

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Prostaglandin-H2 D-isomerase (Homo sapiens (Human)) | BDBM50008805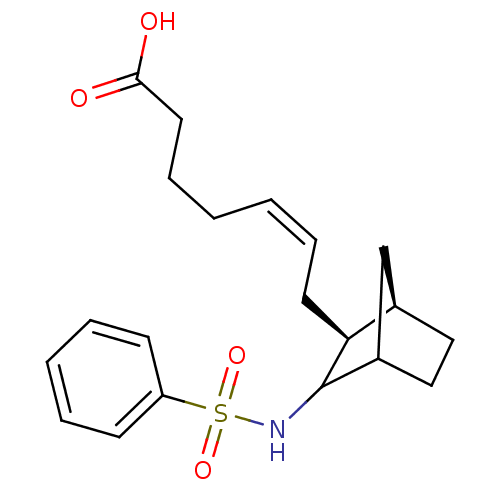 (7-(3-Benzenesulfonylamino-bicyclo[2.2.1]hept-2-yl)...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.340 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi & Co. Curated by PDSP Ki Database | J Pharmacol Exp Ther 298: 411-9 (2001) BindingDB Entry DOI: 10.7270/Q2028Q3C | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin-H2 D-isomerase (GUINEA PIG) | BDBM50008805 (7-(3-Benzenesulfonylamino-bicyclo[2.2.1]hept-2-yl)...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.490 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi & Co. Curated by PDSP Ki Database | J Pharmacol Exp Ther 298: 411-9 (2001) BindingDB Entry DOI: 10.7270/Q2028Q3C | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin-H2 D-isomerase (Homo sapiens (Human)) | BDBM50060462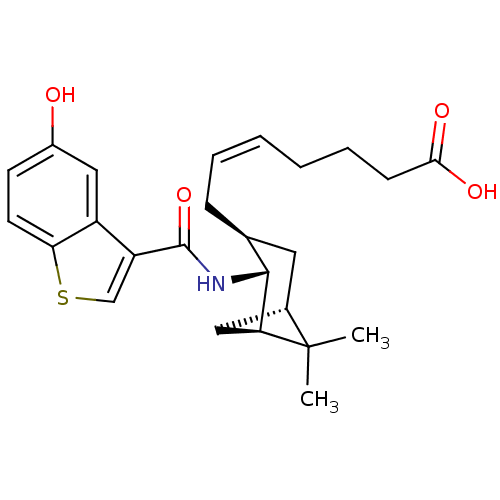 ((Z)-7-{(1R,2R,3S,5S)-2-[(5-Hydroxy-benzo[b]thiophe...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi & Co. Curated by PDSP Ki Database | J Pharmacol Exp Ther 298: 411-9 (2001) BindingDB Entry DOI: 10.7270/Q2028Q3C | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin-H2 D-isomerase (Homo sapiens (Human)) | BDBM50060462 ((Z)-7-{(1R,2R,3S,5S)-2-[(5-Hydroxy-benzo[b]thiophe...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | 24.2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi & Co. Curated by PDSP Ki Database | J Pharmacol Exp Ther 298: 411-9 (2001) BindingDB Entry DOI: 10.7270/Q2028Q3C | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin-H2 D-isomerase (GUINEA PIG) | BDBM50060462 ((Z)-7-{(1R,2R,3S,5S)-2-[(5-Hydroxy-benzo[b]thiophe...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | 193 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi & Co. Curated by PDSP Ki Database | J Pharmacol Exp Ther 298: 411-9 (2001) BindingDB Entry DOI: 10.7270/Q2028Q3C | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin-H2 D-isomerase (Homo sapiens (Human)) | BDBM85347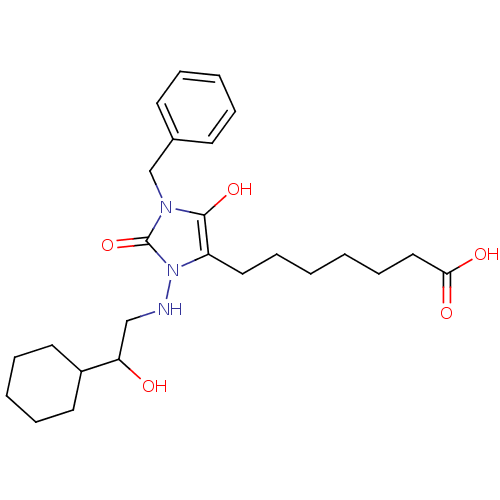 (BWA868C | CAS_122021 | NSC_122021) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Patents | PubMed | 2.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi & Co. Curated by PDSP Ki Database | J Pharmacol Exp Ther 298: 411-9 (2001) BindingDB Entry DOI: 10.7270/Q2028Q3C | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin-H2 D-isomerase (Homo sapiens (Human)) | BDBM50008805 (7-(3-Benzenesulfonylamino-bicyclo[2.2.1]hept-2-yl)...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi & Co. Curated by PDSP Ki Database | J Pharmacol Exp Ther 298: 411-9 (2001) BindingDB Entry DOI: 10.7270/Q2028Q3C | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin-H2 D-isomerase (Mus musculus) | BDBM50009854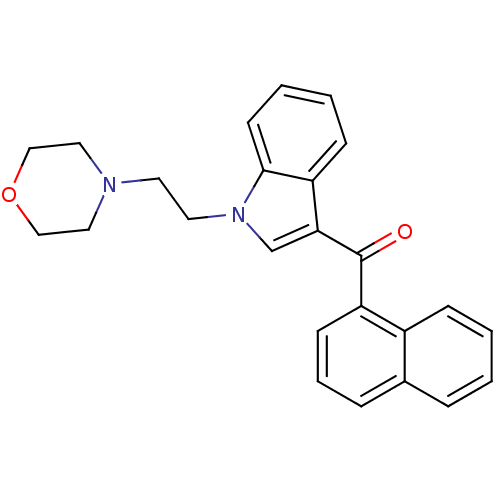 (CHEMBL13078 | [1-(2-Morpholin-4-yl-ethyl)-1H-indol...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Sterling Research Group Curated by ChEMBL | Assay Description Concentration required to inhibit 50% activity of prostaglandin synthetase was determined in vitro in mouse brain microsomes | J Med Chem 34: 1099-110 (1991) BindingDB Entry DOI: 10.7270/Q24M955S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin-H2 D-isomerase (Mus musculus) | BDBM50009864 (CHEMBL274833 | [2-Methyl-1-(2-morpholin-4-yl-ethyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Sterling Research Group Curated by ChEMBL | Assay Description Concentration required to inhibit 50% activity of prostaglandin synthetase was determined in vitro in mouse brain microsomes | J Med Chem 34: 1099-110 (1991) BindingDB Entry DOI: 10.7270/Q24M955S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin-H2 D-isomerase (Mus musculus) | BDBM50009871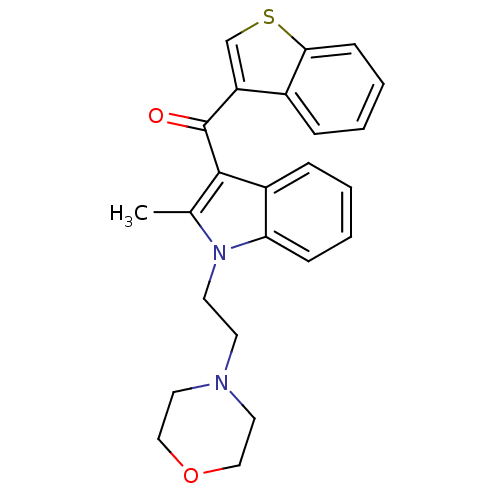 (Benzo[b]thiophen-3-yl-[2-methyl-1-(2-morpholin-4-y...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
Sterling Research Group Curated by ChEMBL | Assay Description Concentration required to inhibit 50% activity of prostaglandin synthetase was determined in vitro in mouse brain microsomes | J Med Chem 34: 1099-110 (1991) BindingDB Entry DOI: 10.7270/Q24M955S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hematopoietic prostaglandin D synthase (Rattus norvegicus) | BDBM50576319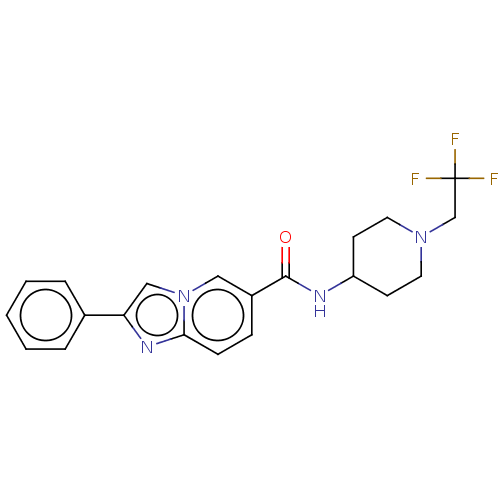 (CHEMBL4868541) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 84 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of HPGDS in rat RBL cells assessed as reduction in PGD2 formation using PGH2 as substrate by mass spectrometry | Citation and Details Article DOI: 10.1016/j.bmcl.2021.128113 BindingDB Entry DOI: 10.7270/Q2B28047 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hematopoietic prostaglandin D synthase (Rattus norvegicus) | BDBM50526485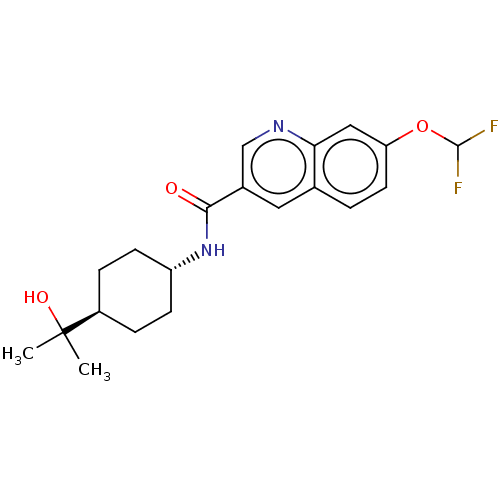 (CHEMBL4473072) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of HPGDS in RBL cells assessed as reduction in A23187-induced PGD2 production preincubated for 30 mins followed by A23187 addition and mea... | Bioorg Med Chem 27: 1456-1478 (2019) Article DOI: 10.1016/j.bmc.2019.02.017 BindingDB Entry DOI: 10.7270/Q2QV3R00 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hematopoietic prostaglandin D synthase (Rattus norvegicus) | BDBM50526486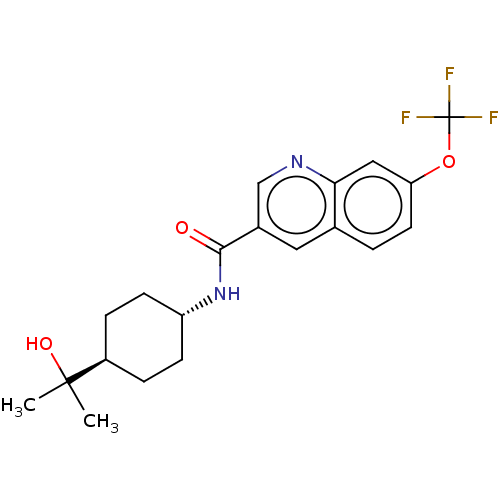 (CHEMBL4526974) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of HPGDS in RBL cells assessed as reduction in A23187-induced PGD2 production preincubated for 30 mins followed by A23187 addition and mea... | Bioorg Med Chem 27: 1456-1478 (2019) Article DOI: 10.1016/j.bmc.2019.02.017 BindingDB Entry DOI: 10.7270/Q2QV3R00 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hematopoietic prostaglandin D synthase (Rattus norvegicus) | BDBM50576314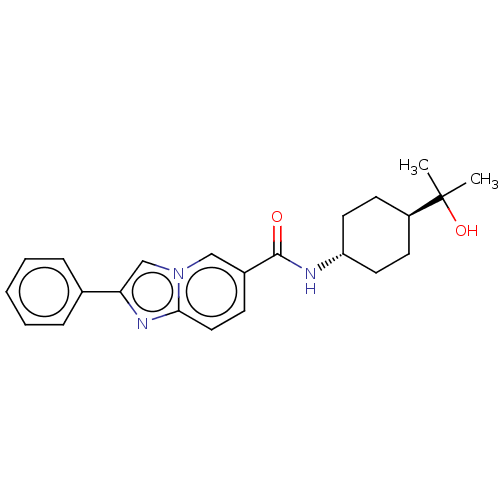 (CHEMBL4864805) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of HPGDS in rat RBL cells assessed as reduction in PGD2 formation using PGH2 as substrate by mass spectrometry | Citation and Details Article DOI: 10.1016/j.bmcl.2021.128113 BindingDB Entry DOI: 10.7270/Q2B28047 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hematopoietic prostaglandin D synthase (Rattus norvegicus) | BDBM50576316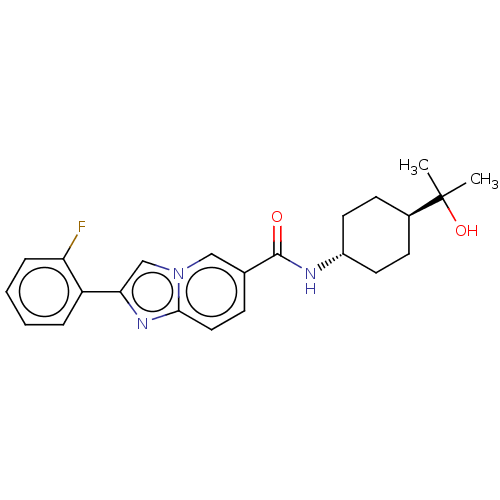 (CHEMBL4870703) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 120 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of HPGDS in rat RBL cells assessed as reduction in PGD2 formation using PGH2 as substrate by mass spectrometry | Citation and Details Article DOI: 10.1016/j.bmcl.2021.128113 BindingDB Entry DOI: 10.7270/Q2B28047 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hematopoietic prostaglandin D synthase (Rattus norvegicus) | BDBM50576315 (CHEMBL4873385) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 160 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of HPGDS in rat RBL cells assessed as reduction in PGD2 formation using PGH2 as substrate by mass spectrometry | Citation and Details Article DOI: 10.1016/j.bmcl.2021.128113 BindingDB Entry DOI: 10.7270/Q2B28047 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin-H2 D-isomerase (Mus musculus) | BDBM50406486 (CHEMBL2092862) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 160 | n/a | n/a | n/a | n/a | n/a | n/a |
Sterling Research Group Curated by ChEMBL | Assay Description Concentration required to inhibit 50% activity of prostaglandin synthetase was determined in vitro in mouse brain microsomes | J Med Chem 34: 1099-110 (1991) BindingDB Entry DOI: 10.7270/Q24M955S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hematopoietic prostaglandin D synthase (Rattus norvegicus) | BDBM50576322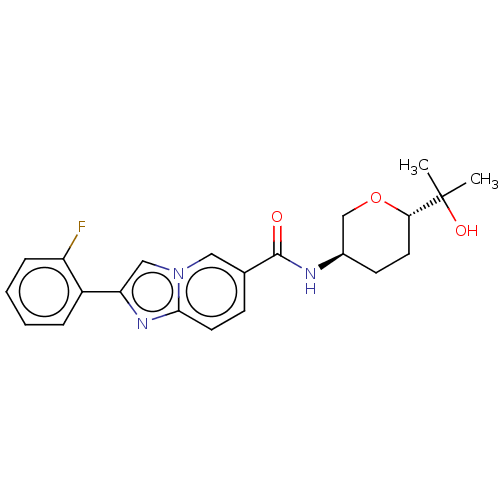 (CHEMBL4859922) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 190 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of HPGDS in rat RBL cells assessed as reduction in PGD2 formation using PGH2 as substrate by mass spectrometry | Citation and Details Article DOI: 10.1016/j.bmcl.2021.128113 BindingDB Entry DOI: 10.7270/Q2B28047 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin-H2 D-isomerase (Mus musculus) | BDBM50009856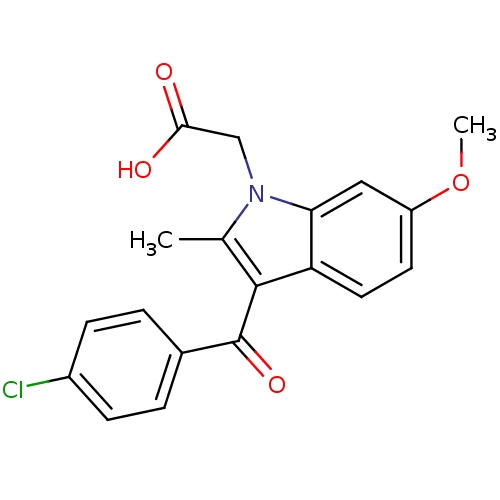 (CHEMBL13376 | Clometacin | [3-(4-Chloro-benzoyl)-6...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
Sterling Research Group Curated by ChEMBL | Assay Description Concentration required to inhibit 50% activity of prostaglandin synthetase was determined in vitro in mouse brain microsomes | J Med Chem 34: 1099-110 (1991) BindingDB Entry DOI: 10.7270/Q24M955S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin-H2 D-isomerase (Mus musculus) | BDBM85511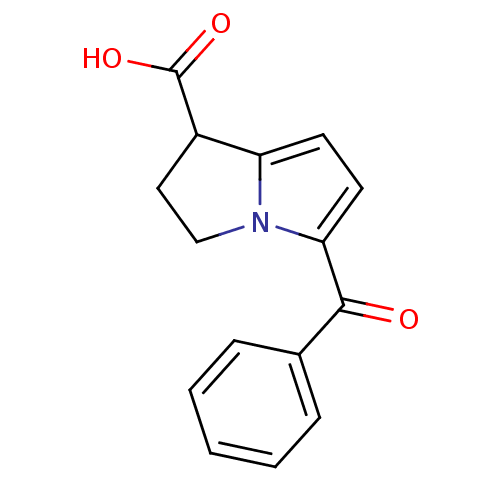 (CAS_74103-07-4 | KETOROLAC | Ketorolac tris salt |...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase DrugBank MCE KEGG PC cid PC sid PDB UniChem Similars | PubMed | n/a | n/a | 230 | n/a | n/a | n/a | n/a | n/a | n/a |
Sterling Research Group Curated by ChEMBL | Assay Description Concentration required to inhibit 50% activity of prostaglandin synthetase was determined in vitro in mouse brain microsomes | J Med Chem 34: 1099-110 (1991) BindingDB Entry DOI: 10.7270/Q24M955S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin-H2 D-isomerase (Mus musculus) | BDBM50009858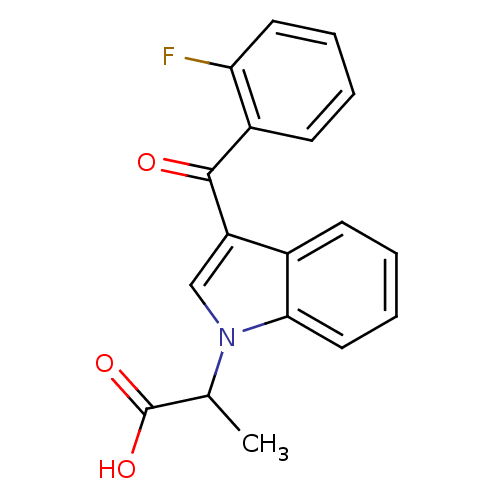 ((R) 2-[3-(2-Fluoro-benzoyl)-indol-1-yl]-propionic ...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
Sterling Research Group Curated by ChEMBL | Assay Description Concentration required to inhibit 50% activity of prostaglandin synthetase was determined in vitro in mouse brain microsomes | J Med Chem 34: 1099-110 (1991) BindingDB Entry DOI: 10.7270/Q24M955S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hematopoietic prostaglandin D synthase (Rattus norvegicus) | BDBM50576323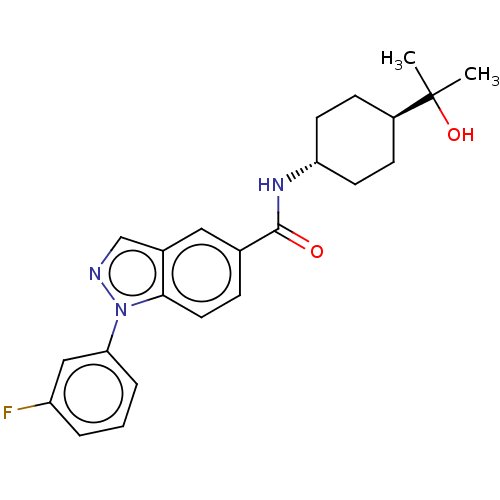 (CHEMBL4866146) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 340 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of HPGDS in rat RBL cells assessed as reduction in PGD2 formation using PGH2 as substrate by mass spectrometry | Citation and Details Article DOI: 10.1016/j.bmcl.2021.128113 BindingDB Entry DOI: 10.7270/Q2B28047 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hematopoietic prostaglandin D synthase (Rattus norvegicus) | BDBM50576320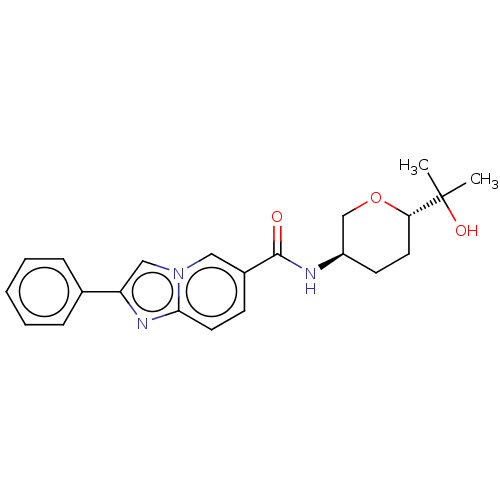 (CHEMBL4847268) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 460 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of HPGDS in rat RBL cells assessed as reduction in PGD2 formation using PGH2 as substrate by mass spectrometry | Citation and Details Article DOI: 10.1016/j.bmcl.2021.128113 BindingDB Entry DOI: 10.7270/Q2B28047 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin-H2 D-isomerase (Mus musculus) | BDBM50009865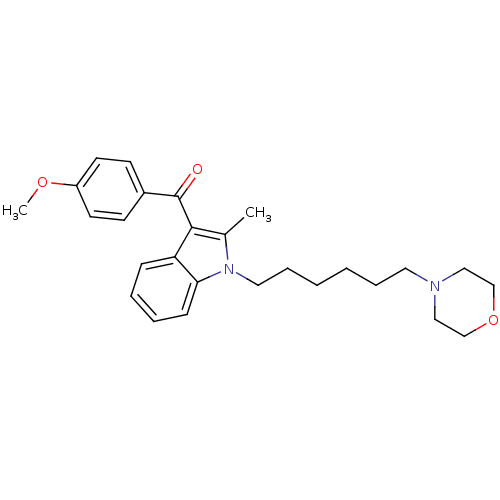 ((4-Methoxy-phenyl)-[2-methyl-1-(6-morpholin-4-yl-h...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 500 | n/a | n/a | n/a | n/a | n/a | n/a |
Sterling Research Group Curated by ChEMBL | Assay Description Concentration required to inhibit 50% activity of prostaglandin synthetase was determined in vitro in mouse brain microsomes | J Med Chem 34: 1099-110 (1991) BindingDB Entry DOI: 10.7270/Q24M955S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin-H2 D-isomerase (Mus musculus) | BDBM17638 (2-{1-[(4-chlorophenyl)carbonyl]-5-methoxy-2-methyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PubMed | n/a | n/a | 500 | n/a | n/a | n/a | n/a | n/a | n/a |
Sterling Research Group Curated by ChEMBL | Assay Description Concentration required to inhibit 50% activity of prostaglandin synthetase was determined in vitro in mouse brain microsomes | J Med Chem 34: 1099-110 (1991) BindingDB Entry DOI: 10.7270/Q24M955S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hematopoietic prostaglandin D synthase (Rattus norvegicus) | BDBM50576325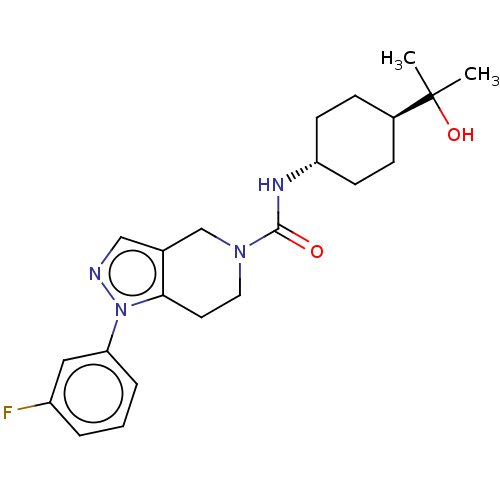 (CHEMBL4852189) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | 510 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of HPGDS in rat RBL cells assessed as reduction in PGD2 formation using PGH2 as substrate by mass spectrometry | Citation and Details Article DOI: 10.1016/j.bmcl.2021.128113 BindingDB Entry DOI: 10.7270/Q2B28047 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin-H2 D-isomerase (Mus musculus) | BDBM50009862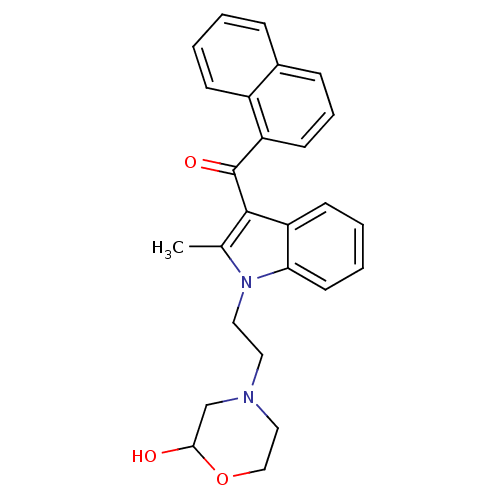 (CHEMBL12566 | {1-[2-(2-Hydroxy-morpholin-4-yl)-eth...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 570 | n/a | n/a | n/a | n/a | n/a | n/a |
Sterling Research Group Curated by ChEMBL | Assay Description Concentration required to inhibit 50% activity of prostaglandin synthetase was determined in mouse vas deferens | J Med Chem 34: 1099-110 (1991) BindingDB Entry DOI: 10.7270/Q24M955S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin-H2 D-isomerase (Mus musculus) | BDBM50009852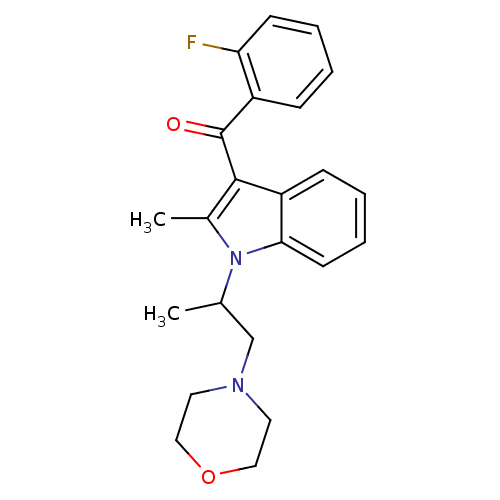 ((2-Fluoro-phenyl)-[2-methyl-1-(1-methyl-2-morpholi...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 700 | n/a | n/a | n/a | n/a | n/a | n/a |
Sterling Research Group Curated by ChEMBL | Assay Description Concentration required to inhibit 50% activity of prostaglandin synthetase was determined in vitro in mouse brain microsomes | J Med Chem 34: 1099-110 (1991) BindingDB Entry DOI: 10.7270/Q24M955S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin-H2 D-isomerase (Mus musculus) | BDBM50368233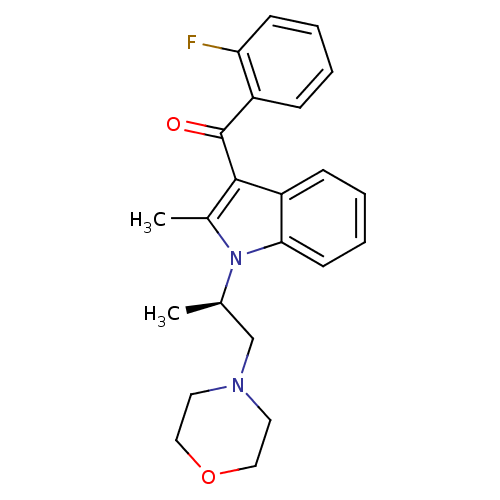 (CHEMBL1907833) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 700 | n/a | n/a | n/a | n/a | n/a | n/a |
Sterling Research Group Curated by ChEMBL | Assay Description Concentration required to inhibit 50% activity of prostaglandin synthetase was determined in vitro in mouse brain microsomes | J Med Chem 34: 1099-110 (1991) BindingDB Entry DOI: 10.7270/Q24M955S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hematopoietic prostaglandin D synthase (Rattus norvegicus) | BDBM50576321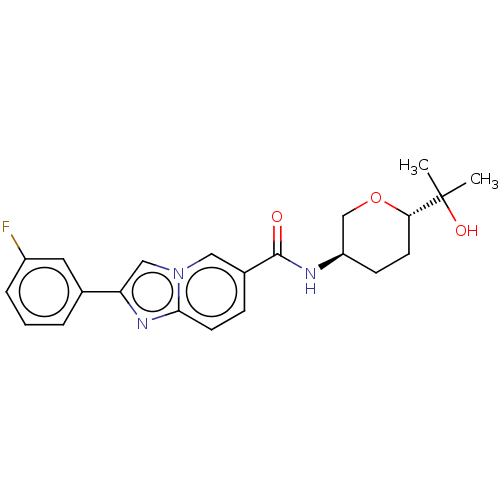 (CHEMBL4872589) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 710 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of HPGDS in rat RBL cells assessed as reduction in PGD2 formation using PGH2 as substrate by mass spectrometry | Citation and Details Article DOI: 10.1016/j.bmcl.2021.128113 BindingDB Entry DOI: 10.7270/Q2B28047 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hematopoietic prostaglandin D synthase (Rattus norvegicus) | BDBM50576324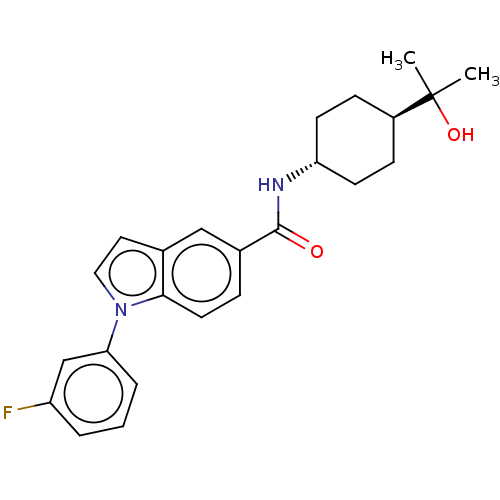 (CHEMBL4868339) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 780 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of HPGDS in rat RBL cells assessed as reduction in PGD2 formation using PGH2 as substrate by mass spectrometry | Citation and Details Article DOI: 10.1016/j.bmcl.2021.128113 BindingDB Entry DOI: 10.7270/Q2B28047 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin-H2 D-isomerase (Mus musculus) | BDBM50009857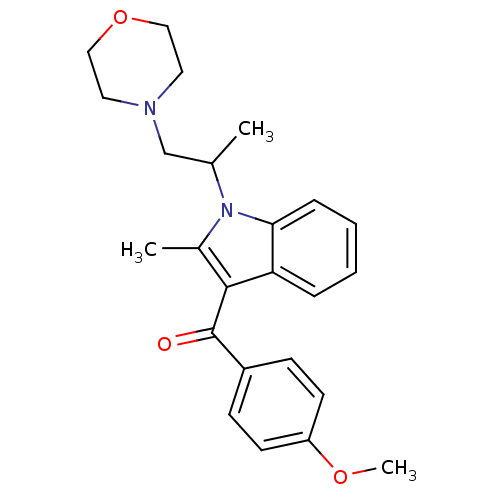 ((4-Methoxy-phenyl)-[2-methyl-1-(1-methyl-2-morphol...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 900 | n/a | n/a | n/a | n/a | n/a | n/a |
Sterling Research Group Curated by ChEMBL | Assay Description Concentration required to inhibit 50% activity of prostaglandin synthetase was determined in vitro in mouse brain microsomes | J Med Chem 34: 1099-110 (1991) BindingDB Entry DOI: 10.7270/Q24M955S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin-H2 D-isomerase (Mus musculus) | BDBM50009853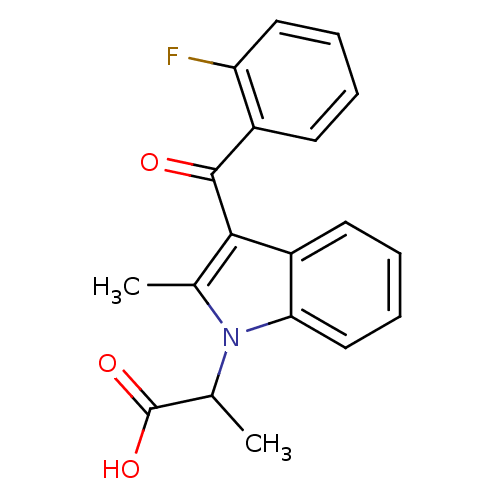 (2-[3-(2-Fluoro-benzoyl)-2-methyl-indol-1-yl]-propi...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Sterling Research Group Curated by ChEMBL | Assay Description Concentration required to inhibit 50% activity of prostaglandin synthetase was determined in vitro in mouse brain microsomes | J Med Chem 34: 1099-110 (1991) BindingDB Entry DOI: 10.7270/Q24M955S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin-H2 D-isomerase (Mus musculus) | BDBM50009869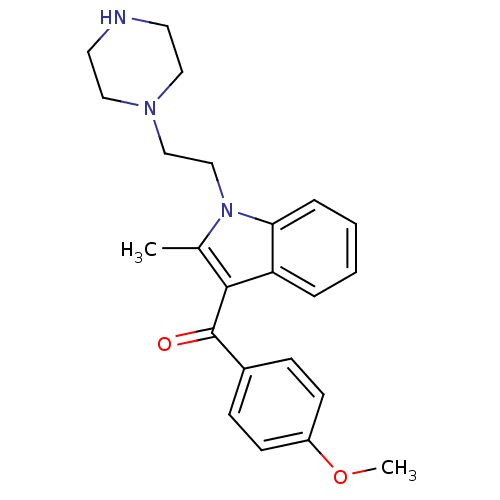 ((4-Methoxy-phenyl)-[2-methyl-1-(2-piperazin-1-yl-e...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 1.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Sterling Research Group Curated by ChEMBL | Assay Description Concentration required to inhibit 50% activity of prostaglandin synthetase was determined in vitro in mouse brain microsomes | J Med Chem 34: 1099-110 (1991) BindingDB Entry DOI: 10.7270/Q24M955S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hematopoietic prostaglandin D synthase (Rattus norvegicus) | BDBM50576317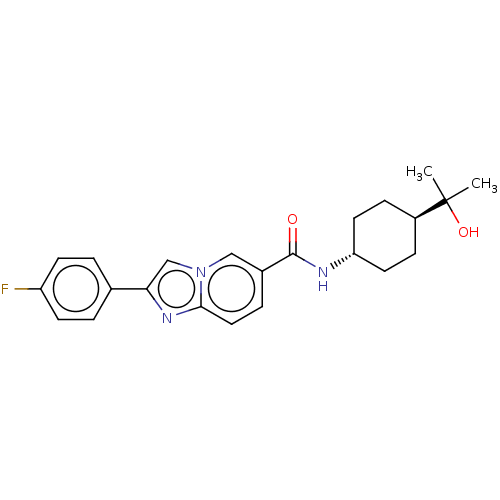 (CHEMBL4849005) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of HPGDS in rat RBL cells assessed as reduction in PGD2 formation using PGH2 as substrate by mass spectrometry | Citation and Details Article DOI: 10.1016/j.bmcl.2021.128113 BindingDB Entry DOI: 10.7270/Q2B28047 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin-H2 D-isomerase (Mus musculus) | BDBM50368232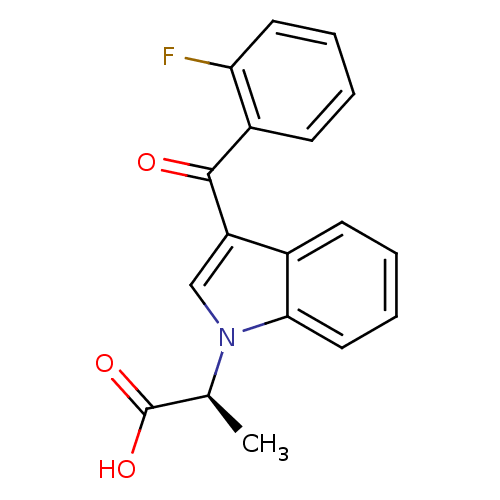 (CHEMBL1907834) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Sterling Research Group Curated by ChEMBL | Assay Description Concentration required to inhibit 50% activity of prostaglandin synthetase was determined in vitro in mouse brain microsomes | J Med Chem 34: 1099-110 (1991) BindingDB Entry DOI: 10.7270/Q24M955S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin-H2 D-isomerase (Mus musculus) | BDBM50008029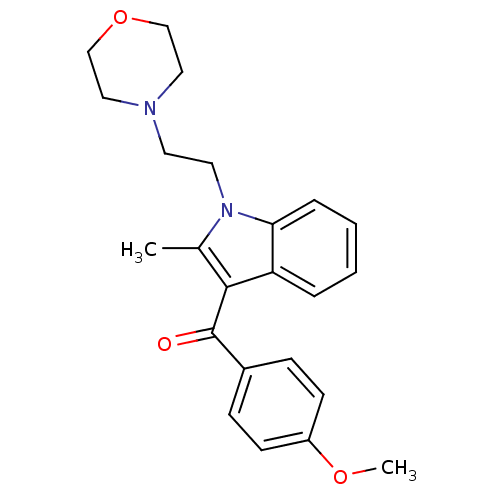 ((4-Methoxy-phenyl)-[2-methyl-1-(2-morpholin-4-yl-e...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 3.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Sterling Research Group Curated by ChEMBL | Assay Description Concentration required to inhibit 50% activity of prostaglandin synthetase was determined in vitro in mouse brain microsomes | J Med Chem 34: 1099-110 (1991) BindingDB Entry DOI: 10.7270/Q24M955S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin-H2 D-isomerase (Mus musculus) | BDBM50008029 ((4-Methoxy-phenyl)-[2-methyl-1-(2-morpholin-4-yl-e...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article | n/a | n/a | 3.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was tested for inhibitory activity against prostaglandin synthetase in mouse brain microsomes | Bioorg Med Chem Lett 5: 381-386 (1995) Article DOI: 10.1016/0960-894X(95)00040-Z BindingDB Entry DOI: 10.7270/Q2571BZN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hematopoietic prostaglandin D synthase (Rattus norvegicus) | BDBM50576318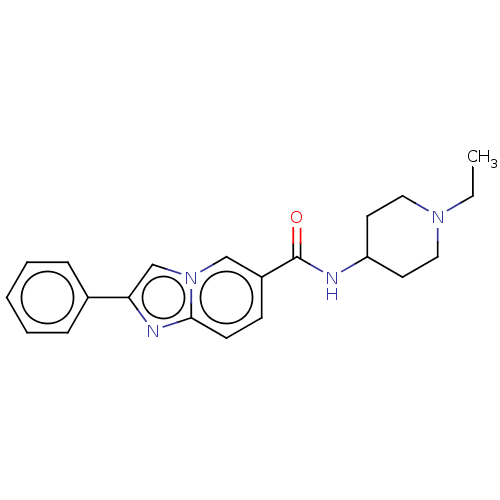 (CHEMBL4868975) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of HPGDS in rat RBL cells assessed as reduction in PGD2 formation using PGH2 as substrate by mass spectrometry | Citation and Details Article DOI: 10.1016/j.bmcl.2021.128113 BindingDB Entry DOI: 10.7270/Q2B28047 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin-H2 D-isomerase (Mus musculus) | BDBM50009863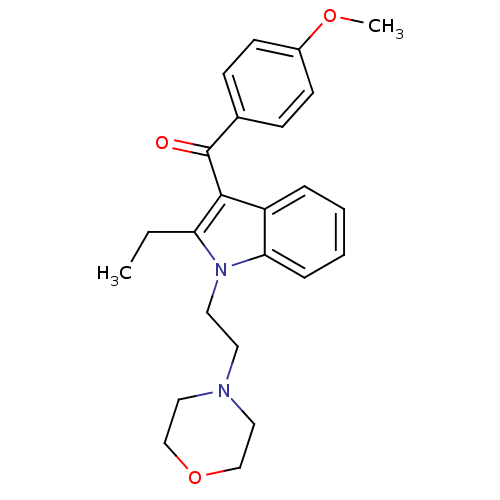 (CHEMBL273372 | [2-Ethyl-1-(2-morpholin-4-yl-ethyl)...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Sterling Research Group Curated by ChEMBL | Assay Description Concentration required to inhibit 50% activity of prostaglandin synthetase was determined in vitro in mouse brain microsomes | J Med Chem 34: 1099-110 (1991) BindingDB Entry DOI: 10.7270/Q24M955S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin-H2 D-isomerase (Mus musculus) | BDBM50009867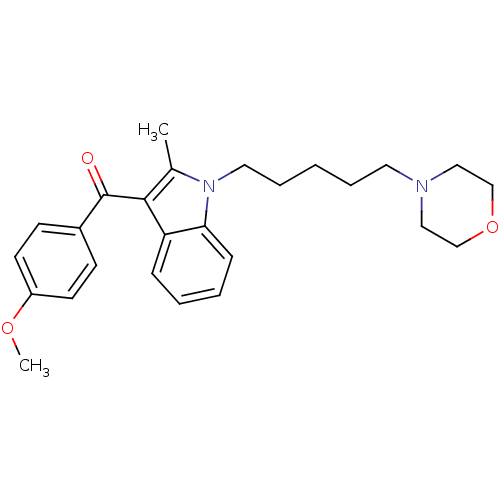 ((4-Methoxy-phenyl)-[2-methyl-1-(5-morpholin-4-yl-p...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Sterling Research Group Curated by ChEMBL | Assay Description Concentration required to inhibit 50% activity of prostaglandin synthetase was determined in vitro in mouse brain microsomes | J Med Chem 34: 1099-110 (1991) BindingDB Entry DOI: 10.7270/Q24M955S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin-H2 D-isomerase (Mus musculus) | BDBM50009868 ((4-Methoxy-phenyl)-[1-(2-morpholin-4-yl-ethyl)-1H-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Sterling Research Group Curated by ChEMBL | Assay Description Concentration required to inhibit 50% activity of prostaglandin synthetase was determined in mouse vas deferens | J Med Chem 34: 1099-110 (1991) BindingDB Entry DOI: 10.7270/Q24M955S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin-H2 D-isomerase (Bos taurus) | BDBM50031087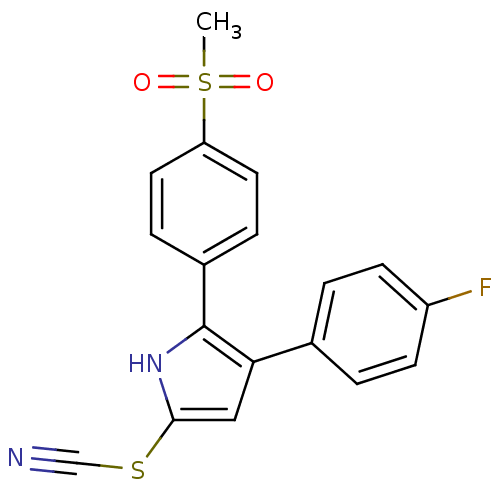 (3-(4-Fluoro-phenyl)-2-(4-methanesulfonyl-phenyl)-5...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 6.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Du Pont Merck Pharmaceutical Company Curated by ChEMBL | Assay Description Inhibitory activity against bovine seminal vesicle prostaglandin synthetase | J Med Chem 37: 988-98 (1994) BindingDB Entry DOI: 10.7270/Q2BP01WP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin-H2 D-isomerase (Mus musculus) | BDBM50368231 (CHEMBL337134) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 8.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Sterling Research Group Curated by ChEMBL | Assay Description Concentration required to inhibit 50% activity of prostaglandin synthetase was determined in vitro in mouse brain microsomes | J Med Chem 34: 1099-110 (1991) BindingDB Entry DOI: 10.7270/Q24M955S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin-H2 D-isomerase (Mus musculus) | BDBM50368234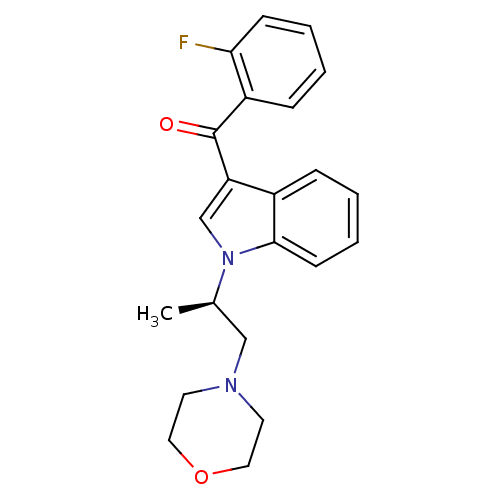 (CHEMBL1907835) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 9.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Sterling Research Group Curated by ChEMBL | Assay Description Concentration required to inhibit 50% activity of prostaglandin synthetase was determined in vitro in mouse brain microsomes | J Med Chem 34: 1099-110 (1991) BindingDB Entry DOI: 10.7270/Q24M955S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin-H2 D-isomerase (Homo sapiens (Human)) | BDBM50615780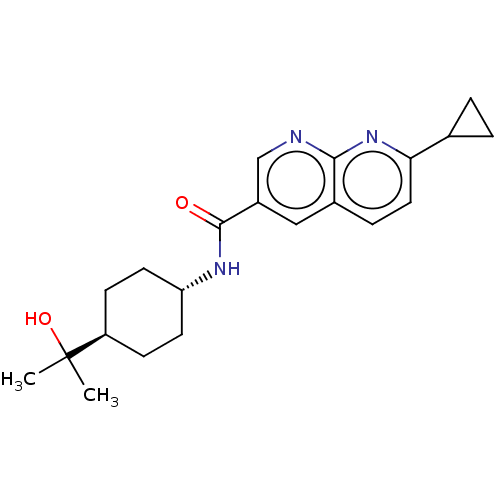 (CHEMBL5285108) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin-H2 D-isomerase (Mus musculus) | BDBM50009866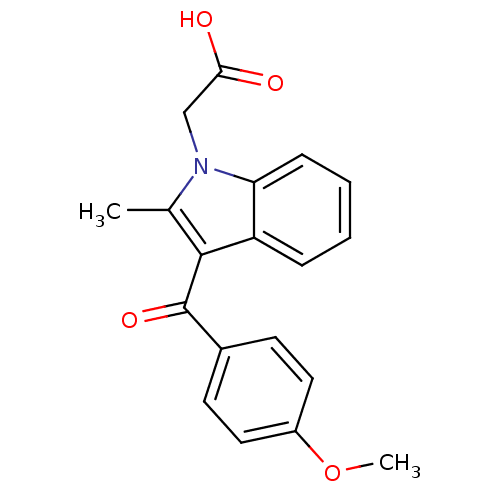 (CHEMBL275312 | [3-(4-Methoxy-benzoyl)-2-methyl-ind...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Sterling Research Group Curated by ChEMBL | Assay Description Concentration required to inhibit 50% activity of prostaglandin synthetase was determined in vitro in mouse brain microsomes | J Med Chem 34: 1099-110 (1991) BindingDB Entry DOI: 10.7270/Q24M955S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin-H2 D-isomerase (Mus musculus) | BDBM50009861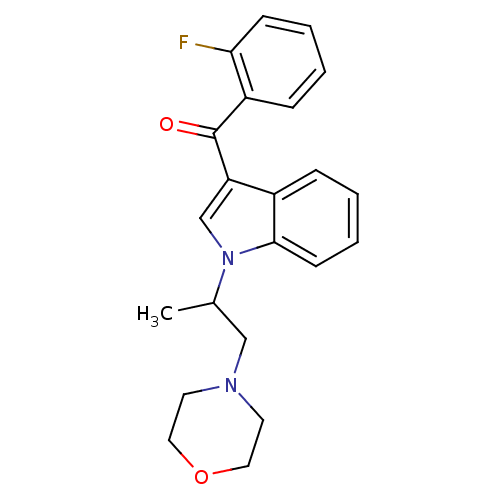 ((2-Fluoro-phenyl)-[1-(1-methyl-2-morpholin-4-yl-et...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Sterling Research Group Curated by ChEMBL | Assay Description Concentration required to inhibit 50% activity of prostaglandin synthetase was determined in vitro in mouse brain microsomes | J Med Chem 34: 1099-110 (1991) BindingDB Entry DOI: 10.7270/Q24M955S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin-H2 D-isomerase (Mus musculus) | BDBM50339185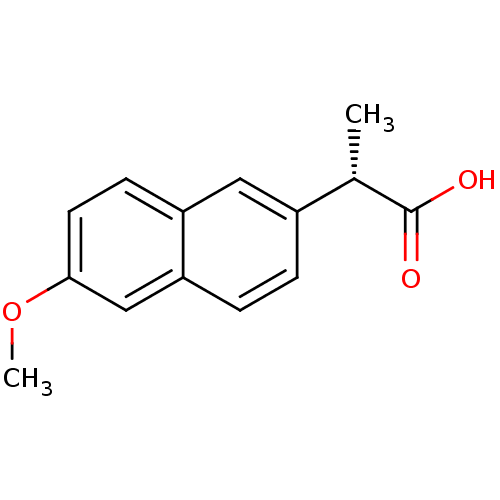 ((2S)-2-(6-methoxynaphthalen-2-yl)propanoic acid | ...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | PubMed | n/a | n/a | 1.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Sterling Research Group Curated by ChEMBL | Assay Description Concentration required to inhibit 50% activity of prostaglandin synthetase was determined in vitro in mouse brain microsomes | J Med Chem 34: 1099-110 (1991) BindingDB Entry DOI: 10.7270/Q24M955S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin-H2 D-isomerase (Mus musculus) | BDBM50009859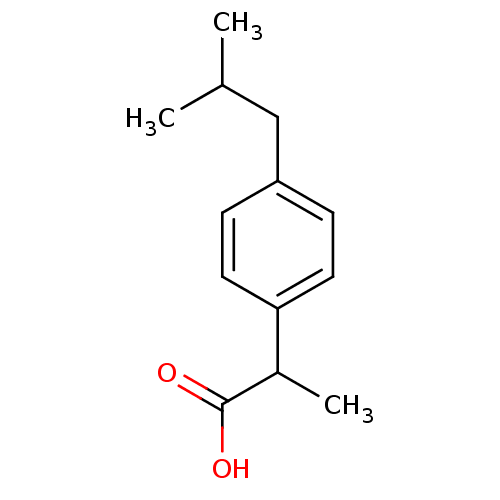 ((+-)-2-(p-isobutylphenyl)propionic acid | (+-)-alp...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | PubMed | n/a | n/a | 1.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Sterling Research Group Curated by ChEMBL | Assay Description Concentration required to inhibit 50% activity of prostaglandin synthetase was determined in vitro in mouse brain microsomes | J Med Chem 34: 1099-110 (1991) BindingDB Entry DOI: 10.7270/Q24M955S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 119 total ) | Next | Last >> |