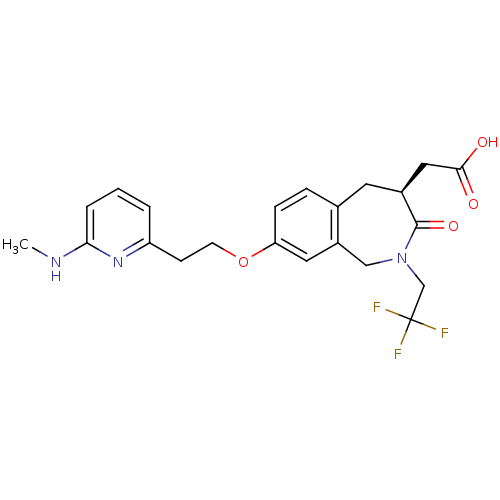 (CHEMBL86992 | [(S)-8-[2-(6-Methylamino-pyridin-2-y...) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents | PubMed | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals Curated by ChEMBL | Assay Description Binding affinity for Vitronectin receptor (alpha V beta 3) | J Med Chem 43: 22-6 (2000) BindingDB Entry DOI: 10.7270/Q290230D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrin alpha-V/beta-3 (Homo sapiens (Human)) | BDBM50083764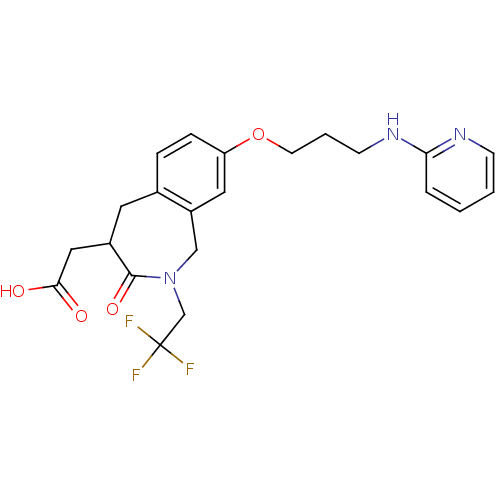 (CHEMBL421533 | [3-Oxo-8-[3-(pyridin-2-ylamino)-pro...) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals Curated by ChEMBL | Assay Description Binding affinity for Vitronectin receptor (alpha V beta 3) | J Med Chem 43: 22-6 (2000) BindingDB Entry DOI: 10.7270/Q290230D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrin alpha-V/beta-3 (Homo sapiens (Human)) | BDBM50078697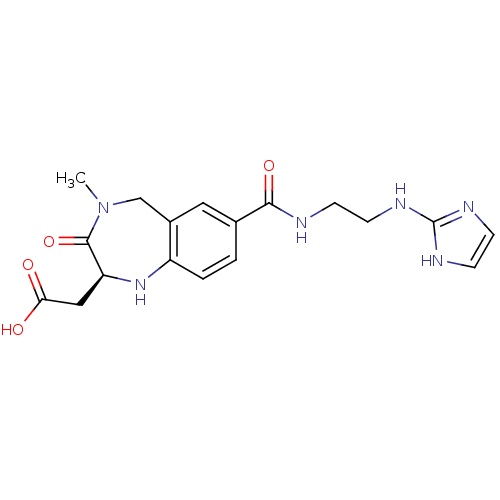 (CHEMBL54138 | {(S)-7-[2-(1H-Imidazol-2-ylamino)-et...) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals Curated by ChEMBL | Assay Description Binding affinity towards human Vitronectin receptor. | Bioorg Med Chem Lett 9: 1801-6 (1999) BindingDB Entry DOI: 10.7270/Q2TT4Q4B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrin alpha-IIb/beta-3 (Homo sapiens (Human)) | BDBM50054826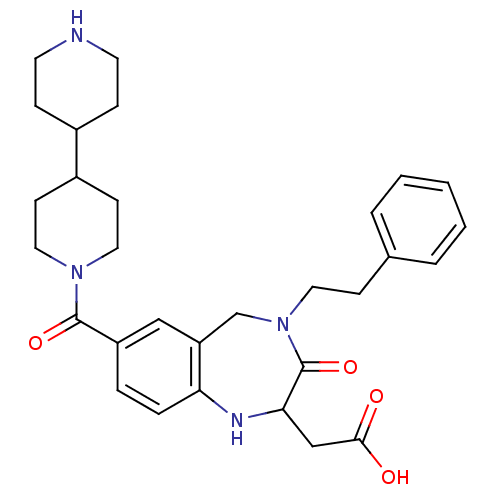 (CHEMBL144474 | [7-([4,4']Bipiperidinyl-1-carbonyl)...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of binding to purified integrin alphaIIb-beta3 of human platelets | J Med Chem 39: 4867-70 (1997) Article DOI: 10.1021/jm960558a BindingDB Entry DOI: 10.7270/Q2GH9H16 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (MOUSE) | BDBM50408676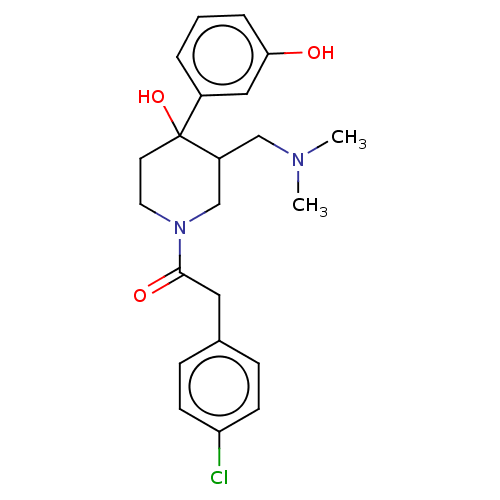 (CHEMBL5272258) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Assay Description Antagonist activity at cloned muscarinic M2 receptor-Gqi5 chimeric protein expressed in CHO cells assessed as acetylcholine-induced change in cytosol... | Citation and Details | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (MOUSE) | BDBM50408673 (CHEMBL5275873) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Assay Description Antagonist activity at cloned muscarinic M1 receptor expressed in CHO cells assessed as acetylcholine-induced change in cytosolic calcium concentrati... | Citation and Details | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrin alpha-V/beta-3 (Homo sapiens (Human)) | BDBM50072493 (CHEMBL108490 | [(S)-7-[(1H-Benzoimidazol-2-ylmethy...) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals Curated by ChEMBL | Assay Description Affinity for alphaIIb-beta3 receptor | Bioorg Med Chem Lett 8: 3165-70 (1999) BindingDB Entry DOI: 10.7270/Q2NG4PSF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrin alpha-IIb/beta-3 (Homo sapiens (Human)) | BDBM50054827 (CHEMBL85094 | SB-208651 | {8-[(4-Carbamimidoyl-phe...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of binding to purified integrin alphaIIb-beta3 of human platelets | J Med Chem 39: 4867-70 (1997) Article DOI: 10.1021/jm960558a BindingDB Entry DOI: 10.7270/Q2GH9H16 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(1A) dopamine receptor (Homo sapiens (Human)) | BDBM86180 (CAS_87075-17-0 | NSC_5018 | SCH 23390 | SCH23390 |...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Patents Similars | Article PubMed | 1.69 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Fudan University | Assay Description The transfection of plasmid and membrane preparations was conducted as described in our previous report, with 0.7 nm [3H] SCH23390 (D1R) or [3H]Spipe... | Chem Biol Drug Des 88: 599-607 (2016) Article DOI: 10.1111/cbdd.12796 BindingDB Entry DOI: 10.7270/Q2QF8RQ5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (MOUSE) | BDBM50408816 (CHEMBL5279890) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Assay Description Antagonist activity at histamine H1 receptor in guinea pig ileum assessed as histamine-induced contractions | Citation and Details | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrin alpha-V/beta-3 (Homo sapiens (Human)) | BDBM50072492 (CHEMBL419180 | {(S)-7-[(1H-Benzoimidazol-2-ylmethy...) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals Curated by ChEMBL | Assay Description Effect against adhesion of HEK 293 cells transfected with human alpha-v beta-3 to vitronectin coated plates | Bioorg Med Chem Lett 8: 3165-70 (1999) BindingDB Entry DOI: 10.7270/Q2NG4PSF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrin alpha-V/beta-3 (Homo sapiens (Human)) | BDBM50083762 (CHEMBL314022 | {2-Methyl-3-oxo-8-[3-(pyridin-2-yla...) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals Curated by ChEMBL | Assay Description Binding affinity for Vitronectin receptor (alpha V beta 3) | J Med Chem 43: 22-6 (2000) BindingDB Entry DOI: 10.7270/Q290230D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (MOUSE) | BDBM50408813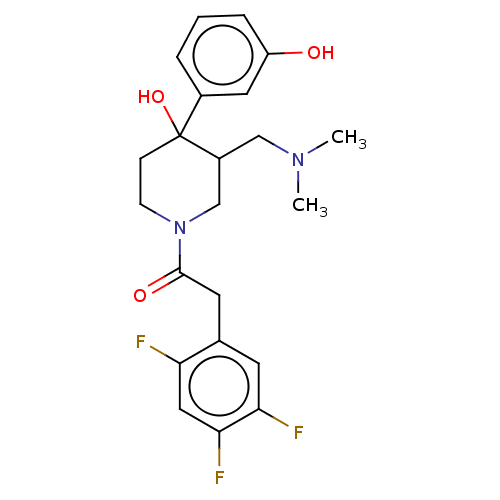 (CHEMBL5273356) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Assay Description Antagonist activity at MTL receptor in rabbit duodenum homogenate | Citation and Details | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrin alpha-V/beta-3 (Homo sapiens (Human)) | BDBM50059133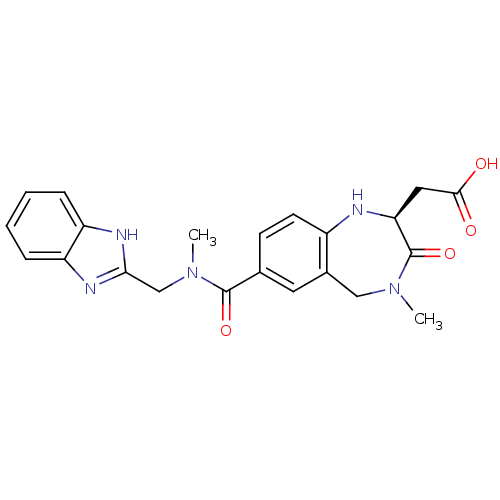 (CHEMBL50106 | SB-223245 | {(S)-7-[(1H-Benzoimidazo...) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals Curated by ChEMBL | Assay Description Binding affinity for Vitronectin receptor (alpha V beta 3) | J Med Chem 43: 22-6 (2000) BindingDB Entry DOI: 10.7270/Q290230D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vascular endothelial growth factor receptor 2 (Homo sapiens (Human)) | BDBM50222500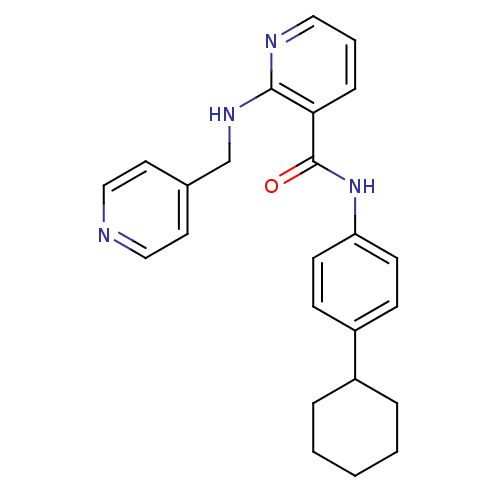 (CHEMBL250482 | N-(4-cyclohexylphenyl)-2-(pyridin-4...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Curated by ChEMBL | Assay Description Inhibition of phosphorylated-Kdr after 30 mins | Bioorg Med Chem Lett 17: 6003-8 (2007) Article DOI: 10.1016/j.bmcl.2007.07.077 BindingDB Entry DOI: 10.7270/Q27P8Z3N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrin alpha-V/beta-3 (Homo sapiens (Human)) | BDBM50059133 (CHEMBL50106 | SB-223245 | {(S)-7-[(1H-Benzoimidazo...) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals Curated by ChEMBL | Assay Description Affinity for vitronectin receptor, integrin alphaV-beta3 | J Med Chem 40: 2289-92 (1997) Article DOI: 10.1021/jm970205r BindingDB Entry DOI: 10.7270/Q22V2F74 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrin alpha-V/beta-3 (Homo sapiens (Human)) | BDBM50059133 (CHEMBL50106 | SB-223245 | {(S)-7-[(1H-Benzoimidazo...) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals Curated by ChEMBL | Assay Description Binding affinity evaluated against vitronectin receptor (alphaV-beta3) receptor | Bioorg Med Chem Lett 8: 3165-70 (1999) BindingDB Entry DOI: 10.7270/Q2NG4PSF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrin alpha-V/beta-3 (Homo sapiens (Human)) | BDBM50059133 (CHEMBL50106 | SB-223245 | {(S)-7-[(1H-Benzoimidazo...) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals Curated by ChEMBL | Assay Description Binding affinity towards human Vitronectin receptor. | Bioorg Med Chem Lett 9: 1801-6 (1999) BindingDB Entry DOI: 10.7270/Q2TT4Q4B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrin alpha-V/beta-3 (Homo sapiens (Human)) | BDBM50078696 (CHEMBL52026 | {(S)-7-[(2-Amino-3H-imidazol-4-ylmet...) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals Curated by ChEMBL | Assay Description Binding affinity towards human alpha IIb beta3 integrin by [3H]-RGD peptide displacement. | Bioorg Med Chem Lett 9: 1801-6 (1999) BindingDB Entry DOI: 10.7270/Q2TT4Q4B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrin alpha-V/beta-3 (Homo sapiens (Human)) | BDBM50078710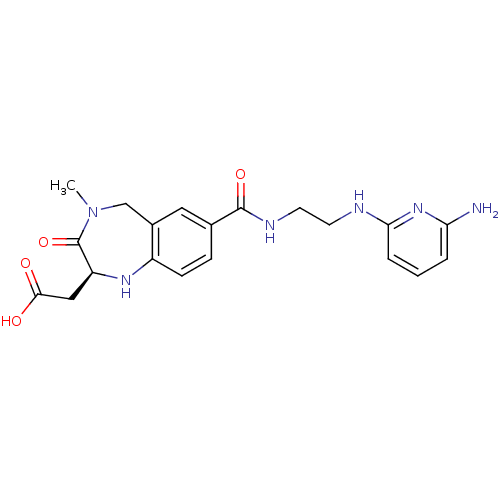 (CHEMBL298782 | {(S)-7-[2-(6-Amino-pyridin-2-ylamin...) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals Curated by ChEMBL | Assay Description Binding affinity towards human Vitronectin receptor. | Bioorg Med Chem Lett 9: 1801-6 (1999) BindingDB Entry DOI: 10.7270/Q2TT4Q4B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrin alpha-V/beta-3 (Homo sapiens (Human)) | BDBM50072468 (CHEMBL108685 | {(S)-4-Methyl-7-[methyl-(7-methyl-1...) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals Curated by ChEMBL | Assay Description Effect against adhesion of HEK 293 cells transfected with human alpha-v beta-3 to vitronectin coated plates | Bioorg Med Chem Lett 8: 3165-70 (1999) BindingDB Entry DOI: 10.7270/Q2NG4PSF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vascular endothelial growth factor receptor 2 (Homo sapiens (Human)) | BDBM50222499 (CHEMBL401153 | N-(3-chlorophenyl)-2-(pyridin-4-ylm...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Curated by ChEMBL | Assay Description Inhibition of phosphorylated-Kdr after 30 mins | Bioorg Med Chem Lett 17: 6003-8 (2007) Article DOI: 10.1016/j.bmcl.2007.07.077 BindingDB Entry DOI: 10.7270/Q27P8Z3N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urotensin-2 receptor (Homo sapiens (Human)) | BDBM50377224 (CHEMBL257767) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals Curated by ChEMBL | Assay Description Displacement of [125I]hU-2 from human recombinant Urotensin 2 receptor expressed in HEK293 cells | Bioorg Med Chem Lett 18: 3500-3 (2008) Article DOI: 10.1016/j.bmcl.2008.05.027 BindingDB Entry DOI: 10.7270/Q29024Q7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urotensin-2 receptor (Homo sapiens (Human)) | BDBM50377219 (CHEMBL402520) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals Curated by ChEMBL | Assay Description Displacement of [125I]hU-2 from human recombinant Urotensin 2 receptor expressed in HEK293 cells | Bioorg Med Chem Lett 18: 3500-3 (2008) Article DOI: 10.1016/j.bmcl.2008.05.027 BindingDB Entry DOI: 10.7270/Q29024Q7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrin alpha-V/beta-3 (Homo sapiens (Human)) | BDBM50059133 (CHEMBL50106 | SB-223245 | {(S)-7-[(1H-Benzoimidazo...) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals Curated by ChEMBL | Assay Description Antagonistic activity for human vitronectin receptor (alphaV-beta3) from platelets | Bioorg Med Chem Lett 8: 3171-6 (1999) BindingDB Entry DOI: 10.7270/Q2B85994 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50463293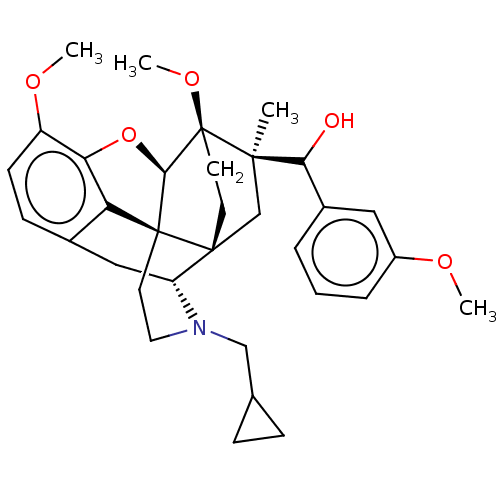 (CHEMBL4242847) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Fudan University Curated by ChEMBL | Assay Description Displacement of [3H]U69,593 from human KOR expressed in CHO cell membranes after 30 mins by liquid scintillation counting analysis | Bioorg Med Chem 26: 4254-4263 (2018) Article DOI: 10.1016/j.bmc.2018.07.020 BindingDB Entry DOI: 10.7270/Q28W3GZJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (MOUSE) | BDBM50408514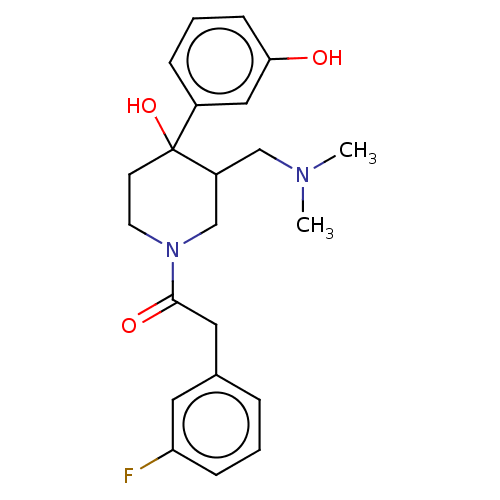 (CHEMBL5281561) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Assay Description Antagonist activity at cloned muscarinic M3 receptor expressed in CHO cells assessed as acetylcholine-induced change in cytosolic calcium concentrati... | Citation and Details | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (MOUSE) | BDBM21015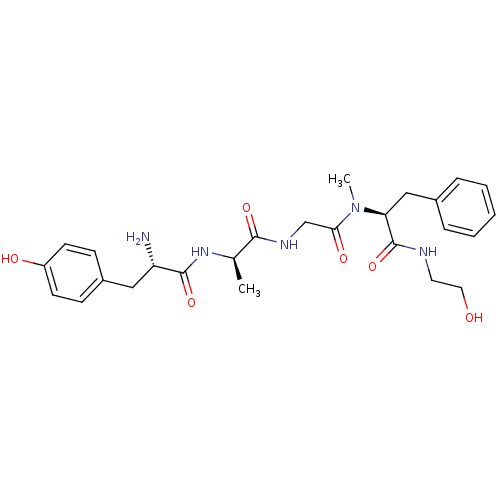 ((2S)-2-{2-[(2R)-2-[(2S)-2-amino-3-(4-hydroxyphenyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid UniChem Similars | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Assay Description Antagonist activity at cloned muscarinic M2 receptor-Gqi5 chimeric protein expressed in CHO cells assessed as acetylcholine-induced change in cytosol... | Citation and Details | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM21008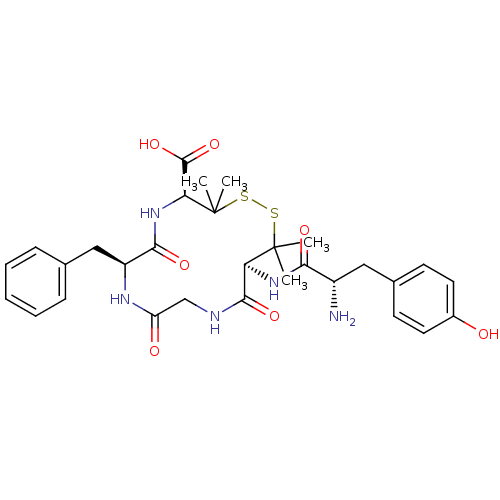 ((4S,7S,13S)-13-[(2S)-2-amino-3-(4-hydroxyphenyl)pr...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Assay Description Antagonist activity at human recombinant EP1 receptor expressed in CHO cells assessed as luciferase activity by Schild assay | Citation and Details | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 2166 total ) | Next | Last >> |