 Found 40 hits with Last Name = 'zhang' and Initial = 'xs'
Found 40 hits with Last Name = 'zhang' and Initial = 'xs' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Histamine H1 receptor
(Homo sapiens (Human)) | BDBM50301397

(CHEMBL571390 | [1-(4-Methyl-benzyl)-piperidin-4-yl...)Show SMILES Cc1ccc(CN2CCC(CC2)C(O)(c2ccccc2)c2ccccc2)cc1 Show InChI InChI=1S/C26H29NO/c1-21-12-14-22(15-13-21)20-27-18-16-25(17-19-27)26(28,23-8-4-2-5-9-23)24-10-6-3-7-11-24/h2-15,25,28H,16-20H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Schering Plough Research Institute
Curated by ChEMBL
| Assay Description
Displacement of [3H]pyrilamine from human recombinant histamine H1 receptor expressed in CHO cell by betaplate scintillation counting |
Bioorg Med Chem Lett 19: 5043-7 (2009)
Article DOI: 10.1016/j.bmcl.2009.07.047
BindingDB Entry DOI: 10.7270/Q2NP24HJ |
More data for this
Ligand-Target Pair | |
Histamine H1 receptor
(Homo sapiens (Human)) | BDBM50301394
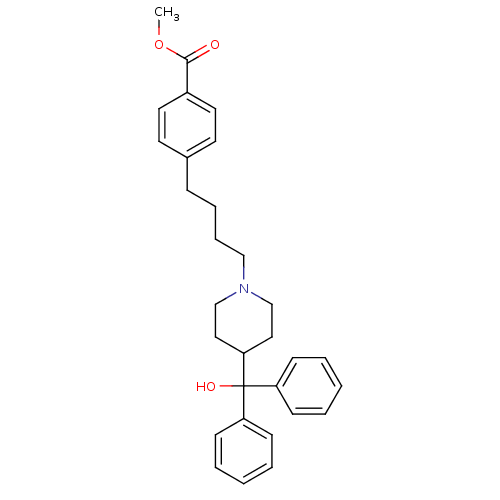
(CHEMBL568892 | methyl 4-(4-(4-(hydroxydiphenylmeth...)Show SMILES COC(=O)c1ccc(CCCCN2CCC(CC2)C(O)(c2ccccc2)c2ccccc2)cc1 Show InChI InChI=1S/C30H35NO3/c1-34-29(32)25-17-15-24(16-18-25)10-8-9-21-31-22-19-28(20-23-31)30(33,26-11-4-2-5-12-26)27-13-6-3-7-14-27/h2-7,11-18,28,33H,8-10,19-23H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Schering Plough Research Institute
Curated by ChEMBL
| Assay Description
Displacement of [3H]pyrilamine from human recombinant histamine H1 receptor expressed in CHO cell by betaplate scintillation counting |
Bioorg Med Chem Lett 19: 5043-7 (2009)
Article DOI: 10.1016/j.bmcl.2009.07.047
BindingDB Entry DOI: 10.7270/Q2NP24HJ |
More data for this
Ligand-Target Pair | |
Histamine H1 receptor
(Homo sapiens (Human)) | BDBM22874
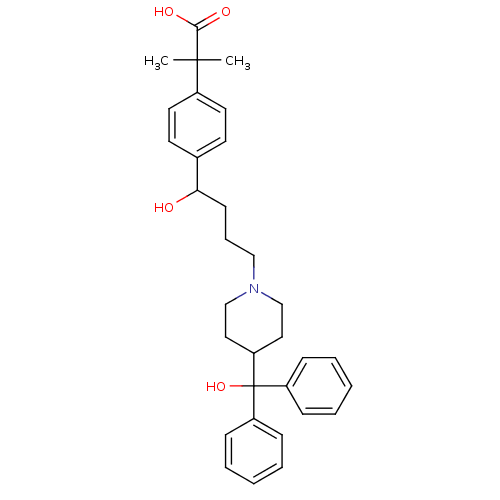
(2-(4-{1-hydroxy-4-[4-(hydroxydiphenylmethyl)piperi...)Show SMILES CC(C)(C(O)=O)c1ccc(cc1)C(O)CCCN1CCC(CC1)C(O)(c1ccccc1)c1ccccc1 Show InChI InChI=1S/C32H39NO4/c1-31(2,30(35)36)25-17-15-24(16-18-25)29(34)14-9-21-33-22-19-28(20-23-33)32(37,26-10-5-3-6-11-26)27-12-7-4-8-13-27/h3-8,10-13,15-18,28-29,34,37H,9,14,19-23H2,1-2H3,(H,35,36) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| 27 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Schering Plough Research Institute
Curated by ChEMBL
| Assay Description
Displacement of [3H]pyrilamine from human recombinant histamine H1 receptor expressed in CHO cell by betaplate scintillation counting |
Bioorg Med Chem Lett 19: 5043-7 (2009)
Article DOI: 10.1016/j.bmcl.2009.07.047
BindingDB Entry DOI: 10.7270/Q2NP24HJ |
More data for this
Ligand-Target Pair | |
Histamine H1 receptor
(Homo sapiens (Human)) | BDBM50301391
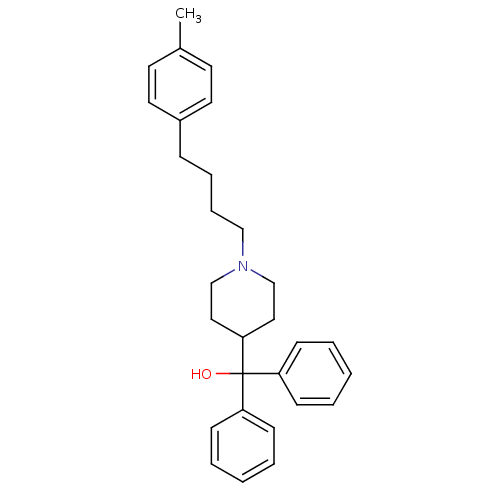
(1-[4-(4-methylphenyl)butyl]-alpha,alpha-diphenyl-4...)Show SMILES Cc1ccc(CCCCN2CCC(CC2)C(O)(c2ccccc2)c2ccccc2)cc1 Show InChI InChI=1S/C29H35NO/c1-24-15-17-25(18-16-24)10-8-9-21-30-22-19-28(20-23-30)29(31,26-11-4-2-5-12-26)27-13-6-3-7-14-27/h2-7,11-18,28,31H,8-10,19-23H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 39 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Schering Plough Research Institute
Curated by ChEMBL
| Assay Description
Displacement of [3H]pyrilamine from human recombinant histamine H1 receptor expressed in CHO cell by betaplate scintillation counting |
Bioorg Med Chem Lett 19: 5043-7 (2009)
Article DOI: 10.1016/j.bmcl.2009.07.047
BindingDB Entry DOI: 10.7270/Q2NP24HJ |
More data for this
Ligand-Target Pair | |
Histamine H1 receptor
(Homo sapiens (Human)) | BDBM50017376

((+/-)1-(4-tert-butylphenyl)-4-(4-(hydroxydiphenylm...)Show SMILES CC(C)(C)c1ccc(cc1)C(O)CCCN1CCC(CC1)C(O)(c1ccccc1)c1ccccc1 Show InChI InChI=1S/C32H41NO2/c1-31(2,3)26-18-16-25(17-19-26)30(34)15-10-22-33-23-20-29(21-24-33)32(35,27-11-6-4-7-12-27)28-13-8-5-9-14-28/h4-9,11-14,16-19,29-30,34-35H,10,15,20-24H2,1-3H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Schering Plough Research Institute
Curated by ChEMBL
| Assay Description
Displacement of [3H]pyrilamine from human recombinant histamine H1 receptor expressed in CHO cell by betaplate scintillation counting |
Bioorg Med Chem Lett 19: 5043-7 (2009)
Article DOI: 10.1016/j.bmcl.2009.07.047
BindingDB Entry DOI: 10.7270/Q2NP24HJ |
More data for this
Ligand-Target Pair | |
Histamine H1 receptor
(Homo sapiens (Human)) | BDBM50301393
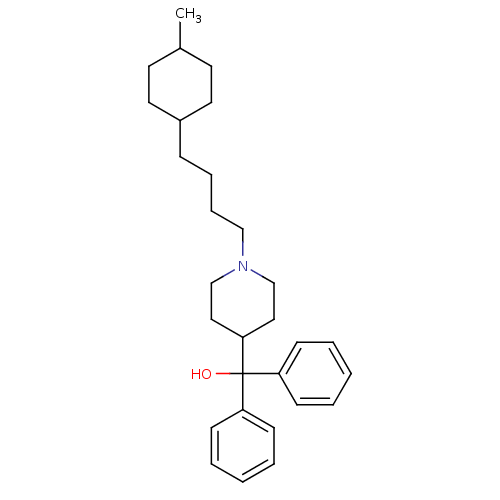
(1-[4-(4-methylcyclohexyl)butyl]alpha,alpha-dipheny...)Show SMILES CC1CCC(CCCCN2CCC(CC2)C(O)(c2ccccc2)c2ccccc2)CC1 |(10.08,-37.25,;8.75,-36.48,;7.41,-37.24,;6.08,-36.46,;6.09,-34.92,;4.76,-34.15,;3.42,-34.91,;2.09,-34.13,;.76,-34.9,;-.57,-34.12,;-1.91,-34.88,;-3.23,-34.11,;-3.23,-32.57,;-1.9,-31.8,;-.57,-32.58,;-4.56,-31.8,;-5.9,-31.03,;-4.56,-30.26,;-3.22,-29.5,;-3.21,-27.96,;-4.55,-27.19,;-5.89,-27.96,;-5.88,-29.5,;-5.89,-32.57,;-7.22,-31.79,;-8.55,-32.56,;-8.56,-34.1,;-7.21,-34.87,;-5.88,-34.1,;7.42,-34.16,;8.75,-34.93,)| Show InChI InChI=1S/C29H41NO/c1-24-15-17-25(18-16-24)10-8-9-21-30-22-19-28(20-23-30)29(31,26-11-4-2-5-12-26)27-13-6-3-7-14-27/h2-7,11-14,24-25,28,31H,8-10,15-23H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 61 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Schering Plough Research Institute
Curated by ChEMBL
| Assay Description
Displacement of [3H]pyrilamine from human recombinant histamine H1 receptor expressed in CHO cell by betaplate scintillation counting |
Bioorg Med Chem Lett 19: 5043-7 (2009)
Article DOI: 10.1016/j.bmcl.2009.07.047
BindingDB Entry DOI: 10.7270/Q2NP24HJ |
More data for this
Ligand-Target Pair | |
Histamine H1 receptor
(Homo sapiens (Human)) | BDBM50301398

(CHEMBL571391 | Diphenyl-[1-(2-p-tolyl-ethyl)-piper...)Show SMILES Cc1ccc(CCN2CCC(CC2)C(O)(c2ccccc2)c2ccccc2)cc1 Show InChI InChI=1S/C27H31NO/c1-22-12-14-23(15-13-22)16-19-28-20-17-26(18-21-28)27(29,24-8-4-2-5-9-24)25-10-6-3-7-11-25/h2-15,26,29H,16-21H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 64 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Schering Plough Research Institute
Curated by ChEMBL
| Assay Description
Displacement of [3H]pyrilamine from human recombinant histamine H1 receptor expressed in CHO cell by betaplate scintillation counting |
Bioorg Med Chem Lett 19: 5043-7 (2009)
Article DOI: 10.1016/j.bmcl.2009.07.047
BindingDB Entry DOI: 10.7270/Q2NP24HJ |
More data for this
Ligand-Target Pair | |
Histamine H1 receptor
(Homo sapiens (Human)) | BDBM50301396

(CHEMBL572034 | Phenyl-[1-(4-p-tolyl-butyl)-piperid...)Show InChI InChI=1S/C23H31NO/c1-19-10-12-20(13-11-19)7-5-6-16-24-17-14-22(15-18-24)23(25)21-8-3-2-4-9-21/h2-4,8-13,22-23,25H,5-7,14-18H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 121 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Schering Plough Research Institute
Curated by ChEMBL
| Assay Description
Displacement of [3H]pyrilamine from human recombinant histamine H1 receptor expressed in CHO cell by betaplate scintillation counting |
Bioorg Med Chem Lett 19: 5043-7 (2009)
Article DOI: 10.1016/j.bmcl.2009.07.047
BindingDB Entry DOI: 10.7270/Q2NP24HJ |
More data for this
Ligand-Target Pair | |
Histamine H1 receptor
(Homo sapiens (Human)) | BDBM50301399
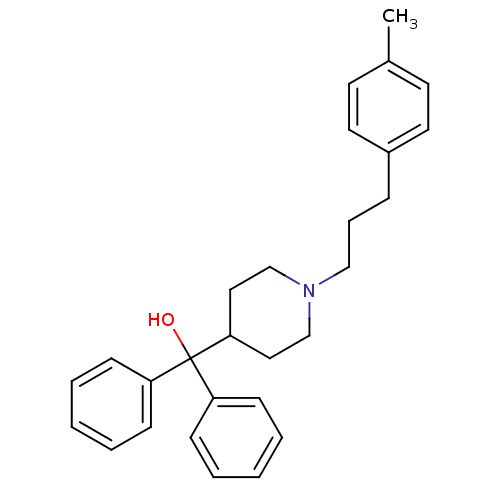
(CHEMBL570695 | Diphenyl-[1-(3-p-tolyl-propyl)-pipe...)Show SMILES Cc1ccc(CCCN2CCC(CC2)C(O)(c2ccccc2)c2ccccc2)cc1 Show InChI InChI=1S/C28H33NO/c1-23-14-16-24(17-15-23)9-8-20-29-21-18-27(19-22-29)28(30,25-10-4-2-5-11-25)26-12-6-3-7-13-26/h2-7,10-17,27,30H,8-9,18-22H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 155 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Schering Plough Research Institute
Curated by ChEMBL
| Assay Description
Displacement of [3H]pyrilamine from human recombinant histamine H1 receptor expressed in CHO cell by betaplate scintillation counting |
Bioorg Med Chem Lett 19: 5043-7 (2009)
Article DOI: 10.1016/j.bmcl.2009.07.047
BindingDB Entry DOI: 10.7270/Q2NP24HJ |
More data for this
Ligand-Target Pair | |
Histamine H1 receptor
(Homo sapiens (Human)) | BDBM50301392
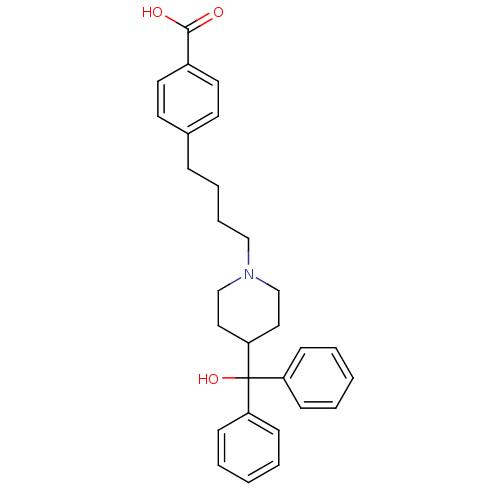
(4-[4-[4-[hydroxy(diphenyl)methyl]-1-piperidinyl]bu...)Show SMILES OC(=O)c1ccc(CCCCN2CCC(CC2)C(O)(c2ccccc2)c2ccccc2)cc1 Show InChI InChI=1S/C29H33NO3/c31-28(32)24-16-14-23(15-17-24)9-7-8-20-30-21-18-27(19-22-30)29(33,25-10-3-1-4-11-25)26-12-5-2-6-13-26/h1-6,10-17,27,33H,7-9,18-22H2,(H,31,32) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 203 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Schering Plough Research Institute
Curated by ChEMBL
| Assay Description
Displacement of [3H]pyrilamine from human recombinant histamine H1 receptor expressed in CHO cell by betaplate scintillation counting |
Bioorg Med Chem Lett 19: 5043-7 (2009)
Article DOI: 10.1016/j.bmcl.2009.07.047
BindingDB Entry DOI: 10.7270/Q2NP24HJ |
More data for this
Ligand-Target Pair | |
Histamine H1 receptor
(Homo sapiens (Human)) | BDBM50017724
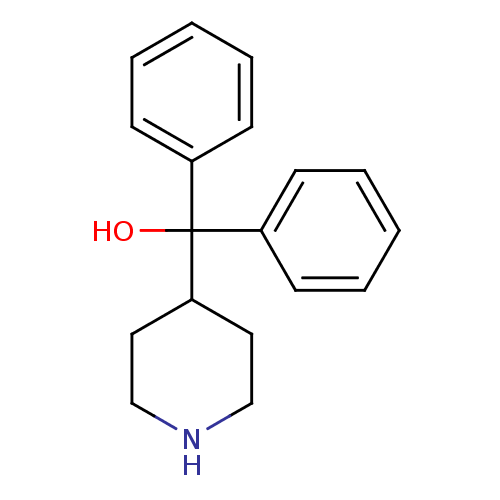
(CHEMBL127508 | Diphenyl-piperidin-4-yl-methanol | ...)Show InChI InChI=1S/C18H21NO/c20-18(15-7-3-1-4-8-15,16-9-5-2-6-10-16)17-11-13-19-14-12-17/h1-10,17,19-20H,11-14H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 659 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Schering Plough Research Institute
Curated by ChEMBL
| Assay Description
Displacement of [3H]pyrilamine from human recombinant histamine H1 receptor expressed in CHO cell by betaplate scintillation counting |
Bioorg Med Chem Lett 19: 5043-7 (2009)
Article DOI: 10.1016/j.bmcl.2009.07.047
BindingDB Entry DOI: 10.7270/Q2NP24HJ |
More data for this
Ligand-Target Pair | |
Histamine H1 receptor
(Homo sapiens (Human)) | BDBM50301395

(1-(4-p-Tolyl-butyl)-piperidine | CHEMBL571073)Show InChI InChI=1S/C16H25N/c1-15-8-10-16(11-9-15)7-3-6-14-17-12-4-2-5-13-17/h8-11H,2-7,12-14H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Schering Plough Research Institute
Curated by ChEMBL
| Assay Description
Displacement of [3H]pyrilamine from human recombinant histamine H1 receptor expressed in CHO cell by betaplate scintillation counting |
Bioorg Med Chem Lett 19: 5043-7 (2009)
Article DOI: 10.1016/j.bmcl.2009.07.047
BindingDB Entry DOI: 10.7270/Q2NP24HJ |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM22874

(2-(4-{1-hydroxy-4-[4-(hydroxydiphenylmethyl)piperi...)Show SMILES CC(C)(C(O)=O)c1ccc(cc1)C(O)CCCN1CCC(CC1)C(O)(c1ccccc1)c1ccccc1 Show InChI InChI=1S/C32H39NO4/c1-31(2,30(35)36)25-17-15-24(16-18-25)29(34)14-9-21-33-22-19-28(20-23-33)32(37,26-10-5-3-6-11-26)27-12-7-4-8-13-27/h3-8,10-13,15-18,28-29,34,37H,9,14,19-23H2,1-2H3,(H,35,36) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >100 | n/a | n/a | n/a | n/a | n/a | n/a |
The Schering Plough Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of human ERG in L929 cells by whole cell patch-clamp assay |
Bioorg Med Chem Lett 19: 5043-7 (2009)
Article DOI: 10.1016/j.bmcl.2009.07.047
BindingDB Entry DOI: 10.7270/Q2NP24HJ |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50017376

((+/-)1-(4-tert-butylphenyl)-4-(4-(hydroxydiphenylm...)Show SMILES CC(C)(C)c1ccc(cc1)C(O)CCCN1CCC(CC1)C(O)(c1ccccc1)c1ccccc1 Show InChI InChI=1S/C32H41NO2/c1-31(2,3)26-18-16-25(17-19-26)30(34)15-10-22-33-23-20-29(21-24-33)32(35,27-11-6-4-7-12-27)28-13-8-5-9-14-28/h4-9,11-14,16-19,29-30,34-35H,10,15,20-24H2,1-3H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 312 | n/a | n/a | n/a | n/a | n/a | n/a |
The Schering Plough Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of human ERG in L929 cells by whole cell patch-clamp assay |
Bioorg Med Chem Lett 19: 5043-7 (2009)
Article DOI: 10.1016/j.bmcl.2009.07.047
BindingDB Entry DOI: 10.7270/Q2NP24HJ |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50017376

((+/-)1-(4-tert-butylphenyl)-4-(4-(hydroxydiphenylm...)Show SMILES CC(C)(C)c1ccc(cc1)C(O)CCCN1CCC(CC1)C(O)(c1ccccc1)c1ccccc1 Show InChI InChI=1S/C32H41NO2/c1-31(2,3)26-18-16-25(17-19-26)30(34)15-10-22-33-23-20-29(21-24-33)32(35,27-11-6-4-7-12-27)28-13-8-5-9-14-28/h4-9,11-14,16-19,29-30,34-35H,10,15,20-24H2,1-3H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 312 | n/a | n/a | n/a | n/a | n/a | n/a |
The Schering Plough Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of human ERG in L929 cells at 10 uM by whole cell patch-clamp assay |
Bioorg Med Chem Lett 19: 5043-7 (2009)
Article DOI: 10.1016/j.bmcl.2009.07.047
BindingDB Entry DOI: 10.7270/Q2NP24HJ |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50301391

(1-[4-(4-methylphenyl)butyl]-alpha,alpha-diphenyl-4...)Show SMILES Cc1ccc(CCCCN2CCC(CC2)C(O)(c2ccccc2)c2ccccc2)cc1 Show InChI InChI=1S/C29H35NO/c1-24-15-17-25(18-16-24)10-8-9-21-30-22-19-28(20-23-30)29(31,26-11-4-2-5-12-26)27-13-6-3-7-14-27/h2-7,11-18,28,31H,8-10,19-23H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 396 | n/a | n/a | n/a | n/a | n/a | n/a |
The Schering Plough Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of human ERG in L929 cells at 10 uM by whole cell patch-clamp assay |
Bioorg Med Chem Lett 19: 5043-7 (2009)
Article DOI: 10.1016/j.bmcl.2009.07.047
BindingDB Entry DOI: 10.7270/Q2NP24HJ |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50301391

(1-[4-(4-methylphenyl)butyl]-alpha,alpha-diphenyl-4...)Show SMILES Cc1ccc(CCCCN2CCC(CC2)C(O)(c2ccccc2)c2ccccc2)cc1 Show InChI InChI=1S/C29H35NO/c1-24-15-17-25(18-16-24)10-8-9-21-30-22-19-28(20-23-30)29(31,26-11-4-2-5-12-26)27-13-6-3-7-14-27/h2-7,11-18,28,31H,8-10,19-23H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 396 | n/a | n/a | n/a | n/a | n/a | n/a |
The Schering Plough Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of human ERG in L929 cells by whole cell patch-clamp assay |
Bioorg Med Chem Lett 19: 5043-7 (2009)
Article DOI: 10.1016/j.bmcl.2009.07.047
BindingDB Entry DOI: 10.7270/Q2NP24HJ |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50423984
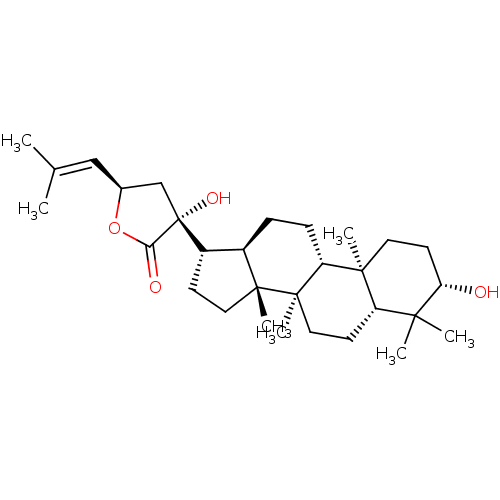
(CHEMBL2313419)Show SMILES [#6]\[#6](-[#6])=[#6]/[#6@H]-1-[#6][C@@]([#8])([#6@H]-2-[#6]-[#6][C@]3([#6])[#6@@H]-2-[#6]-[#6]-[#6@@H]2[C@@]4([#6])[#6]-[#6]-[#6@H](-[#8])C([#6])([#6])[#6@@H]4-[#6]-[#6][C@@]32[#6])[#6](=O)-[#8]1 |r| Show InChI InChI=1S/C30H48O4/c1-18(2)16-19-17-30(33,25(32)34-19)21-10-14-28(6)20(21)8-9-23-27(5)13-12-24(31)26(3,4)22(27)11-15-29(23,28)7/h16,19-24,31,33H,8-15,17H2,1-7H3/t19-,20+,21-,22-,23+,24-,27-,28+,29+,30+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Shenyang Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibition of PTP1B (unknown origin) |
Bioorg Med Chem Lett 23: 297-300 (2012)
Article DOI: 10.1016/j.bmcl.2012.10.097
BindingDB Entry DOI: 10.7270/Q2CJ8FS8 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50301392

(4-[4-[4-[hydroxy(diphenyl)methyl]-1-piperidinyl]bu...)Show SMILES OC(=O)c1ccc(CCCCN2CCC(CC2)C(O)(c2ccccc2)c2ccccc2)cc1 Show InChI InChI=1S/C29H33NO3/c31-28(32)24-16-14-23(15-17-24)9-7-8-20-30-21-18-27(19-22-30)29(33,25-10-3-1-4-11-25)26-12-5-2-6-13-26/h1-6,10-17,27,33H,7-9,18-22H2,(H,31,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
The Schering Plough Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of human ERG in L929 cells at 10 uM by whole cell patch-clamp assay |
Bioorg Med Chem Lett 19: 5043-7 (2009)
Article DOI: 10.1016/j.bmcl.2009.07.047
BindingDB Entry DOI: 10.7270/Q2NP24HJ |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50301392

(4-[4-[4-[hydroxy(diphenyl)methyl]-1-piperidinyl]bu...)Show SMILES OC(=O)c1ccc(CCCCN2CCC(CC2)C(O)(c2ccccc2)c2ccccc2)cc1 Show InChI InChI=1S/C29H33NO3/c31-28(32)24-16-14-23(15-17-24)9-7-8-20-30-21-18-27(19-22-30)29(33,25-10-3-1-4-11-25)26-12-5-2-6-13-26/h1-6,10-17,27,33H,7-9,18-22H2,(H,31,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
The Schering Plough Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of human ERG in L929 cells by whole cell patch-clamp assay |
Bioorg Med Chem Lett 19: 5043-7 (2009)
Article DOI: 10.1016/j.bmcl.2009.07.047
BindingDB Entry DOI: 10.7270/Q2NP24HJ |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50423983

(CHEMBL2313420)Show SMILES C[C@@]12CC[C@@H]([C@H]1CC[C@@H]1[C@@]3(C)CC[C@H](O)C(C)(C)[C@@H]3CC[C@@]21C)C(O)=O |r| Show InChI InChI=1S/C23H38O3/c1-20(2)16-9-13-23(5)17(21(16,3)11-10-18(20)24)7-6-15-14(19(25)26)8-12-22(15,23)4/h14-18,24H,6-13H2,1-5H3,(H,25,26)/t14-,15+,16-,17+,18-,21-,22+,23+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.31E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Shenyang Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibition of PTP1B (unknown origin) |
Bioorg Med Chem Lett 23: 297-300 (2012)
Article DOI: 10.1016/j.bmcl.2012.10.097
BindingDB Entry DOI: 10.7270/Q2CJ8FS8 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50423991
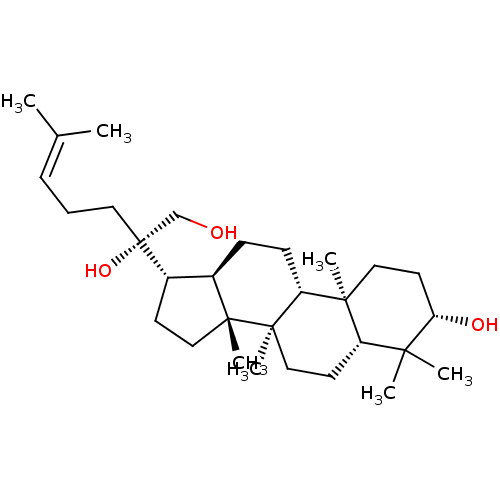
(CHEMBL2313414)Show SMILES [#6]\[#6](-[#6])=[#6]\[#6]-[#6][C@@]([#8])([#6]-[#8])[#6@H]-1-[#6]-[#6][C@]2([#6])[#6@@H]-1-[#6]-[#6]-[#6@@H]1[C@@]3([#6])[#6]-[#6]-[#6@H](-[#8])C([#6])([#6])[#6@@H]3-[#6]-[#6][C@@]21[#6] |r| Show InChI InChI=1S/C30H52O3/c1-20(2)9-8-15-30(33,19-31)22-12-17-28(6)21(22)10-11-24-27(5)16-14-25(32)26(3,4)23(27)13-18-29(24,28)7/h9,21-25,31-33H,8,10-19H2,1-7H3/t21-,22+,23+,24-,25+,27+,28-,29-,30-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.52E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Shenyang Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibition of PTP1B (unknown origin) |
Bioorg Med Chem Lett 23: 297-300 (2012)
Article DOI: 10.1016/j.bmcl.2012.10.097
BindingDB Entry DOI: 10.7270/Q2CJ8FS8 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50423982
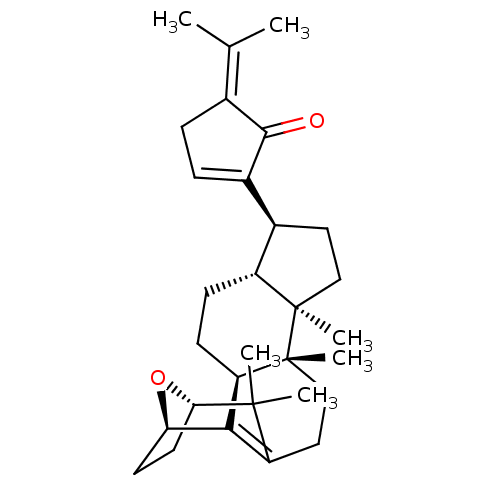
(GYPENSAPOGENIN A)Show SMILES [#6]\[#6](-[#6])=[#6]-1\[#6]-[#6]=[#6](-[#6@H]-2-[#6]-[#6][C@]3([#6])[#6@@H]-2-[#6]-[#6]-[#6@@H]2-[#6]-4=[#6](-[#6]-[#6][C@@]32[#6])C([#6])([#6])[#6@@H]-2-[#6]-[#6]-[#6@H]-4-[#8]-2)-[#6]-1=O |r,c:17,t:5| Show InChI InChI=1S/C30H42O2/c1-17(2)18-7-8-20(27(18)31)19-13-15-29(5)21(19)9-10-23-26-22(14-16-30(23,29)6)28(3,4)25-12-11-24(26)32-25/h8,19,21,23-25H,7,9-16H2,1-6H3/t19-,21-,23-,24-,25+,29-,30-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.97E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Shenyang Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibition of PTP1B (unknown origin) |
Bioorg Med Chem Lett 23: 297-300 (2012)
Article DOI: 10.1016/j.bmcl.2012.10.097
BindingDB Entry DOI: 10.7270/Q2CJ8FS8 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50115732
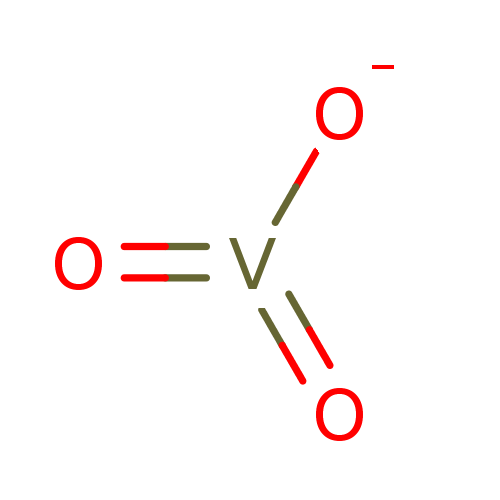
(CHEMBL294467 | Sodium vanadate) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Shenyang Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibition of PTP1B (unknown origin) |
Bioorg Med Chem Lett 23: 297-300 (2012)
Article DOI: 10.1016/j.bmcl.2012.10.097
BindingDB Entry DOI: 10.7270/Q2CJ8FS8 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50423994
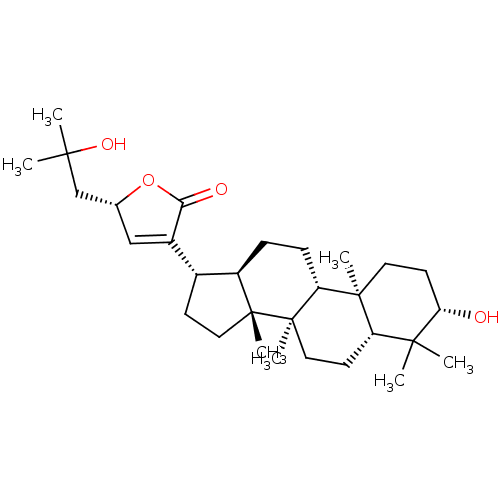
(CHEMBL2313421 | Gypensapogenin E)Show SMILES CC(C)(O)C[C@@H]1OC(=O)C(=C1)[C@H]1CC[C@]2(C)[C@@H]1CC[C@@H]1[C@@]3(C)CC[C@H](O)C(C)(C)[C@@H]3CC[C@@]21C |r,c:9| Show InChI InChI=1S/C30H48O4/c1-26(2,33)17-18-16-20(25(32)34-18)19-10-14-29(6)21(19)8-9-23-28(5)13-12-24(31)27(3,4)22(28)11-15-30(23,29)7/h16,18-19,21-24,31,33H,8-15,17H2,1-7H3/t18-,19-,21-,22+,23-,24+,28+,29-,30-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Shenyang Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibition of PTP1B (unknown origin) |
Bioorg Med Chem Lett 23: 297-300 (2012)
Article DOI: 10.1016/j.bmcl.2012.10.097
BindingDB Entry DOI: 10.7270/Q2CJ8FS8 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50423990
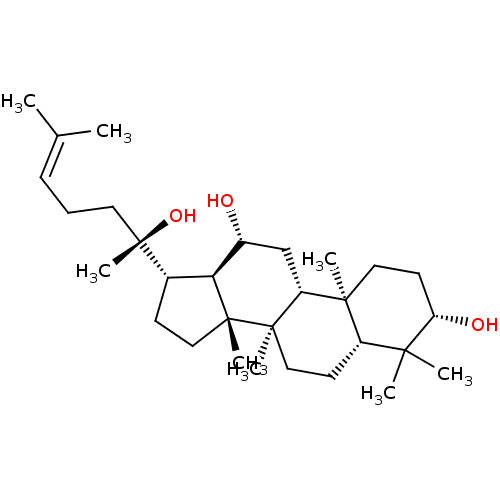
(20(S)-Protopanaxadiol | CHEMBL375563)Show SMILES [#6]\[#6](-[#6])=[#6]\[#6]-[#6][C@]([#6])([#8])[#6@H]-1-[#6]-[#6][C@]2([#6])[#6@@H]-1-[#6@H](-[#8])-[#6]-[#6@@H]1[C@@]3([#6])[#6]-[#6]-[#6@H](-[#8])C([#6])([#6])[#6@@H]3-[#6]-[#6][C@@]21[#6] Show InChI InChI=1S/C30H52O3/c1-19(2)10-9-14-30(8,33)20-11-16-29(7)25(20)21(31)18-23-27(5)15-13-24(32)26(3,4)22(27)12-17-28(23,29)6/h10,20-25,31-33H,9,11-18H2,1-8H3/t20-,21+,22-,23+,24-,25-,27-,28+,29+,30-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.36E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Shenyang Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibition of PTP1B (unknown origin) |
Bioorg Med Chem Lett 23: 297-300 (2012)
Article DOI: 10.1016/j.bmcl.2012.10.097
BindingDB Entry DOI: 10.7270/Q2CJ8FS8 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50423992

(CHEMBL2313423 | Gypensapogenin G)Show SMILES CC(=O)O[C@H]1CC[C@@]2(C)[C@@H](CC[C@]3(C)[C@@H]2CC[C@@H]2[C@H](CC[C@@]32C)C2=C[C@H](CC(C)(C)O)OC2=O)C1(C)C |r,t:26| Show InChI InChI=1S/C32H50O5/c1-19(33)36-26-13-14-30(6)24(29(26,4)5)12-16-32(8)25(30)10-9-23-21(11-15-31(23,32)7)22-17-20(37-27(22)34)18-28(2,3)35/h17,20-21,23-26,35H,9-16,18H2,1-8H3/t20-,21-,23-,24+,25-,26+,30+,31-,32-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.45E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Shenyang Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibition of PTP1B (unknown origin) |
Bioorg Med Chem Lett 23: 297-300 (2012)
Article DOI: 10.1016/j.bmcl.2012.10.097
BindingDB Entry DOI: 10.7270/Q2CJ8FS8 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50423981

(GYPENSAPOGENIN B)Show SMILES [#6]\[#6](-[#6])=[#6]-1\[#6]-[#6](=[#6]-[#6]-1=O)-[#6@H]-1-[#6]-[#6][C@]2([#6])[#6@@H]-1-[#6]-[#6]-[#6@@H]1-[#6]-3=[#6](-[#6]-[#6][C@@]21[#6])C([#6])([#6])[#6@@H]-1-[#6]-[#6]-[#6@H]-3-[#8]-1 |r,c:5,20| Show InChI InChI=1S/C30H42O2/c1-17(2)20-15-18(16-24(20)31)19-11-13-29(5)21(19)7-8-23-27-22(12-14-30(23,29)6)28(3,4)26-10-9-25(27)32-26/h16,19,21,23,25-26H,7-15H2,1-6H3/t19-,21-,23-,25-,26+,29-,30-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.45E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Shenyang Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibition of PTP1B (unknown origin) |
Bioorg Med Chem Lett 23: 297-300 (2012)
Article DOI: 10.1016/j.bmcl.2012.10.097
BindingDB Entry DOI: 10.7270/Q2CJ8FS8 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50423985

(CHEMBL2313418)Show SMILES [#6]\[#6](-[#6])=[#6]/[#6@@H]-1-[#6][C@]([#8])([#6@H]-2-[#6]-[#6][C@]3([#6])[#6@@H]-2-[#6]-[#6]-[#6@@H]2[C@@]4([#6])[#6]-[#6]-[#6@H](-[#8])C([#6])([#6])[#6@@H]4-[#6]-[#6][C@@]32[#6])[#6](=O)-[#8]1 |r| Show InChI InChI=1S/C30H48O4/c1-18(2)16-19-17-30(33,25(32)34-19)21-10-14-28(6)20(21)8-9-23-27(5)13-12-24(31)26(3,4)22(27)11-15-29(23,28)7/h16,19-24,31,33H,8-15,17H2,1-7H3/t19-,20-,21+,22+,23-,24+,27+,28-,29-,30+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.78E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Shenyang Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibition of PTP1B (unknown origin) |
Bioorg Med Chem Lett 23: 297-300 (2012)
Article DOI: 10.1016/j.bmcl.2012.10.097
BindingDB Entry DOI: 10.7270/Q2CJ8FS8 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50423993
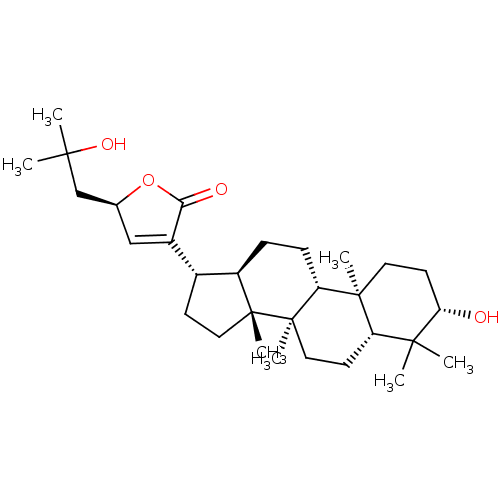
(CHEMBL2313422 | Gypensapogenin F)Show SMILES CC(C)(O)C[C@H]1OC(=O)C(=C1)[C@H]1CC[C@]2(C)[C@@H]1CC[C@@H]1[C@@]3(C)CC[C@H](O)C(C)(C)[C@@H]3CC[C@@]21C |r,c:9| Show InChI InChI=1S/C30H48O4/c1-26(2,33)17-18-16-20(25(32)34-18)19-10-14-29(6)21(19)8-9-23-28(5)13-12-24(31)27(3,4)22(28)11-15-30(23,29)7/h16,18-19,21-24,31,33H,8-15,17H2,1-7H3/t18-,19+,21+,22-,23+,24-,28-,29+,30+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.90E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Shenyang Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibition of PTP1B (unknown origin) |
Bioorg Med Chem Lett 23: 297-300 (2012)
Article DOI: 10.1016/j.bmcl.2012.10.097
BindingDB Entry DOI: 10.7270/Q2CJ8FS8 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50423989

(CHEMBL2313415)Show SMILES C\C(=C\CCC(C)(C)O)[C@H]1CC[C@]2(C)[C@@H]1[C@H](O)C[C@@H]1[C@@]3(C)CC[C@H](O)C(C)(C)[C@@H]3CC[C@@]21C |r| Show InChI InChI=1S/C30H52O3/c1-19(10-9-14-26(2,3)33)20-11-16-30(8)25(20)21(31)18-23-28(6)15-13-24(32)27(4,5)22(28)12-17-29(23,30)7/h10,20-25,31-33H,9,11-18H2,1-8H3/b19-10-/t20-,21-,22+,23-,24+,25+,28+,29-,30-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Shenyang Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibition of PTP1B (unknown origin) |
Bioorg Med Chem Lett 23: 297-300 (2012)
Article DOI: 10.1016/j.bmcl.2012.10.097
BindingDB Entry DOI: 10.7270/Q2CJ8FS8 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50423987

(CHEMBL2313417)Show SMILES CC(=C)[C@@H](O)CC[C@](C)(O)[C@H]1CC[C@]2(C)[C@@H]1[C@H](O)C[C@@H]1[C@@]3(C)CC[C@H](O)C(C)(C)[C@@H]3CC[C@@]21C |r| Show InChI InChI=1S/C30H52O4/c1-18(2)20(31)10-16-30(8,34)19-9-14-29(7)25(19)21(32)17-23-27(5)13-12-24(33)26(3,4)22(27)11-15-28(23,29)6/h19-25,31-34H,1,9-17H2,2-8H3/t19-,20-,21+,22-,23+,24-,25-,27-,28+,29+,30-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Shenyang Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibition of PTP1B (unknown origin) |
Bioorg Med Chem Lett 23: 297-300 (2012)
Article DOI: 10.1016/j.bmcl.2012.10.097
BindingDB Entry DOI: 10.7270/Q2CJ8FS8 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50423986
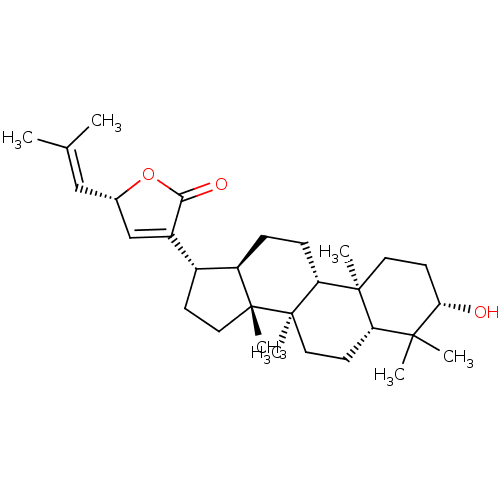
(GYPENSAPOGENIN D)Show SMILES [#6]\[#6](-[#6])=[#6]\[#6@@H]-1-[#8]-[#6](=O)-[#6](=[#6]1)-[#6@H]-1-[#6]-[#6][C@]2([#6])[#6@@H]-1-[#6]-[#6]-[#6@@H]1[C@@]3([#6])[#6]-[#6]-[#6@H](-[#8])C([#6])([#6])[#6@@H]3-[#6]-[#6][C@@]21[#6] |r,c:8| Show InChI InChI=1S/C30H46O3/c1-18(2)16-19-17-21(26(32)33-19)20-10-14-29(6)22(20)8-9-24-28(5)13-12-25(31)27(3,4)23(28)11-15-30(24,29)7/h16-17,19-20,22-25,31H,8-15H2,1-7H3/t19-,20+,22+,23-,24+,25-,28-,29+,30+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Shenyang Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibition of PTP1B (unknown origin) |
Bioorg Med Chem Lett 23: 297-300 (2012)
Article DOI: 10.1016/j.bmcl.2012.10.097
BindingDB Entry DOI: 10.7270/Q2CJ8FS8 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50423988
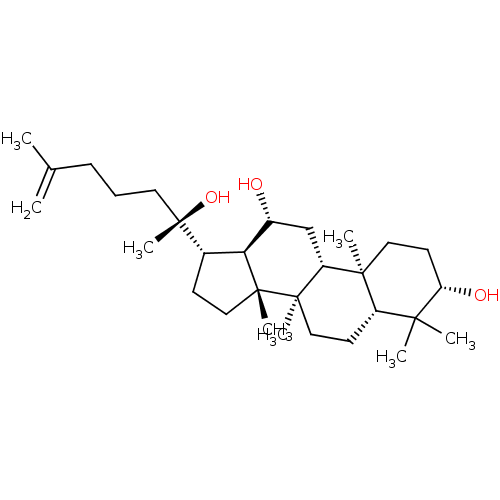
(CHEMBL2313416)Show SMILES CC(=C)CCC[C@](C)(O)[C@H]1CC[C@]2(C)[C@@H]1[C@H](O)C[C@@H]1[C@@]3(C)CC[C@H](O)C(C)(C)[C@@H]3CC[C@@]21C |r| Show InChI InChI=1S/C30H52O3/c1-19(2)10-9-14-30(8,33)20-11-16-29(7)25(20)21(31)18-23-27(5)15-13-24(32)26(3,4)22(27)12-17-28(23,29)6/h20-25,31-33H,1,9-18H2,2-8H3/t20-,21+,22-,23+,24-,25-,27-,28+,29+,30-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Shenyang Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibition of PTP1B (unknown origin) |
Bioorg Med Chem Lett 23: 297-300 (2012)
Article DOI: 10.1016/j.bmcl.2012.10.097
BindingDB Entry DOI: 10.7270/Q2CJ8FS8 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50423980
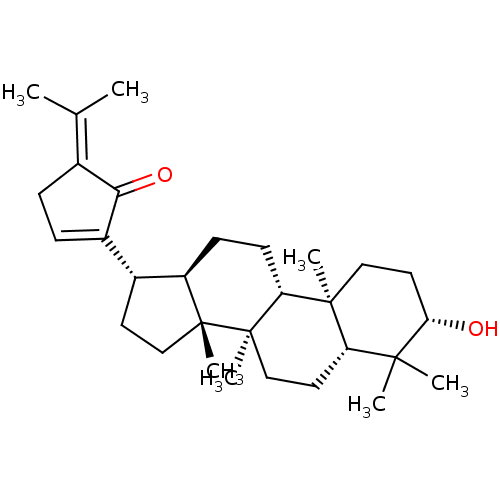
(GYPENSAPOGENIN C)Show SMILES [#6]\[#6](-[#6])=[#6]-1\[#6]-[#6]=[#6](-[#6@H]-2-[#6]-[#6][C@]3([#6])[#6@@H]-2-[#6]-[#6]-[#6@@H]2[C@@]4([#6])[#6]-[#6]-[#6@H](-[#8])C([#6])([#6])[#6@@H]4-[#6]-[#6][C@@]32[#6])-[#6]-1=O |r,t:5| Show InChI InChI=1S/C30H46O2/c1-18(2)19-8-9-21(26(19)32)20-12-16-29(6)22(20)10-11-24-28(5)15-14-25(31)27(3,4)23(28)13-17-30(24,29)7/h9,20,22-25,31H,8,10-17H2,1-7H3/t20-,22-,23+,24-,25+,28+,29-,30-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Shenyang Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibition of PTP1B (unknown origin) |
Bioorg Med Chem Lett 23: 297-300 (2012)
Article DOI: 10.1016/j.bmcl.2012.10.097
BindingDB Entry DOI: 10.7270/Q2CJ8FS8 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50301393

(1-[4-(4-methylcyclohexyl)butyl]alpha,alpha-dipheny...)Show SMILES CC1CCC(CCCCN2CCC(CC2)C(O)(c2ccccc2)c2ccccc2)CC1 |(10.08,-37.25,;8.75,-36.48,;7.41,-37.24,;6.08,-36.46,;6.09,-34.92,;4.76,-34.15,;3.42,-34.91,;2.09,-34.13,;.76,-34.9,;-.57,-34.12,;-1.91,-34.88,;-3.23,-34.11,;-3.23,-32.57,;-1.9,-31.8,;-.57,-32.58,;-4.56,-31.8,;-5.9,-31.03,;-4.56,-30.26,;-3.22,-29.5,;-3.21,-27.96,;-4.55,-27.19,;-5.89,-27.96,;-5.88,-29.5,;-5.89,-32.57,;-7.22,-31.79,;-8.55,-32.56,;-8.56,-34.1,;-7.21,-34.87,;-5.88,-34.1,;7.42,-34.16,;8.75,-34.93,)| Show InChI InChI=1S/C29H41NO/c1-24-15-17-25(18-16-24)10-8-9-21-30-22-19-28(20-23-30)29(31,26-11-4-2-5-12-26)27-13-6-3-7-14-27/h2-7,11-14,24-25,28,31H,8-10,15-23H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
The Schering Plough Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of human ERG in L929 cells at 10 uM by whole cell patch-clamp assay |
Bioorg Med Chem Lett 19: 5043-7 (2009)
Article DOI: 10.1016/j.bmcl.2009.07.047
BindingDB Entry DOI: 10.7270/Q2NP24HJ |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50017724

(CHEMBL127508 | Diphenyl-piperidin-4-yl-methanol | ...)Show InChI InChI=1S/C18H21NO/c20-18(15-7-3-1-4-8-15,16-9-5-2-6-10-16)17-11-13-19-14-12-17/h1-10,17,19-20H,11-14H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
The Schering Plough Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of human ERG in L929 cells at 10 uM by whole cell patch-clamp assay |
Bioorg Med Chem Lett 19: 5043-7 (2009)
Article DOI: 10.1016/j.bmcl.2009.07.047
BindingDB Entry DOI: 10.7270/Q2NP24HJ |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50017724

(CHEMBL127508 | Diphenyl-piperidin-4-yl-methanol | ...)Show InChI InChI=1S/C18H21NO/c20-18(15-7-3-1-4-8-15,16-9-5-2-6-10-16)17-11-13-19-14-12-17/h1-10,17,19-20H,11-14H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
The Schering Plough Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of human ERG in L929 cells by whole cell patch-clamp assay |
Bioorg Med Chem Lett 19: 5043-7 (2009)
Article DOI: 10.1016/j.bmcl.2009.07.047
BindingDB Entry DOI: 10.7270/Q2NP24HJ |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM22874

(2-(4-{1-hydroxy-4-[4-(hydroxydiphenylmethyl)piperi...)Show SMILES CC(C)(C(O)=O)c1ccc(cc1)C(O)CCCN1CCC(CC1)C(O)(c1ccccc1)c1ccccc1 Show InChI InChI=1S/C32H39NO4/c1-31(2,30(35)36)25-17-15-24(16-18-25)29(34)14-9-21-33-22-19-28(20-23-33)32(37,26-10-5-3-6-11-26)27-12-7-4-8-13-27/h3-8,10-13,15-18,28-29,34,37H,9,14,19-23H2,1-2H3,(H,35,36) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
The Schering Plough Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of human ERG in L929 cells at 10 uM by whole cell patch-clamp assay |
Bioorg Med Chem Lett 19: 5043-7 (2009)
Article DOI: 10.1016/j.bmcl.2009.07.047
BindingDB Entry DOI: 10.7270/Q2NP24HJ |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50301393

(1-[4-(4-methylcyclohexyl)butyl]alpha,alpha-dipheny...)Show SMILES CC1CCC(CCCCN2CCC(CC2)C(O)(c2ccccc2)c2ccccc2)CC1 |(10.08,-37.25,;8.75,-36.48,;7.41,-37.24,;6.08,-36.46,;6.09,-34.92,;4.76,-34.15,;3.42,-34.91,;2.09,-34.13,;.76,-34.9,;-.57,-34.12,;-1.91,-34.88,;-3.23,-34.11,;-3.23,-32.57,;-1.9,-31.8,;-.57,-32.58,;-4.56,-31.8,;-5.9,-31.03,;-4.56,-30.26,;-3.22,-29.5,;-3.21,-27.96,;-4.55,-27.19,;-5.89,-27.96,;-5.88,-29.5,;-5.89,-32.57,;-7.22,-31.79,;-8.55,-32.56,;-8.56,-34.1,;-7.21,-34.87,;-5.88,-34.1,;7.42,-34.16,;8.75,-34.93,)| Show InChI InChI=1S/C29H41NO/c1-24-15-17-25(18-16-24)10-8-9-21-30-22-19-28(20-23-30)29(31,26-11-4-2-5-12-26)27-13-6-3-7-14-27/h2-7,11-14,24-25,28,31H,8-10,15-23H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
The Schering Plough Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of human ERG in L929 cells by whole cell patch-clamp assay |
Bioorg Med Chem Lett 19: 5043-7 (2009)
Article DOI: 10.1016/j.bmcl.2009.07.047
BindingDB Entry DOI: 10.7270/Q2NP24HJ |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data