 Found 1176 hits with Last Name = 'zulli' and Initial = 'a'
Found 1176 hits with Last Name = 'zulli' and Initial = 'a' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Cathepsin K
(Homo sapiens (Human)) | BDBM50098576
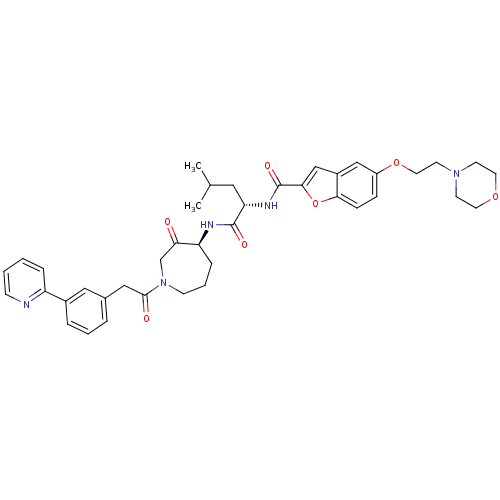
(5-(2-MORPHOLIN-4-YLETHOXY)BENZOFURAN-2-CARBOXYLIC ...)Show SMILES CC(C)C[C@H](NC(=O)c1cc2cc(OCCN3CCOCC3)ccc2o1)C(=O)N[C@H]1CCCN(CC1=O)C(=O)Cc1cccc(c1)-c1ccccn1 Show InChI InChI=1S/C40H47N5O7/c1-27(2)21-34(43-40(49)37-25-30-24-31(11-12-36(30)52-37)51-20-17-44-15-18-50-19-16-44)39(48)42-33-10-6-14-45(26-35(33)46)38(47)23-28-7-5-8-29(22-28)32-9-3-4-13-41-32/h3-5,7-9,11-13,22,24-25,27,33-34H,6,10,14-21,23,26H2,1-2H3,(H,42,48)(H,43,49)/t33-,34-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
PubMed
| 0.00480 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of Human cathepsin K |
J Med Chem 44: 1380-95 (2001)
BindingDB Entry DOI: 10.7270/Q2QR4WDC |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Cathepsin K
(Homo sapiens (Human)) | BDBM19769

((2S)-2-(1-benzofuran-2-ylformamido)-4-methyl-N-[(4...)Show SMILES CC(C)C[C@H](NC(=O)c1cc2ccccc2o1)C(=O)N[C@H]1CCCN(CC1=O)S(=O)(=O)c1ccccn1 |r| Show InChI InChI=1S/C26H30N4O6S/c1-17(2)14-20(29-26(33)23-15-18-8-3-4-10-22(18)36-23)25(32)28-19-9-7-13-30(16-21(19)31)37(34,35)24-11-5-6-12-27-24/h3-6,8,10-12,15,17,19-20H,7,9,13-14,16H2,1-2H3,(H,28,32)(H,29,33)/t19-,20-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
PubMed
| 0.160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of Human cathepsin K |
J Med Chem 44: 1380-95 (2001)
BindingDB Entry DOI: 10.7270/Q2QR4WDC |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50114539
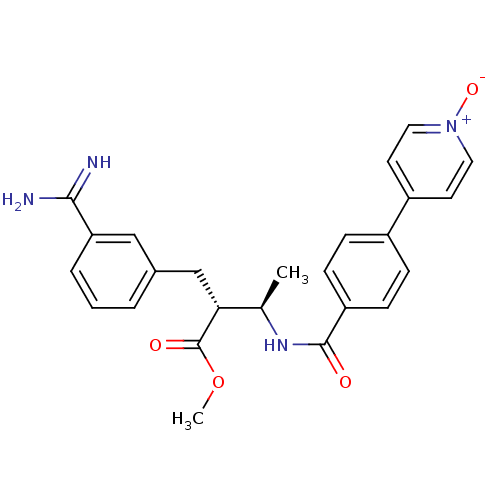
((2R,3R)-2-(3-Carbamimidoyl-benzyl)-3-[4-(1-oxy-pyr...)Show SMILES COC(=O)[C@H](Cc1cccc(c1)C(N)=N)[C@@H](C)NC(=O)c1ccc(cc1)-c1cc[n+]([O-])cc1 Show InChI InChI=1S/C25H26N4O4/c1-16(22(25(31)33-2)15-17-4-3-5-21(14-17)23(26)27)28-24(30)20-8-6-18(7-9-20)19-10-12-29(32)13-11-19/h3-14,16,22H,15H2,1-2H3,(H3,26,27)(H,28,30)/t16-,22-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aventis Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inibition of Coagulation factor Xa |
Bioorg Med Chem Lett 12: 1671-4 (2002)
BindingDB Entry DOI: 10.7270/Q2BV7FXG |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50114534
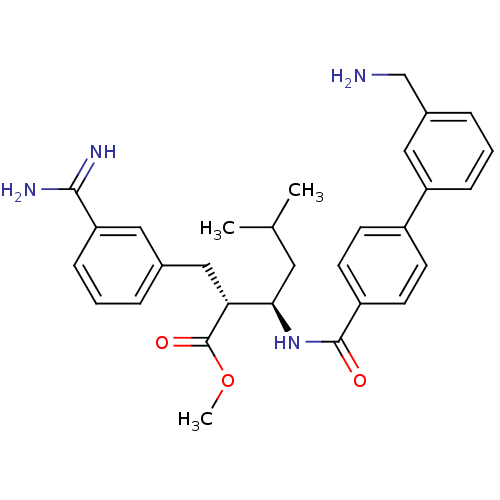
((2R,3R)-3-[(3'-Aminomethyl-biphenyl-4-carbonyl)-am...)Show SMILES COC(=O)[C@H](Cc1cccc(c1)C(N)=N)[C@@H](CC(C)C)NC(=O)c1ccc(cc1)-c1cccc(CN)c1 Show InChI InChI=1S/C30H36N4O3/c1-19(2)14-27(26(30(36)37-3)17-20-6-4-9-25(15-20)28(32)33)34-29(35)23-12-10-22(11-13-23)24-8-5-7-21(16-24)18-31/h4-13,15-16,19,26-27H,14,17-18,31H2,1-3H3,(H3,32,33)(H,34,35)/t26-,27-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aventis Pharmaceuticals
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against human Coagulation factor Xa |
Bioorg Med Chem Lett 12: 1667-70 (2002)
BindingDB Entry DOI: 10.7270/Q2GM86MR |
More data for this
Ligand-Target Pair | |
Procathepsin L
(Homo sapiens (Human)) | BDBM50098576

(5-(2-MORPHOLIN-4-YLETHOXY)BENZOFURAN-2-CARBOXYLIC ...)Show SMILES CC(C)C[C@H](NC(=O)c1cc2cc(OCCN3CCOCC3)ccc2o1)C(=O)N[C@H]1CCCN(CC1=O)C(=O)Cc1cccc(c1)-c1ccccn1 Show InChI InChI=1S/C40H47N5O7/c1-27(2)21-34(43-40(49)37-25-30-24-31(11-12-36(30)52-37)51-20-17-44-15-18-50-19-16-44)39(48)42-33-10-6-14-45(26-35(33)46)38(47)23-28-7-5-8-29(22-28)32-9-3-4-13-41-32/h3-5,7-9,11-13,22,24-25,27,33-34H,6,10,14-21,23,26H2,1-2H3,(H,42,48)(H,43,49)/t33-,34-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PubMed
| 0.490 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibitory activity of the compound against Human cathepsin L |
J Med Chem 44: 1380-95 (2001)
BindingDB Entry DOI: 10.7270/Q2QR4WDC |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50085393
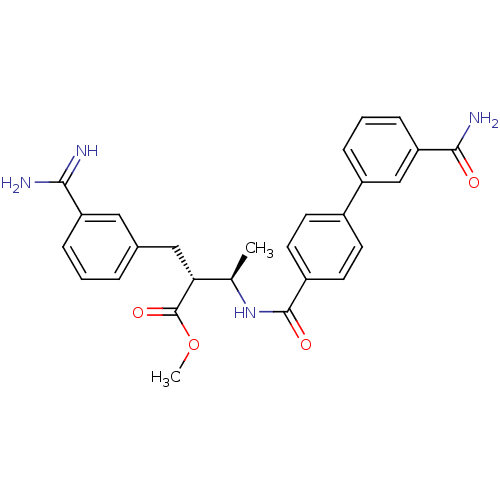
((2R,3R)-2-(3-Carbamimidoyl-benzyl)-3-[(3'-carbamoy...)Show SMILES COC(=O)[C@H](Cc1cccc(c1)C(N)=N)[C@@H](C)NC(=O)c1ccc(cc1)-c1cccc(c1)C(N)=O Show InChI InChI=1S/C27H28N4O4/c1-16(23(27(34)35-2)14-17-5-3-7-21(13-17)24(28)29)31-26(33)19-11-9-18(10-12-19)20-6-4-8-22(15-20)25(30)32/h3-13,15-16,23H,14H2,1-2H3,(H3,28,29)(H2,30,32)(H,31,33)/t16-,23-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aventis Pharmaceuticals
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against human Coagulation factor Xa |
Bioorg Med Chem Lett 12: 1667-70 (2002)
BindingDB Entry DOI: 10.7270/Q2GM86MR |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50114544
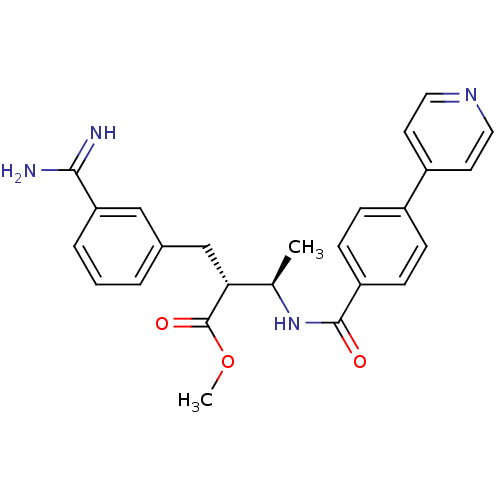
((2R,3R)-2-(3-Carbamimidoyl-benzyl)-3-(4-pyridin-4-...)Show SMILES COC(=O)[C@H](Cc1cccc(c1)C(N)=N)[C@@H](C)NC(=O)c1ccc(cc1)-c1ccncc1 Show InChI InChI=1S/C25H26N4O3/c1-16(22(25(31)32-2)15-17-4-3-5-21(14-17)23(26)27)29-24(30)20-8-6-18(7-9-20)19-10-12-28-13-11-19/h3-14,16,22H,15H2,1-2H3,(H3,26,27)(H,29,30)/t16-,22-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aventis Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inibition of Coagulation factor Xa |
Bioorg Med Chem Lett 12: 1671-4 (2002)
BindingDB Entry DOI: 10.7270/Q2BV7FXG |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50114543

((2R,3R)-2-(3-Carbamimidoyl-benzyl)-3-[4-(1-oxy-pyr...)Show SMILES COC(=O)[C@H](Cc1cccc(c1)C(N)=N)[C@@H](C)NC(=O)c1ccc(cc1)-c1cccc[n+]1[O-] Show InChI InChI=1S/C25H26N4O4/c1-16(21(25(31)33-2)15-17-6-5-7-20(14-17)23(26)27)28-24(30)19-11-9-18(10-12-19)22-8-3-4-13-29(22)32/h3-14,16,21H,15H2,1-2H3,(H3,26,27)(H,28,30)/t16-,21-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.580 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aventis Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inibition of Coagulation factor Xa |
Bioorg Med Chem Lett 12: 1671-4 (2002)
BindingDB Entry DOI: 10.7270/Q2BV7FXG |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50384937

(CHEMBL2036648)Show SMILES C[C@@H]1CCCN1CCCOc1ccc(cc1)C(=O)CN1CCN(CC1)C(=O)c1ccc(F)cc1 |r| Show InChI InChI=1S/C27H34FN3O3/c1-21-4-2-13-30(21)14-3-19-34-25-11-7-22(8-12-25)26(32)20-29-15-17-31(18-16-29)27(33)23-5-9-24(28)10-6-23/h5-12,21H,2-4,13-20H2,1H3/t21-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cephalon, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]NAMH from human histamine H3 receptor expressed in CHO cells |
Bioorg Med Chem Lett 22: 2807-10 (2012)
Article DOI: 10.1016/j.bmcl.2012.02.081
BindingDB Entry DOI: 10.7270/Q26M37VX |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50114536
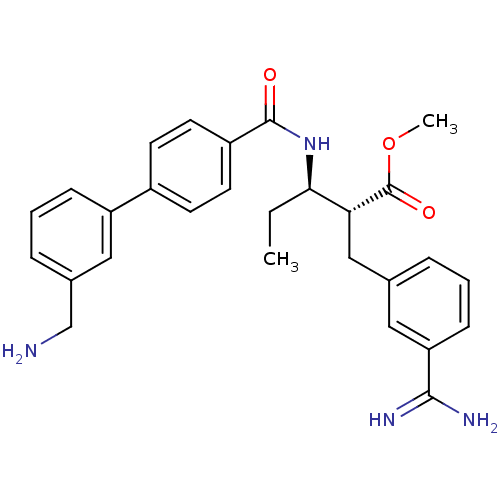
((2R,3R)-3-[(3'-Aminomethyl-biphenyl-4-carbonyl)-am...)Show SMILES CC[C@@H](NC(=O)c1ccc(cc1)-c1cccc(CN)c1)[C@@H](Cc1cccc(c1)C(N)=N)C(=O)OC Show InChI InChI=1S/C28H32N4O3/c1-3-25(24(28(34)35-2)16-18-6-4-9-23(14-18)26(30)31)32-27(33)21-12-10-20(11-13-21)22-8-5-7-19(15-22)17-29/h4-15,24-25H,3,16-17,29H2,1-2H3,(H3,30,31)(H,32,33)/t24-,25-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aventis Pharmaceuticals
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against human Coagulation factor Xa |
Bioorg Med Chem Lett 12: 1667-70 (2002)
BindingDB Entry DOI: 10.7270/Q2GM86MR |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50114540

(3-{4-[(1R,2R)-3-(3-Carbamimidoyl-phenyl)-2-methoxy...)Show SMILES COC(=O)[C@H](Cc1cccc(c1)C(N)=N)[C@@H](C)NC(=O)c1ccc(cc1)-c1ccc[n+](C)c1 Show InChI InChI=1S/C26H28N4O3/c1-17(23(26(32)33-3)15-18-6-4-7-21(14-18)24(27)28)29-25(31)20-11-9-19(10-12-20)22-8-5-13-30(2)16-22/h4-14,16-17,23H,15H2,1-3H3,(H3-,27,28,29,31)/p+1/t17-,23-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aventis Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inibition of Coagulation factor Xa |
Bioorg Med Chem Lett 12: 1671-4 (2002)
BindingDB Entry DOI: 10.7270/Q2BV7FXG |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50114537
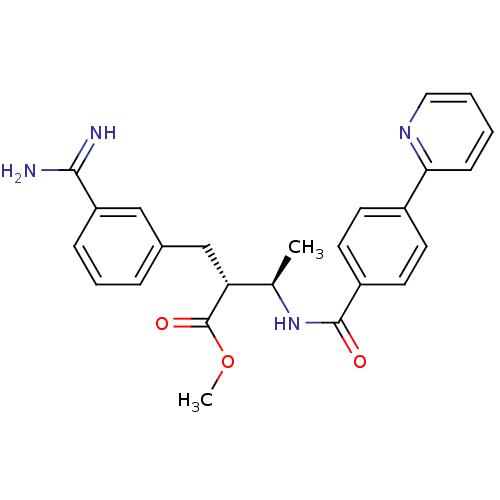
((2R,3R)-2-(3-Carbamimidoyl-benzyl)-3-(4-pyridin-2-...)Show SMILES COC(=O)[C@H](Cc1cccc(c1)C(N)=N)[C@@H](C)NC(=O)c1ccc(cc1)-c1ccccn1 Show InChI InChI=1S/C25H26N4O3/c1-16(21(25(31)32-2)15-17-6-5-7-20(14-17)23(26)27)29-24(30)19-11-9-18(10-12-19)22-8-3-4-13-28-22/h3-14,16,21H,15H2,1-2H3,(H3,26,27)(H,29,30)/t16-,21-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.890 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aventis Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inibition of Coagulation factor Xa |
Bioorg Med Chem Lett 12: 1671-4 (2002)
BindingDB Entry DOI: 10.7270/Q2BV7FXG |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50384940
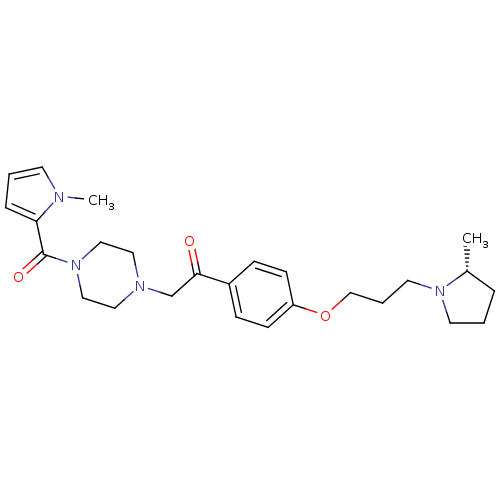
(CHEMBL2036651)Show SMILES C[C@@H]1CCCN1CCCOc1ccc(cc1)C(=O)CN1CCN(CC1)C(=O)c1cccn1C |r| Show InChI InChI=1S/C26H36N4O3/c1-21-6-3-13-29(21)14-5-19-33-23-10-8-22(9-11-23)25(31)20-28-15-17-30(18-16-28)26(32)24-7-4-12-27(24)2/h4,7-12,21H,3,5-6,13-20H2,1-2H3/t21-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cephalon, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]NAMH from human histamine H3 receptor expressed in CHO cells |
Bioorg Med Chem Lett 22: 2807-10 (2012)
Article DOI: 10.1016/j.bmcl.2012.02.081
BindingDB Entry DOI: 10.7270/Q26M37VX |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM12597
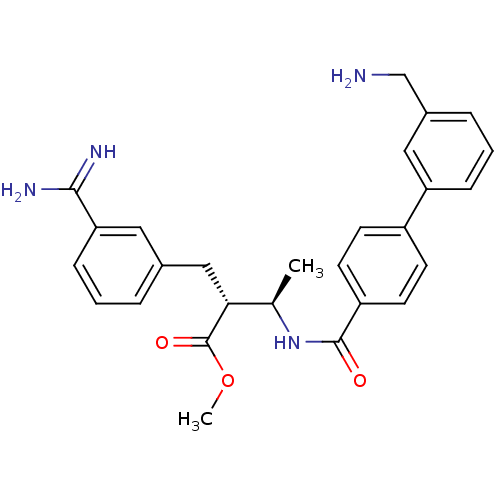
(CHEMBL48046 | RPR128515 | methyl (2R,3R)-2-{3-[ami...)Show SMILES COC(=O)[C@H](Cc1cccc(c1)C(N)=N)[C@@H](C)NC(=O)c1ccc(cc1)-c1cccc(CN)c1 |r| Show InChI InChI=1S/C27H30N4O3/c1-17(24(27(33)34-2)15-18-5-3-8-23(13-18)25(29)30)31-26(32)21-11-9-20(10-12-21)22-7-4-6-19(14-22)16-28/h3-14,17,24H,15-16,28H2,1-2H3,(H3,29,30)(H,31,32)/t17-,24-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PDB
PubMed
| 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aventis Pharmaceuticals
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against human Coagulation factor Xa |
Bioorg Med Chem Lett 12: 1667-70 (2002)
BindingDB Entry DOI: 10.7270/Q2GM86MR |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50114531
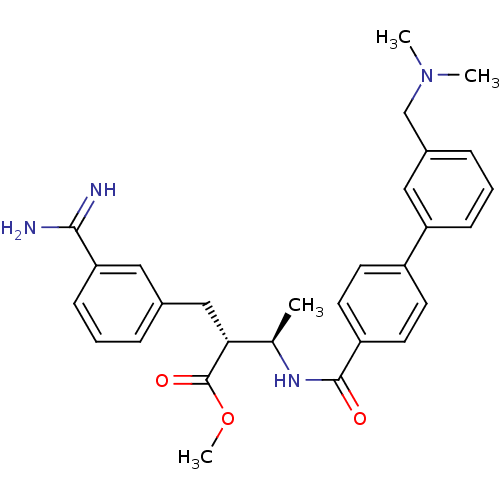
((2R,3R)-2-(3-Carbamimidoyl-benzyl)-3-[(3'-dimethyl...)Show SMILES COC(=O)[C@H](Cc1cccc(c1)C(N)=N)[C@@H](C)NC(=O)c1ccc(cc1)-c1cccc(CN(C)C)c1 Show InChI InChI=1S/C29H34N4O3/c1-19(26(29(35)36-4)17-20-7-5-10-25(15-20)27(30)31)32-28(34)23-13-11-22(12-14-23)24-9-6-8-21(16-24)18-33(2)3/h5-16,19,26H,17-18H2,1-4H3,(H3,30,31)(H,32,34)/t19-,26-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aventis Pharmaceuticals
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against human Coagulation factor Xa |
Bioorg Med Chem Lett 12: 1667-70 (2002)
BindingDB Entry DOI: 10.7270/Q2GM86MR |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50114548
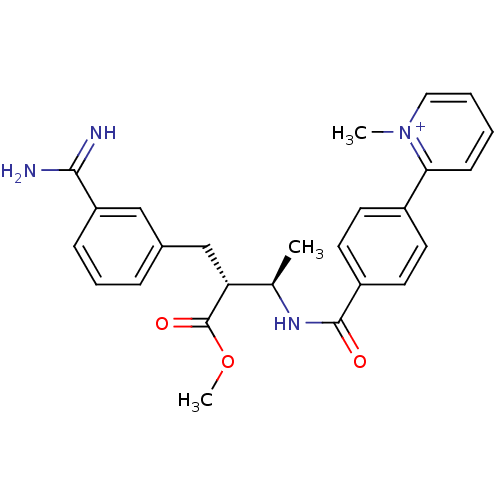
(2-{4-[(1R,2R)-3-(3-Carbamimidoyl-phenyl)-2-methoxy...)Show SMILES COC(=O)[C@H](Cc1cccc(c1)C(N)=N)[C@@H](C)NC(=O)c1ccc(cc1)-c1cccc[n+]1C Show InChI InChI=1S/C26H28N4O3/c1-17(22(26(32)33-3)16-18-7-6-8-21(15-18)24(27)28)29-25(31)20-12-10-19(11-13-20)23-9-4-5-14-30(23)2/h4-15,17,22H,16H2,1-3H3,(H3-,27,28,29,31)/p+1/t17-,22-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aventis Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inibition of Coagulation factor Xa |
Bioorg Med Chem Lett 12: 1671-4 (2002)
BindingDB Entry DOI: 10.7270/Q2BV7FXG |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50384941

(CHEMBL2036652)Show SMILES C[C@@H]1CCCN1CCCOc1ccc(cc1)C(=O)CN1CCN(CC1)C(=O)c1cc2ccccc2s1 |r| Show InChI InChI=1S/C29H35N3O3S/c1-22-6-4-13-31(22)14-5-19-35-25-11-9-23(10-12-25)26(33)21-30-15-17-32(18-16-30)29(34)28-20-24-7-2-3-8-27(24)36-28/h2-3,7-12,20,22H,4-6,13-19,21H2,1H3/t22-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cephalon, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]NAMH from human histamine H3 receptor expressed in CHO cells |
Bioorg Med Chem Lett 22: 2807-10 (2012)
Article DOI: 10.1016/j.bmcl.2012.02.081
BindingDB Entry DOI: 10.7270/Q26M37VX |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50353165
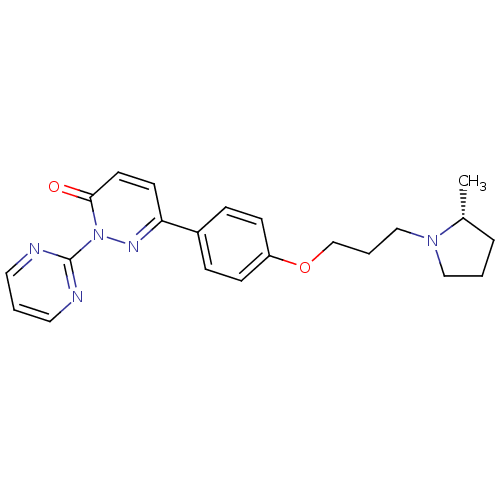
(CHEMBL1829472)Show SMILES C[C@@H]1CCCN1CCCOc1ccc(cc1)-c1ccc(=O)n(n1)-c1ncccn1 |r| Show InChI InChI=1S/C22H25N5O2/c1-17-5-2-14-26(17)15-4-16-29-19-8-6-18(7-9-19)20-10-11-21(28)27(25-20)22-23-12-3-13-24-22/h3,6-13,17H,2,4-5,14-16H2,1H3/t17-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cephalon, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]NAMH from human cloned histamine 3 receptor expressed in CHO cells |
Bioorg Med Chem Lett 21: 5493-7 (2011)
Article DOI: 10.1016/j.bmcl.2011.06.108
BindingDB Entry DOI: 10.7270/Q2KP8359 |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50350022
(CHEMBL1813057)Show SMILES C[C@@H]1CCCN1CCCOc1ccc(cc1)-c1ccc(=O)n(C)n1 |r| Show InChI InChI=1S/C19H25N3O2/c1-15-5-3-12-22(15)13-4-14-24-17-8-6-16(7-9-17)18-10-11-19(23)21(2)20-18/h6-11,15H,3-5,12-14H2,1-2H3/t15-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cephalon, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]NAMH from human cloned histamine 3 receptor expressed in CHO cells |
Bioorg Med Chem Lett 21: 5493-7 (2011)
Article DOI: 10.1016/j.bmcl.2011.06.108
BindingDB Entry DOI: 10.7270/Q2KP8359 |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Homo sapiens (Human)) | BDBM50098580
(Benzofuran-2-carboxylic acid {3-methyl-1-[2-oxo-3-...)Show SMILES CC(C)C[C@H](NC(=O)c1cc2ccccc2o1)C(=O)NCC(=O)CNS(=O)(=O)c1ccccn1 Show InChI InChI=1S/C23H26N4O6S/c1-15(2)11-18(27-23(30)20-12-16-7-3-4-8-19(16)33-20)22(29)25-13-17(28)14-26-34(31,32)21-9-5-6-10-24-21/h3-10,12,15,18,26H,11,13-14H2,1-2H3,(H,25,29)(H,27,30)/t18-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of Human cathepsin K |
J Med Chem 44: 1380-95 (2001)
BindingDB Entry DOI: 10.7270/Q2QR4WDC |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50364972
(CHEMBL1950738)Show InChI InChI=1S/C19H24N4O2/c1-22-19(24)8-6-17(21-22)14-5-7-18(20-13-14)25-16-9-11-23(12-10-16)15-3-2-4-15/h5-8,13,15-16H,2-4,9-12H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cephalon, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]NAMH from human histamine H3 receptor expressed in CHO cells |
Bioorg Med Chem Lett 22: 1504-9 (2012)
Article DOI: 10.1016/j.bmcl.2012.01.026
BindingDB Entry DOI: 10.7270/Q2W37WSF |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50364957
(CHEMBL1950643)Show SMILES O=c1ccc(n[nH]1)-c1ccc(OC2CCN(CC2)C2CCC2)cc1 Show InChI InChI=1S/C19H23N3O2/c23-19-9-8-18(20-21-19)14-4-6-16(7-5-14)24-17-10-12-22(13-11-17)15-2-1-3-15/h4-9,15,17H,1-3,10-13H2,(H,21,23) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cephalon, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]NAMH from human histamine H3 receptor expressed in CHO cells |
Bioorg Med Chem Lett 22: 1504-9 (2012)
Article DOI: 10.1016/j.bmcl.2012.01.026
BindingDB Entry DOI: 10.7270/Q2W37WSF |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50360899
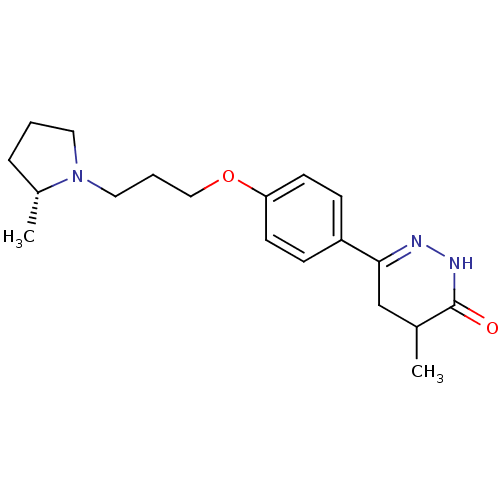
(CHEMBL1935110)Show SMILES C[C@@H]1CCCN1CCCOc1ccc(cc1)C1=NNC(=O)C(C)C1 |r,t:18| Show InChI InChI=1S/C19H27N3O2/c1-14-13-18(20-21-19(14)23)16-6-8-17(9-7-16)24-12-4-11-22-10-3-5-15(22)2/h6-9,14-15H,3-5,10-13H2,1-2H3,(H,21,23)/t14?,15-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cephalon, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]NAMH from human histamine H3 receptor expressed in CHO cells |
Bioorg Med Chem Lett 22: 194-8 (2011)
Article DOI: 10.1016/j.bmcl.2011.11.037
BindingDB Entry DOI: 10.7270/Q25Q4WJV |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50384928

(CHEMBL2036635)Show SMILES C[C@@H]1CCCN1CCCOc1ccc(cc1)C(=O)CN1CCN(CC1)C(=O)c1ccccc1 |r| Show InChI InChI=1S/C27H35N3O3/c1-22-7-5-14-29(22)15-6-20-33-25-12-10-23(11-13-25)26(31)21-28-16-18-30(19-17-28)27(32)24-8-3-2-4-9-24/h2-4,8-13,22H,5-7,14-21H2,1H3/t22-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cephalon, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]NAMH from human histamine H3 receptor expressed in CHO cells |
Bioorg Med Chem Lett 22: 2807-10 (2012)
Article DOI: 10.1016/j.bmcl.2012.02.081
BindingDB Entry DOI: 10.7270/Q26M37VX |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50360897
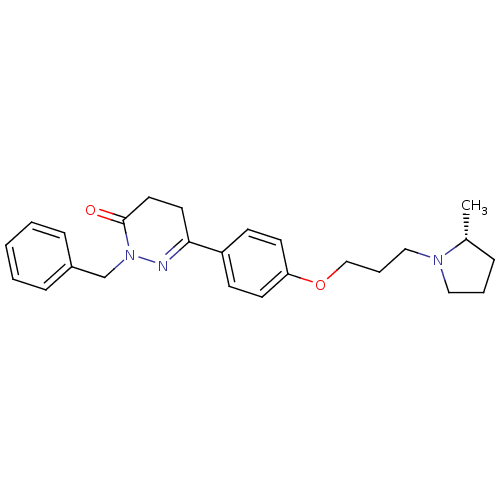
(CHEMBL1935108)Show SMILES C[C@@H]1CCCN1CCCOc1ccc(cc1)C1=NN(Cc2ccccc2)C(=O)CC1 |r,t:18| Show InChI InChI=1S/C25H31N3O2/c1-20-7-5-16-27(20)17-6-18-30-23-12-10-22(11-13-23)24-14-15-25(29)28(26-24)19-21-8-3-2-4-9-21/h2-4,8-13,20H,5-7,14-19H2,1H3/t20-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cephalon, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]NAMH from human histamine H3 receptor expressed in CHO cells |
Bioorg Med Chem Lett 22: 194-8 (2011)
Article DOI: 10.1016/j.bmcl.2011.11.037
BindingDB Entry DOI: 10.7270/Q25Q4WJV |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Homo sapiens (Human)) | BDBM50098584
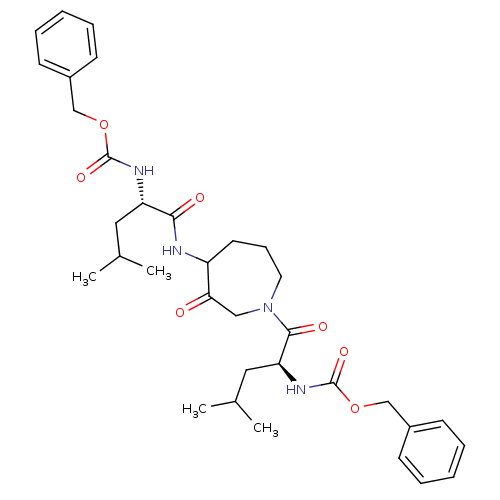
(CHEMBL31947 | {1-[4-(2-Benzyloxycarbonylamino-4-me...)Show SMILES CC(C)C[C@H](NC(=O)OCc1ccccc1)C(=O)NC1CCCN(CC1=O)C(=O)[C@H](CC(C)C)NC(=O)OCc1ccccc1 Show InChI InChI=1S/C34H46N4O7/c1-23(2)18-28(36-33(42)44-21-25-12-7-5-8-13-25)31(40)35-27-16-11-17-38(20-30(27)39)32(41)29(19-24(3)4)37-34(43)45-22-26-14-9-6-10-15-26/h5-10,12-15,23-24,27-29H,11,16-22H2,1-4H3,(H,35,40)(H,36,42)(H,37,43)/t27?,28-,29-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of Human cathepsin K |
J Med Chem 44: 1380-95 (2001)
BindingDB Entry DOI: 10.7270/Q2QR4WDC |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50114547

((2R,3R)-2-(3-Carbamimidoyl-benzyl)-3-[4-(1-oxy-pyr...)Show SMILES COC(=O)[C@H](Cc1cccc(c1)C(N)=N)[C@@H](C)NC(=O)c1ccc(cc1)-c1ccc[n+]([O-])c1 Show InChI InChI=1S/C25H26N4O4/c1-16(22(25(31)33-2)14-17-5-3-6-20(13-17)23(26)27)28-24(30)19-10-8-18(9-11-19)21-7-4-12-29(32)15-21/h3-13,15-16,22H,14H2,1-2H3,(H3,26,27)(H,28,30)/t16-,22-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aventis Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inibition of Coagulation factor Xa |
Bioorg Med Chem Lett 12: 1671-4 (2002)
BindingDB Entry DOI: 10.7270/Q2BV7FXG |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50114528
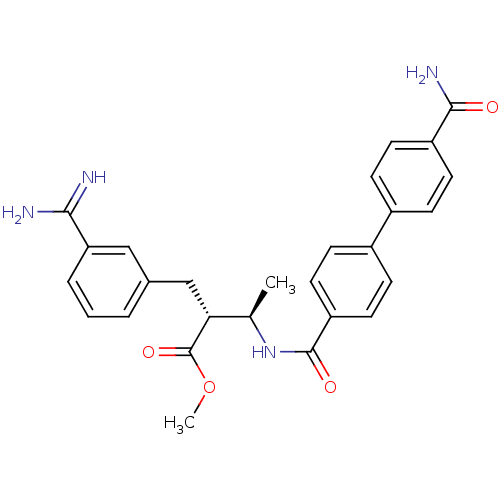
((2R,3R)-2-(3-Carbamimidoyl-benzyl)-3-[(4'-carbamoy...)Show SMILES COC(=O)[C@H](Cc1cccc(c1)C(N)=N)[C@@H](C)NC(=O)c1ccc(cc1)-c1ccc(cc1)C(N)=O Show InChI InChI=1S/C27H28N4O4/c1-16(23(27(34)35-2)15-17-4-3-5-22(14-17)24(28)29)31-26(33)21-12-8-19(9-13-21)18-6-10-20(11-7-18)25(30)32/h3-14,16,23H,15H2,1-2H3,(H3,28,29)(H2,30,32)(H,31,33)/t16-,23-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aventis Pharmaceuticals
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against human Coagulation factor Xa |
Bioorg Med Chem Lett 12: 1667-70 (2002)
BindingDB Entry DOI: 10.7270/Q2GM86MR |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50350021
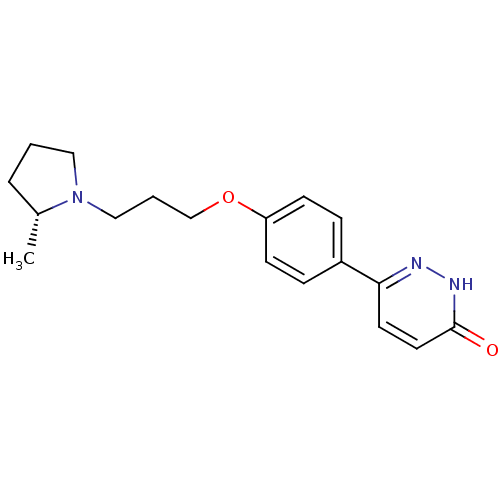
(CHEMBL1813067)Show SMILES C[C@@H]1CCCN1CCCOc1ccc(cc1)-c1ccc(=O)[nH]n1 |r| Show InChI InChI=1S/C18H23N3O2/c1-14-4-2-11-21(14)12-3-13-23-16-7-5-15(6-8-16)17-9-10-18(22)20-19-17/h5-10,14H,2-4,11-13H2,1H3,(H,20,22)/t14-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cephalon, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]NAMH from human cloned histamine 3 receptor expressed in CHO cells |
Bioorg Med Chem Lett 21: 5493-7 (2011)
Article DOI: 10.1016/j.bmcl.2011.06.108
BindingDB Entry DOI: 10.7270/Q2KP8359 |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50384939

(CHEMBL2036650)Show SMILES C[C@@H]1CCCN1CCCOc1ccc(cc1)C(=O)CN1CCN(CC1)C(=O)c1ccco1 |r| Show InChI InChI=1S/C25H33N3O4/c1-20-5-2-11-27(20)12-4-18-31-22-9-7-21(8-10-22)23(29)19-26-13-15-28(16-14-26)25(30)24-6-3-17-32-24/h3,6-10,17,20H,2,4-5,11-16,18-19H2,1H3/t20-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cephalon, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]NAMH from human histamine H3 receptor expressed in CHO cells |
Bioorg Med Chem Lett 22: 2807-10 (2012)
Article DOI: 10.1016/j.bmcl.2012.02.081
BindingDB Entry DOI: 10.7270/Q26M37VX |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50384935

(CHEMBL2036647)Show SMILES C[C@@H]1CCCN1CCCOc1ccc(cc1)C(=O)CN1CCN(CC1)C(=O)C(F)(F)F |r| Show InChI InChI=1S/C22H30F3N3O3/c1-17-4-2-9-27(17)10-3-15-31-19-7-5-18(6-8-19)20(29)16-26-11-13-28(14-12-26)21(30)22(23,24)25/h5-8,17H,2-4,9-16H2,1H3/t17-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cephalon, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]NAMH from human histamine H3 receptor expressed in CHO cells |
Bioorg Med Chem Lett 22: 2807-10 (2012)
Article DOI: 10.1016/j.bmcl.2012.02.081
BindingDB Entry DOI: 10.7270/Q26M37VX |
More data for this
Ligand-Target Pair | |
Procathepsin L
(Homo sapiens (Human)) | BDBM19769

((2S)-2-(1-benzofuran-2-ylformamido)-4-methyl-N-[(4...)Show SMILES CC(C)C[C@H](NC(=O)c1cc2ccccc2o1)C(=O)N[C@H]1CCCN(CC1=O)S(=O)(=O)c1ccccn1 |r| Show InChI InChI=1S/C26H30N4O6S/c1-17(2)14-20(29-26(33)23-15-18-8-3-4-10-22(18)36-23)25(32)28-19-9-7-13-30(16-21(19)31)37(34,35)24-11-5-6-12-27-24/h3-6,8,10-12,15,17,19-20H,7,9,13-14,16H2,1-2H3,(H,28,32)(H,29,33)/t19-,20-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PubMed
| 2.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibitory activity of the compound against Human cathepsin L |
J Med Chem 44: 1380-95 (2001)
BindingDB Entry DOI: 10.7270/Q2QR4WDC |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Rattus norvegicus (rat)) | BDBM50364957

(CHEMBL1950643)Show SMILES O=c1ccc(n[nH]1)-c1ccc(OC2CCN(CC2)C2CCC2)cc1 Show InChI InChI=1S/C19H23N3O2/c23-19-9-8-18(20-21-19)14-4-6-16(7-5-14)24-17-10-12-22(13-11-17)15-2-1-3-15/h4-9,15,17H,1-3,10-13H2,(H,21,23) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 2.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cephalon, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]NAMH from rat histamine H3 receptor expressed in CHO cells |
Bioorg Med Chem Lett 22: 1504-9 (2012)
Article DOI: 10.1016/j.bmcl.2012.01.026
BindingDB Entry DOI: 10.7270/Q2W37WSF |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Homo sapiens (Human)) | BDBM50098579
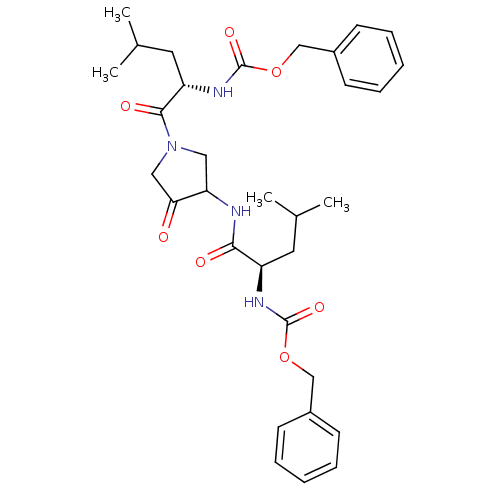
(CHEMBL29483 | {1-[3-(2-Benzyloxycarbonylamino-4-me...)Show SMILES CC(C)C[C@@H](NC(=O)OCc1ccccc1)C(=O)NC1CN(CC1=O)C(=O)[C@H](CC(C)C)NC(=O)OCc1ccccc1 Show InChI InChI=1S/C32H42N4O7/c1-21(2)15-25(34-31(40)42-19-23-11-7-5-8-12-23)29(38)33-27-17-36(18-28(27)37)30(39)26(16-22(3)4)35-32(41)43-20-24-13-9-6-10-14-24/h5-14,21-22,25-27H,15-20H2,1-4H3,(H,33,38)(H,34,40)(H,35,41)/t25-,26+,27?/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 2.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of Human cathepsin K |
J Med Chem 44: 1380-95 (2001)
BindingDB Entry DOI: 10.7270/Q2QR4WDC |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50352798
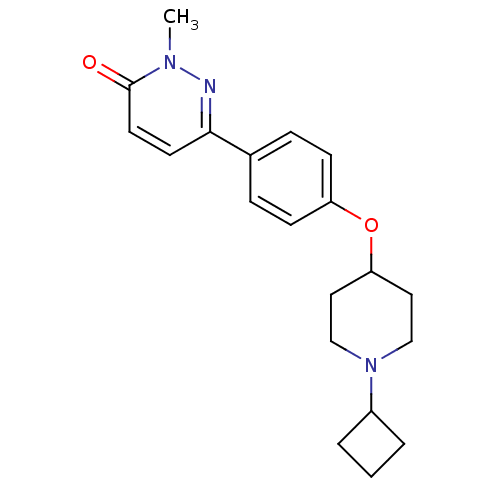
(CHEMBL1823402)Show InChI InChI=1S/C20H25N3O2/c1-22-20(24)10-9-19(21-22)15-5-7-17(8-6-15)25-18-11-13-23(14-12-18)16-3-2-4-16/h5-10,16,18H,2-4,11-14H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 2.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cephalon, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]NAMH from human histamine H3 receptor expressed in CHO cells |
Bioorg Med Chem Lett 22: 1504-9 (2012)
Article DOI: 10.1016/j.bmcl.2012.01.026
BindingDB Entry DOI: 10.7270/Q2W37WSF |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50353181
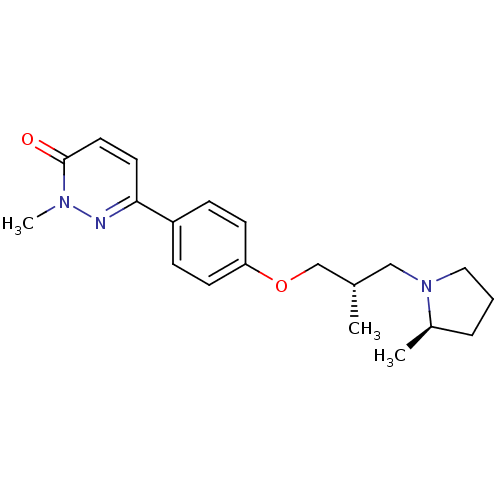
(CHEMBL1829485)Show SMILES C[C@H](COc1ccc(cc1)-c1ccc(=O)n(C)n1)CN1CCC[C@H]1C |r| Show InChI InChI=1S/C20H27N3O2/c1-15(13-23-12-4-5-16(23)2)14-25-18-8-6-17(7-9-18)19-10-11-20(24)22(3)21-19/h6-11,15-16H,4-5,12-14H2,1-3H3/t15-,16+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 2.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cephalon, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]NAMH from human cloned histamine 3 receptor expressed in CHO cells |
Bioorg Med Chem Lett 21: 5493-7 (2011)
Article DOI: 10.1016/j.bmcl.2011.06.108
BindingDB Entry DOI: 10.7270/Q2KP8359 |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50350030
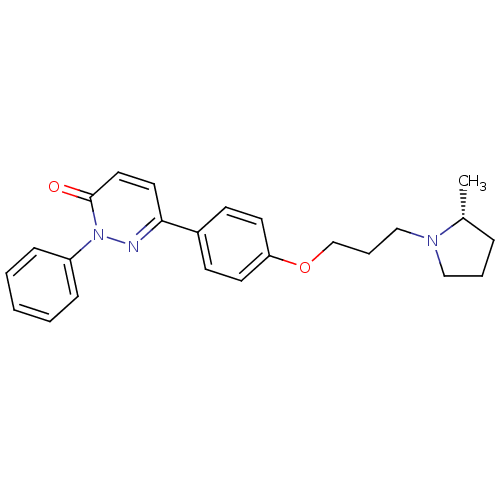
(CHEMBL1813060)Show SMILES C[C@@H]1CCCN1CCCOc1ccc(cc1)-c1ccc(=O)n(n1)-c1ccccc1 |r| Show InChI InChI=1S/C24H27N3O2/c1-19-7-5-16-26(19)17-6-18-29-22-12-10-20(11-13-22)23-14-15-24(28)27(25-23)21-8-3-2-4-9-21/h2-4,8-15,19H,5-7,16-18H2,1H3/t19-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 2.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cephalon, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]NAMH from human cloned histamine 3 receptor expressed in CHO cells |
Bioorg Med Chem Lett 21: 5493-7 (2011)
Article DOI: 10.1016/j.bmcl.2011.06.108
BindingDB Entry DOI: 10.7270/Q2KP8359 |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50384932
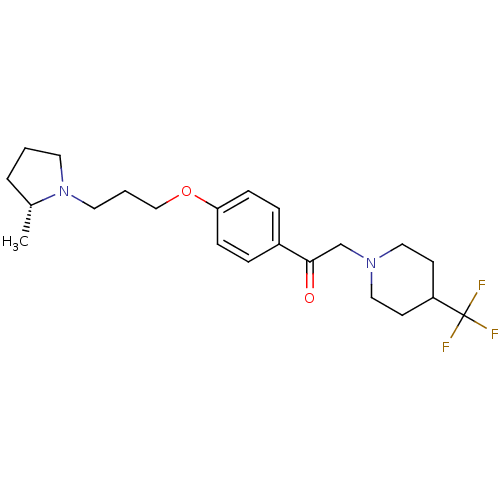
(CHEMBL2036642)Show SMILES C[C@@H]1CCCN1CCCOc1ccc(cc1)C(=O)CN1CCC(CC1)C(F)(F)F |r| Show InChI InChI=1S/C22H31F3N2O2/c1-17-4-2-11-27(17)12-3-15-29-20-7-5-18(6-8-20)21(28)16-26-13-9-19(10-14-26)22(23,24)25/h5-8,17,19H,2-4,9-16H2,1H3/t17-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cephalon, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]NAMH from human histamine H3 receptor expressed in CHO cells |
Bioorg Med Chem Lett 22: 2807-10 (2012)
Article DOI: 10.1016/j.bmcl.2012.02.081
BindingDB Entry DOI: 10.7270/Q26M37VX |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Homo sapiens (Human)) | BDBM50098575
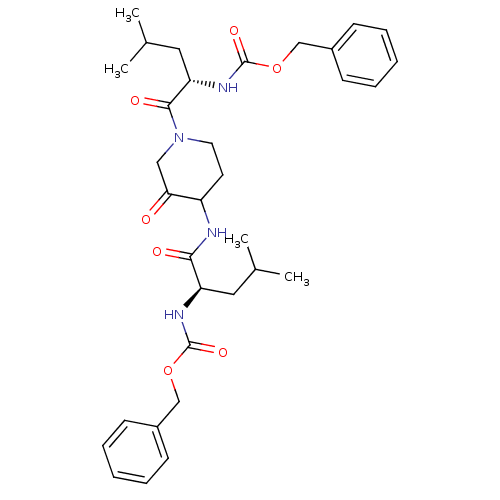
(CHEMBL281086 | {1-[4-(2-Benzyloxycarbonylamino-4-m...)Show SMILES CC(C)C[C@@H](NC(=O)OCc1ccccc1)C(=O)NC1CCN(CC1=O)C(=O)[C@H](CC(C)C)NC(=O)OCc1ccccc1 Show InChI InChI=1S/C33H44N4O7/c1-22(2)17-27(35-32(41)43-20-24-11-7-5-8-12-24)30(39)34-26-15-16-37(19-29(26)38)31(40)28(18-23(3)4)36-33(42)44-21-25-13-9-6-10-14-25/h5-14,22-23,26-28H,15-21H2,1-4H3,(H,34,39)(H,35,41)(H,36,42)/t26?,27-,28+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 2.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of Human cathepsin K |
J Med Chem 44: 1380-95 (2001)
BindingDB Entry DOI: 10.7270/Q2QR4WDC |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50352799
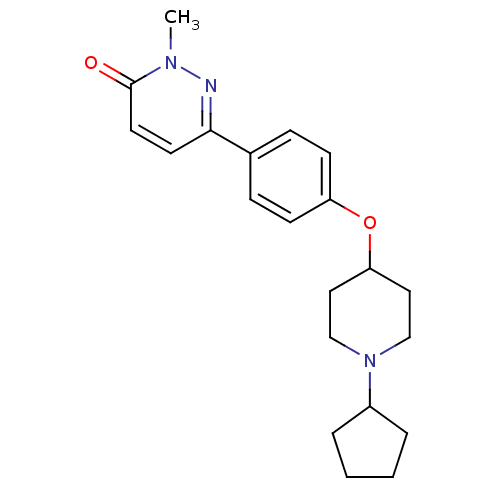
(CHEMBL1823403)Show InChI InChI=1S/C21H27N3O2/c1-23-21(25)11-10-20(22-23)16-6-8-18(9-7-16)26-19-12-14-24(15-13-19)17-4-2-3-5-17/h6-11,17,19H,2-5,12-15H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 2.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cephalon, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]NAMH from human histamine H3 receptor expressed in CHO cells |
Bioorg Med Chem Lett 22: 1504-9 (2012)
Article DOI: 10.1016/j.bmcl.2012.01.026
BindingDB Entry DOI: 10.7270/Q2W37WSF |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50114542
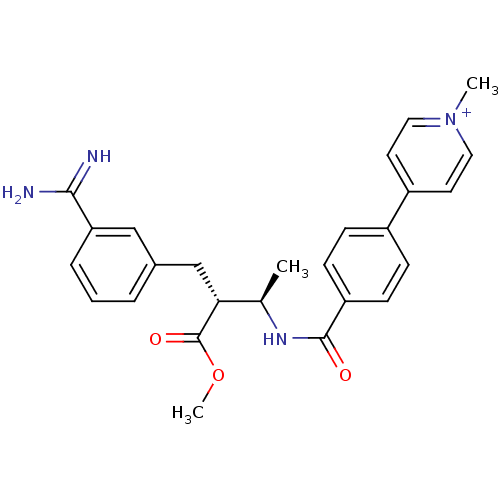
(4-{4-[(1R,2R)-3-(3-Carbamimidoyl-phenyl)-2-methoxy...)Show SMILES COC(=O)[C@H](Cc1cccc(c1)C(N)=N)[C@@H](C)NC(=O)c1ccc(cc1)-c1cc[n+](C)cc1 Show InChI InChI=1S/C26H28N4O3/c1-17(23(26(32)33-3)16-18-5-4-6-22(15-18)24(27)28)29-25(31)21-9-7-19(8-10-21)20-11-13-30(2)14-12-20/h4-15,17,23H,16H2,1-3H3,(H3-,27,28,29,31)/p+1/t17-,23-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 2.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aventis Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inibition of Coagulation factor Xa |
Bioorg Med Chem Lett 12: 1671-4 (2002)
BindingDB Entry DOI: 10.7270/Q2BV7FXG |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50364958
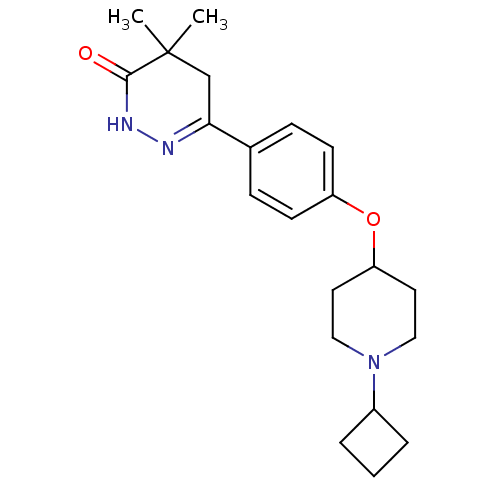
(CHEMBL1950743)Show SMILES CC1(C)CC(=NNC1=O)c1ccc(OC2CCN(CC2)C2CCC2)cc1 |c:4| Show InChI InChI=1S/C21H29N3O2/c1-21(2)14-19(22-23-20(21)25)15-6-8-17(9-7-15)26-18-10-12-24(13-11-18)16-4-3-5-16/h6-9,16,18H,3-5,10-14H2,1-2H3,(H,23,25) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 2.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cephalon, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]NAMH from human histamine H3 receptor expressed in CHO cells |
Bioorg Med Chem Lett 22: 1504-9 (2012)
Article DOI: 10.1016/j.bmcl.2012.01.026
BindingDB Entry DOI: 10.7270/Q2W37WSF |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50384934

(CHEMBL2036646)Show SMILES C[C@@H]1CCCN1CCCOc1ccc(cc1)C(=O)CN1CCN(CC1)C(C)=O |r| Show InChI InChI=1S/C22H33N3O3/c1-18-5-3-10-24(18)11-4-16-28-21-8-6-20(7-9-21)22(27)17-23-12-14-25(15-13-23)19(2)26/h6-9,18H,3-5,10-17H2,1-2H3/t18-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cephalon, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]NAMH from human histamine H3 receptor expressed in CHO cells |
Bioorg Med Chem Lett 22: 2807-10 (2012)
Article DOI: 10.1016/j.bmcl.2012.02.081
BindingDB Entry DOI: 10.7270/Q26M37VX |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50353167
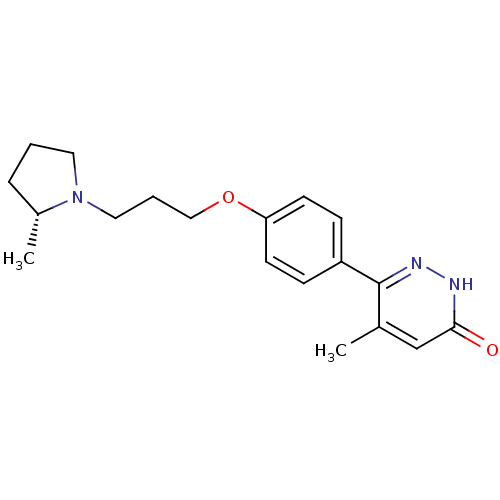
(CHEMBL1829475)Show SMILES C[C@@H]1CCCN1CCCOc1ccc(cc1)-c1n[nH]c(=O)cc1C |r| Show InChI InChI=1S/C19H25N3O2/c1-14-13-18(23)20-21-19(14)16-6-8-17(9-7-16)24-12-4-11-22-10-3-5-15(22)2/h6-9,13,15H,3-5,10-12H2,1-2H3,(H,20,23)/t15-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 2.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cephalon, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]NAMH from human histamine H3 receptor expressed in CHO cells |
Bioorg Med Chem Lett 22: 194-8 (2011)
Article DOI: 10.1016/j.bmcl.2011.11.037
BindingDB Entry DOI: 10.7270/Q25Q4WJV |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50353167

(CHEMBL1829475)Show SMILES C[C@@H]1CCCN1CCCOc1ccc(cc1)-c1n[nH]c(=O)cc1C |r| Show InChI InChI=1S/C19H25N3O2/c1-14-13-18(23)20-21-19(14)16-6-8-17(9-7-16)24-12-4-11-22-10-3-5-15(22)2/h6-9,13,15H,3-5,10-12H2,1-2H3,(H,20,23)/t15-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 2.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cephalon, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]NAMH from human cloned histamine 3 receptor expressed in CHO cells |
Bioorg Med Chem Lett 21: 5493-7 (2011)
Article DOI: 10.1016/j.bmcl.2011.06.108
BindingDB Entry DOI: 10.7270/Q2KP8359 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50114538
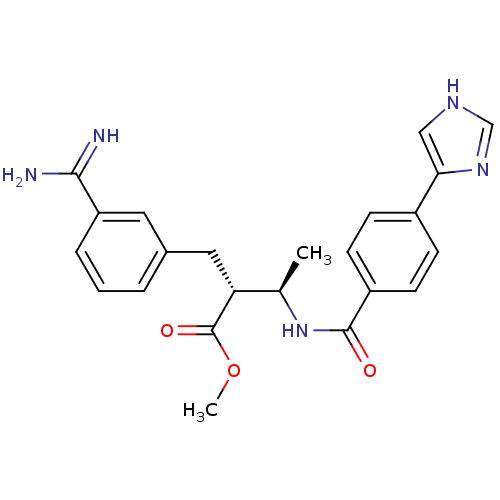
((2R,3R)-2-(3-Carbamimidoyl-benzyl)-3-[4-(1H-imidaz...)Show SMILES COC(=O)[C@H](Cc1cccc(c1)C(N)=N)[C@@H](C)NC(=O)c1ccc(cc1)-c1c[nH]cn1 Show InChI InChI=1S/C23H25N5O3/c1-14(19(23(30)31-2)11-15-4-3-5-18(10-15)21(24)25)28-22(29)17-8-6-16(7-9-17)20-12-26-13-27-20/h3-10,12-14,19H,11H2,1-2H3,(H3,24,25)(H,26,27)(H,28,29)/t14-,19-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aventis Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inibition of Coagulation factor Xa |
Bioorg Med Chem Lett 12: 1671-4 (2002)
BindingDB Entry DOI: 10.7270/Q2BV7FXG |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50384944

(CHEMBL2036657)Show SMILES C[C@@H]1CCCN1CCCOc1ccc(cc1)C(=O)CN1CCN(CC1)C(=O)Oc1ccc(F)cc1 |r| Show InChI InChI=1S/C27H34FN3O4/c1-21-4-2-13-30(21)14-3-19-34-24-9-5-22(6-10-24)26(32)20-29-15-17-31(18-16-29)27(33)35-25-11-7-23(28)8-12-25/h5-12,21H,2-4,13-20H2,1H3/t21-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cephalon, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]NAMH from human histamine H3 receptor expressed in CHO cells |
Bioorg Med Chem Lett 22: 2807-10 (2012)
Article DOI: 10.1016/j.bmcl.2012.02.081
BindingDB Entry DOI: 10.7270/Q26M37VX |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50353164
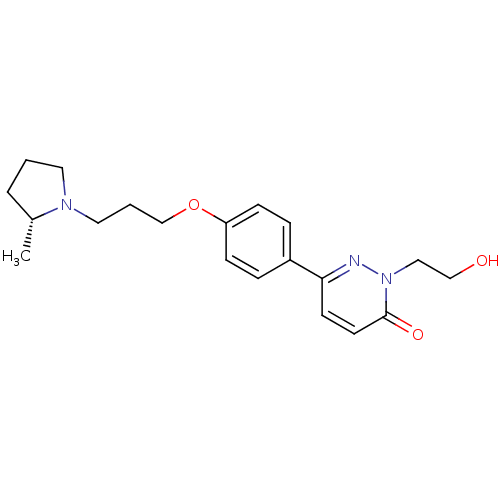
(CHEMBL1829469)Show SMILES C[C@@H]1CCCN1CCCOc1ccc(cc1)-c1ccc(=O)n(CCO)n1 |r| Show InChI InChI=1S/C20H27N3O3/c1-16-4-2-11-22(16)12-3-15-26-18-7-5-17(6-8-18)19-9-10-20(25)23(21-19)13-14-24/h5-10,16,24H,2-4,11-15H2,1H3/t16-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cephalon, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]NAMH from human cloned histamine 3 receptor expressed in CHO cells |
Bioorg Med Chem Lett 21: 5493-7 (2011)
Article DOI: 10.1016/j.bmcl.2011.06.108
BindingDB Entry DOI: 10.7270/Q2KP8359 |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50353163
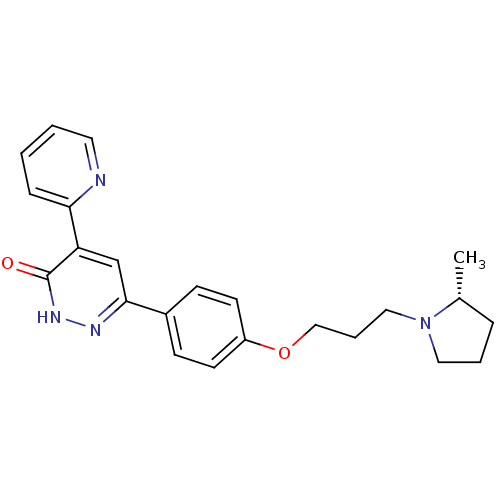
(CHEMBL1829474)Show SMILES C[C@@H]1CCCN1CCCOc1ccc(cc1)-c1cc(-c2ccccn2)c(=O)[nH]n1 |r| Show InChI InChI=1S/C23H26N4O2/c1-17-6-4-13-27(17)14-5-15-29-19-10-8-18(9-11-19)22-16-20(23(28)26-25-22)21-7-2-3-12-24-21/h2-3,7-12,16-17H,4-6,13-15H2,1H3,(H,26,28)/t17-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 3.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cephalon, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]NAMH from human cloned histamine 3 receptor expressed in CHO cells |
Bioorg Med Chem Lett 21: 5493-7 (2011)
Article DOI: 10.1016/j.bmcl.2011.06.108
BindingDB Entry DOI: 10.7270/Q2KP8359 |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Rattus norvegicus (rat)) | BDBM50352798

(CHEMBL1823402)Show InChI InChI=1S/C20H25N3O2/c1-22-20(24)10-9-19(21-22)15-5-7-17(8-6-15)25-18-11-13-23(14-12-18)16-3-2-4-16/h5-10,16,18H,2-4,11-14H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 3.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cephalon, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]NAMH from rat histamine H3 receptor expressed in CHO cells |
Bioorg Med Chem Lett 22: 1504-9 (2012)
Article DOI: 10.1016/j.bmcl.2012.01.026
BindingDB Entry DOI: 10.7270/Q2W37WSF |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data