

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50400528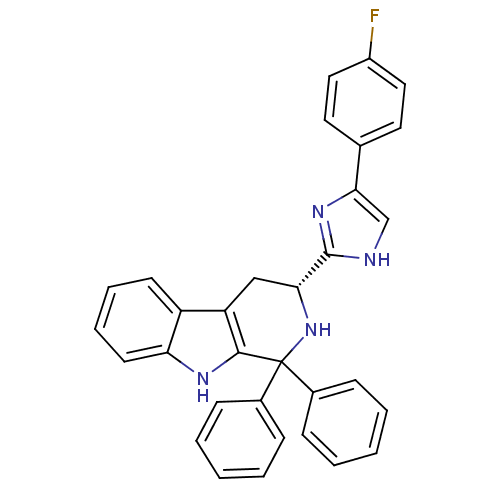 (CHEMBL2204934) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 44 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Displacement of radiolabeled MK-499 from human ERG channel | ACS Med Chem Lett 3: 484-489 (2012) Article DOI: 10.1021/ml300063m BindingDB Entry DOI: 10.7270/Q2V9897C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50400518 (CHEMBL2204942) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Displacement of radiolabeled MK-499 from human ERG channel | ACS Med Chem Lett 3: 484-489 (2012) Article DOI: 10.1021/ml300063m BindingDB Entry DOI: 10.7270/Q2V9897C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50389590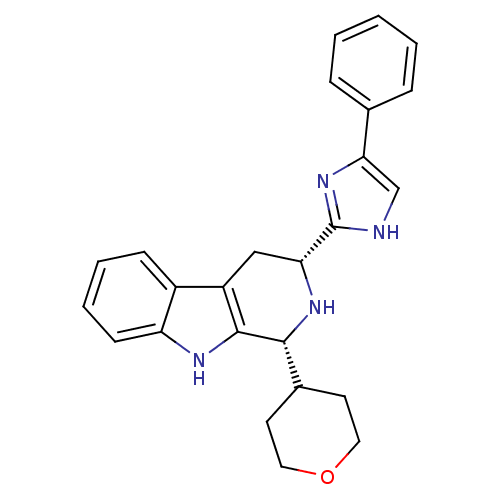 (CHEMBL2069502) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 369 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Displacement of radiolabeled MK-499 from human ERG channel | ACS Med Chem Lett 3: 484-489 (2012) Article DOI: 10.1021/ml300063m BindingDB Entry DOI: 10.7270/Q2V9897C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50400519 (CHEMBL2204941) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 377 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Displacement of radiolabeled MK-499 from human ERG channel | ACS Med Chem Lett 3: 484-489 (2012) Article DOI: 10.1021/ml300063m BindingDB Entry DOI: 10.7270/Q2V9897C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50400520 (CHEMBL2204932) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 385 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Displacement of radiolabeled MK-499 from human ERG channel | ACS Med Chem Lett 3: 484-489 (2012) Article DOI: 10.1021/ml300063m BindingDB Entry DOI: 10.7270/Q2V9897C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50400526 (CHEMBL2204937) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 462 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Displacement of radiolabeled MK-499 from human ERG channel | ACS Med Chem Lett 3: 484-489 (2012) Article DOI: 10.1021/ml300063m BindingDB Entry DOI: 10.7270/Q2V9897C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50400525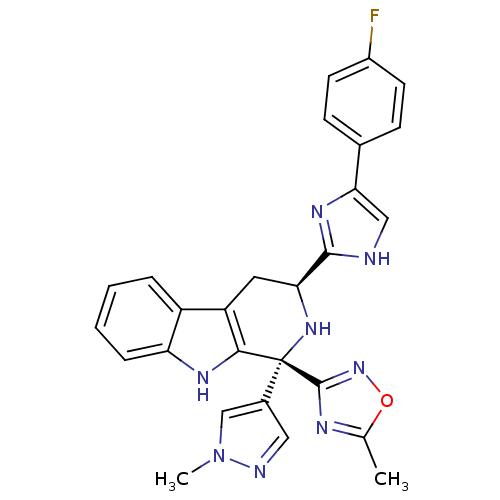 (CHEMBL2204938) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 543 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Displacement of radiolabeled MK-499 from human ERG channel | ACS Med Chem Lett 3: 484-489 (2012) Article DOI: 10.1021/ml300063m BindingDB Entry DOI: 10.7270/Q2V9897C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50400523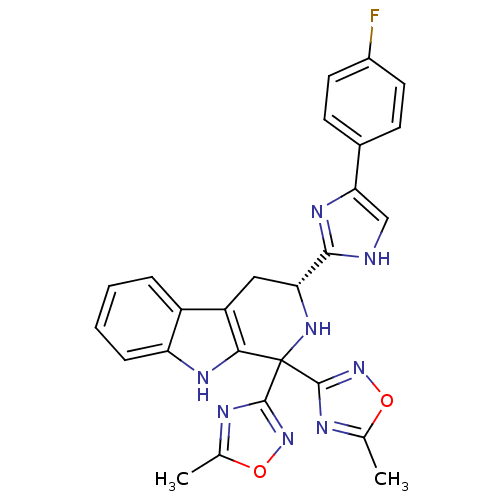 (CHEMBL2204940) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Displacement of radiolabeled MK-499 from human ERG channel | ACS Med Chem Lett 3: 484-489 (2012) Article DOI: 10.1021/ml300063m BindingDB Entry DOI: 10.7270/Q2V9897C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50400527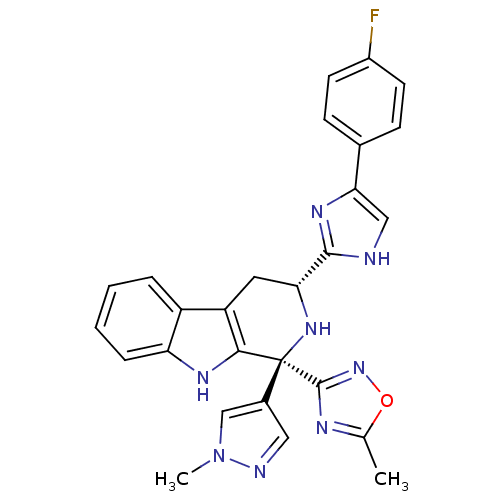 (CHEMBL2204936) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.64E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Displacement of radiolabeled MK-499 from human ERG channel | ACS Med Chem Lett 3: 484-489 (2012) Article DOI: 10.1021/ml300063m BindingDB Entry DOI: 10.7270/Q2V9897C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50400517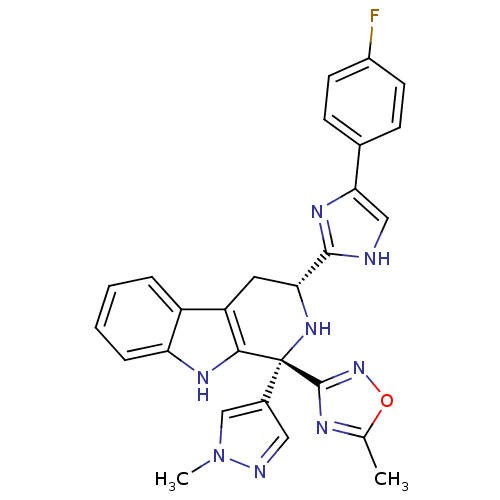 (CHEMBL2204935) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Displacement of radiolabeled MK-499 from human ERG channel | ACS Med Chem Lett 3: 484-489 (2012) Article DOI: 10.1021/ml300063m BindingDB Entry DOI: 10.7270/Q2V9897C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50400522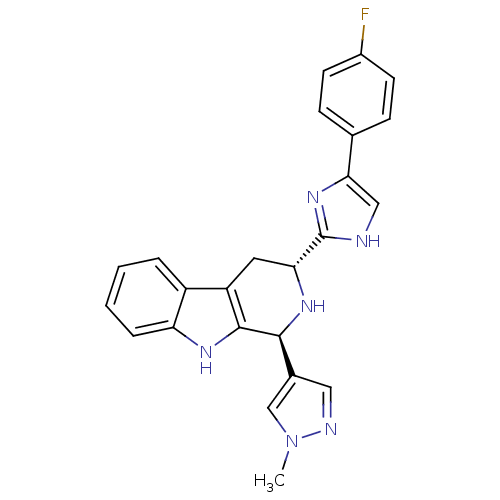 (CHEMBL2204931) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.19E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Displacement of radiolabeled MK-499 from human ERG channel | ACS Med Chem Lett 3: 484-489 (2012) Article DOI: 10.1021/ml300063m BindingDB Entry DOI: 10.7270/Q2V9897C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50400521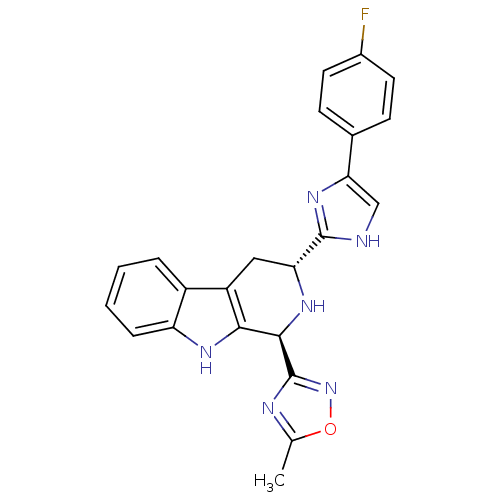 (CHEMBL2204933) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.21E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Displacement of radiolabeled MK-499 from human ERG channel | ACS Med Chem Lett 3: 484-489 (2012) Article DOI: 10.1021/ml300063m BindingDB Entry DOI: 10.7270/Q2V9897C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50400524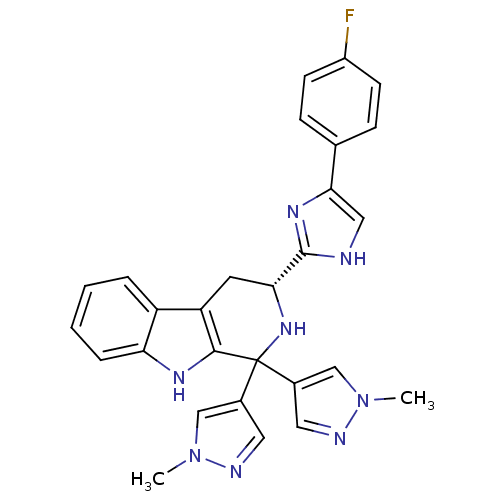 (CHEMBL2204939) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.62E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Displacement of radiolabeled MK-499 from human ERG channel | ACS Med Chem Lett 3: 484-489 (2012) Article DOI: 10.1021/ml300063m BindingDB Entry DOI: 10.7270/Q2V9897C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metallo-beta-lactamase type 2 (Klebsiella pneumoniae) | BDBM262675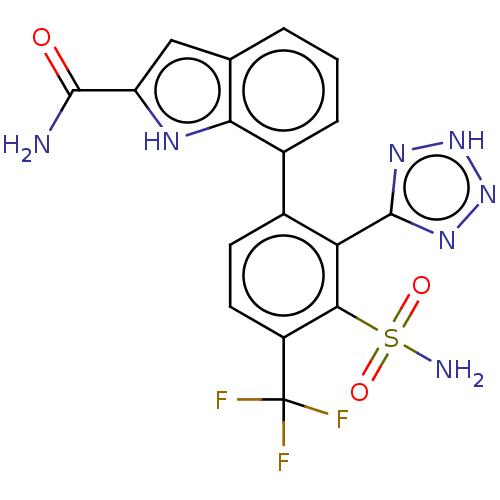 (7-(3-Sulfamoyl-2-(2H-tetrazol-5-yl)- 4-(trifluorom...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.0250 | n/a | n/a | n/a | n/a | 7.0 | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description The Class B enzyme activities were measured in the presence of the test inhibitor in a fluorescence assay against a commercially available substrate ... | US Patent US9708336 (2017) BindingDB Entry DOI: 10.7270/Q2FN186K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metallo-beta-lactamase type 2 (Klebsiella pneumoniae) | BDBM262700 (3-(2-amino-1,3-benzothiazol- 7-yl)-2-(1H-tetrazol-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.0500 | n/a | n/a | n/a | n/a | 7.0 | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description The Class B enzyme activities were measured in the presence of the test inhibitor in a fluorescence assay against a commercially available substrate ... | US Patent US9708336 (2017) BindingDB Entry DOI: 10.7270/Q2FN186K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| VIM-1 metallo-beta-lactamase (Klebsiella pneumoniae) | BDBM262700 (3-(2-amino-1,3-benzothiazol- 7-yl)-2-(1H-tetrazol-...) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.0620 | n/a | n/a | n/a | n/a | 7.0 | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description The Class B enzyme activities were measured in the presence of the test inhibitor in a fluorescence assay against a commercially available substrate ... | US Patent US9708336 (2017) BindingDB Entry DOI: 10.7270/Q2FN186K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| VIM-1 metallo-beta-lactamase (Klebsiella pneumoniae) | BDBM262696 (3-(2-amino-1,3-benzothiazol- 6-yl)-2-(1H-tetrazol-...) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.0630 | n/a | n/a | n/a | n/a | 7.0 | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description The Class B enzyme activities were measured in the presence of the test inhibitor in a fluorescence assay against a commercially available substrate ... | US Patent US9708336 (2017) BindingDB Entry DOI: 10.7270/Q2FN186K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| VIM-1 metallo-beta-lactamase (Klebsiella pneumoniae) | BDBM262972 (4-[4-[3-sulfamoyl-2-(1H-tetrazol-5- yl)-4- (triflu...) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.0810 | n/a | n/a | n/a | n/a | 7.0 | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description The Class B enzyme activities were measured in the presence of the test inhibitor in a fluorescence assay against a commercially available substrate ... | US Patent US9708336 (2017) BindingDB Entry DOI: 10.7270/Q2FN186K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| VIM-1 metallo-beta-lactamase (Klebsiella pneumoniae) | BDBM262672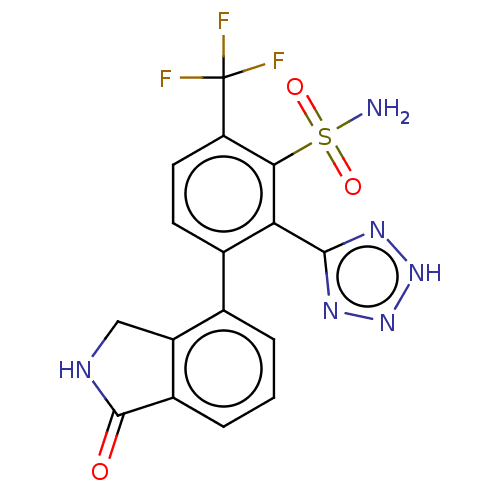 (3-(1-Oxoisoindolin-4-yl)-2-(2H- tetrazol-5-yl)-6- ...) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.0900 | n/a | n/a | n/a | n/a | 7.0 | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description The Class B enzyme activities were measured in the presence of the test inhibitor in a fluorescence assay against a commercially available substrate ... | US Patent US9708336 (2017) BindingDB Entry DOI: 10.7270/Q2FN186K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metallo-beta-lactamase type 2 (Klebsiella pneumoniae) | BDBM262715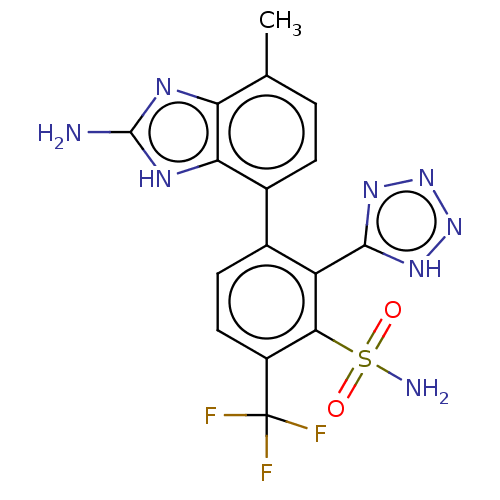 (3-(2-amino-4-methyl-1H-benzo[d]imidazol-7-yl)-2-(1...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.0980 | n/a | n/a | n/a | n/a | 7.0 | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description The Class B enzyme activities were measured in the presence of the test inhibitor in a fluorescence assay against a commercially available substrate ... | US Patent US9708336 (2017) BindingDB Entry DOI: 10.7270/Q2FN186K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| VIM-1 metallo-beta-lactamase (Klebsiella pneumoniae) | BDBM262898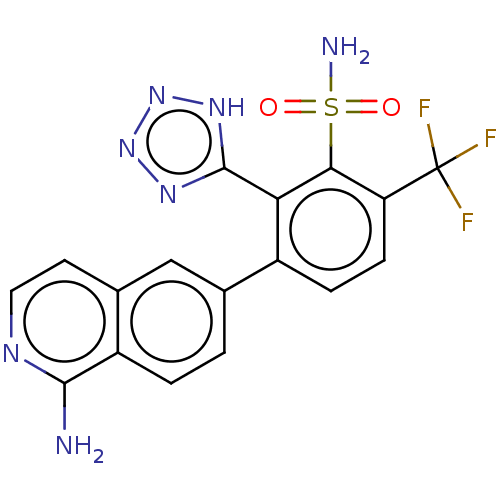 (3-(1-aminoisoquinolin-6-yl)-2- (1H-tetrazol-5-yl)-...) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.108 | n/a | n/a | n/a | n/a | 7.0 | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description The Class B enzyme activities were measured in the presence of the test inhibitor in a fluorescence assay against a commercially available substrate ... | US Patent US9708336 (2017) BindingDB Entry DOI: 10.7270/Q2FN186K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| VIM-1 metallo-beta-lactamase (Klebsiella pneumoniae) | BDBM262926 (3-[4-(4-piperidyl)-1-piperidyl]- 2-(1H-tetrazol-5-...) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.110 | n/a | n/a | n/a | n/a | 7.0 | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description The Class B enzyme activities were measured in the presence of the test inhibitor in a fluorescence assay against a commercially available substrate ... | US Patent US9708336 (2017) BindingDB Entry DOI: 10.7270/Q2FN186K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metallo-beta-lactamase type 2 (Klebsiella pneumoniae) | BDBM262701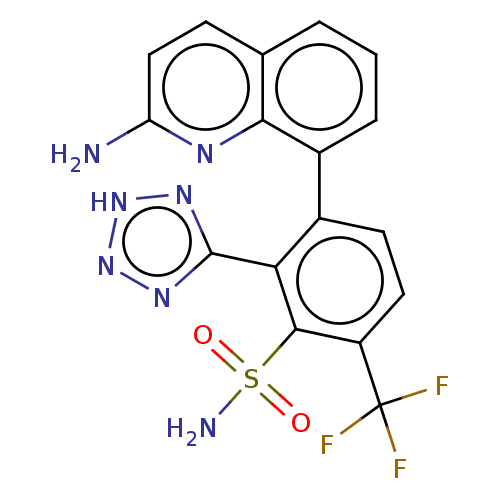 (3-(2-amino-8-quinolyl)-2-(2H- tetrazol-5-yl)-6- (t...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.115 | n/a | n/a | n/a | n/a | 7.0 | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description The Class B enzyme activities were measured in the presence of the test inhibitor in a fluorescence assay against a commercially available substrate ... | US Patent US9708336 (2017) BindingDB Entry DOI: 10.7270/Q2FN186K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| VIM-1 metallo-beta-lactamase (Klebsiella pneumoniae) | BDBM262703 (3-(2-aminoquinazolin-5-yl)-2- (2H-tetrazol-5-yl)-6...) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.122 | n/a | n/a | n/a | n/a | 7.0 | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description The Class B enzyme activities were measured in the presence of the test inhibitor in a fluorescence assay against a commercially available substrate ... | US Patent US9708336 (2017) BindingDB Entry DOI: 10.7270/Q2FN186K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metallo-beta-lactamase type 2 (Klebsiella pneumoniae) | BDBM262703 (3-(2-aminoquinazolin-5-yl)-2- (2H-tetrazol-5-yl)-6...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.125 | n/a | n/a | n/a | n/a | 7.0 | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description The Class B enzyme activities were measured in the presence of the test inhibitor in a fluorescence assay against a commercially available substrate ... | US Patent US9708336 (2017) BindingDB Entry DOI: 10.7270/Q2FN186K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metallo-beta-lactamase type 2 (Klebsiella pneumoniae) | BDBM262688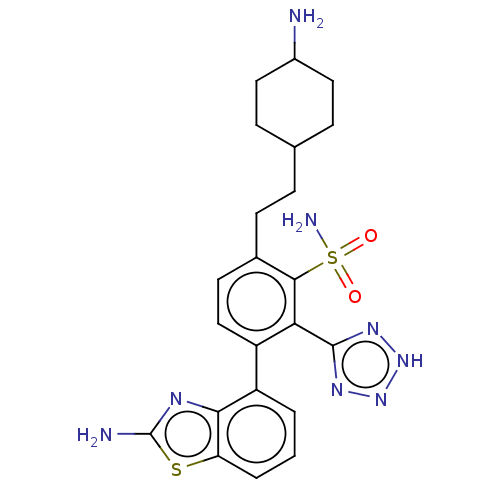 (3-(2-Aminobenzo[d]thiazol-4-yl)-6- (2-(4-aminocycl...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.133 | n/a | n/a | n/a | n/a | 7.0 | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description The Class B enzyme activities were measured in the presence of the test inhibitor in a fluorescence assay against a commercially available substrate ... | US Patent US9708336 (2017) BindingDB Entry DOI: 10.7270/Q2FN186K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| VIM-1 metallo-beta-lactamase (Klebsiella pneumoniae) | BDBM262641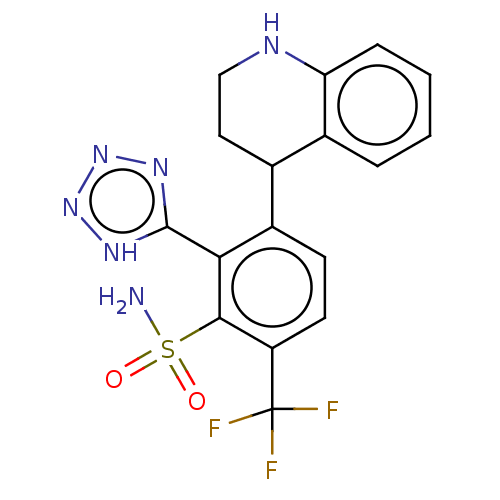 (3-(1,2,3,4-Tetrahydroquinolin-4-yl)-2-(1H-tetrazol...) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.133 | n/a | n/a | n/a | n/a | 7.0 | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description The Class B enzyme activities were measured in the presence of the test inhibitor in a fluorescence assay against a commercially available substrate ... | US Patent US9708336 (2017) BindingDB Entry DOI: 10.7270/Q2FN186K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| VIM-1 metallo-beta-lactamase (Klebsiella pneumoniae) | BDBM262634 (3-(Quinolin-5-yl)-2-(2H-tetrazol-5-yl)-6-(trifluor...) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.134 | n/a | n/a | n/a | n/a | 7.0 | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description The Class B enzyme activities were measured in the presence of the test inhibitor in a fluorescence assay against a commercially available substrate ... | US Patent US9708336 (2017) BindingDB Entry DOI: 10.7270/Q2FN186K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| VIM-1 metallo-beta-lactamase (Klebsiella pneumoniae) | BDBM262661 (5-(3-Sulfamoyl-2-(2H-tetrazol-5-yl)- 4-(trifluorom...) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.138 | n/a | n/a | n/a | n/a | 7.0 | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description The Class B enzyme activities were measured in the presence of the test inhibitor in a fluorescence assay against a commercially available substrate ... | US Patent US9708336 (2017) BindingDB Entry DOI: 10.7270/Q2FN186K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 3 (MOUSE) | BDBM50400525 (CHEMBL2204938) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.140 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of mouse SSTR3 transfected in CHO cells assessed as inhibition of SRIF-induced reduction of cAMP accumulation after 45 mins | ACS Med Chem Lett 3: 484-489 (2012) Article DOI: 10.1021/ml300063m BindingDB Entry DOI: 10.7270/Q2V9897C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metallo-beta-lactamase type 2 (Klebsiella pneumoniae) | BDBM262698 (3-(2-amino-3H-benzimidazol- 4-yl)-2-(1H-tetrazol-5...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.142 | n/a | n/a | n/a | n/a | 7.0 | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description The Class B enzyme activities were measured in the presence of the test inhibitor in a fluorescence assay against a commercially available substrate ... | US Patent US9708336 (2017) BindingDB Entry DOI: 10.7270/Q2FN186K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| VIM-1 metallo-beta-lactamase (Klebsiella pneumoniae) | BDBM262855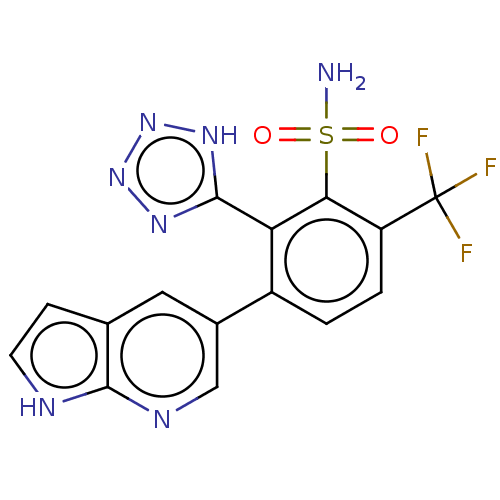 (3-(1H-pyrrolo[2,3-b]pyridin-5- yl)-2-(1H-tetrazol-...) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.144 | n/a | n/a | n/a | n/a | 7.0 | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description The Class B enzyme activities were measured in the presence of the test inhibitor in a fluorescence assay against a commercially available substrate ... | US Patent US9708336 (2017) BindingDB Entry DOI: 10.7270/Q2FN186K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metallo-beta-lactamase type 2 (Klebsiella pneumoniae) | BDBM262705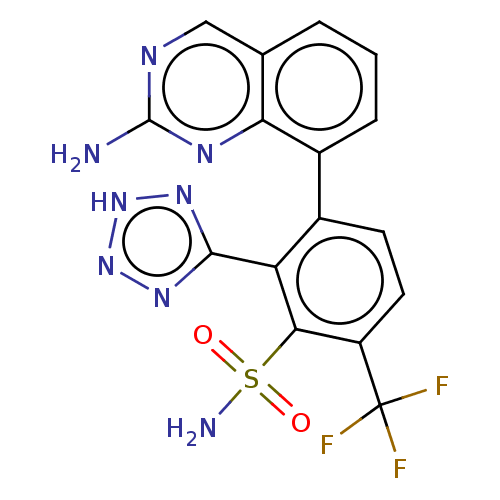 (3-(2-aminoquinazolin-8-yl)-2- (2H-tetrazol-5-yl)-6...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.145 | n/a | n/a | n/a | n/a | 7.0 | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description The Class B enzyme activities were measured in the presence of the test inhibitor in a fluorescence assay against a commercially available substrate ... | US Patent US9708336 (2017) BindingDB Entry DOI: 10.7270/Q2FN186K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| VIM-1 metallo-beta-lactamase (Klebsiella pneumoniae) | BDBM262675 (7-(3-Sulfamoyl-2-(2H-tetrazol-5-yl)- 4-(trifluorom...) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.146 | n/a | n/a | n/a | n/a | 7.0 | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description The Class B enzyme activities were measured in the presence of the test inhibitor in a fluorescence assay against a commercially available substrate ... | US Patent US9708336 (2017) BindingDB Entry DOI: 10.7270/Q2FN186K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| VIM-1 metallo-beta-lactamase (Klebsiella pneumoniae) | BDBM262890 (3-(2-amino-4-fluoro-1,3- benzothiazol-6-yl)-2-(1H-...) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.147 | n/a | n/a | n/a | n/a | 7.0 | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description The Class B enzyme activities were measured in the presence of the test inhibitor in a fluorescence assay against a commercially available substrate ... | US Patent US9708336 (2017) BindingDB Entry DOI: 10.7270/Q2FN186K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metallo-beta-lactamase type 2 (Klebsiella pneumoniae) | BDBM262571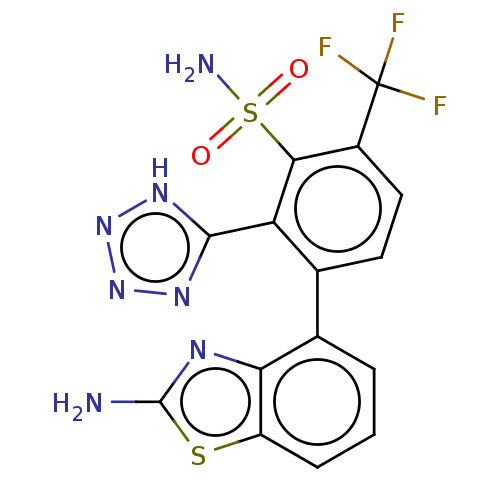 (3-(2-amino-1,3-benzothiazol-4-yl)-2-(2H-tetrazol-5...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | MCE PC cid PC sid UniChem | US Patent | n/a | n/a | 0.153 | n/a | n/a | n/a | n/a | 7.0 | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description The Class B enzyme activities were measured in the presence of the test inhibitor in a fluorescence assay against a commercially available substrate ... | US Patent US9708336 (2017) BindingDB Entry DOI: 10.7270/Q2FN186K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metallo-beta-lactamase type 2 (Klebsiella pneumoniae) | BDBM262972 (4-[4-[3-sulfamoyl-2-(1H-tetrazol-5- yl)-4- (triflu...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.157 | n/a | n/a | n/a | n/a | 7.0 | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description The Class B enzyme activities were measured in the presence of the test inhibitor in a fluorescence assay against a commercially available substrate ... | US Patent US9708336 (2017) BindingDB Entry DOI: 10.7270/Q2FN186K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| VIM-1 metallo-beta-lactamase (Klebsiella pneumoniae) | BDBM262715 (3-(2-amino-4-methyl-1H-benzo[d]imidazol-7-yl)-2-(1...) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.161 | n/a | n/a | n/a | n/a | 7.0 | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description The Class B enzyme activities were measured in the presence of the test inhibitor in a fluorescence assay against a commercially available substrate ... | US Patent US9708336 (2017) BindingDB Entry DOI: 10.7270/Q2FN186K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| VIM-1 metallo-beta-lactamase (Klebsiella pneumoniae) | BDBM262421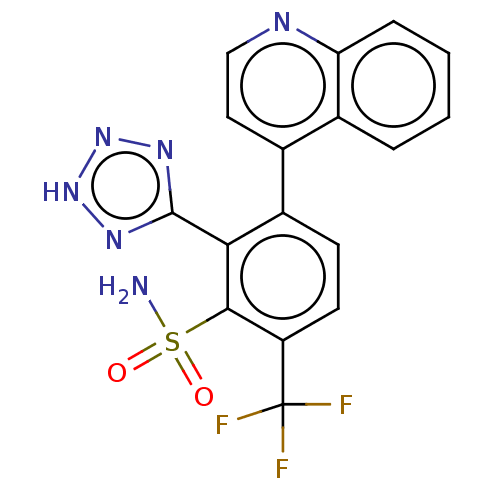 (US9708336, 273) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.165 | n/a | n/a | n/a | n/a | 7.0 | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description The Class B enzyme activities were measured in the presence of the test inhibitor in a fluorescence assay against a commercially available substrate ... | US Patent US9708336 (2017) BindingDB Entry DOI: 10.7270/Q2FN186K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| VIM-1 metallo-beta-lactamase (Klebsiella pneumoniae) | BDBM262857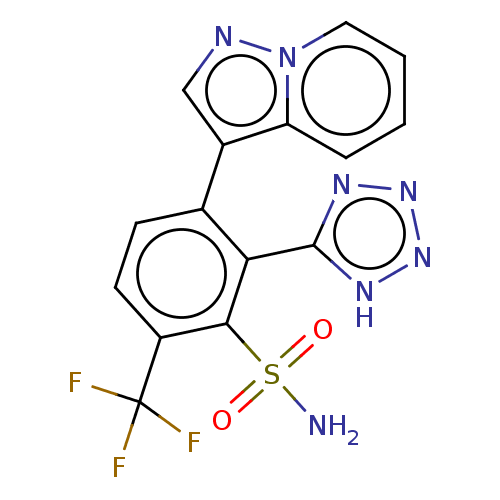 (3-pyrazolo[1,5-a]pyridin-3-yl-2- (1H-tetrazol-5-yl...) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.168 | n/a | n/a | n/a | n/a | 7.0 | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description The Class B enzyme activities were measured in the presence of the test inhibitor in a fluorescence assay against a commercially available substrate ... | US Patent US9708336 (2017) BindingDB Entry DOI: 10.7270/Q2FN186K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| VIM-1 metallo-beta-lactamase (Klebsiella pneumoniae) | BDBM262595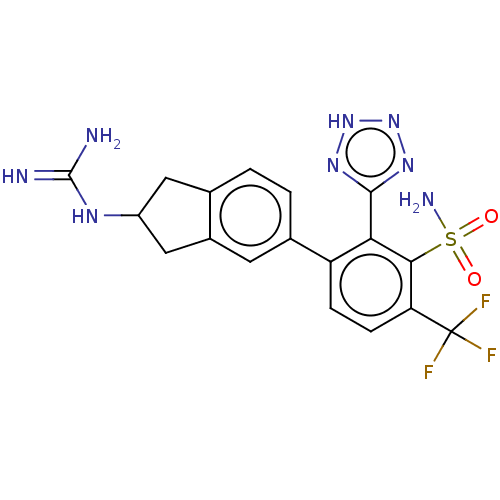 (3-(2-guanidino-2,3-dihydro-1H-inden-5- yl)-2-(2H-t...) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.171 | n/a | n/a | n/a | n/a | 7.0 | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description The Class B enzyme activities were measured in the presence of the test inhibitor in a fluorescence assay against a commercially available substrate ... | US Patent US9708336 (2017) BindingDB Entry DOI: 10.7270/Q2FN186K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| VIM-1 metallo-beta-lactamase (Klebsiella pneumoniae) | BDBM262892 (3-(2-aminoquinolin-6-yl)-2-(1H- tetrazol-5-yl)-6- ...) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.175 | n/a | n/a | n/a | n/a | 7.0 | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description The Class B enzyme activities were measured in the presence of the test inhibitor in a fluorescence assay against a commercially available substrate ... | US Patent US9708336 (2017) BindingDB Entry DOI: 10.7270/Q2FN186K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| VIM-1 metallo-beta-lactamase (Klebsiella pneumoniae) | BDBM262582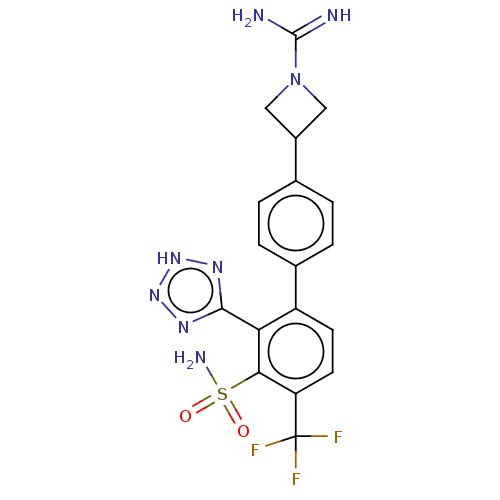 (3-(3′-sulfamoyl-2′-(2H-tetrazol-5-yl)-...) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.175 | n/a | n/a | n/a | n/a | 7.0 | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description The Class B enzyme activities were measured in the presence of the test inhibitor in a fluorescence assay against a commercially available substrate ... | US Patent US9708336 (2017) BindingDB Entry DOI: 10.7270/Q2FN186K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| VIM-1 metallo-beta-lactamase (Klebsiella pneumoniae) | BDBM262702 (3-(6-amino-2-methyl-3- pyridyl)-2-(2H-tetrazol-5-y...) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.177 | n/a | n/a | n/a | n/a | 7.0 | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description The Class B enzyme activities were measured in the presence of the test inhibitor in a fluorescence assay against a commercially available substrate ... | US Patent US9708336 (2017) BindingDB Entry DOI: 10.7270/Q2FN186K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| VIM-1 metallo-beta-lactamase (Klebsiella pneumoniae) | BDBM262705 (3-(2-aminoquinazolin-8-yl)-2- (2H-tetrazol-5-yl)-6...) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.178 | n/a | n/a | n/a | n/a | 7.0 | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description The Class B enzyme activities were measured in the presence of the test inhibitor in a fluorescence assay against a commercially available substrate ... | US Patent US9708336 (2017) BindingDB Entry DOI: 10.7270/Q2FN186K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| VIM-1 metallo-beta-lactamase (Klebsiella pneumoniae) | BDBM262651 (US9708336, 503) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.183 | n/a | n/a | n/a | n/a | 7.0 | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description The Class B enzyme activities were measured in the presence of the test inhibitor in a fluorescence assay against a commercially available substrate ... | US Patent US9708336 (2017) BindingDB Entry DOI: 10.7270/Q2FN186K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| VIM-1 metallo-beta-lactamase (Klebsiella pneumoniae) | BDBM262902 (3-[4-(2-aminoethyl)piperidin-1- yl]-2-(1H-tetrazol...) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.191 | n/a | n/a | n/a | n/a | 7.0 | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description The Class B enzyme activities were measured in the presence of the test inhibitor in a fluorescence assay against a commercially available substrate ... | US Patent US9708336 (2017) BindingDB Entry DOI: 10.7270/Q2FN186K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metallo-beta-lactamase type 2 (Klebsiella pneumoniae) | BDBM262634 (3-(Quinolin-5-yl)-2-(2H-tetrazol-5-yl)-6-(trifluor...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.192 | n/a | n/a | n/a | n/a | 7.0 | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description The Class B enzyme activities were measured in the presence of the test inhibitor in a fluorescence assay against a commercially available substrate ... | US Patent US9708336 (2017) BindingDB Entry DOI: 10.7270/Q2FN186K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metallo-beta-lactamase type 2 (Klebsiella pneumoniae) | BDBM262686 (3-(4-(2-Aminobenzo[d]thiazol-4-yl)- 2-sulfamoyl-3-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.192 | n/a | n/a | n/a | n/a | 7.0 | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description The Class B enzyme activities were measured in the presence of the test inhibitor in a fluorescence assay against a commercially available substrate ... | US Patent US9708336 (2017) BindingDB Entry DOI: 10.7270/Q2FN186K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| VIM-1 metallo-beta-lactamase (Klebsiella pneumoniae) | BDBM262630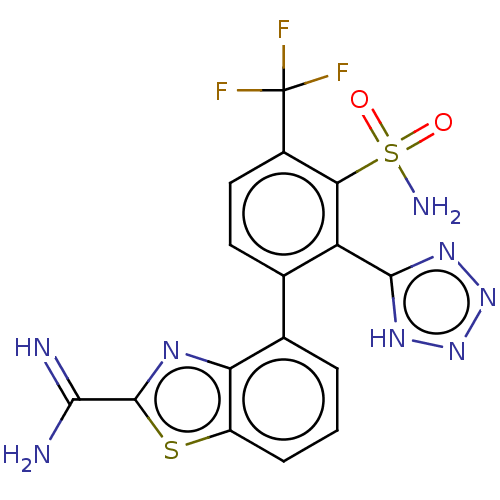 (4-(3-Sulfamoyl-2-(1H-tetrazol-5-yl)-4-(trifluorome...) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.194 | n/a | n/a | n/a | n/a | 7.0 | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description The Class B enzyme activities were measured in the presence of the test inhibitor in a fluorescence assay against a commercially available substrate ... | US Patent US9708336 (2017) BindingDB Entry DOI: 10.7270/Q2FN186K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 3220 total ) | Next | Last >> |