

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Muscarinic acetylcholine receptor M3 (Homo sapiens (Human)) | BDBM50194640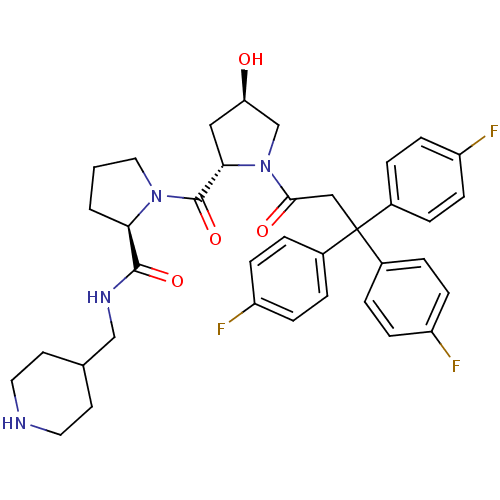 ((2R)-1-((2S,4R)-4-hydroxy-1-[3,3,3-tris(4-fluoroph...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arkansas for Medical Sciences Curated by ChEMBL | Assay Description Binding affinity to human M3 muscarinic receptor | J Med Chem 56: 1693-703 (2013) Article DOI: 10.1021/jm301774u BindingDB Entry DOI: 10.7270/Q2765GN2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome b (Sus scrofa) | BDBM50487138 (CHEMBL2251795) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.570 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Central China Normal University Curated by ChEMBL | Assay Description Inhibition of Sus scrofa (pig) heart cytochrome bc1 complex using DBH2 as substrate by spectrophotometric analysis | J Am Chem Soc 132: 185-94 (2010) Article DOI: 10.1021/ja905756c BindingDB Entry DOI: 10.7270/Q27W6G35 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M2 (Homo sapiens (Human)) | BDBM50111346 ((2-Amino-3-methyl-phenyl)-{4-[4-(3-chloro-benzenes...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.890 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arkansas for Medical Sciences Curated by ChEMBL | Assay Description Binding affinity to human M2 muscarinic receptor | J Med Chem 56: 1693-703 (2013) Article DOI: 10.1021/jm301774u BindingDB Entry DOI: 10.7270/Q2765GN2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M4 (Homo sapiens (Human)) | BDBM50426284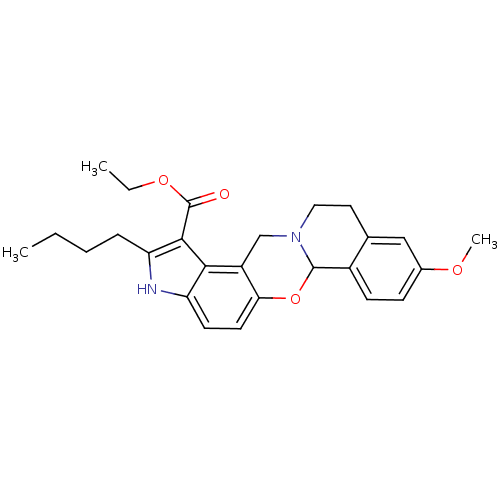 (CHEMBL326450) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arkansas for Medical Sciences Curated by ChEMBL | Assay Description Binding affinity to human M4 muscarinic receptor | J Med Chem 56: 1693-703 (2013) Article DOI: 10.1021/jm301774u BindingDB Entry DOI: 10.7270/Q2765GN2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome b (Sus scrofa) | BDBM50487139 (CHEMBL2251793) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Central China Normal University Curated by ChEMBL | Assay Description Inhibition of Sus scrofa (pig) heart cytochrome bc1 complex using DBH2 as substrate by spectrophotometric analysis | J Am Chem Soc 132: 185-94 (2010) Article DOI: 10.1021/ja905756c BindingDB Entry DOI: 10.7270/Q27W6G35 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50572486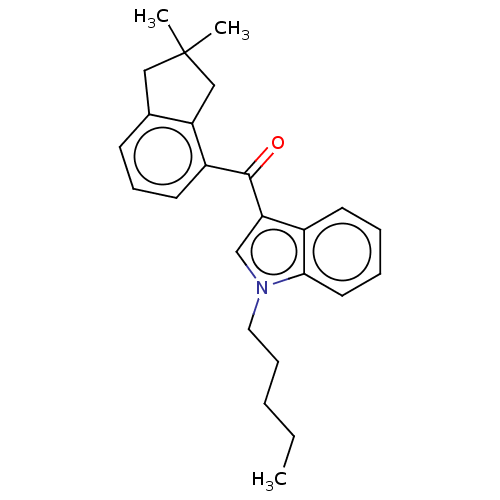 (CHEMBL4860950) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]-CP55940 from human CB2 receptor expressed in CHO cells incubated for 90 mins by liquid scintillation spectrophotometry relative ... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00442 BindingDB Entry DOI: 10.7270/Q2B56PHQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50572486 (CHEMBL4860950) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]-CP55940 from human CB1 receptor expressed in CHO cells incubated for 90 mins by liquid scintillation spectrophotometry relative ... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00442 BindingDB Entry DOI: 10.7270/Q2B56PHQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50353747 (CHEMBL561013 | JWH-018) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 2.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]-CP55940 from human CB2 receptor expressed in CHO cells incubated for 90 mins by liquid scintillation spectrophotometry relative ... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00442 BindingDB Entry DOI: 10.7270/Q2B56PHQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome b (Sus scrofa) | BDBM50487144 (CHEMBL2251794) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 3.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Central China Normal University Curated by ChEMBL | Assay Description Inhibition of Sus scrofa (pig) heart cytochrome bc1 complex using DBH2 as substrate by spectrophotometric analysis | J Am Chem Soc 132: 185-94 (2010) Article DOI: 10.1021/ja905756c BindingDB Entry DOI: 10.7270/Q27W6G35 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome b (Sus scrofa) | BDBM50487137 (CHEBI:78780 | DNDI1724943 | PYRACLOSTROBIN) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | 3.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Central China Normal University Curated by ChEMBL | Assay Description Inhibition of Sus scrofa (pig) heart cytochrome bc1 complex using DBH2 as substrate by spectrophotometric analysis | J Am Chem Soc 132: 185-94 (2010) Article DOI: 10.1021/ja905756c BindingDB Entry DOI: 10.7270/Q27W6G35 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome b (Sus scrofa) | BDBM50487146 (CHEMBL2251796) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 3.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Central China Normal University Curated by ChEMBL | Assay Description Inhibition of Sus scrofa (pig) heart cytochrome bc1 complex using DBH2 as substrate by spectrophotometric analysis | J Am Chem Soc 132: 185-94 (2010) Article DOI: 10.1021/ja905756c BindingDB Entry DOI: 10.7270/Q27W6G35 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50572487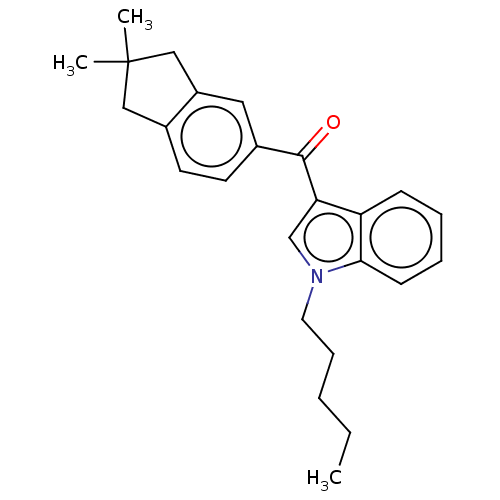 (CHEMBL4856192) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 4.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]-CP55940 from human CB2 receptor expressed in CHO cells incubated for 90 mins by liquid scintillation spectrophotometry relative ... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00442 BindingDB Entry DOI: 10.7270/Q2B56PHQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M3 (Homo sapiens (Human)) | BDBM50368127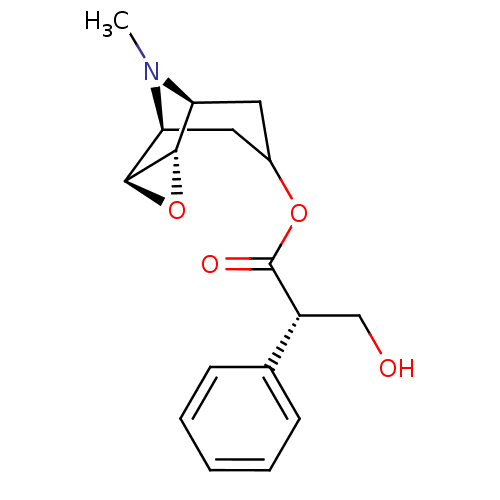 (Isoptpo Hyoscine | SCOPOLAMINE) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | 6.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arkansas for Medical Sciences Curated by ChEMBL | Assay Description Displacement of [3H]NMS from human M3 muscarinic receptor expressed in CHO-K1 cells | J Med Chem 56: 1693-703 (2013) Article DOI: 10.1021/jm301774u BindingDB Entry DOI: 10.7270/Q2765GN2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M1 (Homo sapiens (Human)) | BDBM50368127 (Isoptpo Hyoscine | SCOPOLAMINE) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | 7.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arkansas for Medical Sciences Curated by ChEMBL | Assay Description Displacement of [3H]NMS from human M1 muscarinic receptor expressed in CHO-K1 cells | J Med Chem 56: 1693-703 (2013) Article DOI: 10.1021/jm301774u BindingDB Entry DOI: 10.7270/Q2765GN2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50353747 (CHEMBL561013 | JWH-018) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]-CP55940 from human CB1 receptor expressed in CHO cells incubated for 90 mins by liquid scintillation spectrophotometry relative ... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00442 BindingDB Entry DOI: 10.7270/Q2B56PHQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M1 (Homo sapiens (Human)) | BDBM39341 (11-[(4-methylpiperazin-1-yl)acetyl]-5,11-dihydro-6...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid UniChem Similars | Article PubMed | 9.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arkansas for Medical Sciences Curated by ChEMBL | Assay Description Binding affinity to human M1 muscarinic receptor | J Med Chem 56: 1693-703 (2013) Article DOI: 10.1021/jm301774u BindingDB Entry DOI: 10.7270/Q2765GN2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M2 (Homo sapiens (Human)) | BDBM50368127 (Isoptpo Hyoscine | SCOPOLAMINE) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | 9.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arkansas for Medical Sciences Curated by ChEMBL | Assay Description Displacement of [3H]NMS from human M2 muscarinic receptor expressed in CHO-K1 cells | J Med Chem 56: 1693-703 (2013) Article DOI: 10.1021/jm301774u BindingDB Entry DOI: 10.7270/Q2765GN2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome b (Sus scrofa) | BDBM50487145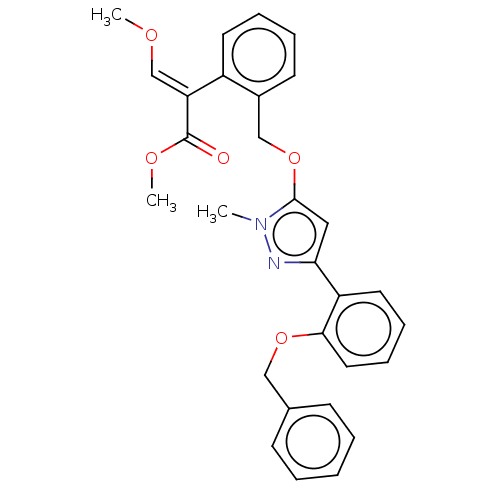 (CHEMBL2251799) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Central China Normal University Curated by ChEMBL | Assay Description Inhibition of Sus scrofa (pig) heart cytochrome bc1 complex using DBH2 as substrate by spectrophotometric analysis | J Am Chem Soc 132: 185-94 (2010) Article DOI: 10.1021/ja905756c BindingDB Entry DOI: 10.7270/Q27W6G35 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50572485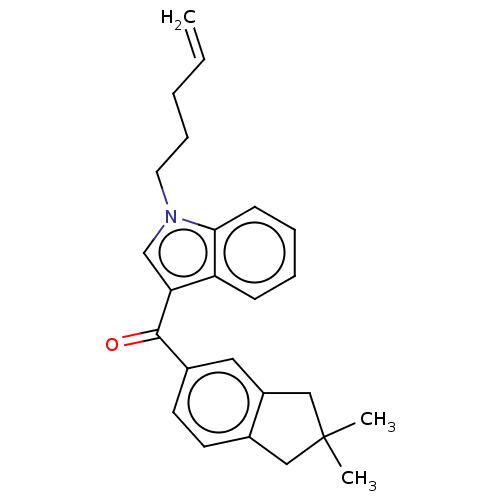 (CHEMBL4855206) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]-CP55940 from human CB2 receptor expressed in CHO cells incubated for 90 mins by liquid scintillation spectrophotometry relative ... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00442 BindingDB Entry DOI: 10.7270/Q2B56PHQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M5 (Homo sapiens (Human)) | BDBM50368127 (Isoptpo Hyoscine | SCOPOLAMINE) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | 18 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arkansas for Medical Sciences Curated by ChEMBL | Assay Description Displacement of [3H]NMS from human M5 muscarinic receptor expressed in CHO-K1 cells | J Med Chem 56: 1693-703 (2013) Article DOI: 10.1021/jm301774u BindingDB Entry DOI: 10.7270/Q2765GN2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M1 (Homo sapiens (Human)) | BDBM50426256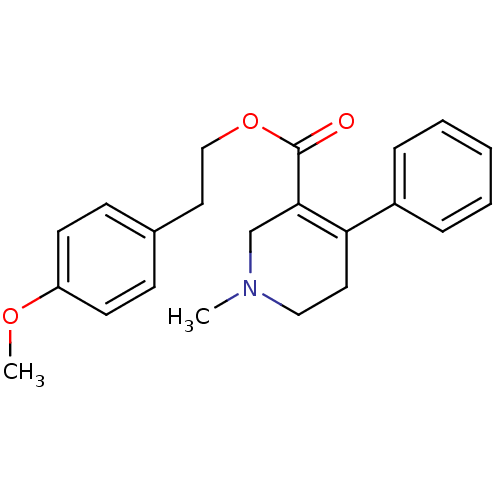 (CHEMBL2312358) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arkansas for Medical Sciences Curated by ChEMBL | Assay Description Displacement of [3H]NMS from human M1 muscarinic receptor expressed in CHO-K1 cells | J Med Chem 56: 1693-703 (2013) Article DOI: 10.1021/jm301774u BindingDB Entry DOI: 10.7270/Q2765GN2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome b (Sus scrofa) | BDBM50487142 (CHEMBL2251798) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Central China Normal University Curated by ChEMBL | Assay Description Inhibition of Sus scrofa (pig) heart cytochrome bc1 complex using DBH2 as substrate by spectrophotometric analysis | J Am Chem Soc 132: 185-94 (2010) Article DOI: 10.1021/ja905756c BindingDB Entry DOI: 10.7270/Q27W6G35 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50572489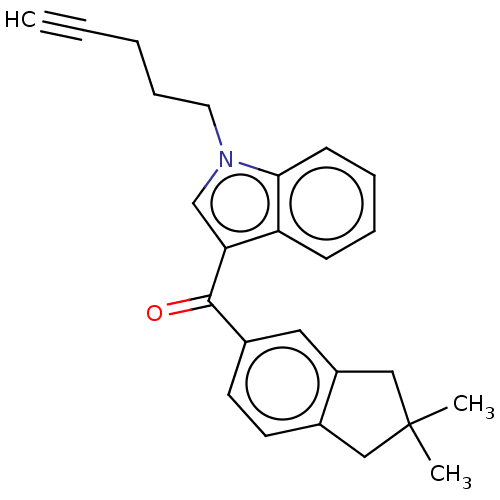 (CHEMBL4877815) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 24 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]-CP55940 from human CB2 receptor expressed in CHO cells incubated for 90 mins by liquid scintillation spectrophotometry relative ... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00442 BindingDB Entry DOI: 10.7270/Q2B56PHQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50553588 (CHEMBL4747163) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 24 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]CP-55940 from CB2 receptor (unknown origin) by competitive binding assay | Citation and Details Article DOI: 10.1016/j.bmcl.2020.127501 BindingDB Entry DOI: 10.7270/Q2NV9NW2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome b (Sus scrofa) | BDBM50487143 (CHEMBL2251797) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 29 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Central China Normal University Curated by ChEMBL | Assay Description Inhibition of Sus scrofa (pig) heart cytochrome bc1 complex using DBH2 as substrate by spectrophotometric analysis | J Am Chem Soc 132: 185-94 (2010) Article DOI: 10.1021/ja905756c BindingDB Entry DOI: 10.7270/Q27W6G35 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M5 (Homo sapiens (Human)) | BDBM50426256 (CHEMBL2312358) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arkansas for Medical Sciences Curated by ChEMBL | Assay Description Displacement of [3H]NMS from human M5 muscarinic receptor expressed in CHO-K1 cells | J Med Chem 56: 1693-703 (2013) Article DOI: 10.1021/jm301774u BindingDB Entry DOI: 10.7270/Q2765GN2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M4 (Homo sapiens (Human)) | BDBM50368127 (Isoptpo Hyoscine | SCOPOLAMINE) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | 37 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arkansas for Medical Sciences Curated by ChEMBL | Assay Description Displacement of [3H]NMS from human M4 muscarinic receptor expressed in CHO-K1 cells | J Med Chem 56: 1693-703 (2013) Article DOI: 10.1021/jm301774u BindingDB Entry DOI: 10.7270/Q2765GN2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50572487 (CHEMBL4856192) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 39 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]-CP55940 from human CB1 receptor expressed in CHO cells incubated for 90 mins by liquid scintillation spectrophotometry relative ... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00442 BindingDB Entry DOI: 10.7270/Q2B56PHQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M4 (Homo sapiens (Human)) | BDBM50194640 ((2R)-1-((2S,4R)-4-hydroxy-1-[3,3,3-tris(4-fluoroph...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 42 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arkansas for Medical Sciences Curated by ChEMBL | Assay Description Binding affinity to human M4 muscarinic receptor | J Med Chem 56: 1693-703 (2013) Article DOI: 10.1021/jm301774u BindingDB Entry DOI: 10.7270/Q2765GN2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M5 (Homo sapiens (Human)) | BDBM50426253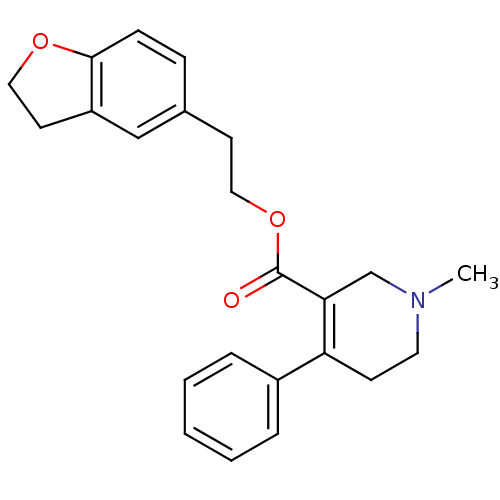 (CHEMBL2312361) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arkansas for Medical Sciences Curated by ChEMBL | Assay Description Displacement of [3H]NMS from human M5 muscarinic receptor expressed in CHO-K1 cells | J Med Chem 56: 1693-703 (2013) Article DOI: 10.1021/jm301774u BindingDB Entry DOI: 10.7270/Q2765GN2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M1 (Homo sapiens (Human)) | BDBM50426264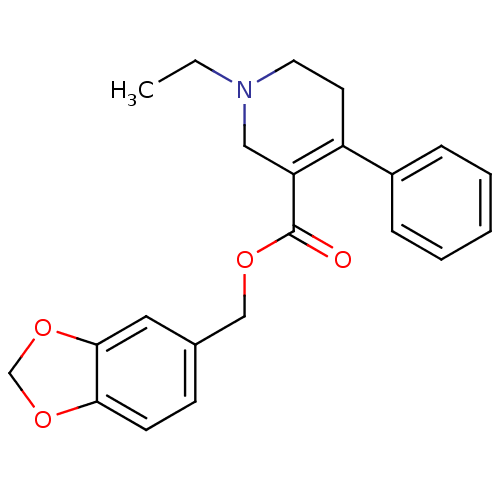 (CHEMBL2312378) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arkansas for Medical Sciences Curated by ChEMBL | Assay Description Displacement of [3H]NMS from human M1 muscarinic receptor expressed in CHO-K1 cells | J Med Chem 56: 1693-703 (2013) Article DOI: 10.1021/jm301774u BindingDB Entry DOI: 10.7270/Q2765GN2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M4 (Homo sapiens (Human)) | BDBM50111346 ((2-Amino-3-methyl-phenyl)-{4-[4-(3-chloro-benzenes...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 61 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arkansas for Medical Sciences Curated by ChEMBL | Assay Description Binding affinity to human M4 muscarinic receptor | J Med Chem 56: 1693-703 (2013) Article DOI: 10.1021/jm301774u BindingDB Entry DOI: 10.7270/Q2765GN2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50572488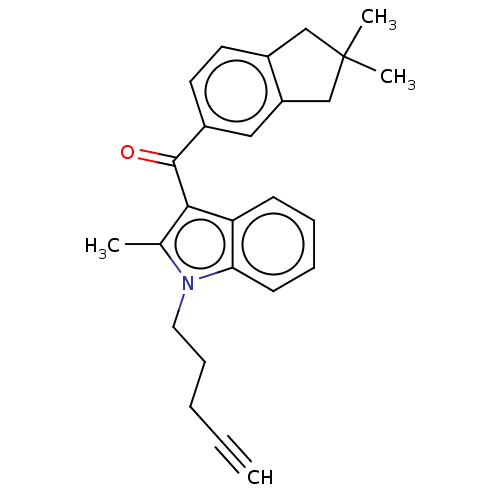 (CHEMBL4866238) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 63 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]-CP55940 from human CB2 receptor expressed in CHO cells incubated for 90 mins by liquid scintillation spectrophotometry relative ... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00442 BindingDB Entry DOI: 10.7270/Q2B56PHQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Relaxin-3 receptor 1 (Homo sapiens (Human)) | BDBM50581019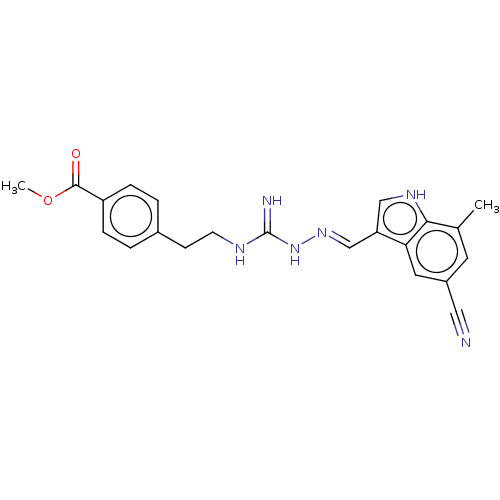 (CHEMBL5089949) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | 69 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [125I]R3/I5 from human RXFP3 expressed in human CHO-K1 cells incubated for 1 hr by microplate scintillation counting analysis | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01081 BindingDB Entry DOI: 10.7270/Q2ZK5MJP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M5 (Homo sapiens (Human)) | BDBM50426244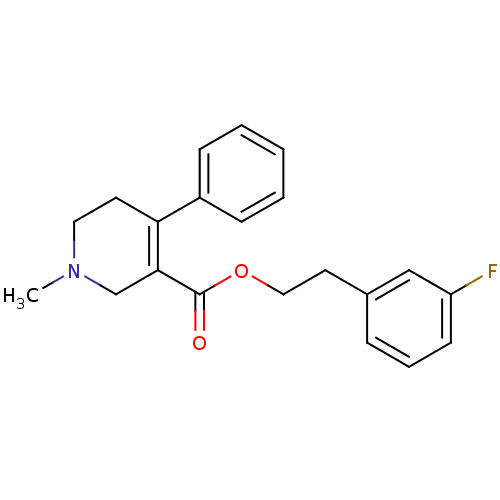 (CHEMBL2312370) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arkansas for Medical Sciences Curated by ChEMBL | Assay Description Displacement of [3H]NMS from human M5 muscarinic receptor expressed in CHO-K1 cells | J Med Chem 56: 1693-703 (2013) Article DOI: 10.1021/jm301774u BindingDB Entry DOI: 10.7270/Q2765GN2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M2 (Homo sapiens (Human)) | BDBM50018056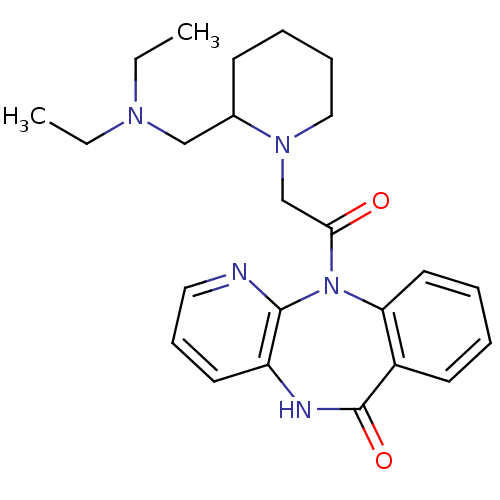 ((AF-DX 116) 11-[2-(2-Diethylaminomethyl-piperidin-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 81 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arkansas for Medical Sciences Curated by ChEMBL | Assay Description Binding affinity to human M2 muscarinic receptor | J Med Chem 56: 1693-703 (2013) Article DOI: 10.1021/jm301774u BindingDB Entry DOI: 10.7270/Q2765GN2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M5 (Homo sapiens (Human)) | BDBM50111346 ((2-Amino-3-methyl-phenyl)-{4-[4-(3-chloro-benzenes...) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 85 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arkansas for Medical Sciences Curated by ChEMBL | Assay Description Binding affinity to human M5 muscarinic receptor | J Med Chem 56: 1693-703 (2013) Article DOI: 10.1021/jm301774u BindingDB Entry DOI: 10.7270/Q2765GN2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome b (Sus scrofa) | BDBM50487141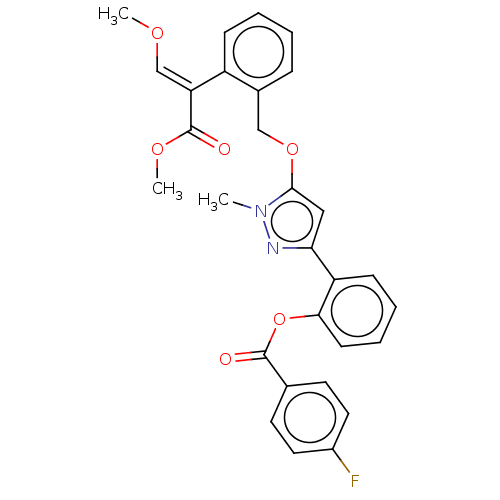 (CHEMBL2251800) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 98 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Central China Normal University Curated by ChEMBL | Assay Description Inhibition of Sus scrofa (pig) heart cytochrome bc1 complex using DBH2 as substrate by spectrophotometric analysis | J Am Chem Soc 132: 185-94 (2010) Article DOI: 10.1021/ja905756c BindingDB Entry DOI: 10.7270/Q27W6G35 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M1 (Homo sapiens (Human)) | BDBM50426271 (CHEMBL2312392) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arkansas for Medical Sciences Curated by ChEMBL | Assay Description Displacement of [3H]NMS from human M1 muscarinic receptor expressed in CHO-K1 cells | J Med Chem 56: 1693-703 (2013) Article DOI: 10.1021/jm301774u BindingDB Entry DOI: 10.7270/Q2765GN2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M4 (Homo sapiens (Human)) | BDBM39341 (11-[(4-methylpiperazin-1-yl)acetyl]-5,11-dihydro-6...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid UniChem Similars | Article PubMed | 105 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arkansas for Medical Sciences Curated by ChEMBL | Assay Description Binding affinity to human M4 muscarinic receptor | J Med Chem 56: 1693-703 (2013) Article DOI: 10.1021/jm301774u BindingDB Entry DOI: 10.7270/Q2765GN2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50553585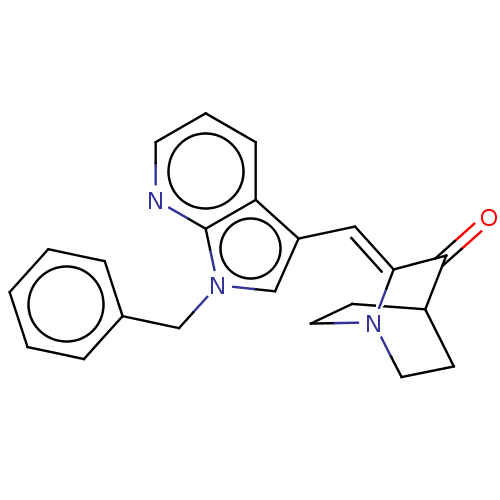 (CHEMBL4741229) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]CP-55940 from CB2 receptor (unknown origin) by competitive binding assay | Citation and Details Article DOI: 10.1016/j.bmcl.2020.127501 BindingDB Entry DOI: 10.7270/Q2NV9NW2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M5 (Homo sapiens (Human)) | BDBM50426246 (CHEMBL2312368) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arkansas for Medical Sciences Curated by ChEMBL | Assay Description Displacement of [3H]NMS from human M5 muscarinic receptor expressed in CHO-K1 cells | J Med Chem 56: 1693-703 (2013) Article DOI: 10.1021/jm301774u BindingDB Entry DOI: 10.7270/Q2765GN2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50553586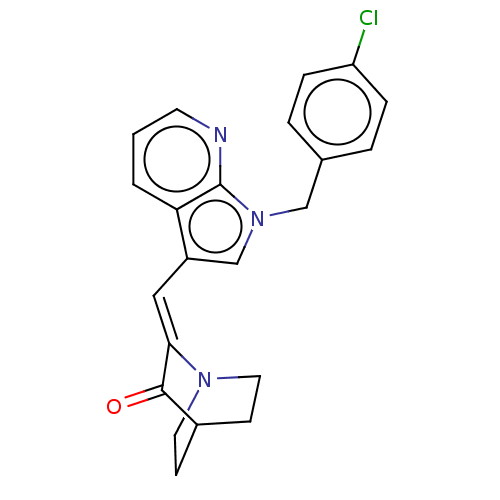 (CHEMBL4794483) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 115 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]CP-55940 from CB2 receptor (unknown origin) by competitive binding assay | Citation and Details Article DOI: 10.1016/j.bmcl.2020.127501 BindingDB Entry DOI: 10.7270/Q2NV9NW2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M5 (Homo sapiens (Human)) | BDBM50426264 (CHEMBL2312378) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arkansas for Medical Sciences Curated by ChEMBL | Assay Description Displacement of [3H]NMS from human M5 muscarinic receptor expressed in CHO-K1 cells | J Med Chem 56: 1693-703 (2013) Article DOI: 10.1021/jm301774u BindingDB Entry DOI: 10.7270/Q2765GN2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-3/beta-4 (Homo sapiens (Human)) | BDBM50450976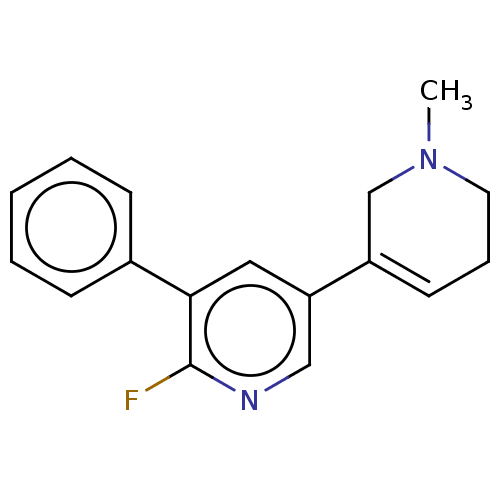 (CHEMBL4210672) | PDB KEGG UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 123 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Michigan Curated by ChEMBL | Assay Description Antagonist activity at human alpha3beta4 nAChR expressed in Xenopus laevis oocytes assessed as reduction in acetylcholine-induced peak current at -60... | Bioorg Med Chem Lett 27: 4350-4353 (2017) Article DOI: 10.1016/j.bmcl.2017.08.025 BindingDB Entry DOI: 10.7270/Q2H70JDP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M5 (Homo sapiens (Human)) | BDBM39341 (11-[(4-methylpiperazin-1-yl)acetyl]-5,11-dihydro-6...) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid UniChem Similars | Article PubMed | 126 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arkansas for Medical Sciences Curated by ChEMBL | Assay Description Binding affinity to human M5 muscarinic receptor | J Med Chem 56: 1693-703 (2013) Article DOI: 10.1021/jm301774u BindingDB Entry DOI: 10.7270/Q2765GN2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50572490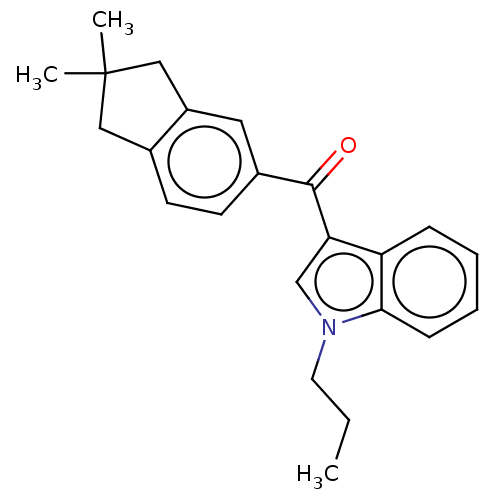 (CHEMBL4872576) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]-CP55940 from human CB2 receptor expressed in CHO cells incubated for 90 mins by liquid scintillation spectrophotometry relative ... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00442 BindingDB Entry DOI: 10.7270/Q2B56PHQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M5 (Homo sapiens (Human)) | BDBM50426259 (CHEMBL2312383) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arkansas for Medical Sciences Curated by ChEMBL | Assay Description Displacement of [3H]NMS from human M5 muscarinic receptor expressed in CHO-K1 cells | J Med Chem 56: 1693-703 (2013) Article DOI: 10.1021/jm301774u BindingDB Entry DOI: 10.7270/Q2765GN2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M1 (Homo sapiens (Human)) | BDBM50426258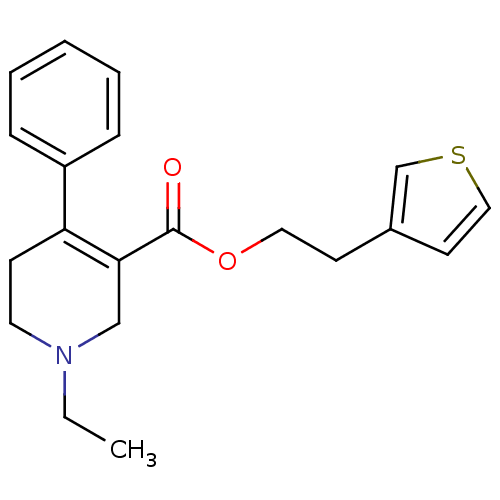 (CHEMBL2312384) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arkansas for Medical Sciences Curated by ChEMBL | Assay Description Displacement of [3H]NMS from human M1 muscarinic receptor expressed in CHO-K1 cells | J Med Chem 56: 1693-703 (2013) Article DOI: 10.1021/jm301774u BindingDB Entry DOI: 10.7270/Q2765GN2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M5 (Homo sapiens (Human)) | BDBM50426276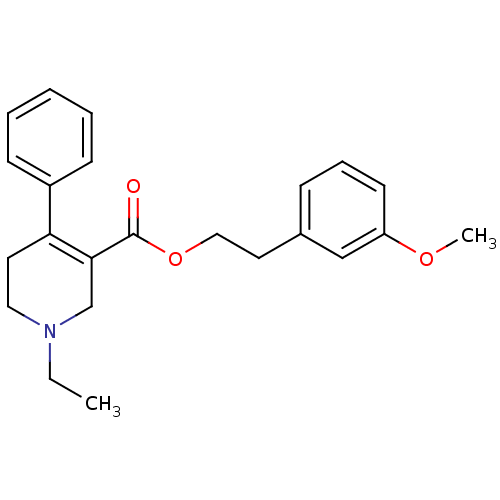 (CHEMBL2312387) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arkansas for Medical Sciences Curated by ChEMBL | Assay Description Displacement of [3H]NMS from human M5 muscarinic receptor expressed in CHO-K1 cells | J Med Chem 56: 1693-703 (2013) Article DOI: 10.1021/jm301774u BindingDB Entry DOI: 10.7270/Q2765GN2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 358 total ) | Next | Last >> |