

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Tyrosine-protein kinase ABL1 [229-512] (Homo sapiens (Human)) | BDBM13216 (BMS-354825 | CHEMBL1421 | DASATINIB | N-(2-Chloro-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | n/a | 0.0300 | n/a | n/a | n/a | n/a | n/a |
Stony Brook University | Assay Description Inhibitor selectivity profiles were obtained through Luceome Biotechnologies (Tuscon, AZ). Each inhibitor was screened at 0.5 μM against a panel... | ACS Chem Biol 11: 1296-304 (2016) Article DOI: 10.1021/acschembio.5b01018 BindingDB Entry DOI: 10.7270/Q2MW2FXD | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Tyrosine-protein kinase ABL1 [229-512] (Homo sapiens (Human)) | BDBM50237710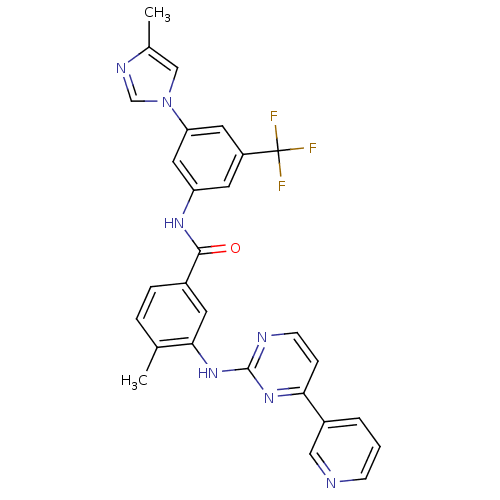 (4-methyl-N-[3-(4-methyl-1H-imidazol-1-yl)-5-(trifl...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a |
Stony Brook University | Assay Description Inhibitor selectivity profiles were obtained through Luceome Biotechnologies (Tuscon, AZ). Each inhibitor was screened at 0.5 μM against phospho... | ACS Chem Biol 11: 1296-304 (2016) Article DOI: 10.1021/acschembio.5b01018 BindingDB Entry DOI: 10.7270/Q2MW2FXD | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Tyrosine-protein kinase ABL1 [229-512] (Homo sapiens (Human)) | BDBM185671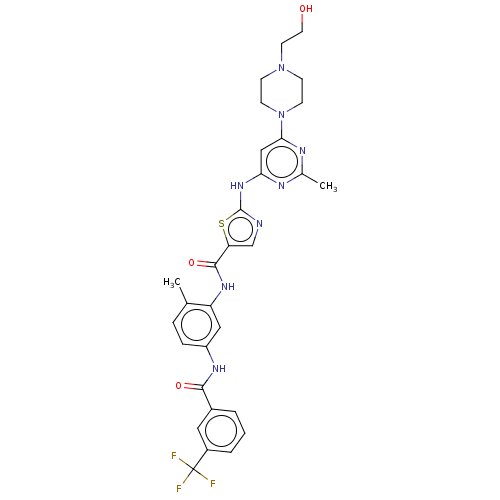 (DAS-DFGO-II) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | 2.90 | n/a | n/a | n/a | n/a | n/a |
Stony Brook University | Assay Description Inhibitor selectivity profiles were obtained through Luceome Biotechnologies (Tuscon, AZ). Each inhibitor was screened at 0.5 μM against a panel... | ACS Chem Biol 11: 1296-304 (2016) Article DOI: 10.1021/acschembio.5b01018 BindingDB Entry DOI: 10.7270/Q2MW2FXD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase ABL1 [229-512] (Homo sapiens (Human)) | BDBM185672 (DAS-CHO-I) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a |
Stony Brook University | Assay Description Inhibitor selectivity profiles were obtained through Luceome Biotechnologies (Tuscon, AZ). Each inhibitor was screened at 0.5 μM against a panel... | ACS Chem Biol 11: 1296-304 (2016) Article DOI: 10.1021/acschembio.5b01018 BindingDB Entry DOI: 10.7270/Q2MW2FXD | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Tyrosine-protein kinase ABL1 [229-512] (Homo sapiens (Human)) | BDBM185673 (DAS-CHO-II) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | 0.320 | n/a | n/a | n/a | n/a | n/a |
Stony Brook University | Assay Description Inhibitor selectivity profiles were obtained through Luceome Biotechnologies (Tuscon, AZ). Each inhibitor was screened at 0.5 μM against a panel... | ACS Chem Biol 11: 1296-304 (2016) Article DOI: 10.1021/acschembio.5b01018 BindingDB Entry DOI: 10.7270/Q2MW2FXD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase ABL1 [229-512] (Homo sapiens (Human)) | BDBM13530 (4-[(4-methylpiperazin-1-yl)methyl]-N-[4-methyl-3-[...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a |
Stony Brook University | Assay Description Inhibitor selectivity profiles were obtained through Luceome Biotechnologies (Tuscon, AZ). Each inhibitor was screened at 0.5 μM against a panel... | ACS Chem Biol 11: 1296-304 (2016) Article DOI: 10.1021/acschembio.5b01018 BindingDB Entry DOI: 10.7270/Q2MW2FXD | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Tyrosine-protein kinase ABL1 [229-512] (Homo sapiens (Human)) | BDBM50237710 (4-methyl-N-[3-(4-methyl-1H-imidazol-1-yl)-5-(trifl...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a |
Stony Brook University | Assay Description Inhibitor selectivity profiles were obtained through Luceome Biotechnologies (Tuscon, AZ). Each inhibitor was screened at 0.5 μM against a panel... | ACS Chem Biol 11: 1296-304 (2016) Article DOI: 10.1021/acschembio.5b01018 BindingDB Entry DOI: 10.7270/Q2MW2FXD | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Tyrosine-protein kinase ABL1 [229-512] (Homo sapiens (Human)) | BDBM185674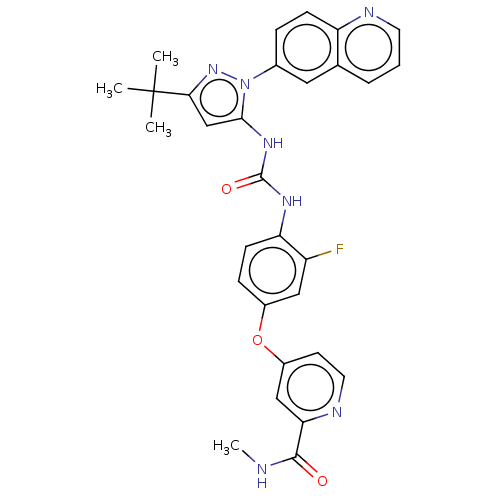 (4-[4-[(5-tert-butyl-2-quinolin-6-ylpyrazol-3-yl)ca...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | n/a | 7.20 | n/a | n/a | n/a | n/a | n/a |
Stony Brook University | Assay Description Inhibitor selectivity profiles were obtained through Luceome Biotechnologies (Tuscon, AZ). Each inhibitor was screened at 0.5 μM against a panel... | ACS Chem Biol 11: 1296-304 (2016) Article DOI: 10.1021/acschembio.5b01018 BindingDB Entry DOI: 10.7270/Q2MW2FXD | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Tyrosine-protein kinase ABL1 [229-512] (Homo sapiens (Human)) | BDBM50322535 (3-(imidazo[1,2-b]pyridazin-3-ylethynyl)-4-methyl-N...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a |
Stony Brook University | Assay Description Inhibitor selectivity profiles were obtained through Luceome Biotechnologies (Tuscon, AZ). Each inhibitor was screened at 0.5 μM against a panel... | ACS Chem Biol 11: 1296-304 (2016) Article DOI: 10.1021/acschembio.5b01018 BindingDB Entry DOI: 10.7270/Q2MW2FXD | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Tyrosine-protein kinase ABL1 [229-512] (Homo sapiens (Human)) | BDBM185675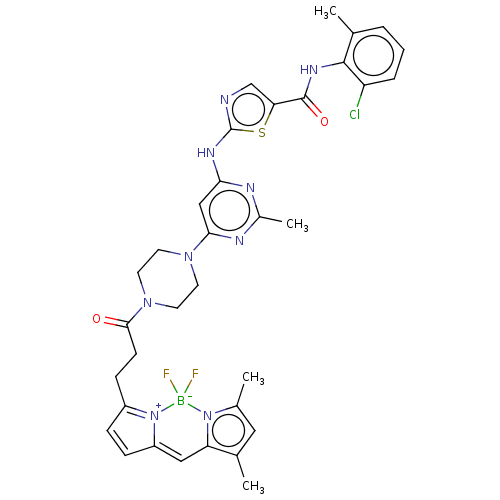 (DAS-BODIPY) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | 0.150 | n/a | n/a | n/a | 8.0 | n/a |
Stony Brook University | Assay Description Reaction volumes of 50 μL were used in 96-well plates. Buffer A (1×, 34 μL; 100 mM Tris, pH 8, 10 mM MgCl2) was added to a single row, foll... | ACS Chem Biol 11: 1296-304 (2016) Article DOI: 10.1021/acschembio.5b01018 BindingDB Entry DOI: 10.7270/Q2MW2FXD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase ABL1 [229-512] (Homo sapiens (Human)) | BDBM185676 (DAS-DFGO-II-BODIPY) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | 0.75 | n/a | n/a | n/a | 8.0 | n/a |
Stony Brook University | Assay Description Reaction volumes of 50 μL were used in 96-well plates. Buffer A (1×, 34 μL; 100 mM Tris, pH 8, 10 mM MgCl2) was added to a single row, foll... | ACS Chem Biol 11: 1296-304 (2016) Article DOI: 10.1021/acschembio.5b01018 BindingDB Entry DOI: 10.7270/Q2MW2FXD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase ABL1 [229-512] (Homo sapiens (Human)) | BDBM185670 (DAS-DFGO-I | N-(2-chloro-6-methylphenyl)-2-[[6-[4-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a |
Stony Brook University | Assay Description Inhibitor selectivity profiles were obtained through Luceome Biotechnologies (Tuscon, AZ). Each inhibitor was screened at 0.5 μM against phospho... | ACS Chem Biol 11: 1296-304 (2016) Article DOI: 10.1021/acschembio.5b01018 BindingDB Entry DOI: 10.7270/Q2MW2FXD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase ABL1 [229-512] (Homo sapiens (Human)) | BDBM185671 (DAS-DFGO-II) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a |
Stony Brook University | Assay Description Inhibitor selectivity profiles were obtained through Luceome Biotechnologies (Tuscon, AZ). Each inhibitor was screened at 0.5 μM against phospho... | ACS Chem Biol 11: 1296-304 (2016) Article DOI: 10.1021/acschembio.5b01018 BindingDB Entry DOI: 10.7270/Q2MW2FXD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase ABL1 [229-512] (Homo sapiens (Human)) | BDBM13530 (4-[(4-methylpiperazin-1-yl)methyl]-N-[4-methyl-3-[...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | n/a | 24 | n/a | n/a | n/a | n/a | n/a |
Stony Brook University | Assay Description Inhibitor selectivity profiles were obtained through Luceome Biotechnologies (Tuscon, AZ). Each inhibitor was screened at 0.5 μM against phospho... | ACS Chem Biol 11: 1296-304 (2016) Article DOI: 10.1021/acschembio.5b01018 BindingDB Entry DOI: 10.7270/Q2MW2FXD | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Tyrosine-protein kinase ABL1 [229-512] (Homo sapiens (Human)) | BDBM185670 (DAS-DFGO-I | N-(2-chloro-6-methylphenyl)-2-[[6-[4-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | n/a | 2.20 | n/a | n/a | n/a | n/a | n/a |
Stony Brook University | Assay Description Inhibitor selectivity profiles were obtained through Luceome Biotechnologies (Tuscon, AZ). Each inhibitor was screened at 0.5 μM against a panel... | ACS Chem Biol 11: 1296-304 (2016) Article DOI: 10.1021/acschembio.5b01018 BindingDB Entry DOI: 10.7270/Q2MW2FXD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||