 Found 253 hits with Last Name = 'zou' and Initial = 'p'
Found 253 hits with Last Name = 'zou' and Initial = 'p' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
E3 ubiquitin-protein ligase Mdm2
(Homo sapiens (Human)) | BDBM50436682
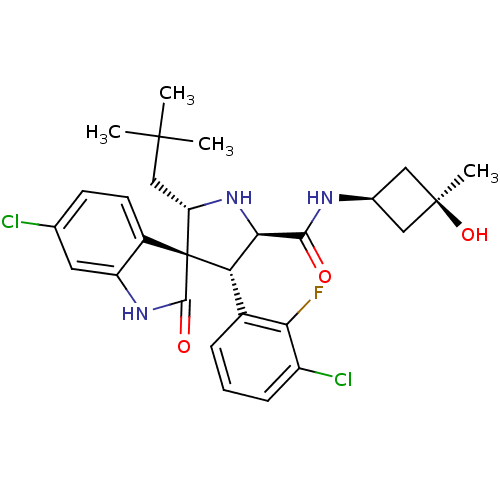
(CHEMBL2396674)Show SMILES CC(C)(C)C[C@@H]1N[C@H]([C@H](c2cccc(Cl)c2F)[C@]11C(=O)Nc2cc(Cl)ccc12)C(=O)N[C@H]1C[C@@](C)(O)C1 |r,wU:33.38,17.19,8.8,31.34,5.4,wD:33.37,7.31,(42.41,-31.09,;41.21,-30.13,;41.45,-28.61,;42.69,-29.73,;39.78,-30.69,;38.58,-29.72,;38.65,-28.19,;37.21,-27.64,;36.25,-28.84,;34.91,-28.07,;33.58,-28.84,;32.23,-28.08,;32.23,-26.52,;33.57,-25.75,;33.56,-24.21,;34.91,-26.52,;34.9,-24.97,;37.09,-30.13,;38,-31.39,;39.54,-31.39,;37.09,-32.64,;35.61,-32.16,;34.28,-32.93,;32.95,-32.16,;31.61,-32.93,;32.95,-30.62,;34.28,-29.85,;35.61,-30.61,;37.21,-26.1,;35.88,-25.33,;38.55,-25.33,;40.09,-25.32,;41.18,-24.24,;42.26,-25.32,;43.59,-24.55,;43.6,-26.09,;41.19,-26.42,)| Show InChI InChI=1S/C28H32Cl2FN3O3/c1-26(2,3)13-20-28(17-9-8-14(29)10-19(17)33-25(28)36)21(16-6-5-7-18(30)22(16)31)23(34-20)24(35)32-15-11-27(4,37)12-15/h5-10,15,20-21,23,34,37H,11-13H2,1-4H3,(H,32,35)(H,33,36)/t15-,20-,21-,23+,27+,28+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.440 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Michigan
Curated by ChEMBL
| Assay Description
Binding affinity to recombinant human His-tagged MDM2 (1 to 118 amino acids) using p53-based PMDM6-F as probe after 15 mins by competition assay |
J Med Chem 56: 5553-61 (2014)
Article DOI: 10.1021/jm4005708
BindingDB Entry DOI: 10.7270/Q2SJ1N2D |
More data for this
Ligand-Target Pair | |
E3 ubiquitin-protein ligase Mdm2
(Homo sapiens (Human)) | BDBM50436688
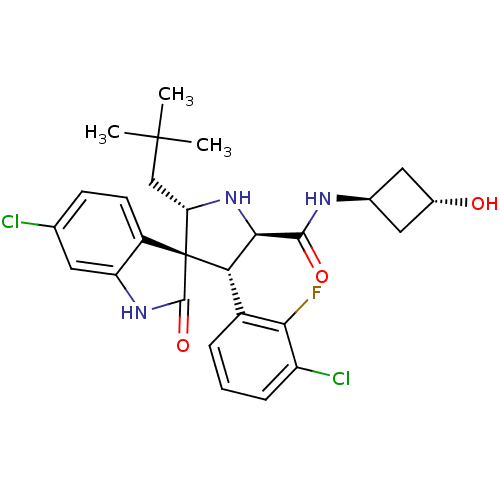
(CHEMBL2398473)Show SMILES CC(C)(C)C[C@@H]1N[C@H]([C@H](c2cccc(Cl)c2F)[C@]11C(=O)Nc2cc(Cl)ccc12)C(=O)N[C@H]1C[C@H](O)C1 |r,wU:17.19,8.8,31.34,5.4,wD:7.31,33.37,(42.17,-8.02,;40.97,-7.05,;41.2,-5.53,;42.45,-6.65,;39.53,-7.61,;38.33,-6.65,;38.41,-5.11,;36.97,-4.56,;36.01,-5.76,;34.66,-4.99,;33.33,-5.77,;31.99,-5.01,;31.98,-3.45,;33.32,-2.67,;33.32,-1.13,;34.66,-3.44,;34.66,-1.9,;36.85,-7.05,;37.76,-8.31,;39.3,-8.31,;36.85,-9.57,;35.37,-9.09,;34.04,-9.86,;32.71,-9.09,;31.37,-9.86,;32.71,-7.54,;34.04,-6.77,;35.37,-7.54,;36.97,-3.02,;35.64,-2.25,;38.31,-2.26,;39.64,-3.03,;41.12,-2.65,;41.52,-4.14,;42.85,-4.91,;40.03,-4.53,)| Show InChI InChI=1S/C27H30Cl2FN3O3/c1-26(2,3)12-20-27(17-8-7-13(28)9-19(17)32-25(27)36)21(16-5-4-6-18(29)22(16)30)23(33-20)24(35)31-14-10-15(34)11-14/h4-9,14-15,20-21,23,33-34H,10-12H2,1-3H3,(H,31,35)(H,32,36)/t14-,15-,20-,21-,23+,27+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.610 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Michigan
Curated by ChEMBL
| Assay Description
Binding affinity to recombinant human His-tagged MDM2 (1 to 118 amino acids) using p53-based PMDM6-F as probe after 15 mins by competition assay |
J Med Chem 56: 5553-61 (2014)
Article DOI: 10.1021/jm4005708
BindingDB Entry DOI: 10.7270/Q2SJ1N2D |
More data for this
Ligand-Target Pair | |
E3 ubiquitin-protein ligase Mdm2
(Homo sapiens (Human)) | BDBM50436685

(CHEMBL2398476)Show SMILES CC(C)(C)C[C@@H]1N[C@H]([C@H](c2cccc(Cl)c2F)[C@]11C(=O)Nc2cc(Cl)ccc12)C(=O)N[C@H]1CC[C@H](O)C1 |r| Show InChI InChI=1S/C28H32Cl2FN3O3/c1-27(2,3)13-21-28(18-10-7-14(29)11-20(18)33-26(28)37)22(17-5-4-6-19(30)23(17)31)24(34-21)25(36)32-15-8-9-16(35)12-15/h4-7,10-11,15-16,21-22,24,34-35H,8-9,12-13H2,1-3H3,(H,32,36)(H,33,37)/t15-,16-,21-,22-,24+,28+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.620 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Michigan
Curated by ChEMBL
| Assay Description
Binding affinity to recombinant human His-tagged MDM2 (1 to 118 amino acids) using p53-based PMDM6-F as probe after 15 mins by competition assay |
J Med Chem 56: 5553-61 (2014)
Article DOI: 10.1021/jm4005708
BindingDB Entry DOI: 10.7270/Q2SJ1N2D |
More data for this
Ligand-Target Pair | |
E3 ubiquitin-protein ligase Mdm2
(Homo sapiens (Human)) | BDBM50436681
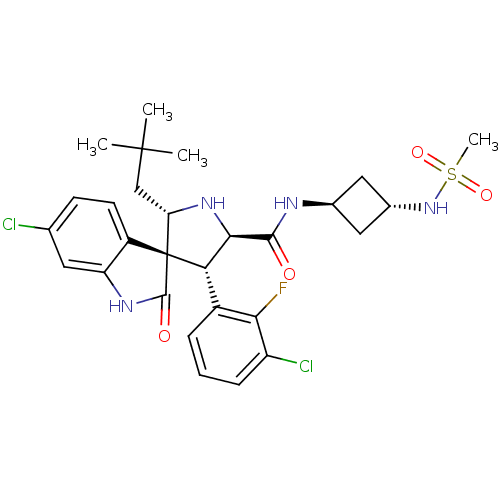
(CHEMBL2398479)Show SMILES CC(C)(C)C[C@@H]1N[C@H]([C@H](c2cccc(Cl)c2F)[C@]11C(=O)Nc2cc(Cl)ccc12)C(=O)N[C@H]1C[C@@H](C1)NS(C)(=O)=O |r,wU:17.19,8.8,31.34,5.4,wD:7.31,33.39,(12.69,-41.86,;11.49,-40.9,;11.72,-39.38,;12.96,-40.5,;10.05,-41.46,;8.85,-40.49,;8.93,-38.96,;7.48,-38.41,;6.52,-39.61,;5.18,-38.84,;3.85,-39.62,;2.51,-38.85,;2.5,-37.3,;3.84,-36.52,;3.84,-34.98,;5.18,-37.29,;5.17,-35.74,;7.37,-40.9,;8.28,-42.16,;9.82,-42.16,;7.36,-43.41,;5.89,-42.93,;4.56,-43.71,;3.22,-42.93,;1.89,-43.7,;3.22,-41.39,;4.55,-40.62,;5.89,-41.38,;7.49,-36.87,;6.16,-36.1,;8.83,-36.11,;10.16,-36.88,;11.64,-36.5,;12.03,-37.98,;10.54,-38.38,;13.36,-38.76,;14.7,-37.99,;14.71,-36.45,;16.24,-37.99,;15.47,-39.33,)| Show InChI InChI=1S/C28H33Cl2FN4O4S/c1-27(2,3)13-21-28(18-9-8-14(29)10-20(18)33-26(28)37)22(17-6-5-7-19(30)23(17)31)24(34-21)25(36)32-15-11-16(12-15)35-40(4,38)39/h5-10,15-16,21-22,24,34-35H,11-13H2,1-4H3,(H,32,36)(H,33,37)/t15-,16-,21-,22-,24+,28+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.620 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Michigan
Curated by ChEMBL
| Assay Description
Binding affinity to recombinant human His-tagged MDM2 (1 to 118 amino acids) using p53-based PMDM6-F as probe after 15 mins by competition assay |
J Med Chem 56: 5553-61 (2014)
Article DOI: 10.1021/jm4005708
BindingDB Entry DOI: 10.7270/Q2SJ1N2D |
More data for this
Ligand-Target Pair | |
E3 ubiquitin-protein ligase Mdm2
(Homo sapiens (Human)) | BDBM50436689

(CHEMBL2398472)Show SMILES CC(C)(C)C[C@@H]1N[C@H]([C@H](c2cccc(Cl)c2F)[C@]11C(=O)Nc2cc(Cl)ccc12)C(=O)NCC[C@H](O)CO |r| Show InChI InChI=1S/C27H32Cl2FN3O4/c1-26(2,3)12-20-27(17-8-7-14(28)11-19(17)32-25(27)37)21(16-5-4-6-18(29)22(16)30)23(33-20)24(36)31-10-9-15(35)13-34/h4-8,11,15,20-21,23,33-35H,9-10,12-13H2,1-3H3,(H,31,36)(H,32,37)/t15-,20-,21-,23+,27+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.860 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Michigan
Curated by ChEMBL
| Assay Description
Binding affinity to recombinant human His-tagged MDM2 (1 to 118 amino acids) using p53-based PMDM6-F as probe after 15 mins by competition assay |
J Med Chem 56: 5553-61 (2014)
Article DOI: 10.1021/jm4005708
BindingDB Entry DOI: 10.7270/Q2SJ1N2D |
More data for this
Ligand-Target Pair | |
E3 ubiquitin-protein ligase Mdm2
(Homo sapiens (Human)) | BDBM50436686

(CHEMBL2398475)Show SMILES CC(C)(C)C[C@@H]1N[C@H]([C@H](c2cccc(Cl)c2F)[C@]11C(=O)Nc2cc(Cl)ccc12)C(=O)N[C@H]1CC[C@@H](O)C1 |r| Show InChI InChI=1S/C28H32Cl2FN3O3/c1-27(2,3)13-21-28(18-10-7-14(29)11-20(18)33-26(28)37)22(17-5-4-6-19(30)23(17)31)24(34-21)25(36)32-15-8-9-16(35)12-15/h4-7,10-11,15-16,21-22,24,34-35H,8-9,12-13H2,1-3H3,(H,32,36)(H,33,37)/t15-,16+,21-,22-,24+,28+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.940 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Michigan
Curated by ChEMBL
| Assay Description
Binding affinity to recombinant human His-tagged MDM2 (1 to 118 amino acids) using p53-based PMDM6-F as probe after 15 mins by competition assay |
J Med Chem 56: 5553-61 (2014)
Article DOI: 10.1021/jm4005708
BindingDB Entry DOI: 10.7270/Q2SJ1N2D |
More data for this
Ligand-Target Pair | |
E3 ubiquitin-protein ligase Mdm2
(Homo sapiens (Human)) | BDBM50436683

(CHEMBL2398478)Show SMILES CC(C)(C)C[C@@H]1N[C@H]([C@H](c2cccc(Cl)c2F)[C@]11C(=O)Nc2cc(Cl)ccc12)C(=O)N[C@H]1C[C@](C)(O)C1 |r,wU:33.37,17.19,8.8,31.34,5.4,wD:33.38,7.31,(28.66,-31.03,;27.45,-30.07,;27.69,-28.55,;28.93,-29.66,;26.02,-30.63,;24.82,-29.66,;24.89,-28.12,;23.45,-27.58,;22.49,-28.78,;21.15,-28.01,;19.82,-28.78,;18.48,-28.02,;18.47,-26.46,;19.81,-25.69,;19.81,-24.15,;21.15,-26.46,;21.14,-24.91,;23.33,-30.07,;24.24,-31.33,;25.78,-31.33,;23.33,-32.58,;21.86,-32.1,;20.52,-32.87,;19.19,-32.1,;17.86,-32.87,;19.19,-30.56,;20.52,-29.79,;21.86,-30.55,;23.46,-26.04,;22.13,-25.26,;24.79,-25.27,;26.33,-25.26,;27.42,-24.18,;28.5,-25.26,;29.83,-24.48,;29.84,-26.02,;27.43,-26.36,)| Show InChI InChI=1S/C28H32Cl2FN3O3/c1-26(2,3)13-20-28(17-9-8-14(29)10-19(17)33-25(28)36)21(16-6-5-7-18(30)22(16)31)23(34-20)24(35)32-15-11-27(4,37)12-15/h5-10,15,20-21,23,34,37H,11-13H2,1-4H3,(H,32,35)(H,33,36)/t15-,20-,21-,23+,27-,28+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.970 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Michigan
Curated by ChEMBL
| Assay Description
Binding affinity to recombinant human His-tagged MDM2 (1 to 118 amino acids) using p53-based PMDM6-F as probe after 15 mins by competition assay |
J Med Chem 56: 5553-61 (2014)
Article DOI: 10.1021/jm4005708
BindingDB Entry DOI: 10.7270/Q2SJ1N2D |
More data for this
Ligand-Target Pair | |
E3 ubiquitin-protein ligase Mdm2
(Homo sapiens (Human)) | BDBM50436687
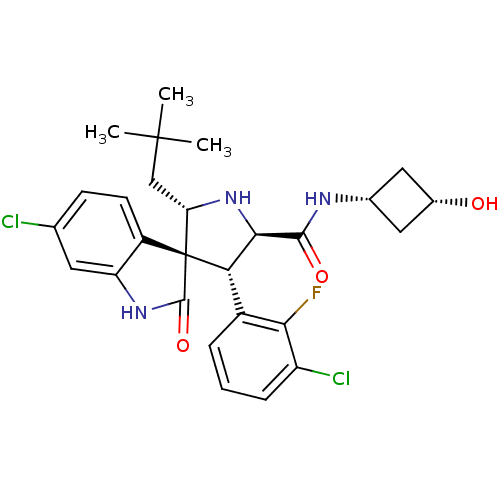
(CHEMBL2398474)Show SMILES CC(C)(C)C[C@@H]1N[C@H]([C@H](c2cccc(Cl)c2F)[C@]11C(=O)Nc2cc(Cl)ccc12)C(=O)N[C@@H]1C[C@H](O)C1 |r,wU:17.19,8.8,31.34,5.4,33.37,wD:7.31,(13.04,-18.98,;11.84,-18.02,;12.07,-16.5,;13.31,-17.62,;10.4,-18.58,;9.2,-17.61,;9.28,-16.08,;7.83,-15.53,;6.87,-16.73,;5.53,-15.96,;4.2,-16.73,;2.86,-15.97,;2.85,-14.41,;4.19,-13.64,;4.19,-12.1,;5.53,-14.41,;5.52,-12.86,;7.72,-18.02,;8.63,-19.28,;10.17,-19.28,;7.71,-20.53,;6.24,-20.05,;4.91,-20.82,;3.57,-20.05,;2.24,-20.82,;3.57,-18.51,;4.9,-17.74,;6.24,-18.5,;7.84,-13.99,;6.51,-13.22,;9.18,-13.22,;10.51,-14,;10.89,-15.5,;12.38,-15.1,;13.71,-15.88,;11.99,-13.62,)| Show InChI InChI=1S/C27H30Cl2FN3O3/c1-26(2,3)12-20-27(17-8-7-13(28)9-19(17)32-25(27)36)21(16-5-4-6-18(29)22(16)30)23(33-20)24(35)31-14-10-15(34)11-14/h4-9,14-15,20-21,23,33-34H,10-12H2,1-3H3,(H,31,35)(H,32,36)/t14-,15+,20-,21-,23+,27+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Michigan
Curated by ChEMBL
| Assay Description
Binding affinity to recombinant human His-tagged MDM2 (1 to 118 amino acids) using p53-based PMDM6-F as probe after 15 mins by competition assay |
J Med Chem 56: 5553-61 (2014)
Article DOI: 10.1021/jm4005708
BindingDB Entry DOI: 10.7270/Q2SJ1N2D |
More data for this
Ligand-Target Pair | |
E3 ubiquitin-protein ligase Mdm2
(Homo sapiens (Human)) | BDBM50436684

(CHEMBL2398477)Show SMILES CC(C)(C)C[C@@H]1N[C@H]([C@H](c2cccc(Cl)c2F)[C@]11C(=O)Nc2cc(Cl)ccc12)C(=O)NCC(C)(C)O |r| Show InChI InChI=1S/C27H32Cl2FN3O3/c1-25(2,3)12-19-27(16-10-9-14(28)11-18(16)32-24(27)35)20(15-7-6-8-17(29)21(15)30)22(33-19)23(34)31-13-26(4,5)36/h6-11,19-20,22,33,36H,12-13H2,1-5H3,(H,31,34)(H,32,35)/t19-,20-,22+,27+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Michigan
Curated by ChEMBL
| Assay Description
Binding affinity to recombinant human His-tagged MDM2 (1 to 118 amino acids) using p53-based PMDM6-F as probe after 15 mins by competition assay |
J Med Chem 56: 5553-61 (2014)
Article DOI: 10.1021/jm4005708
BindingDB Entry DOI: 10.7270/Q2SJ1N2D |
More data for this
Ligand-Target Pair | |
E3 ubiquitin-protein ligase Mdm2
(Homo sapiens (Human)) | BDBM50300121
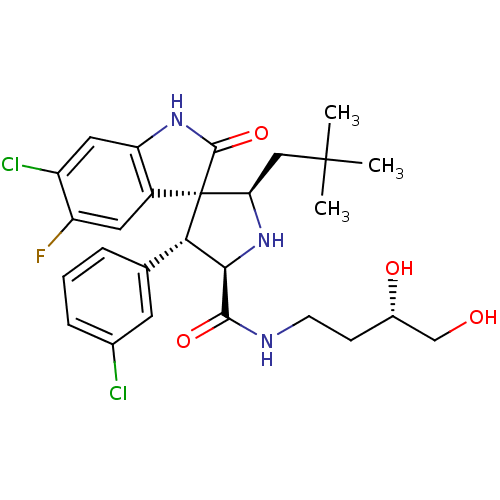
((2'R,3S,4'R,5'R)-6-chloro-4'-(3-chlorophenyl)-N-((...)Show SMILES CC(C)(C)C[C@H]1N[C@H]([C@H](c2cccc(Cl)c2)[C@@]11C(=O)Nc2cc(Cl)c(F)cc12)C(=O)NCC[C@H](O)CO |r| Show InChI InChI=1S/C27H32Cl2FN3O4/c1-26(2,3)12-21-27(17-10-19(30)18(29)11-20(17)32-25(27)37)22(14-5-4-6-15(28)9-14)23(33-21)24(36)31-8-7-16(35)13-34/h4-6,9-11,16,21-23,33-35H,7-8,12-13H2,1-3H3,(H,31,36)(H,32,37)/t16-,21+,22-,23+,27-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Michigan
Curated by ChEMBL
| Assay Description
Binding affinity to recombinant human His-tagged MDM2 (1 to 118 amino acids) using p53-based PMDM6-F as probe after 15 mins by competition assay |
J Med Chem 56: 5553-61 (2014)
Article DOI: 10.1021/jm4005708
BindingDB Entry DOI: 10.7270/Q2SJ1N2D |
More data for this
Ligand-Target Pair | |
Ribonuclease pancreatic
(Homo sapiens (Human)) | BDBM50402338
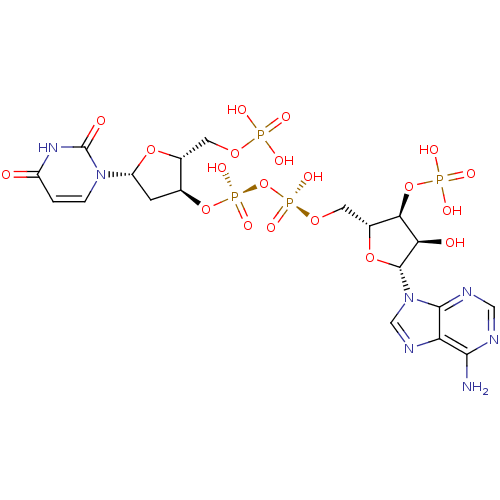
(CHEMBL401150)Show SMILES Nc1ncnc2n(cnc12)[C@@H]1O[C@H](CO[P@@](O)(=O)O[P@@](O)(=O)O[C@H]2C[C@@H](O[C@@H]2COP(O)(O)=O)n2ccc(=O)[nH]c2=O)[C@@H](OP(O)(O)=O)[C@H]1O |r| Show InChI InChI=1S/C19H27N7O20P4/c20-16-13-17(22-6-21-16)26(7-23-13)18-14(28)15(45-48(33,34)35)10(43-18)5-41-49(36,37)46-50(38,39)44-8-3-12(25-2-1-11(27)24-19(25)29)42-9(8)4-40-47(30,31)32/h1-2,6-10,12,14-15,18,28H,3-5H2,(H,36,37)(H,38,39)(H2,20,21,22)(H,24,27,29)(H2,30,31,32)(H2,33,34,35)/t8-,9+,10+,12+,14+,15+,18+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 27 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Thessaly
Curated by ChEMBL
| Assay Description
Inhibition of RNase A |
Bioorg Med Chem 20: 7184-93 (2012)
Article DOI: 10.1016/j.bmc.2012.09.067
BindingDB Entry DOI: 10.7270/Q2M61MDG |
More data for this
Ligand-Target Pair | |
Non-secretory ribonuclease
(Homo sapiens (Human)) | BDBM50402338

(CHEMBL401150)Show SMILES Nc1ncnc2n(cnc12)[C@@H]1O[C@H](CO[P@@](O)(=O)O[P@@](O)(=O)O[C@H]2C[C@@H](O[C@@H]2COP(O)(O)=O)n2ccc(=O)[nH]c2=O)[C@@H](OP(O)(O)=O)[C@H]1O |r| Show InChI InChI=1S/C19H27N7O20P4/c20-16-13-17(22-6-21-16)26(7-23-13)18-14(28)15(45-48(33,34)35)10(43-18)5-41-49(36,37)46-50(38,39)44-8-3-12(25-2-1-11(27)24-19(25)29)42-9(8)4-40-47(30,31)32/h1-2,6-10,12,14-15,18,28H,3-5H2,(H,36,37)(H,38,39)(H2,20,21,22)(H,24,27,29)(H2,30,31,32)(H2,33,34,35)/t8-,9+,10+,12+,14+,15+,18+/m0/s1 | PDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Thessaly
Curated by ChEMBL
| Assay Description
Inhibition of EDN |
Bioorg Med Chem 20: 7184-93 (2012)
Article DOI: 10.1016/j.bmc.2012.09.067
BindingDB Entry DOI: 10.7270/Q2M61MDG |
More data for this
Ligand-Target Pair | |
Ribonuclease pancreatic
(Bison bison (American bison)) | BDBM50402337
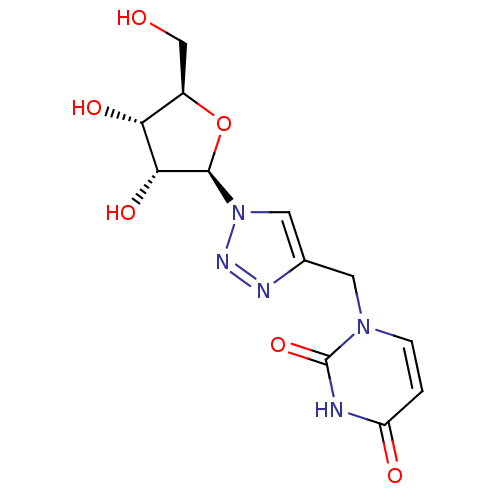
(CHEMBL2206660)Show SMILES OC[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1cc(Cn2ccc(=O)[nH]c2=O)nn1 |r| Show InChI InChI=1S/C12H15N5O6/c18-5-7-9(20)10(21)11(23-7)17-4-6(14-15-17)3-16-2-1-8(19)13-12(16)22/h1-2,4,7,9-11,18,20-21H,3,5H2,(H,13,19,22)/t7-,9-,10-,11-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 240 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Thessaly
Curated by ChEMBL
| Assay Description
Inhibition of bovine pancreatic RNase A |
Bioorg Med Chem 20: 7184-93 (2012)
Article DOI: 10.1016/j.bmc.2012.09.067
BindingDB Entry DOI: 10.7270/Q2M61MDG |
More data for this
Ligand-Target Pair | |
Glycogen phosphorylase, muscle form
(Oryctolagus cuniculus (rabbit)) | BDBM50386288
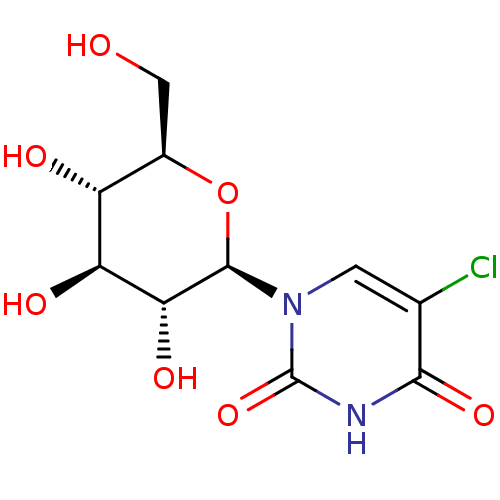
(CHEMBL2041081)Show SMILES OC[C@H]1O[C@H]([C@H](O)[C@@H](O)[C@@H]1O)n1cc(Cl)c(=O)[nH]c1=O |r| Show InChI InChI=1S/C10H13ClN2O7/c11-3-1-13(10(19)12-8(3)18)9-7(17)6(16)5(15)4(2-14)20-9/h1,4-7,9,14-17H,2H2,(H,12,18,19)/t4-,5-,6+,7-,9-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 1.02E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Thessaly
Curated by ChEMBL
| Assay Description
Competitive inhibition of rabbit skeletal muscle glycogen phosphorylase b using Glc-1-P as substrate |
Eur J Med Chem 54: 740-9 (2012)
Article DOI: 10.1016/j.ejmech.2012.06.029
BindingDB Entry DOI: 10.7270/Q2VX0HKH |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Glycogen phosphorylase, muscle form
(Homo sapiens (Human)) | BDBM50386288

(CHEMBL2041081)Show SMILES OC[C@H]1O[C@H]([C@H](O)[C@@H](O)[C@@H]1O)n1cc(Cl)c(=O)[nH]c1=O |r| Show InChI InChI=1S/C10H13ClN2O7/c11-3-1-13(10(19)12-8(3)18)9-7(17)6(16)5(15)4(2-14)20-9/h1,4-7,9,14-17H,2H2,(H,12,18,19)/t4-,5-,6+,7-,9-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 1.02E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Thessaly
Curated by ChEMBL
| Assay Description
Inhibition of glycogen phosphorylase b |
Eur J Med Chem 54: 740-9 (2012)
Article DOI: 10.1016/j.ejmech.2012.06.029
BindingDB Entry DOI: 10.7270/Q2VX0HKH |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Glycogen phosphorylase, muscle form
(Homo sapiens (Human)) | BDBM50386290
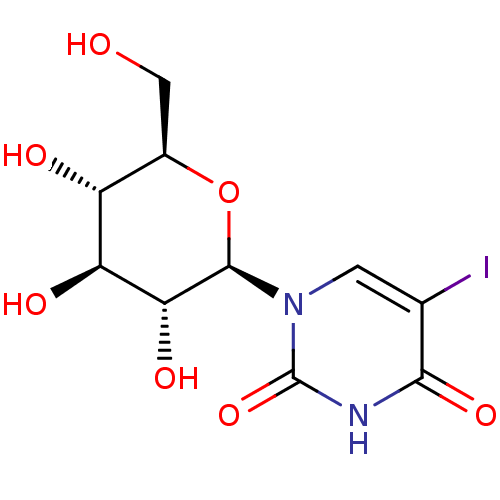
(CHEMBL2040853)Show SMILES OC[C@H]1O[C@H]([C@H](O)[C@@H](O)[C@@H]1O)n1cc(I)c(=O)[nH]c1=O |r| Show InChI InChI=1S/C10H13IN2O7/c11-3-1-13(10(19)12-8(3)18)9-7(17)6(16)5(15)4(2-14)20-9/h1,4-7,9,14-17H,2H2,(H,12,18,19)/t4-,5-,6+,7-,9-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 1.94E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Thessaly
Curated by ChEMBL
| Assay Description
Inhibition of glycogen phosphorylase b |
Eur J Med Chem 54: 740-9 (2012)
Article DOI: 10.1016/j.ejmech.2012.06.029
BindingDB Entry DOI: 10.7270/Q2VX0HKH |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Glycogen phosphorylase, muscle form
(Homo sapiens (Human)) | BDBM50386289
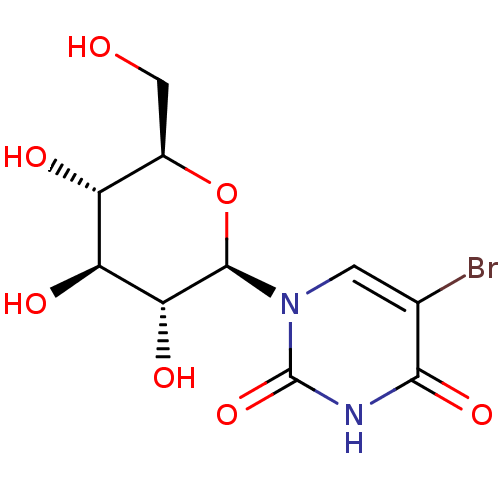
(CHEMBL2041082)Show SMILES OC[C@H]1O[C@H]([C@H](O)[C@@H](O)[C@@H]1O)n1cc(Br)c(=O)[nH]c1=O |r| Show InChI InChI=1S/C10H13BrN2O7/c11-3-1-13(10(19)12-8(3)18)9-7(17)6(16)5(15)4(2-14)20-9/h1,4-7,9,14-17H,2H2,(H,12,18,19)/t4-,5-,6+,7-,9-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 3.27E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Thessaly
Curated by ChEMBL
| Assay Description
Inhibition of glycogen phosphorylase b |
Eur J Med Chem 54: 740-9 (2012)
Article DOI: 10.1016/j.ejmech.2012.06.029
BindingDB Entry DOI: 10.7270/Q2VX0HKH |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Glycogen phosphorylase, muscle form
(Oryctolagus cuniculus (rabbit)) | BDBM50386283

(CHEMBL2041078)Show SMILES OC[C@H]1O[C@H]([C@H](O)[C@@H](O)[C@@H]1O)n1cc(C#C)c(=O)[nH]c1=O |r| Show InChI InChI=1S/C12H14N2O7/c1-2-5-3-14(12(20)13-10(5)19)11-9(18)8(17)7(16)6(4-15)21-11/h1,3,6-9,11,15-18H,4H2,(H,13,19,20)/t6-,7-,8+,9-,11-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 4.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Thessaly
Curated by ChEMBL
| Assay Description
Competitive inhibition of rabbit skeletal muscle glycogen phosphorylase b using Glc-1-P as substrate |
Eur J Med Chem 54: 740-9 (2012)
Article DOI: 10.1016/j.ejmech.2012.06.029
BindingDB Entry DOI: 10.7270/Q2VX0HKH |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Glycogen phosphorylase, muscle form
(Oryctolagus cuniculus (rabbit)) | BDBM50386286

(CHEMBL2041079)Show SMILES CCCc1cc2cn([C@@H]3O[C@H](CO)[C@@H](O)[C@H](O)[C@H]3O)c(=O)nc2o1 |r| Show InChI InChI=1S/C15H20N2O7/c1-2-3-8-4-7-5-17(15(22)16-13(7)23-8)14-12(21)11(20)10(19)9(6-18)24-14/h4-5,9-12,14,18-21H,2-3,6H2,1H3/t9-,10-,11+,12-,14-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 3.24E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Thessaly
Curated by ChEMBL
| Assay Description
Competitive inhibition of rabbit skeletal muscle glycogen phosphorylase b using Glc-1-P as substrate |
Eur J Med Chem 54: 740-9 (2012)
Article DOI: 10.1016/j.ejmech.2012.06.029
BindingDB Entry DOI: 10.7270/Q2VX0HKH |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Glycogen phosphorylase, muscle form
(Oryctolagus cuniculus (rabbit)) | BDBM50386287
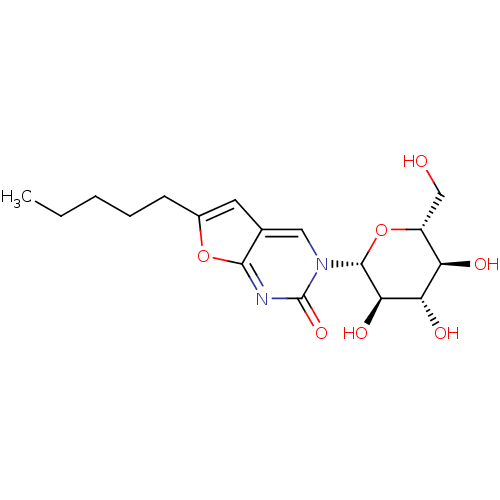
(CHEMBL2041080)Show SMILES CCCCCc1cc2cn([C@@H]3O[C@H](CO)[C@@H](O)[C@H](O)[C@H]3O)c(=O)nc2o1 |r| Show InChI InChI=1S/C17H24N2O7/c1-2-3-4-5-10-6-9-7-19(17(24)18-15(9)25-10)16-14(23)13(22)12(21)11(8-20)26-16/h6-7,11-14,16,20-23H,2-5,8H2,1H3/t11-,12-,13+,14-,16-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 3.34E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Thessaly
Curated by ChEMBL
| Assay Description
Competitive inhibition of rabbit skeletal muscle glycogen phosphorylase b using Glc-1-P as substrate |
Eur J Med Chem 54: 740-9 (2012)
Article DOI: 10.1016/j.ejmech.2012.06.029
BindingDB Entry DOI: 10.7270/Q2VX0HKH |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Glycogen phosphorylase, muscle form
(Oryctolagus cuniculus (rabbit)) | BDBM50386284

(CHEMBL2041076)Show SMILES CCCC#Cc1cn([C@@H]2O[C@H](CO)[C@@H](O)[C@H](O)[C@H]2O)c(=O)[nH]c1=O |r| Show InChI InChI=1S/C15H20N2O7/c1-2-3-4-5-8-6-17(15(23)16-13(8)22)14-12(21)11(20)10(19)9(7-18)24-14/h6,9-12,14,18-21H,2-3,7H2,1H3,(H,16,22,23)/t9-,10-,11+,12-,14-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 3.03E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Thessaly
Curated by ChEMBL
| Assay Description
Competitive inhibition of rabbit skeletal muscle glycogen phosphorylase b using Glc-1-P as substrate |
Eur J Med Chem 54: 740-9 (2012)
Article DOI: 10.1016/j.ejmech.2012.06.029
BindingDB Entry DOI: 10.7270/Q2VX0HKH |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Angiogenin
(Homo sapiens (Human)) | BDBM50402338

(CHEMBL401150)Show SMILES Nc1ncnc2n(cnc12)[C@@H]1O[C@H](CO[P@@](O)(=O)O[P@@](O)(=O)O[C@H]2C[C@@H](O[C@@H]2COP(O)(O)=O)n2ccc(=O)[nH]c2=O)[C@@H](OP(O)(O)=O)[C@H]1O |r| Show InChI InChI=1S/C19H27N7O20P4/c20-16-13-17(22-6-21-16)26(7-23-13)18-14(28)15(45-48(33,34)35)10(43-18)5-41-49(36,37)46-50(38,39)44-8-3-12(25-2-1-11(27)24-19(25)29)42-9(8)4-40-47(30,31)32/h1-2,6-10,12,14-15,18,28H,3-5H2,(H,36,37)(H,38,39)(H2,20,21,22)(H,24,27,29)(H2,30,31,32)(H2,33,34,35)/t8-,9+,10+,12+,14+,15+,18+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 3.60E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Thessaly
Curated by ChEMBL
| Assay Description
Inhibition of ANG |
Bioorg Med Chem 20: 7184-93 (2012)
Article DOI: 10.1016/j.bmc.2012.09.067
BindingDB Entry DOI: 10.7270/Q2M61MDG |
More data for this
Ligand-Target Pair | |
Glycogen phosphorylase, muscle form
(Oryctolagus cuniculus (rabbit)) | BDBM50351158
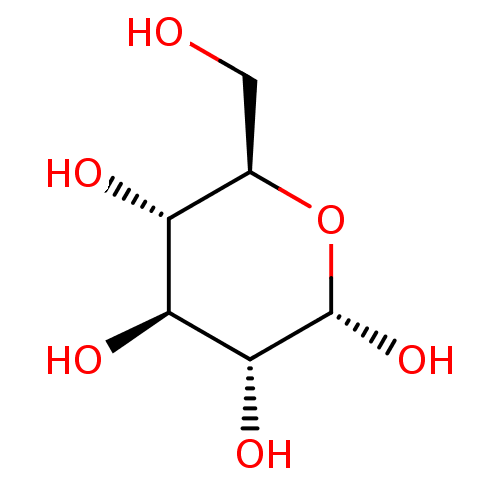
(CHEMBL423707)Show SMILES OC[C@H]1O[C@H](O)[C@H](O)[C@@H](O)[C@@H]1O |r| Show InChI InChI=1S/C6H12O6/c7-1-2-3(8)4(9)5(10)6(11)12-2/h2-11H,1H2/t2-,3-,4+,5-,6+/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.70E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Thessaly
Curated by ChEMBL
| Assay Description
Competitive inhibition of rabbit skeletal muscle glycogen phosphorylase b using Glc-1-P as substrate |
Eur J Med Chem 54: 740-9 (2012)
Article DOI: 10.1016/j.ejmech.2012.06.029
BindingDB Entry DOI: 10.7270/Q2VX0HKH |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha
(Homo sapiens (Human)) | BDBM50136383
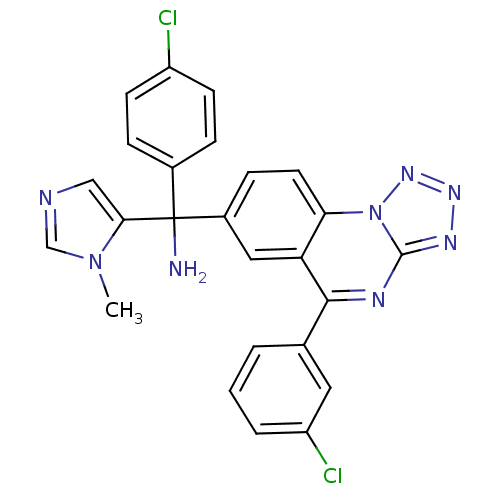
((4-chlorophenyl)(5-(3-chlorophenyl)tetrazolo[1,5-a...)Show SMILES Cn1cncc1C(N)(c1ccc(Cl)cc1)c1ccc2c(c1)c(nc1nnnn21)-c1cccc(Cl)c1 Show InChI InChI=1S/C25H18Cl2N8/c1-34-14-29-13-22(34)25(28,16-5-8-18(26)9-6-16)17-7-10-21-20(12-17)23(15-3-2-4-19(27)11-15)30-24-31-32-33-35(21)24/h2-14H,28H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Medicinal Chemistry Department Johnson & Johnson Pharmaceutical Research & Development (J&JPRD)
Curated by ChEMBL
| Assay Description
Inhibition of Geranylgeranylprotein transferase-I catalyzed incorporation of [3H]-GGPP into biotinYRASNRSCAIL peptide |
Bioorg Med Chem Lett 13: 4365-9 (2003)
BindingDB Entry DOI: 10.7270/Q21R6PX5 |
More data for this
Ligand-Target Pair | |
Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha
(Homo sapiens (Human)) | BDBM50136383

((4-chlorophenyl)(5-(3-chlorophenyl)tetrazolo[1,5-a...)Show SMILES Cn1cncc1C(N)(c1ccc(Cl)cc1)c1ccc2c(c1)c(nc1nnnn21)-c1cccc(Cl)c1 Show InChI InChI=1S/C25H18Cl2N8/c1-34-14-29-13-22(34)25(28,16-5-8-18(26)9-6-16)17-7-10-21-20(12-17)23(15-3-2-4-19(27)11-15)30-24-31-32-33-35(21)24/h2-14H,28H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Medicinal Chemistry Department Johnson & Johnson Pharmaceutical Research & Development (J&JPRD)
Curated by ChEMBL
| Assay Description
Inhibition of [3H]-FPP incorporation into biotinylated laminB peptide by farnesyl transferase |
Bioorg Med Chem Lett 13: 4365-9 (2003)
BindingDB Entry DOI: 10.7270/Q21R6PX5 |
More data for this
Ligand-Target Pair | |
Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha
(Homo sapiens (Human)) | BDBM50126335
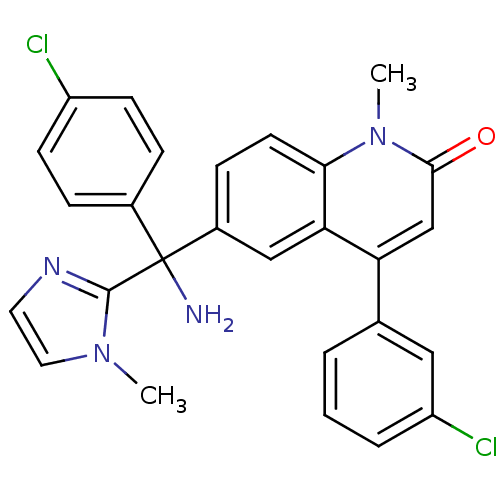
(6-[(R)-Amino-(4-chloro-phenyl)-(3-methyl-3H-imidaz...)Show SMILES Cn1ccnc1C(N)(c1ccc(Cl)cc1)c1ccc2n(C)c(=O)cc(-c3cccc(Cl)c3)c2c1 Show InChI InChI=1S/C27H22Cl2N4O/c1-32-13-12-31-26(32)27(30,18-6-9-20(28)10-7-18)19-8-11-24-23(15-19)22(16-25(34)33(24)2)17-4-3-5-21(29)14-17/h3-16H,30H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
UniChem
Patents
Similars
| MMDB
PubMed
| n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
Medicinal Chemistry Department Johnson & Johnson Pharmaceutical Research & Development (J&JPRD)
Curated by ChEMBL
| Assay Description
Inhibition of [3H]-FPP incorporation into biotinylated laminB peptide by farnesyl transferase |
Bioorg Med Chem Lett 13: 4365-9 (2003)
BindingDB Entry DOI: 10.7270/Q21R6PX5 |
More data for this
Ligand-Target Pair | |
Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha
(Homo sapiens (Human)) | BDBM50126335

(6-[(R)-Amino-(4-chloro-phenyl)-(3-methyl-3H-imidaz...)Show SMILES Cn1ccnc1C(N)(c1ccc(Cl)cc1)c1ccc2n(C)c(=O)cc(-c3cccc(Cl)c3)c2c1 Show InChI InChI=1S/C27H22Cl2N4O/c1-32-13-12-31-26(32)27(30,18-6-9-20(28)10-7-18)19-8-11-24-23(15-19)22(16-25(34)33(24)2)17-4-3-5-21(29)14-17/h3-16H,30H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
UniChem
Patents
Similars
| MMDB
PubMed
| n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
Medicinal Chemistry Department Johnson & Johnson Pharmaceutical Research & Development (J&JPRD)
Curated by ChEMBL
| Assay Description
Inhibition of [3H]-FPP incorporation into biotinylated lamin B peptide by farnesyl transferase |
Bioorg Med Chem Lett 13: 4361-4 (2003)
BindingDB Entry DOI: 10.7270/Q25H7FPB |
More data for this
Ligand-Target Pair | |
Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha
(Homo sapiens (Human)) | BDBM50136377

(4-(3-Chloro-phenyl)-6-[(4-chloro-phenyl)-(4-methyl...)Show SMILES Cn1cnnc1C(c1ccc(Cl)cc1)c1ccc2n(C)c(=O)cc(-c3cccc(Cl)c3)c2c1 Show InChI InChI=1S/C26H20Cl2N4O/c1-31-15-29-30-26(31)25(16-6-9-19(27)10-7-16)18-8-11-23-22(13-18)21(14-24(33)32(23)2)17-4-3-5-20(28)12-17/h3-15,25H,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Medicinal Chemistry Department Johnson & Johnson Pharmaceutical Research & Development (J&JPRD)
Curated by ChEMBL
| Assay Description
Inhibition of [3H]-FPP incorporation into biotinylated lamin B peptide by farnesyl transferase |
Bioorg Med Chem Lett 13: 4361-4 (2003)
BindingDB Entry DOI: 10.7270/Q25H7FPB |
More data for this
Ligand-Target Pair | |
Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha
(Homo sapiens (Human)) | BDBM50136375

(6-[Amino-(4-chloro-phenyl)-(4-methyl-4H-[1,2,4]tri...)Show SMILES Cn1cnnc1C(N)(c1ccc(Cl)cc1)c1ccc2n(C)c(=O)cc(-c3cccc(Cl)c3)c2c1 Show InChI InChI=1S/C26H21Cl2N5O/c1-32-15-30-31-25(32)26(29,17-6-9-19(27)10-7-17)18-8-11-23-22(13-18)21(14-24(34)33(23)2)16-4-3-5-20(28)12-16/h3-15H,29H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Medicinal Chemistry Department Johnson & Johnson Pharmaceutical Research & Development (J&JPRD)
Curated by ChEMBL
| Assay Description
Inhibition of [3H]-FPP incorporation into biotinylated lamin B peptide by farnesyl transferase |
Bioorg Med Chem Lett 13: 4361-4 (2003)
BindingDB Entry DOI: 10.7270/Q25H7FPB |
More data for this
Ligand-Target Pair | |
Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha
(Homo sapiens (Human)) | BDBM50136385
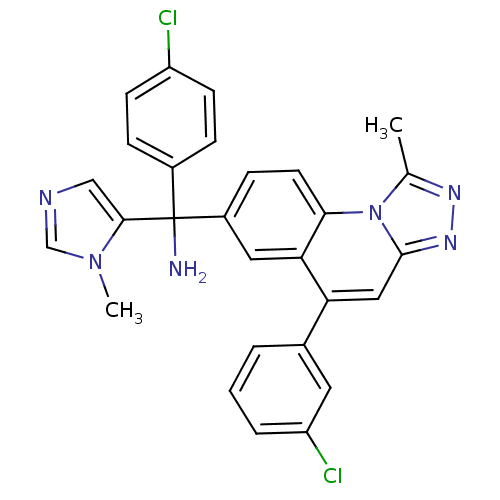
(C-(4-Chloro-phenyl)-C-[5-(3-chloro-phenyl)-1-methy...)Show SMILES Cc1nnc2cc(-c3cccc(Cl)c3)c3cc(ccc3n12)C(N)(c1cncn1C)c1ccc(Cl)cc1 Show InChI InChI=1S/C28H22Cl2N6/c1-17-33-34-27-14-23(18-4-3-5-22(30)12-18)24-13-20(8-11-25(24)36(17)27)28(31,26-15-32-16-35(26)2)19-6-9-21(29)10-7-19/h3-16H,31H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Medicinal Chemistry Department Johnson & Johnson Pharmaceutical Research & Development (J&JPRD)
Curated by ChEMBL
| Assay Description
Inhibition of [3H]-FPP incorporation into biotinylated laminB peptide by farnesyl transferase |
Bioorg Med Chem Lett 13: 4365-9 (2003)
BindingDB Entry DOI: 10.7270/Q21R6PX5 |
More data for this
Ligand-Target Pair | |
Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha
(Homo sapiens (Human)) | BDBM50136384

((4-chlorophenyl)(5-(3-chlorophenyl)tetrazolo[1,5-a...)Show SMILES Cn1cncc1C(N)(c1ccc(Cl)cc1)c1ccc2c(c1)c(cc1nnnn21)-c1cccc(Cl)c1 Show InChI InChI=1S/C26H19Cl2N7/c1-34-15-30-14-24(34)26(29,17-5-8-19(27)9-6-17)18-7-10-23-22(12-18)21(13-25-31-32-33-35(23)25)16-3-2-4-20(28)11-16/h2-15H,29H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| PubMed
| n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Medicinal Chemistry Department Johnson & Johnson Pharmaceutical Research & Development (J&JPRD)
Curated by ChEMBL
| Assay Description
Inhibition of [3H]-FPP incorporation into biotinylated laminB peptide by farnesyl transferase |
Bioorg Med Chem Lett 13: 4365-9 (2003)
BindingDB Entry DOI: 10.7270/Q21R6PX5 |
More data for this
Ligand-Target Pair | |
Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha
(Homo sapiens (Human)) | BDBM50136379
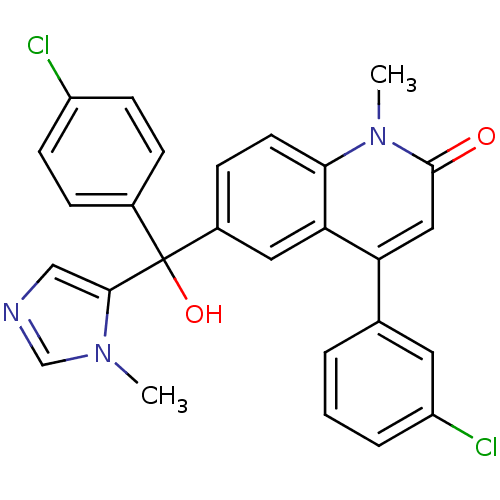
(4-(3-Chloro-phenyl)-6-[(4-chloro-phenyl)-hydroxy-(...)Show SMILES Cn1cncc1C(O)(c1ccc(Cl)cc1)c1ccc2n(C)c(=O)cc(-c3cccc(Cl)c3)c2c1 Show InChI InChI=1S/C27H21Cl2N3O2/c1-31-16-30-15-25(31)27(34,18-6-9-20(28)10-7-18)19-8-11-24-23(13-19)22(14-26(33)32(24)2)17-4-3-5-21(29)12-17/h3-16,34H,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Medicinal Chemistry Department Johnson & Johnson Pharmaceutical Research & Development (J&JPRD)
Curated by ChEMBL
| Assay Description
Inhibition of [3H]-FPP incorporation into biotinylated lamin B peptide by farnesyl transferase |
Bioorg Med Chem Lett 13: 4361-4 (2003)
BindingDB Entry DOI: 10.7270/Q25H7FPB |
More data for this
Ligand-Target Pair | |
Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha
(Homo sapiens (Human)) | BDBM50136384

((4-chlorophenyl)(5-(3-chlorophenyl)tetrazolo[1,5-a...)Show SMILES Cn1cncc1C(N)(c1ccc(Cl)cc1)c1ccc2c(c1)c(cc1nnnn21)-c1cccc(Cl)c1 Show InChI InChI=1S/C26H19Cl2N7/c1-34-15-30-14-24(34)26(29,17-5-8-19(27)9-6-17)18-7-10-23-22(12-18)21(13-25-31-32-33-35(23)25)16-3-2-4-20(28)11-16/h2-15H,29H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| PubMed
| n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Medicinal Chemistry Department Johnson & Johnson Pharmaceutical Research & Development (J&JPRD)
Curated by ChEMBL
| Assay Description
Inhibition of Geranylgeranylprotein transferase-I catalyzed incorporation of [3H]-GGPP into biotinYRASNRSCAIL peptide |
Bioorg Med Chem Lett 13: 4365-9 (2003)
BindingDB Entry DOI: 10.7270/Q21R6PX5 |
More data for this
Ligand-Target Pair | |
Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha
(Homo sapiens (Human)) | BDBM50136376
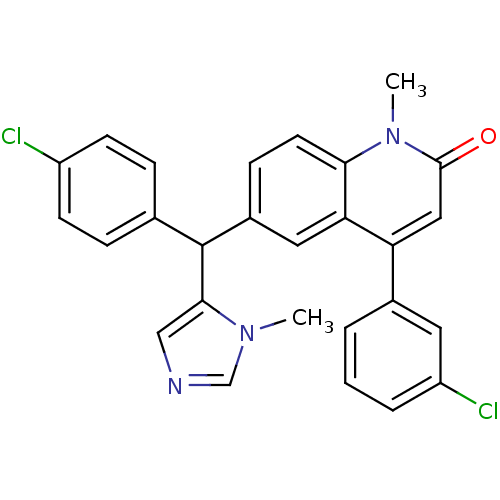
(4-(3-Chloro-phenyl)-6-[(4-chloro-phenyl)-(3-methyl...)Show SMILES Cn1cncc1C(c1ccc(Cl)cc1)c1ccc2n(C)c(=O)cc(-c3cccc(Cl)c3)c2c1 Show InChI InChI=1S/C27H21Cl2N3O/c1-31-16-30-15-25(31)27(17-6-9-20(28)10-7-17)19-8-11-24-23(13-19)22(14-26(33)32(24)2)18-4-3-5-21(29)12-18/h3-16,27H,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Medicinal Chemistry Department Johnson & Johnson Pharmaceutical Research & Development (J&JPRD)
Curated by ChEMBL
| Assay Description
Inhibition of [3H]-FPP incorporation into biotinylated lamin B peptide by farnesyl transferase |
Bioorg Med Chem Lett 13: 4361-4 (2003)
BindingDB Entry DOI: 10.7270/Q25H7FPB |
More data for this
Ligand-Target Pair | |
Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha
(Homo sapiens (Human)) | BDBM50136389

(C-(4-Chloro-phenyl)-C-[5-(3-chloro-phenyl)-[1,2,4]...)Show SMILES Cn1cncc1C(N)(c1ccc(Cl)cc1)c1ccc2c(c1)c(nc1nncn21)-c1cccc(Cl)c1 Show InChI InChI=1S/C26H19Cl2N7/c1-34-14-30-13-23(34)26(29,17-5-8-19(27)9-6-17)18-7-10-22-21(12-18)24(16-3-2-4-20(28)11-16)32-25-33-31-15-35(22)25/h2-15H,29H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Medicinal Chemistry Department Johnson & Johnson Pharmaceutical Research & Development (J&JPRD)
Curated by ChEMBL
| Assay Description
Inhibition of [3H]-FPP incorporation into biotinylated laminB peptide by farnesyl transferase |
Bioorg Med Chem Lett 13: 4365-9 (2003)
BindingDB Entry DOI: 10.7270/Q21R6PX5 |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor
(Homo sapiens (Human)) | BDBM82258

(CAS_114798-26-4 | Losartan | NSC_3961)Show SMILES CCCCc1nc(Cl)c(CO)n1Cc1ccc(cc1)-c1ccccc1-c1nnn[nH]1 Show InChI InChI=1S/C22H23ClN6O/c1-2-3-8-20-24-21(23)19(14-30)29(20)13-15-9-11-16(12-10-15)17-6-4-5-7-18(17)22-25-27-28-26-22/h4-7,9-12,30H,2-3,8,13-14H2,1H3,(H,25,26,27,28) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a |
National Hellenic Research Foundation
Curated by ChEMBL
| Assay Description
Displacement of [125I-Sar1-Ile8]Ang2 from human AT1 receptor expressed in HEK293 cell membrane incubated for 1 hr by gamma counting method |
Bioorg Med Chem 24: 4444-4451 (2016)
Article DOI: 10.1016/j.bmc.2016.07.047
BindingDB Entry DOI: 10.7270/Q20P11ZV |
More data for this
Ligand-Target Pair | |
Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha
(Homo sapiens (Human)) | BDBM50136384

((4-chlorophenyl)(5-(3-chlorophenyl)tetrazolo[1,5-a...)Show SMILES Cn1cncc1C(N)(c1ccc(Cl)cc1)c1ccc2c(c1)c(cc1nnnn21)-c1cccc(Cl)c1 Show InChI InChI=1S/C26H19Cl2N7/c1-34-15-30-14-24(34)26(29,17-5-8-19(27)9-6-17)18-7-10-23-22(12-18)21(13-25-31-32-33-35(23)25)16-3-2-4-20(28)11-16/h2-15H,29H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| PubMed
| n/a | n/a | 3.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Medicinal Chemistry Department Johnson & Johnson Pharmaceutical Research & Development (J&JPRD)
Curated by ChEMBL
| Assay Description
Inhibition of Geranylgeranylprotein transferase-I catalyzed incorporation of [3H]-GGPP into biotinYRASNRSCAIL peptide |
Bioorg Med Chem Lett 13: 4365-9 (2003)
BindingDB Entry DOI: 10.7270/Q21R6PX5 |
More data for this
Ligand-Target Pair | |
Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha
(Homo sapiens (Human)) | BDBM50136380

(4-(3-Chloro-phenyl)-6-[1-(4-chloro-phenyl)-1-(3-me...)Show SMILES Cn1cncc1C(C)(c1ccc(Cl)cc1)c1ccc2n(C)c(=O)cc(-c3cccc(Cl)c3)c2c1 Show InChI InChI=1S/C28H23Cl2N3O/c1-28(26-16-31-17-32(26)2,19-7-10-21(29)11-8-19)20-9-12-25-24(14-20)23(15-27(34)33(25)3)18-5-4-6-22(30)13-18/h4-17H,1-3H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Medicinal Chemistry Department Johnson & Johnson Pharmaceutical Research & Development (J&JPRD)
Curated by ChEMBL
| Assay Description
Inhibition of [3H]-FPP incorporation into biotinylated lamin B peptide by farnesyl transferase |
Bioorg Med Chem Lett 13: 4361-4 (2003)
BindingDB Entry DOI: 10.7270/Q25H7FPB |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha
(Homo sapiens (Human)) | BDBM50136378
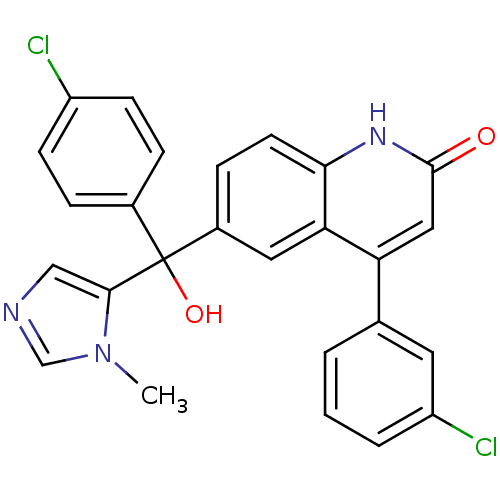
(4-(3-Chloro-phenyl)-6-[(4-chloro-phenyl)-hydroxy-(...)Show SMILES Cn1cncc1C(O)(c1ccc(Cl)cc1)c1ccc2[nH]c(=O)cc(-c3cccc(Cl)c3)c2c1 Show InChI InChI=1S/C26H19Cl2N3O2/c1-31-15-29-14-24(31)26(33,17-5-8-19(27)9-6-17)18-7-10-23-22(12-18)21(13-25(32)30-23)16-3-2-4-20(28)11-16/h2-15,33H,1H3,(H,30,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Medicinal Chemistry Department Johnson & Johnson Pharmaceutical Research & Development (J&JPRD)
Curated by ChEMBL
| Assay Description
Inhibition of [3H]-FPP incorporation into biotinylated lamin B peptide by farnesyl transferase |
Bioorg Med Chem Lett 13: 4361-4 (2003)
BindingDB Entry DOI: 10.7270/Q25H7FPB |
More data for this
Ligand-Target Pair | |
Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha
(Homo sapiens (Human)) | BDBM50136386

(C-(4-Chloro-phenyl)-C-[5-(3-chloro-phenyl)-imidazo...)Show SMILES Cn1cncc1C(N)(c1ccc(Cl)cc1)c1ccc2c(c1)c(nc1nccn21)-c1cccc(Cl)c1 Show InChI InChI=1S/C27H20Cl2N6/c1-34-16-31-15-24(34)27(30,18-5-8-20(28)9-6-18)19-7-10-23-22(14-19)25(17-3-2-4-21(29)13-17)33-26-32-11-12-35(23)26/h2-16H,30H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Medicinal Chemistry Department Johnson & Johnson Pharmaceutical Research & Development (J&JPRD)
Curated by ChEMBL
| Assay Description
Inhibition of [3H]-FPP incorporation into biotinylated laminB peptide by farnesyl transferase |
Bioorg Med Chem Lett 13: 4365-9 (2003)
BindingDB Entry DOI: 10.7270/Q21R6PX5 |
More data for this
Ligand-Target Pair | |
Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha
(Homo sapiens (Human)) | BDBM50136382

(4-(3-Chloro-phenyl)-6-[1-(4-chloro-phenyl)-1-(4-me...)Show SMILES Cn1cnnc1C(C)(c1ccc(Cl)cc1)c1ccc2n(C)c(=O)cc(-c3cccc(Cl)c3)c2c1 Show InChI InChI=1S/C27H22Cl2N4O/c1-27(26-31-30-16-32(26)2,18-7-10-20(28)11-8-18)19-9-12-24-23(14-19)22(15-25(34)33(24)3)17-5-4-6-21(29)13-17/h4-16H,1-3H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Medicinal Chemistry Department Johnson & Johnson Pharmaceutical Research & Development (J&JPRD)
Curated by ChEMBL
| Assay Description
Inhibition of [3H]-FPP incorporation into biotinylated lamin B peptide by farnesyl transferase |
Bioorg Med Chem Lett 13: 4361-4 (2003)
BindingDB Entry DOI: 10.7270/Q25H7FPB |
More data for this
Ligand-Target Pair | |
Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha
(Homo sapiens (Human)) | BDBM50136381

(4-(3-Chloro-phenyl)-6-[(4-chloro-phenyl)-hydroxy-(...)Show SMILES Cn1cnnc1C(O)(c1ccc(Cl)cc1)c1ccc2n(C)c(=O)cc(-c3cccc(Cl)c3)c2c1 Show InChI InChI=1S/C26H20Cl2N4O2/c1-31-15-29-30-25(31)26(34,17-6-9-19(27)10-7-17)18-8-11-23-22(13-18)21(14-24(33)32(23)2)16-4-3-5-20(28)12-16/h3-15,34H,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Medicinal Chemistry Department Johnson & Johnson Pharmaceutical Research & Development (J&JPRD)
Curated by ChEMBL
| Assay Description
Inhibition of [3H]-FPP incorporation into biotinylated lamin B peptide by farnesyl transferase |
Bioorg Med Chem Lett 13: 4361-4 (2003)
BindingDB Entry DOI: 10.7270/Q25H7FPB |
More data for this
Ligand-Target Pair | |
E3 ubiquitin-protein ligase Mdm2
(Homo sapiens (Human)) | BDBM50436682

(CHEMBL2396674)Show SMILES CC(C)(C)C[C@@H]1N[C@H]([C@H](c2cccc(Cl)c2F)[C@]11C(=O)Nc2cc(Cl)ccc12)C(=O)N[C@H]1C[C@@](C)(O)C1 |r,wU:33.38,17.19,8.8,31.34,5.4,wD:33.37,7.31,(42.41,-31.09,;41.21,-30.13,;41.45,-28.61,;42.69,-29.73,;39.78,-30.69,;38.58,-29.72,;38.65,-28.19,;37.21,-27.64,;36.25,-28.84,;34.91,-28.07,;33.58,-28.84,;32.23,-28.08,;32.23,-26.52,;33.57,-25.75,;33.56,-24.21,;34.91,-26.52,;34.9,-24.97,;37.09,-30.13,;38,-31.39,;39.54,-31.39,;37.09,-32.64,;35.61,-32.16,;34.28,-32.93,;32.95,-32.16,;31.61,-32.93,;32.95,-30.62,;34.28,-29.85,;35.61,-30.61,;37.21,-26.1,;35.88,-25.33,;38.55,-25.33,;40.09,-25.32,;41.18,-24.24,;42.26,-25.32,;43.59,-24.55,;43.6,-26.09,;41.19,-26.42,)| Show InChI InChI=1S/C28H32Cl2FN3O3/c1-26(2,3)13-20-28(17-9-8-14(29)10-19(17)33-25(28)36)21(16-6-5-7-18(30)22(16)31)23(34-20)24(35)32-15-11-27(4,37)12-15/h5-10,15,20-21,23,34,37H,11-13H2,1-4H3,(H,32,35)(H,33,36)/t15-,20-,21-,23+,27+,28+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.80 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Michigan
Curated by ChEMBL
| Assay Description
Binding affinity to recombinant human His-tagged MDM2 (1 to 118 amino acids) using p53-based PMDM6-F as probe after 15 to 30 mins by fluorescence pol... |
J Med Chem 56: 5553-61 (2014)
Article DOI: 10.1021/jm4005708
BindingDB Entry DOI: 10.7270/Q2SJ1N2D |
More data for this
Ligand-Target Pair | |
Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha
(Homo sapiens (Human)) | BDBM50136387

(C-(4-Chloro-phenyl)-C-[5-(3-chloro-phenyl)-imidazo...)Show SMILES Cn1cncc1C(N)(c1ccc(Cl)cc1)c1ccc2c(c1)c(cc1nccn21)-c1cccc(Cl)c1 Show InChI InChI=1S/C28H21Cl2N5/c1-34-17-32-16-26(34)28(31,19-5-8-21(29)9-6-19)20-7-10-25-24(14-20)23(15-27-33-11-12-35(25)27)18-3-2-4-22(30)13-18/h2-17H,31H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Medicinal Chemistry Department Johnson & Johnson Pharmaceutical Research & Development (J&JPRD)
Curated by ChEMBL
| Assay Description
Inhibition of [3H]-FPP incorporation into biotinylated laminB peptide by farnesyl transferase |
Bioorg Med Chem Lett 13: 4365-9 (2003)
BindingDB Entry DOI: 10.7270/Q21R6PX5 |
More data for this
Ligand-Target Pair | |
E3 ubiquitin-protein ligase Mdm2
(Homo sapiens (Human)) | BDBM50436688

(CHEMBL2398473)Show SMILES CC(C)(C)C[C@@H]1N[C@H]([C@H](c2cccc(Cl)c2F)[C@]11C(=O)Nc2cc(Cl)ccc12)C(=O)N[C@H]1C[C@H](O)C1 |r,wU:17.19,8.8,31.34,5.4,wD:7.31,33.37,(42.17,-8.02,;40.97,-7.05,;41.2,-5.53,;42.45,-6.65,;39.53,-7.61,;38.33,-6.65,;38.41,-5.11,;36.97,-4.56,;36.01,-5.76,;34.66,-4.99,;33.33,-5.77,;31.99,-5.01,;31.98,-3.45,;33.32,-2.67,;33.32,-1.13,;34.66,-3.44,;34.66,-1.9,;36.85,-7.05,;37.76,-8.31,;39.3,-8.31,;36.85,-9.57,;35.37,-9.09,;34.04,-9.86,;32.71,-9.09,;31.37,-9.86,;32.71,-7.54,;34.04,-6.77,;35.37,-7.54,;36.97,-3.02,;35.64,-2.25,;38.31,-2.26,;39.64,-3.03,;41.12,-2.65,;41.52,-4.14,;42.85,-4.91,;40.03,-4.53,)| Show InChI InChI=1S/C27H30Cl2FN3O3/c1-26(2,3)12-20-27(17-8-7-13(28)9-19(17)32-25(27)36)21(16-5-4-6-18(29)22(16)30)23(33-20)24(35)31-14-10-15(34)11-14/h4-9,14-15,20-21,23,33-34H,10-12H2,1-3H3,(H,31,35)(H,32,36)/t14-,15-,20-,21-,23+,27+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7.10 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Michigan
Curated by ChEMBL
| Assay Description
Binding affinity to recombinant human His-tagged MDM2 (1 to 118 amino acids) using p53-based PMDM6-F as probe after 15 to 30 mins by fluorescence pol... |
J Med Chem 56: 5553-61 (2014)
Article DOI: 10.1021/jm4005708
BindingDB Entry DOI: 10.7270/Q2SJ1N2D |
More data for this
Ligand-Target Pair | |
E3 ubiquitin-protein ligase Mdm2
(Homo sapiens (Human)) | BDBM50436681

(CHEMBL2398479)Show SMILES CC(C)(C)C[C@@H]1N[C@H]([C@H](c2cccc(Cl)c2F)[C@]11C(=O)Nc2cc(Cl)ccc12)C(=O)N[C@H]1C[C@@H](C1)NS(C)(=O)=O |r,wU:17.19,8.8,31.34,5.4,wD:7.31,33.39,(12.69,-41.86,;11.49,-40.9,;11.72,-39.38,;12.96,-40.5,;10.05,-41.46,;8.85,-40.49,;8.93,-38.96,;7.48,-38.41,;6.52,-39.61,;5.18,-38.84,;3.85,-39.62,;2.51,-38.85,;2.5,-37.3,;3.84,-36.52,;3.84,-34.98,;5.18,-37.29,;5.17,-35.74,;7.37,-40.9,;8.28,-42.16,;9.82,-42.16,;7.36,-43.41,;5.89,-42.93,;4.56,-43.71,;3.22,-42.93,;1.89,-43.7,;3.22,-41.39,;4.55,-40.62,;5.89,-41.38,;7.49,-36.87,;6.16,-36.1,;8.83,-36.11,;10.16,-36.88,;11.64,-36.5,;12.03,-37.98,;10.54,-38.38,;13.36,-38.76,;14.7,-37.99,;14.71,-36.45,;16.24,-37.99,;15.47,-39.33,)| Show InChI InChI=1S/C28H33Cl2FN4O4S/c1-27(2,3)13-21-28(18-9-8-14(29)10-20(18)33-26(28)37)22(17-6-5-7-19(30)23(17)31)24(34-21)25(36)32-15-11-16(12-15)35-40(4,38)39/h5-10,15-16,21-22,24,34-35H,11-13H2,1-4H3,(H,32,36)(H,33,37)/t15-,16-,21-,22-,24+,28+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7.60 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Michigan
Curated by ChEMBL
| Assay Description
Binding affinity to recombinant human His-tagged MDM2 (1 to 118 amino acids) using p53-based PMDM6-F as probe after 15 to 30 mins by fluorescence pol... |
J Med Chem 56: 5553-61 (2014)
Article DOI: 10.1021/jm4005708
BindingDB Entry DOI: 10.7270/Q2SJ1N2D |
More data for this
Ligand-Target Pair | |
E3 ubiquitin-protein ligase Mdm2
(Homo sapiens (Human)) | BDBM50436687

(CHEMBL2398474)Show SMILES CC(C)(C)C[C@@H]1N[C@H]([C@H](c2cccc(Cl)c2F)[C@]11C(=O)Nc2cc(Cl)ccc12)C(=O)N[C@@H]1C[C@H](O)C1 |r,wU:17.19,8.8,31.34,5.4,33.37,wD:7.31,(13.04,-18.98,;11.84,-18.02,;12.07,-16.5,;13.31,-17.62,;10.4,-18.58,;9.2,-17.61,;9.28,-16.08,;7.83,-15.53,;6.87,-16.73,;5.53,-15.96,;4.2,-16.73,;2.86,-15.97,;2.85,-14.41,;4.19,-13.64,;4.19,-12.1,;5.53,-14.41,;5.52,-12.86,;7.72,-18.02,;8.63,-19.28,;10.17,-19.28,;7.71,-20.53,;6.24,-20.05,;4.91,-20.82,;3.57,-20.05,;2.24,-20.82,;3.57,-18.51,;4.9,-17.74,;6.24,-18.5,;7.84,-13.99,;6.51,-13.22,;9.18,-13.22,;10.51,-14,;10.89,-15.5,;12.38,-15.1,;13.71,-15.88,;11.99,-13.62,)| Show InChI InChI=1S/C27H30Cl2FN3O3/c1-26(2,3)12-20-27(17-8-7-13(28)9-19(17)32-25(27)36)21(16-5-4-6-18(29)22(16)30)23(33-20)24(35)31-14-10-15(34)11-14/h4-9,14-15,20-21,23,33-34H,10-12H2,1-3H3,(H,31,35)(H,32,36)/t14-,15+,20-,21-,23+,27+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8.20 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Michigan
Curated by ChEMBL
| Assay Description
Binding affinity to recombinant human His-tagged MDM2 (1 to 118 amino acids) using p53-based PMDM6-F as probe after 15 to 30 mins by fluorescence pol... |
J Med Chem 56: 5553-61 (2014)
Article DOI: 10.1021/jm4005708
BindingDB Entry DOI: 10.7270/Q2SJ1N2D |
More data for this
Ligand-Target Pair | |
E3 ubiquitin-protein ligase Mdm2
(Homo sapiens (Human)) | BDBM50436685

(CHEMBL2398476)Show SMILES CC(C)(C)C[C@@H]1N[C@H]([C@H](c2cccc(Cl)c2F)[C@]11C(=O)Nc2cc(Cl)ccc12)C(=O)N[C@H]1CC[C@H](O)C1 |r| Show InChI InChI=1S/C28H32Cl2FN3O3/c1-27(2,3)13-21-28(18-10-7-14(29)11-20(18)33-26(28)37)22(17-5-4-6-19(30)23(17)31)24(34-21)25(36)32-15-8-9-16(35)12-15/h4-7,10-11,15-16,21-22,24,34-35H,8-9,12-13H2,1-3H3,(H,32,36)(H,33,37)/t15-,16-,21-,22-,24+,28+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8.40 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Michigan
Curated by ChEMBL
| Assay Description
Binding affinity to recombinant human His-tagged MDM2 (1 to 118 amino acids) using p53-based PMDM6-F as probe after 15 to 30 mins by fluorescence pol... |
J Med Chem 56: 5553-61 (2014)
Article DOI: 10.1021/jm4005708
BindingDB Entry DOI: 10.7270/Q2SJ1N2D |
More data for this
Ligand-Target Pair | |
E3 ubiquitin-protein ligase Mdm2
(Homo sapiens (Human)) | BDBM50436686

(CHEMBL2398475)Show SMILES CC(C)(C)C[C@@H]1N[C@H]([C@H](c2cccc(Cl)c2F)[C@]11C(=O)Nc2cc(Cl)ccc12)C(=O)N[C@H]1CC[C@@H](O)C1 |r| Show InChI InChI=1S/C28H32Cl2FN3O3/c1-27(2,3)13-21-28(18-10-7-14(29)11-20(18)33-26(28)37)22(17-5-4-6-19(30)23(17)31)24(34-21)25(36)32-15-8-9-16(35)12-15/h4-7,10-11,15-16,21-22,24,34-35H,8-9,12-13H2,1-3H3,(H,32,36)(H,33,37)/t15-,16+,21-,22-,24+,28+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8.80 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Michigan
Curated by ChEMBL
| Assay Description
Binding affinity to recombinant human His-tagged MDM2 (1 to 118 amino acids) using p53-based PMDM6-F as probe after 15 to 30 mins by fluorescence pol... |
J Med Chem 56: 5553-61 (2014)
Article DOI: 10.1021/jm4005708
BindingDB Entry DOI: 10.7270/Q2SJ1N2D |
More data for this
Ligand-Target Pair | |
E3 ubiquitin-protein ligase Mdm2
(Homo sapiens (Human)) | BDBM50436689

(CHEMBL2398472)Show SMILES CC(C)(C)C[C@@H]1N[C@H]([C@H](c2cccc(Cl)c2F)[C@]11C(=O)Nc2cc(Cl)ccc12)C(=O)NCC[C@H](O)CO |r| Show InChI InChI=1S/C27H32Cl2FN3O4/c1-26(2,3)12-20-27(17-8-7-14(28)11-19(17)32-25(27)37)21(16-5-4-6-18(29)22(16)30)23(33-20)24(36)31-10-9-15(35)13-34/h4-8,11,15,20-21,23,33-35H,9-10,12-13H2,1-3H3,(H,31,36)(H,32,37)/t15-,20-,21-,23+,27+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 9.80 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Michigan
Curated by ChEMBL
| Assay Description
Binding affinity to recombinant human His-tagged MDM2 (1 to 118 amino acids) using p53-based PMDM6-F as probe after 15 to 30 mins by fluorescence pol... |
J Med Chem 56: 5553-61 (2014)
Article DOI: 10.1021/jm4005708
BindingDB Entry DOI: 10.7270/Q2SJ1N2D |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data