

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Nuclear receptor ROR-gamma (Homo sapiens (Human)) | BDBM50466897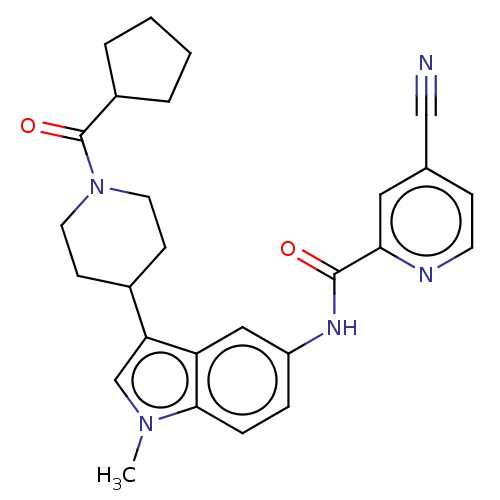 (CHEMBL4287715) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Karo Bio AB (now Karo Pharma AB) Curated by ChEMBL | Assay Description Displacement of [3H]-25-hydroxycholesterol from human N-terminal 6His-tagged-RORC2 LBD (259 to 483 residues) expressed in Escherichia coli (DE3) cell... | J Med Chem 61: 10415-10439 (2018) Article DOI: 10.1021/acs.jmedchem.8b00392 BindingDB Entry DOI: 10.7270/Q27P922T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor ROR-gamma (Homo sapiens (Human)) | BDBM50466891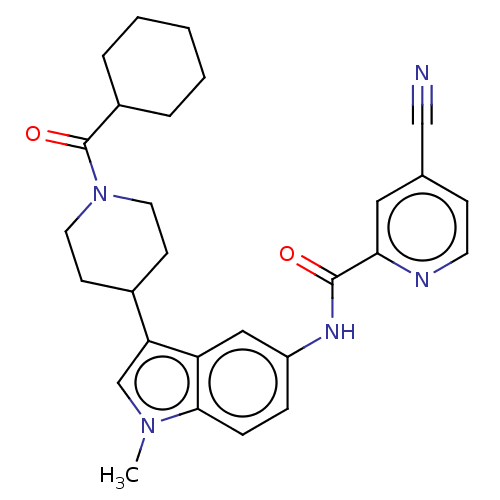 (CHEMBL4281109) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | PDB Article PubMed | 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Karo Bio AB (now Karo Pharma AB) Curated by ChEMBL | Assay Description Displacement of [3H]-25-hydroxycholesterol from human N-terminal 6His-tagged-RORC2 LBD (259 to 483 residues) expressed in Escherichia coli (DE3) cell... | J Med Chem 61: 10415-10439 (2018) Article DOI: 10.1021/acs.jmedchem.8b00392 BindingDB Entry DOI: 10.7270/Q27P922T | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Nuclear receptor ROR-gamma (Homo sapiens (Human)) | BDBM50466893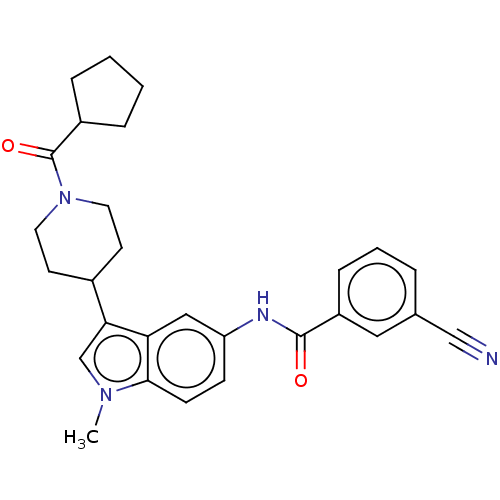 (CHEMBL4291727) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 50 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Karo Bio AB (now Karo Pharma AB) Curated by ChEMBL | Assay Description Displacement of [3H]-25-hydroxycholesterol from human N-terminal 6His-tagged-RORC2 LBD (259 to 483 residues) expressed in Escherichia coli (DE3) cell... | J Med Chem 61: 10415-10439 (2018) Article DOI: 10.1021/acs.jmedchem.8b00392 BindingDB Entry DOI: 10.7270/Q27P922T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor ROR-gamma (Homo sapiens (Human)) | BDBM50466892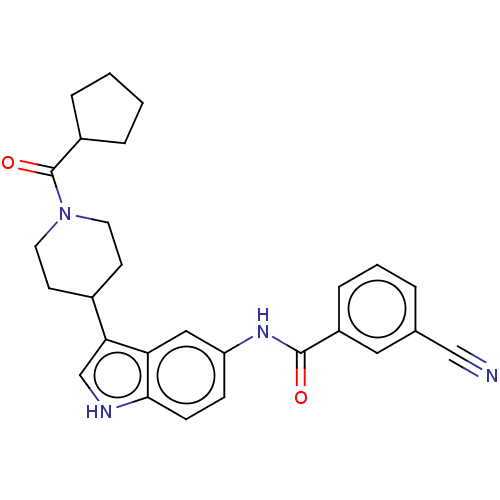 (CHEMBL4283871) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Karo Bio AB (now Karo Pharma AB) Curated by ChEMBL | Assay Description Displacement of [3H]-25-hydroxycholesterol from human N-terminal 6His-tagged-RORC2 LBD (259 to 483 residues) expressed in Escherichia coli (DE3) cell... | J Med Chem 61: 10415-10439 (2018) Article DOI: 10.1021/acs.jmedchem.8b00392 BindingDB Entry DOI: 10.7270/Q27P922T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor ROR-gamma (Homo sapiens (Human)) | BDBM50466913 (CHEMBL4289304) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.90E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Karo Bio AB (now Karo Pharma AB) Curated by ChEMBL | Assay Description Displacement of [3H]-25-hydroxycholesterol from human N-terminal 6His-tagged-RORC2 LBD (259 to 483 residues) expressed in Escherichia coli (DE3) cell... | J Med Chem 61: 10415-10439 (2018) Article DOI: 10.1021/acs.jmedchem.8b00392 BindingDB Entry DOI: 10.7270/Q27P922T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor ROR-gamma (Homo sapiens (Human)) | BDBM189904 (US10227346, Example 29 | US10426135, Example 29 | ...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. US Patent | Assay Description The activity of compound of the invention can be determined by a co-activator recruitment by TR-FRET (time-resolved fluorescence resonance energy tra... | US Patent US10426135 (2019) BindingDB Entry DOI: 10.7270/Q23N25QB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor ROR-gamma (Homo sapiens (Human)) | BDBM189904 (US10227346, Example 29 | US10426135, Example 29 | ...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
Universita di Napoli | Assay Description The activity of compound of the invention can be determined by a co-activator recruitment by TR-FRET (time-resolved fluorescence resonance energy tra... | J Med Chem 51: 1764-70 (2008) BindingDB Entry DOI: 10.7270/Q2SF2ZGV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor ROR-gamma (Homo sapiens (Human)) | BDBM189904 (US10227346, Example 29 | US10426135, Example 29 | ...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | 7.5 | 25 |
Pfizer Inc. US Patent | Assay Description Specifically, in one embodiment the aforementioned assay was performed as outlined below. The assay was carried out in black polystyrene, 384-well pl... | US Patent US9670201 (2017) BindingDB Entry DOI: 10.7270/Q28G8HWT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor ROR-gamma (Homo sapiens (Human)) | BDBM411418 (N-(3-((3R,4R)-1-((R)-2-cyclopentylpropanoyl)- 3-me...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. US Patent | Assay Description In general, the assay is based on the interaction between N-terminally Six-Histidine-tagged-RORC2 ligand binding domain (6-His-RORC2 LBD), expressed ... | US Patent US10385036 (2019) BindingDB Entry DOI: 10.7270/Q24X5B40 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor ROR-gamma (Homo sapiens (Human)) | BDBM411412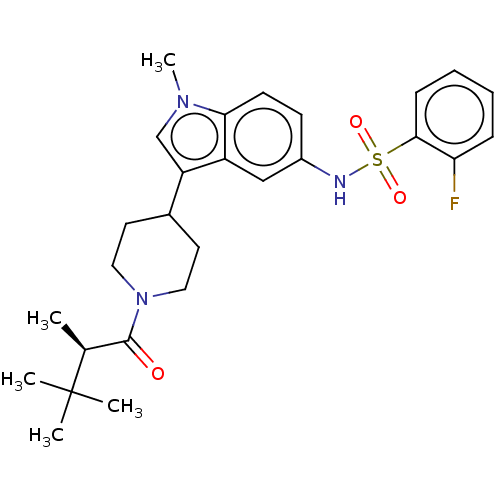 ((R)-2-fluoro-N-(1-methyl-3-(1-(2,3,3- trimethylbut...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. US Patent | Assay Description In general, the assay is based on the interaction between N-terminally Six-Histidine-tagged-RORC2 ligand binding domain (6-His-RORC2 LBD), expressed ... | US Patent US10385036 (2019) BindingDB Entry DOI: 10.7270/Q24X5B40 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor ROR-gamma (Homo sapiens (Human)) | BDBM411419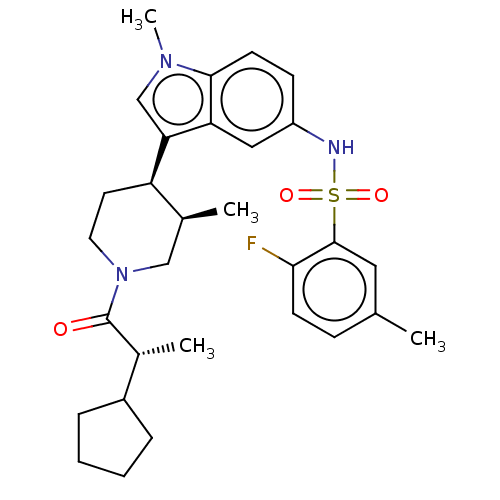 (N-(3-((3R,4R)-1-((R)-2-cyclopentylpropanoyl)- 3-me...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. US Patent | Assay Description In general, the assay is based on the interaction between N-terminally Six-Histidine-tagged-RORC2 ligand binding domain (6-His-RORC2 LBD), expressed ... | US Patent US10385036 (2019) BindingDB Entry DOI: 10.7270/Q24X5B40 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor ROR-gamma (Homo sapiens (Human)) | BDBM411417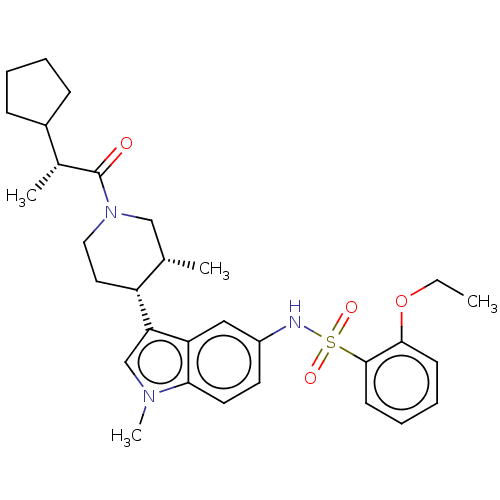 (N-(3-((3R,4R)-1-((R)-2-cyclopentylpropanoyl)- 3-me...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. US Patent | Assay Description In general, the assay is based on the interaction between N-terminally Six-Histidine-tagged-RORC2 ligand binding domain (6-His-RORC2 LBD), expressed ... | US Patent US10385036 (2019) BindingDB Entry DOI: 10.7270/Q24X5B40 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor ROR-gamma (Homo sapiens (Human)) | BDBM190325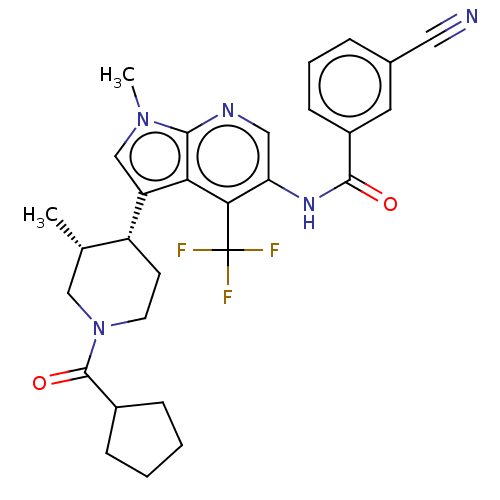 (US10227346, Example 81 | US10426135, Example 81 | ...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Universita di Napoli | Assay Description The activity of compound of the invention can be determined by a co-activator recruitment by TR-FRET (time-resolved fluorescence resonance energy tra... | J Med Chem 51: 1764-70 (2008) BindingDB Entry DOI: 10.7270/Q2SF2ZGV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor ROR-gamma (Homo sapiens (Human)) | BDBM190325 (US10227346, Example 81 | US10426135, Example 81 | ...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. US Patent | Assay Description The activity of compound of the invention can be determined by a co-activator recruitment by TR-FRET (time-resolved fluorescence resonance energy tra... | US Patent US10426135 (2019) BindingDB Entry DOI: 10.7270/Q23N25QB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor ROR-gamma (Homo sapiens (Human)) | BDBM190325 (US10227346, Example 81 | US10426135, Example 81 | ...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | 7.5 | 25 |
Pfizer Inc. US Patent | Assay Description Specifically, in one embodiment the aforementioned assay was performed as outlined below. The assay was carried out in black polystyrene, 384-well pl... | US Patent US9670201 (2017) BindingDB Entry DOI: 10.7270/Q28G8HWT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor ROR-gamma (Homo sapiens (Human)) | BDBM411410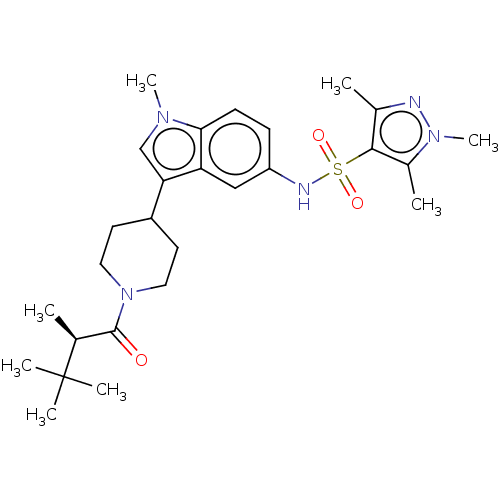 ((R)-1,3,5-trimethyl-N-(1-methyl-3-(1-(2,3,3- trime...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. US Patent | Assay Description In general, the assay is based on the interaction between N-terminally Six-Histidine-tagged-RORC2 ligand binding domain (6-His-RORC2 LBD), expressed ... | US Patent US10385036 (2019) BindingDB Entry DOI: 10.7270/Q24X5B40 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor ROR-gamma (Homo sapiens (Human)) | BDBM189884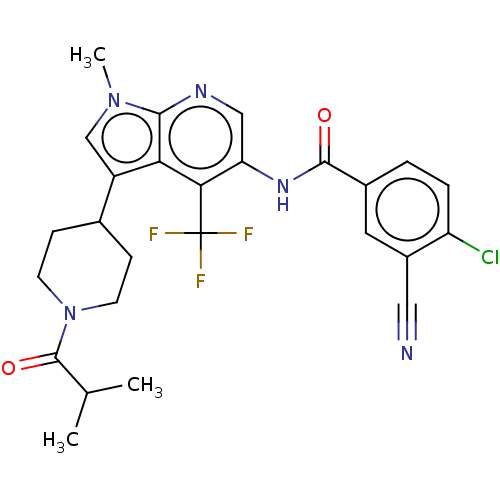 (US10227346, Example 11 | US10426135, Example 11 | ...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. US Patent | Assay Description The activity of compound of the invention can be determined by a co-activator recruitment by TR-FRET (time-resolved fluorescence resonance energy tra... | US Patent US10426135 (2019) BindingDB Entry DOI: 10.7270/Q23N25QB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor ROR-gamma (Homo sapiens (Human)) | BDBM189884 (US10227346, Example 11 | US10426135, Example 11 | ...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | 7.5 | 25 |
Pfizer Inc. US Patent | Assay Description Specifically, in one embodiment the aforementioned assay was performed as outlined below. The assay was carried out in black polystyrene, 384-well pl... | US Patent US9670201 (2017) BindingDB Entry DOI: 10.7270/Q28G8HWT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor ROR-gamma (Homo sapiens (Human)) | BDBM189884 (US10227346, Example 11 | US10426135, Example 11 | ...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Universita di Napoli | Assay Description The activity of compound of the invention can be determined by a co-activator recruitment by TR-FRET (time-resolved fluorescence resonance energy tra... | J Med Chem 51: 1764-70 (2008) BindingDB Entry DOI: 10.7270/Q2SF2ZGV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor ROR-gamma (Homo sapiens (Human)) | BDBM411411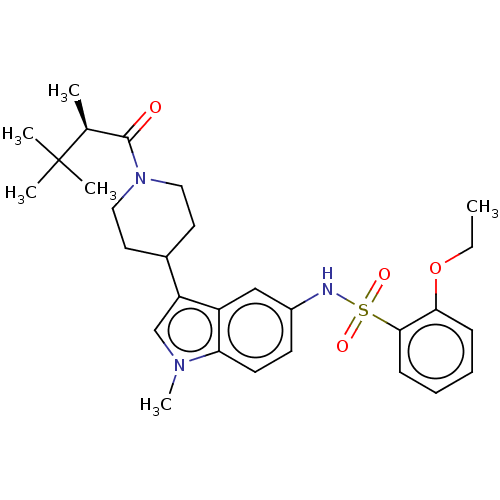 ((R)-2-ethoxy-N-(1-methyl-3-(1-(2,3,3- trimethylbut...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. US Patent | Assay Description In general, the assay is based on the interaction between N-terminally Six-Histidine-tagged-RORC2 ligand binding domain (6-His-RORC2 LBD), expressed ... | US Patent US10385036 (2019) BindingDB Entry DOI: 10.7270/Q24X5B40 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor ROR-gamma (Homo sapiens (Human)) | BDBM368656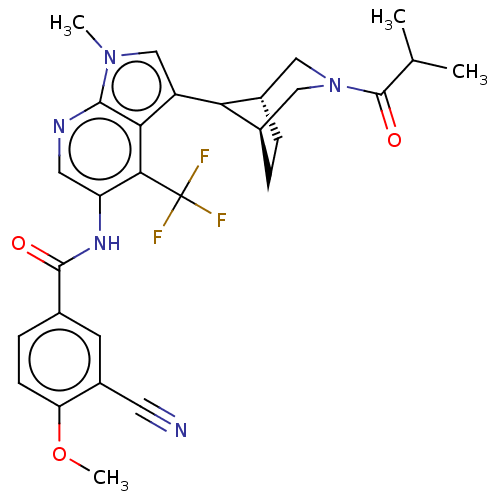 (3-cyano-N-(3-((1R,5S,8r)-3-isobutyryl-3- azabicycl...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Universita di Napoli | Assay Description The activity of compound of the invention can be determined by a co-activator recruitment by TR-FRET (time-resolved fluorescence resonance energy tra... | J Med Chem 51: 1764-70 (2008) BindingDB Entry DOI: 10.7270/Q2SF2ZGV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor ROR-gamma (Homo sapiens (Human)) | BDBM368656 (3-cyano-N-(3-((1R,5S,8r)-3-isobutyryl-3- azabicycl...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. US Patent | Assay Description The activity of compound of the invention can be determined by a co-activator recruitment by TR-FRET (time-resolved fluorescence resonance energy tra... | US Patent US10426135 (2019) BindingDB Entry DOI: 10.7270/Q23N25QB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor ROR-gamma (Homo sapiens (Human)) | BDBM190322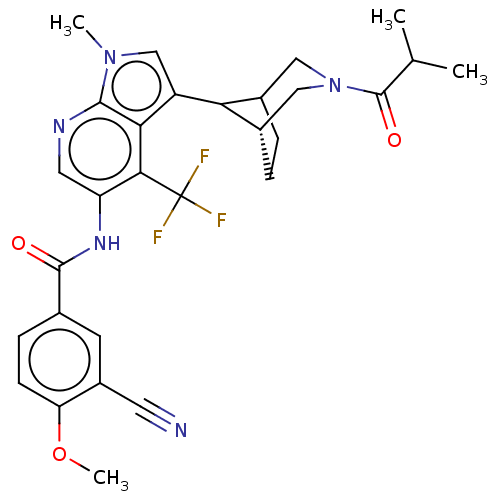 (US9670201, 78 3-cyano-N-(3-((1R,5S,8r)-3-isobutyry...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | 7.5 | 25 |
Pfizer Inc. US Patent | Assay Description Specifically, in one embodiment the aforementioned assay was performed as outlined below. The assay was carried out in black polystyrene, 384-well pl... | US Patent US9670201 (2017) BindingDB Entry DOI: 10.7270/Q28G8HWT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor ROR-gamma (Homo sapiens (Human)) | BDBM411409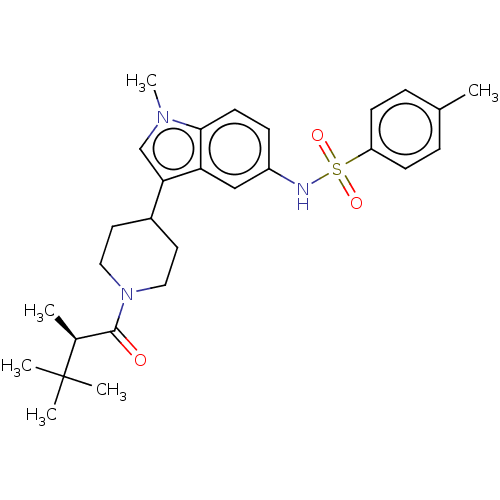 ((R)-4-methyl-N-(1-methyl-3-(1-(2,3,3- trimethylbut...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. US Patent | Assay Description In general, the assay is based on the interaction between N-terminally Six-Histidine-tagged-RORC2 ligand binding domain (6-His-RORC2 LBD), expressed ... | US Patent US10385036 (2019) BindingDB Entry DOI: 10.7270/Q24X5B40 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor ROR-gamma (Homo sapiens (Human)) | BDBM411415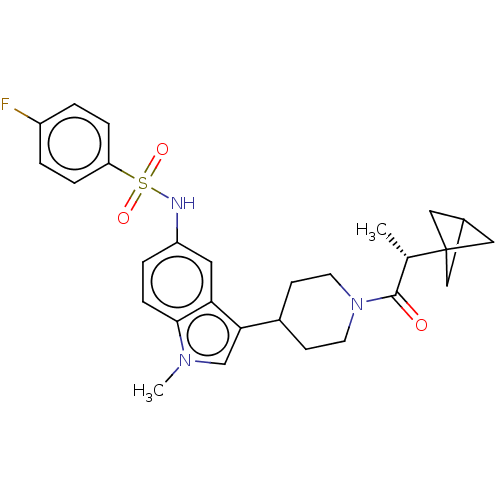 ((R)-N-(3-(1-(2-(bicyclo[1.1.1]pentan-1- yl)propano...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. US Patent | Assay Description In general, the assay is based on the interaction between N-terminally Six-Histidine-tagged-RORC2 ligand binding domain (6-His-RORC2 LBD), expressed ... | US Patent US10385036 (2019) BindingDB Entry DOI: 10.7270/Q24X5B40 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor ROR-gamma (Homo sapiens (Human)) | BDBM411416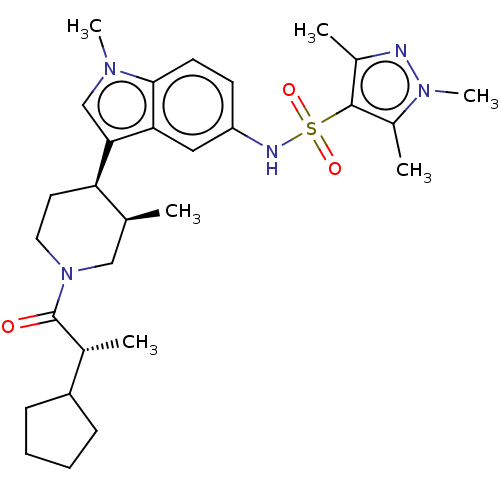 (N-(3-((3R,4R)-1-((R)-2-cyclopentylpropanoyl)- 3-me...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. US Patent | Assay Description In general, the assay is based on the interaction between N-terminally Six-Histidine-tagged-RORC2 ligand binding domain (6-His-RORC2 LBD), expressed ... | US Patent US10385036 (2019) BindingDB Entry DOI: 10.7270/Q24X5B40 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor ROR-gamma (Homo sapiens (Human)) | BDBM190346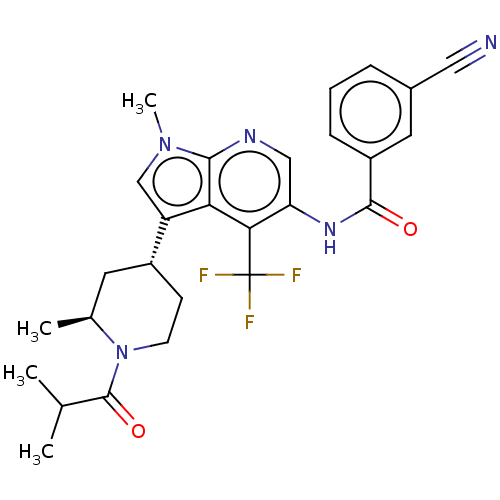 (US10227346, Example 95 | US10426135, Example 95 | ...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. US Patent | Assay Description The activity of compound of the invention can be determined by a co-activator recruitment by TR-FRET (time-resolved fluorescence resonance energy tra... | US Patent US10426135 (2019) BindingDB Entry DOI: 10.7270/Q23N25QB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor ROR-gamma (Homo sapiens (Human)) | BDBM189895 (US10227346, Example 22 | US10426135, Example 22 | ...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. US Patent | Assay Description The activity of compound of the invention can be determined by a co-activator recruitment by TR-FRET (time-resolved fluorescence resonance energy tra... | US Patent US10426135 (2019) BindingDB Entry DOI: 10.7270/Q23N25QB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor ROR-gamma (Homo sapiens (Human)) | BDBM190346 (US10227346, Example 95 | US10426135, Example 95 | ...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Universita di Napoli | Assay Description The activity of compound of the invention can be determined by a co-activator recruitment by TR-FRET (time-resolved fluorescence resonance energy tra... | J Med Chem 51: 1764-70 (2008) BindingDB Entry DOI: 10.7270/Q2SF2ZGV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor ROR-gamma (Homo sapiens (Human)) | BDBM189895 (US10227346, Example 22 | US10426135, Example 22 | ...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Universita di Napoli | Assay Description The activity of compound of the invention can be determined by a co-activator recruitment by TR-FRET (time-resolved fluorescence resonance energy tra... | J Med Chem 51: 1764-70 (2008) BindingDB Entry DOI: 10.7270/Q2SF2ZGV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor ROR-gamma (Homo sapiens (Human)) | BDBM190346 (US10227346, Example 95 | US10426135, Example 95 | ...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2.70 | n/a | n/a | n/a | n/a | 7.5 | 25 |
Pfizer Inc. US Patent | Assay Description Specifically, in one embodiment the aforementioned assay was performed as outlined below. The assay was carried out in black polystyrene, 384-well pl... | US Patent US9670201 (2017) BindingDB Entry DOI: 10.7270/Q28G8HWT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor ROR-gamma (Homo sapiens (Human)) | BDBM189895 (US10227346, Example 22 | US10426135, Example 22 | ...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2.70 | n/a | n/a | n/a | n/a | 7.5 | 25 |
Pfizer Inc. US Patent | Assay Description Specifically, in one embodiment the aforementioned assay was performed as outlined below. The assay was carried out in black polystyrene, 384-well pl... | US Patent US9670201 (2017) BindingDB Entry DOI: 10.7270/Q28G8HWT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor ROR-gamma (Homo sapiens (Human)) | BDBM189905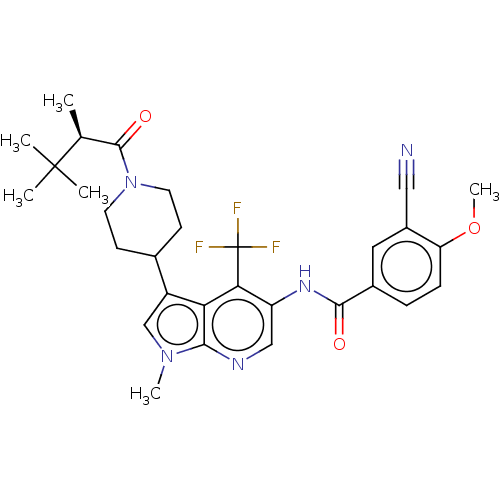 (US10227346, Example 30 | US10426135, Example 30 | ...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. US Patent | Assay Description The activity of compound of the invention can be determined by a co-activator recruitment by TR-FRET (time-resolved fluorescence resonance energy tra... | US Patent US10426135 (2019) BindingDB Entry DOI: 10.7270/Q23N25QB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor ROR-gamma (Homo sapiens (Human)) | BDBM189905 (US10227346, Example 30 | US10426135, Example 30 | ...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2.80 | n/a | n/a | n/a | n/a | 7.5 | 25 |
Pfizer Inc. US Patent | Assay Description Specifically, in one embodiment the aforementioned assay was performed as outlined below. The assay was carried out in black polystyrene, 384-well pl... | US Patent US9670201 (2017) BindingDB Entry DOI: 10.7270/Q28G8HWT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor ROR-gamma (Homo sapiens (Human)) | BDBM189905 (US10227346, Example 30 | US10426135, Example 30 | ...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Universita di Napoli | Assay Description The activity of compound of the invention can be determined by a co-activator recruitment by TR-FRET (time-resolved fluorescence resonance energy tra... | J Med Chem 51: 1764-70 (2008) BindingDB Entry DOI: 10.7270/Q2SF2ZGV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor ROR-gamma (Homo sapiens (Human)) | BDBM411400 ((S)-N-(3-(1-(2,3-dimethylbutanoyl)pipendin-4-yl)-1...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 3.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. US Patent | Assay Description In general, the assay is based on the interaction between N-terminally Six-Histidine-tagged-RORC2 ligand binding domain (6-His-RORC2 LBD), expressed ... | US Patent US10385036 (2019) BindingDB Entry DOI: 10.7270/Q24X5B40 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor ROR-gamma (Homo sapiens (Human)) | BDBM189874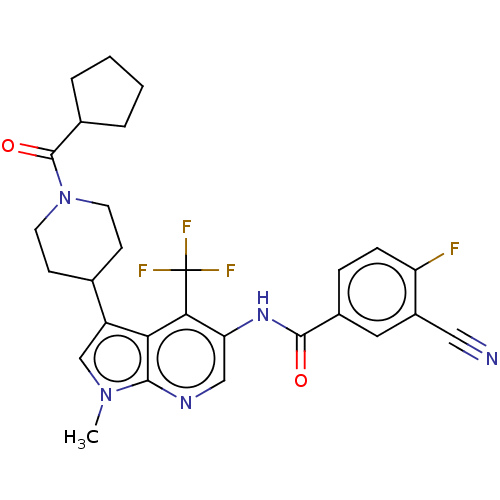 (US10227346, Example 3 | US10426135, Example 3 | US...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. US Patent | Assay Description The activity of compound of the invention can be determined by a co-activator recruitment by TR-FRET (time-resolved fluorescence resonance energy tra... | US Patent US10426135 (2019) BindingDB Entry DOI: 10.7270/Q23N25QB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor ROR-gamma (Homo sapiens (Human)) | BDBM189874 (US10227346, Example 3 | US10426135, Example 3 | US...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Universita di Napoli | Assay Description The activity of compound of the invention can be determined by a co-activator recruitment by TR-FRET (time-resolved fluorescence resonance energy tra... | J Med Chem 51: 1764-70 (2008) BindingDB Entry DOI: 10.7270/Q2SF2ZGV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor ROR-gamma (Homo sapiens (Human)) | BDBM189874 (US10227346, Example 3 | US10426135, Example 3 | US...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 3.20 | n/a | n/a | n/a | n/a | 7.5 | 25 |
Pfizer Inc. US Patent | Assay Description Specifically, in one embodiment the aforementioned assay was performed as outlined below. The assay was carried out in black polystyrene, 384-well pl... | US Patent US9670201 (2017) BindingDB Entry DOI: 10.7270/Q28G8HWT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor ROR-gamma (Homo sapiens (Human)) | BDBM50466890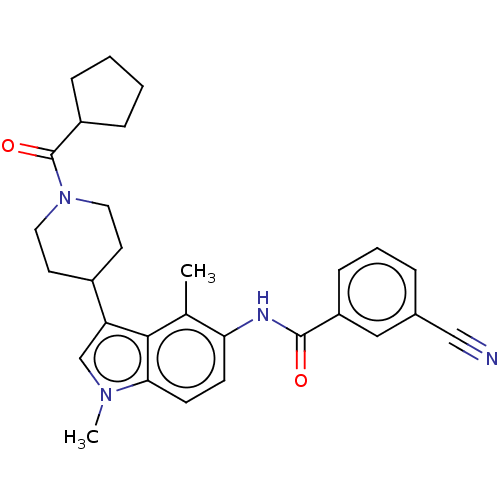 (CHEMBL4290728) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | PDB Article PubMed | n/a | n/a | 3.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Karo Bio AB (now Karo Pharma AB) Curated by ChEMBL | Assay Description Inverse agonist activity at RORC2 in human Th17 cells assessed as inhibition of IL17A production after 6 days by sandwich ELISA | J Med Chem 61: 10415-10439 (2018) Article DOI: 10.1021/acs.jmedchem.8b00392 BindingDB Entry DOI: 10.7270/Q27P922T | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Nuclear receptor ROR-gamma (Homo sapiens (Human)) | BDBM189882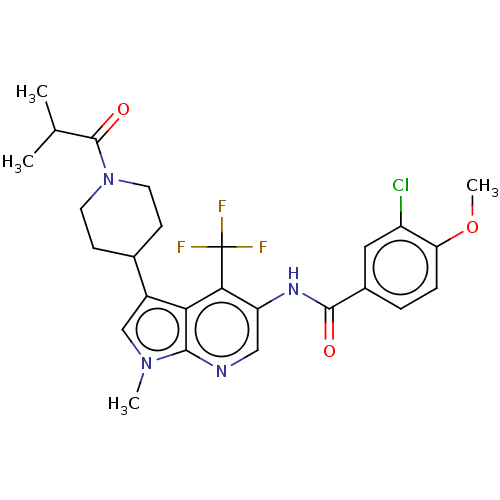 (US10227346, Example 10 | US10426135, Example 10 | ...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 3.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. US Patent | Assay Description The activity of compound of the invention can be determined by a co-activator recruitment by TR-FRET (time-resolved fluorescence resonance energy tra... | US Patent US10426135 (2019) BindingDB Entry DOI: 10.7270/Q23N25QB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor ROR-gamma (Homo sapiens (Human)) | BDBM189882 (US10227346, Example 10 | US10426135, Example 10 | ...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 3.5 | n/a | n/a | n/a | n/a | 7.5 | 25 |
Pfizer Inc. US Patent | Assay Description Specifically, in one embodiment the aforementioned assay was performed as outlined below. The assay was carried out in black polystyrene, 384-well pl... | US Patent US9670201 (2017) BindingDB Entry DOI: 10.7270/Q28G8HWT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor ROR-gamma (Homo sapiens (Human)) | BDBM189882 (US10227346, Example 10 | US10426135, Example 10 | ...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 3.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Universita di Napoli | Assay Description The activity of compound of the invention can be determined by a co-activator recruitment by TR-FRET (time-resolved fluorescence resonance energy tra... | J Med Chem 51: 1764-70 (2008) BindingDB Entry DOI: 10.7270/Q2SF2ZGV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor ROR-gamma (Homo sapiens (Human)) | BDBM411403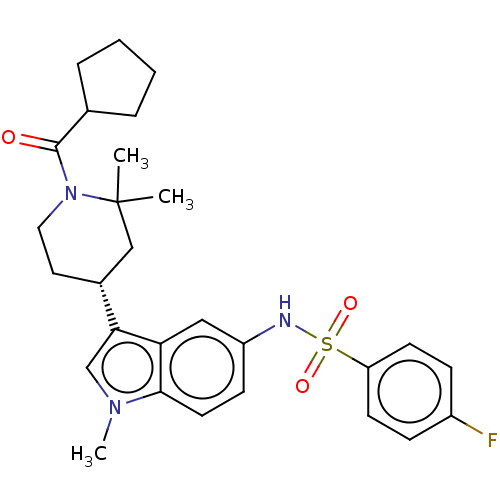 ((R)-N-(3-(1-(cyclopentanecarbonyl)-2,2-dimethylpip...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 3.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. US Patent | Assay Description In general, the assay is based on the interaction between N-terminally Six-Histidine-tagged-RORC2 ligand binding domain (6-His-RORC2 LBD), expressed ... | US Patent US10385036 (2019) BindingDB Entry DOI: 10.7270/Q24X5B40 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor ROR-gamma (Homo sapiens (Human)) | BDBM189891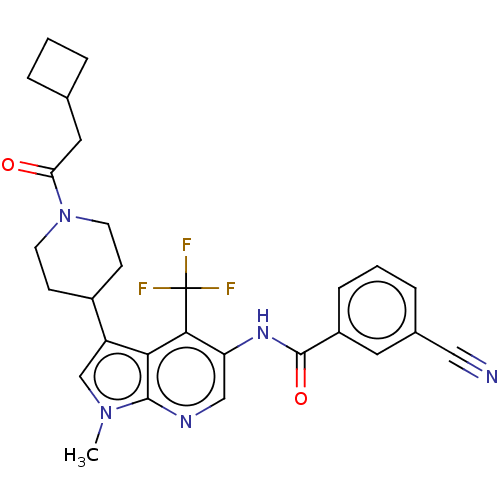 (US10227346, Example 18 | US10426135, Example 18 | ...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 3.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Universita di Napoli | Assay Description The activity of compound of the invention can be determined by a co-activator recruitment by TR-FRET (time-resolved fluorescence resonance energy tra... | J Med Chem 51: 1764-70 (2008) BindingDB Entry DOI: 10.7270/Q2SF2ZGV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor ROR-gamma (Homo sapiens (Human)) | BDBM189891 (US10227346, Example 18 | US10426135, Example 18 | ...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 3.80 | n/a | n/a | n/a | n/a | 7.5 | 25 |
Pfizer Inc. US Patent | Assay Description Specifically, in one embodiment the aforementioned assay was performed as outlined below. The assay was carried out in black polystyrene, 384-well pl... | US Patent US9670201 (2017) BindingDB Entry DOI: 10.7270/Q28G8HWT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor ROR-gamma (Homo sapiens (Human)) | BDBM189891 (US10227346, Example 18 | US10426135, Example 18 | ...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 3.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. US Patent | Assay Description The activity of compound of the invention can be determined by a co-activator recruitment by TR-FRET (time-resolved fluorescence resonance energy tra... | US Patent US10426135 (2019) BindingDB Entry DOI: 10.7270/Q23N25QB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor ROR-gamma (Homo sapiens (Human)) | BDBM189888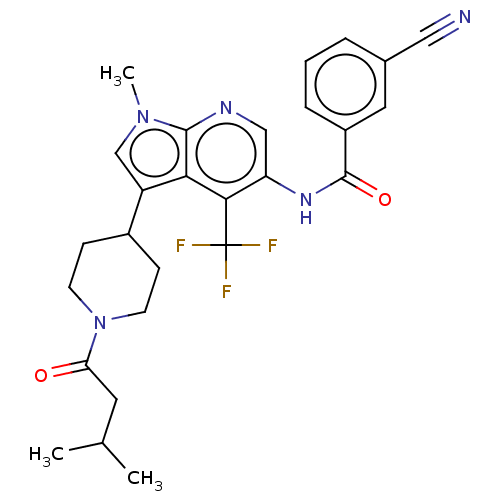 (US10227346, Example 15 | US10426135, Example 15 | ...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 3.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. US Patent | Assay Description The activity of compound of the invention can be determined by a co-activator recruitment by TR-FRET (time-resolved fluorescence resonance energy tra... | US Patent US10426135 (2019) BindingDB Entry DOI: 10.7270/Q23N25QB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor ROR-gamma (Homo sapiens (Human)) | BDBM189888 (US10227346, Example 15 | US10426135, Example 15 | ...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 3.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Universita di Napoli | Assay Description The activity of compound of the invention can be determined by a co-activator recruitment by TR-FRET (time-resolved fluorescence resonance energy tra... | J Med Chem 51: 1764-70 (2008) BindingDB Entry DOI: 10.7270/Q2SF2ZGV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor ROR-gamma (Homo sapiens (Human)) | BDBM189888 (US10227346, Example 15 | US10426135, Example 15 | ...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 3.90 | n/a | n/a | n/a | n/a | 7.5 | 25 |
Pfizer Inc. US Patent | Assay Description Specifically, in one embodiment the aforementioned assay was performed as outlined below. The assay was carried out in black polystyrene, 384-well pl... | US Patent US9670201 (2017) BindingDB Entry DOI: 10.7270/Q28G8HWT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 463 total ) | Next | Last >> |