

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Renin (Homo sapiens (Human)) | BDBM50340417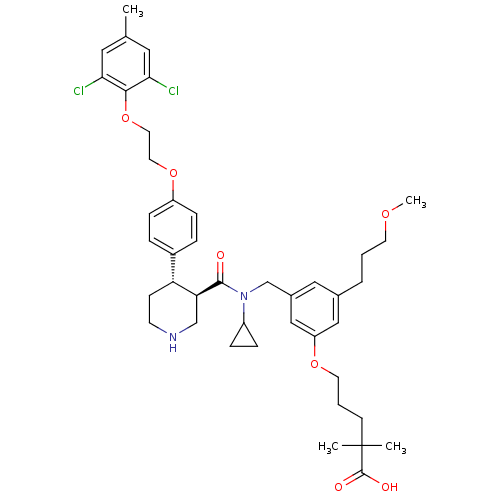 (5-(3-(((3R,4S)-N-cyclopropyl-4-(4-(2-(2,6-dichloro...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.0100 | n/a | n/a | n/a | n/a | 7.4 | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Inhibition of human recombinant renin in PBS buffer using tetradecapeptide at pH 7.4 | Bioorg Med Chem Lett 21: 2430-6 (2011) Article DOI: 10.1016/j.bmcl.2011.02.067 BindingDB Entry DOI: 10.7270/Q2G16157 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50340421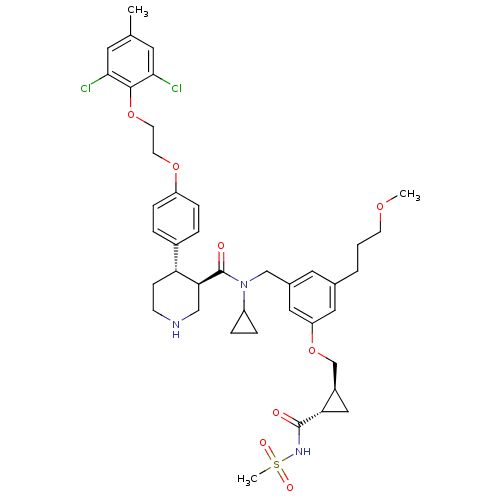 ((3R,4S)-N-cyclopropyl-4-(4-(2-(2,6-dichloro-4-meth...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.0100 | n/a | n/a | n/a | n/a | 7.4 | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Inhibition of human recombinant renin in PBS buffer using tetradecapeptide at pH 7.4 | Bioorg Med Chem Lett 21: 2430-6 (2011) Article DOI: 10.1016/j.bmcl.2011.02.067 BindingDB Entry DOI: 10.7270/Q2G16157 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50340419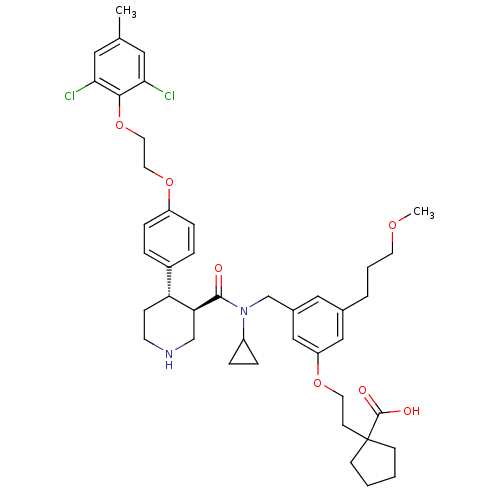 (1-(2-(3-(((3R,4S)-N-cyclopropyl-4-(4-(2-(2,6-dichl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.0100 | n/a | n/a | n/a | n/a | 7.4 | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Inhibition of human recombinant renin in PBS buffer using tetradecapeptide at pH 7.4 | Bioorg Med Chem Lett 21: 2430-6 (2011) Article DOI: 10.1016/j.bmcl.2011.02.067 BindingDB Entry DOI: 10.7270/Q2G16157 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50340407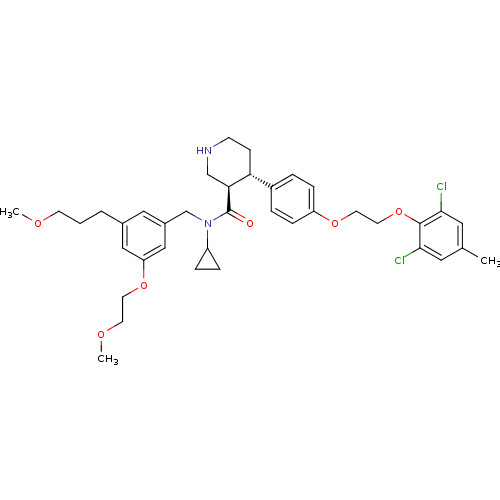 ((3R,4S)-N-cyclopropyl-4-(4-(2-(2,6-dichloro-4-meth...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Inhibition of renin in human plasma using Q-FRET substrate pretreated for 10 mins before substrate addition measured after 1 hr | Bioorg Med Chem Lett 21: 3970-5 (2011) Article DOI: 10.1016/j.bmcl.2011.05.013 BindingDB Entry DOI: 10.7270/Q2PN9608 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50346987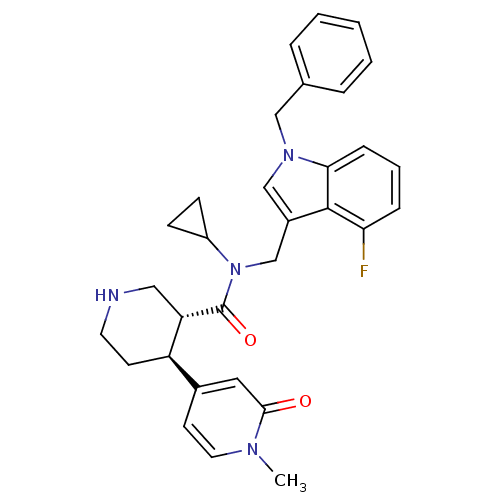 (CHEMBL1796073) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Inhibition of human recombinant renin using Q-FRET substrate after 3 hrs by fluorescence assay | Bioorg Med Chem Lett 21: 3970-5 (2011) Article DOI: 10.1016/j.bmcl.2011.05.013 BindingDB Entry DOI: 10.7270/Q2PN9608 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50340416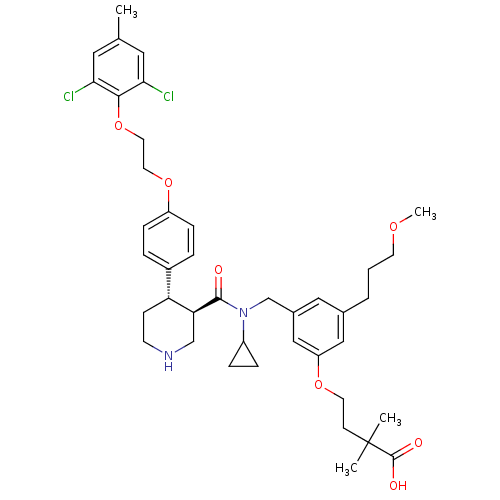 (4-(3-(((3R,4S)-N-cyclopropyl-4-(4-(2-(2,6-dichloro...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.0200 | n/a | n/a | n/a | n/a | 7.4 | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Inhibition of human recombinant renin in PBS buffer using tetradecapeptide at pH 7.4 | Bioorg Med Chem Lett 21: 2430-6 (2011) Article DOI: 10.1016/j.bmcl.2011.02.067 BindingDB Entry DOI: 10.7270/Q2G16157 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50340418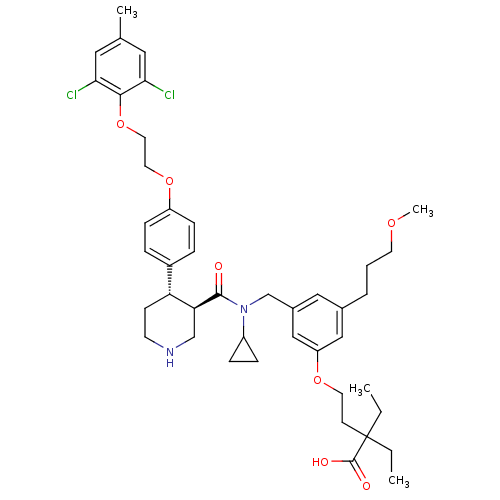 (4-(3-(((3R,4S)-N-cyclopropyl-4-(4-(2-(2,6-dichloro...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.0200 | n/a | n/a | n/a | n/a | 7.4 | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Inhibition of human recombinant renin in PBS buffer using tetradecapeptide at pH 7.4 | Bioorg Med Chem Lett 21: 2430-6 (2011) Article DOI: 10.1016/j.bmcl.2011.02.067 BindingDB Entry DOI: 10.7270/Q2G16157 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50340407 ((3R,4S)-N-cyclopropyl-4-(4-(2-(2,6-dichloro-4-meth...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.0210 | n/a | n/a | n/a | n/a | 7.4 | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Inhibition of human recombinant renin in PBS buffer using tetradecapeptide at pH 7.4 | Bioorg Med Chem Lett 21: 2430-6 (2011) Article DOI: 10.1016/j.bmcl.2011.02.067 BindingDB Entry DOI: 10.7270/Q2G16157 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50340410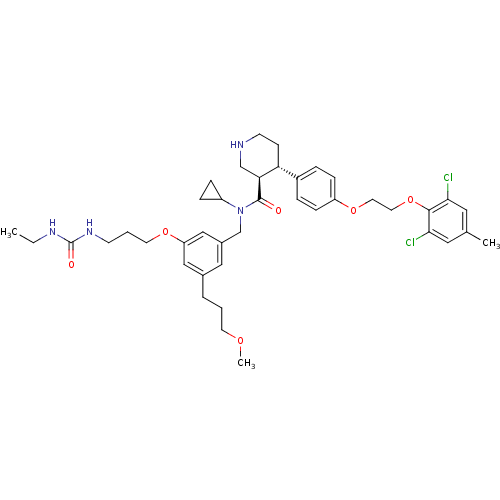 ((3R,4S)-N-cyclopropyl-4-(4-(2-(2,6-dichloro-4-meth...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.0220 | n/a | n/a | n/a | n/a | 7.4 | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Inhibition of human recombinant renin in PBS buffer using tetradecapeptide at pH 7.4 | Bioorg Med Chem Lett 21: 2430-6 (2011) Article DOI: 10.1016/j.bmcl.2011.02.067 BindingDB Entry DOI: 10.7270/Q2G16157 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50077669 ((S)-2-Benzyl-N-[(S)-1-((1S,2R,3S)-1-cyclohexylmeth...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB PubMed | n/a | n/a | 0.0250 | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd Curated by ChEMBL | Assay Description In vitro inhibitory activity against purified recombinant human renin | Bioorg Med Chem Lett 9: 1397-402 (1999) BindingDB Entry DOI: 10.7270/Q24F1PWN | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50340413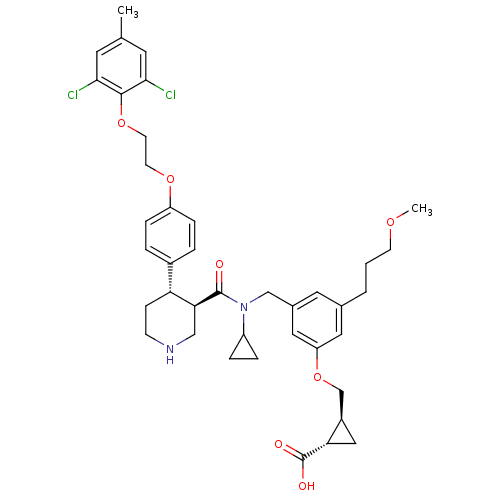 ((1S,2S)-2-((3-(((3R,4S)-N-cyclopropyl-4-(4-(2-(2,6...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.0270 | n/a | n/a | n/a | n/a | 7.4 | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Inhibition of human recombinant renin in PBS buffer using tetradecapeptide at pH 7.4 | Bioorg Med Chem Lett 21: 2430-6 (2011) Article DOI: 10.1016/j.bmcl.2011.02.067 BindingDB Entry DOI: 10.7270/Q2G16157 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50340406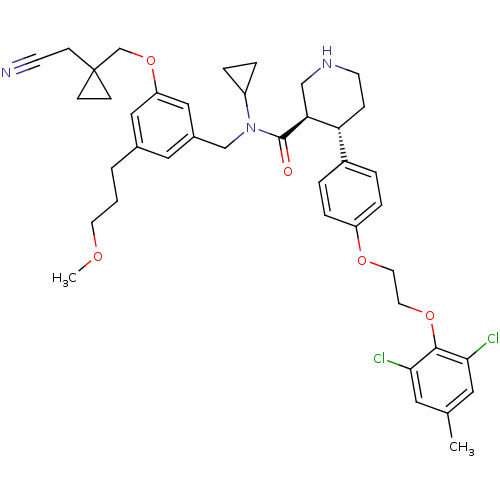 ((3R,4S)-N-(3-((1-(cyanomethyl)cyclopropyl)methoxy)...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.0280 | n/a | n/a | n/a | n/a | 7.4 | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Inhibition of human recombinant renin in PBS buffer using tetradecapeptide at pH 7.4 | Bioorg Med Chem Lett 21: 2430-6 (2011) Article DOI: 10.1016/j.bmcl.2011.02.067 BindingDB Entry DOI: 10.7270/Q2G16157 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50340409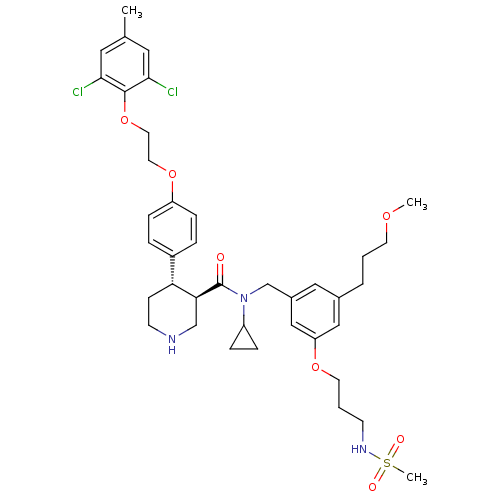 ((3R,4S)-N-cyclopropyl-4-(4-(2-(2,6-dichloro-4-meth...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.0280 | n/a | n/a | n/a | n/a | 7.4 | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Inhibition of human recombinant renin in PBS buffer using tetradecapeptide at pH 7.4 | Bioorg Med Chem Lett 21: 2430-6 (2011) Article DOI: 10.1016/j.bmcl.2011.02.067 BindingDB Entry DOI: 10.7270/Q2G16157 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50340422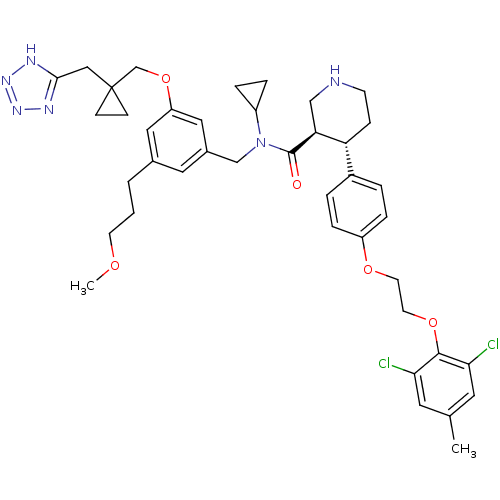 ((3R,4S)-N-(3-((1-((2H-tetrazol-5-yl)methyl)cyclopr...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.0300 | n/a | n/a | n/a | n/a | 7.4 | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Inhibition of human recombinant renin in PBS buffer using tetradecapeptide at pH 7.4 | Bioorg Med Chem Lett 21: 2430-6 (2011) Article DOI: 10.1016/j.bmcl.2011.02.067 BindingDB Entry DOI: 10.7270/Q2G16157 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50355509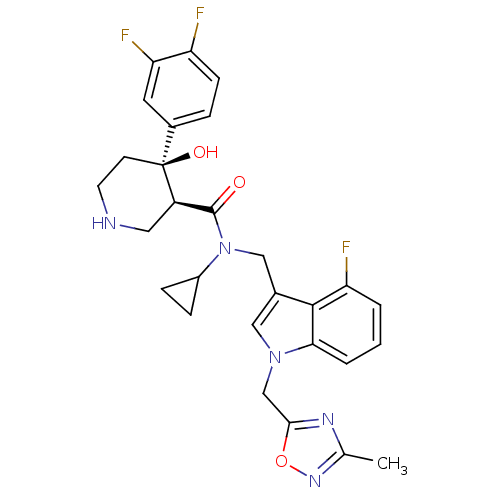 (CHEMBL1910311) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Inhibition of human recombinant renin using DNP-Lys-His-Pro-Phe-His-Leu-Val-Ile-His-D,L-Amp as Q-FRET substrate after 3 hrs by fluorescence assay | Bioorg Med Chem Lett 21: 3976-81 (2011) Article DOI: 10.1016/j.bmcl.2011.05.014 BindingDB Entry DOI: 10.7270/Q21N81HN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50403370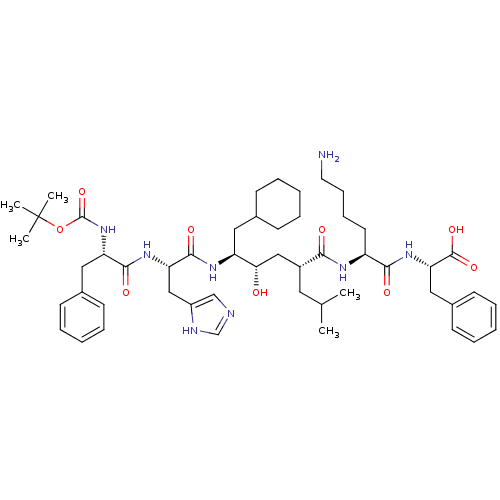 (CHEMBL2052021 | CP-71362) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was tested for its inhibitory activity against dog plasma renin | Bioorg Med Chem Lett 4: 589-592 (1994) Article DOI: 10.1016/S0960-894X(01)80160-8 BindingDB Entry DOI: 10.7270/Q2KD1XVS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50330345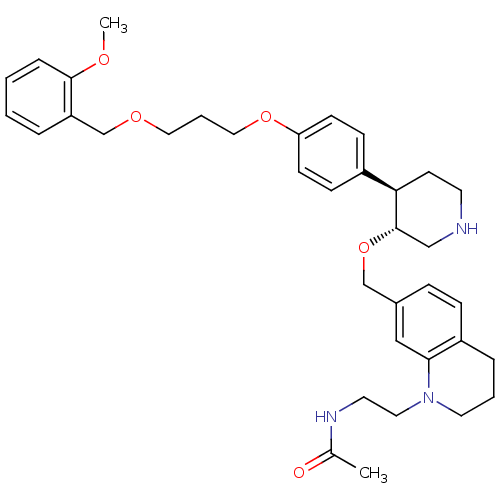 (CHEMBL1276275 | N-(2-(7-(((3R,4R)-4-(4-(3-(2-metho...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.0390 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharmaceuticals Corp Curated by ChEMBL | Assay Description Inhibition of human recombinant renin in buffer | J Med Chem 53: 7490-520 (2010) Article DOI: 10.1021/jm901885s BindingDB Entry DOI: 10.7270/Q2S75GKG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50330345 (CHEMBL1276275 | N-(2-(7-(((3R,4R)-4-(4-(3-(2-metho...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.0390 | n/a | n/a | n/a | n/a | n/a | n/a |
Actelion Pharmaceuticals Ltd. Curated by ChEMBL | Assay Description Inhibition of human recombinant rennin in buffer assessed as accumulation of angiotensin 1 using human tetradecapeptide by immunoassay | J Med Chem 52: 3689-702 (2009) Article DOI: 10.1021/jm900022f BindingDB Entry DOI: 10.7270/Q2PC3391 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50195783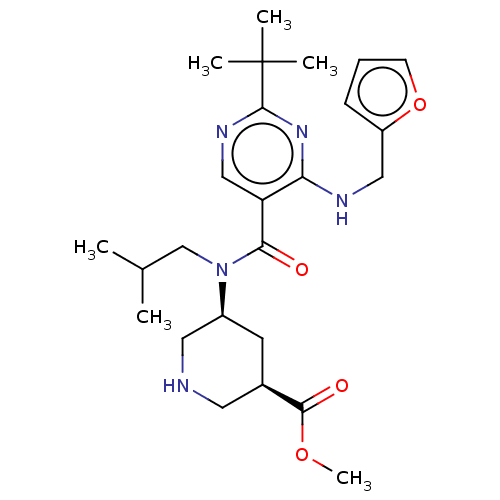 (CHEMBL3907708) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.0390 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Inhibition of recombinant human preprorenin expressed in human 293F cells using angiotensinogen as substrate preincubated for 10 mins followed by sub... | ACS Med Chem Lett 7: 933-938 (2016) Article DOI: 10.1021/acsmedchemlett.6b00251 BindingDB Entry DOI: 10.7270/Q2SF2Z3X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50195783 (CHEMBL3907708) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.0390 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Inhibition of recombinant human preprorenin expressed in human 293F cells using angiotensinogen as substrate preincubated for 10 mins followed by sub... | ACS Med Chem Lett 7: 933-938 (2016) Article DOI: 10.1021/acsmedchemlett.6b00251 BindingDB Entry DOI: 10.7270/Q2SF2Z3X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50355536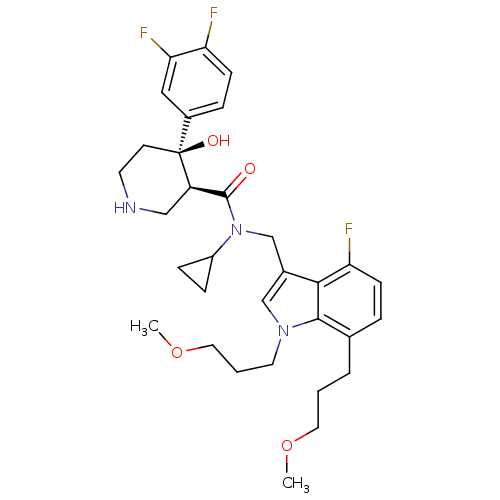 (CHEMBL1910302) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.0400 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Inhibition of human recombinant renin using DNP-Lys-His-Pro-Phe-His-Leu-Val-Ile-His-D,L-Amp as Q-FRET substrate after 3 hrs by fluorescence assay | Bioorg Med Chem Lett 21: 3976-81 (2011) Article DOI: 10.1016/j.bmcl.2011.05.014 BindingDB Entry DOI: 10.7270/Q21N81HN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50355517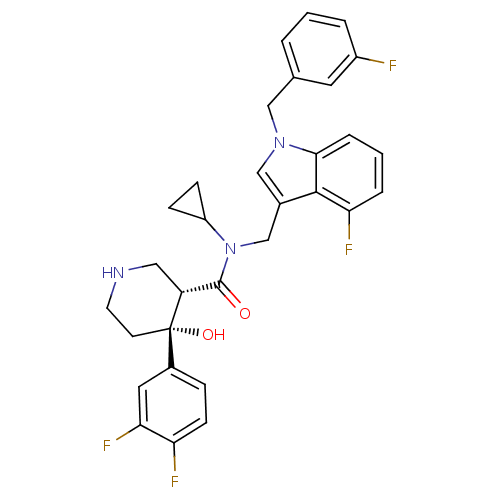 (CHEMBL1910319) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.0400 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Inhibition of human recombinant renin using DNP-Lys-His-Pro-Phe-His-Leu-Val-Ile-His-D,L-Amp as Q-FRET substrate after 3 hrs by fluorescence assay | Bioorg Med Chem Lett 21: 3976-81 (2011) Article DOI: 10.1016/j.bmcl.2011.05.014 BindingDB Entry DOI: 10.7270/Q21N81HN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50340414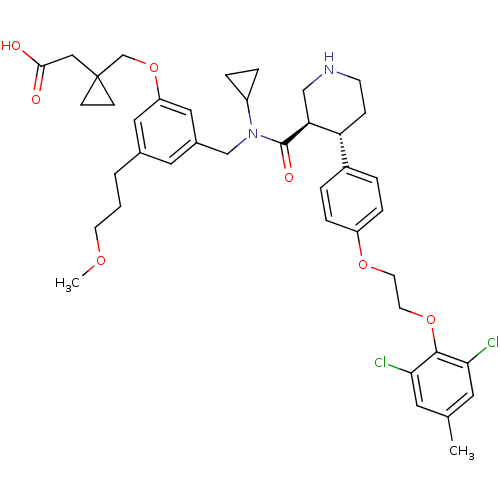 (2-(1-((3-(((3R,4S)-N-cyclopropyl-4-(4-(2-(2,6-dich...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.0400 | n/a | n/a | n/a | n/a | 7.4 | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Inhibition of human recombinant renin in PBS buffer using tetradecapeptide at pH 7.4 | Bioorg Med Chem Lett 21: 2430-6 (2011) Article DOI: 10.1016/j.bmcl.2011.02.067 BindingDB Entry DOI: 10.7270/Q2G16157 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50327295 (CHEMBL1256492 | N-(2-(4'-(1-amino-3-(4-(2-(2,6-dic...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.0400 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Center for Therapeutic Research Curated by ChEMBL | Assay Description Inhibition of human recombinant renin in phosphate buffer | Bioorg Med Chem Lett 20: 5822-6 (2010) Article DOI: 10.1016/j.bmcl.2010.07.127 BindingDB Entry DOI: 10.7270/Q2ZP46BP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50355522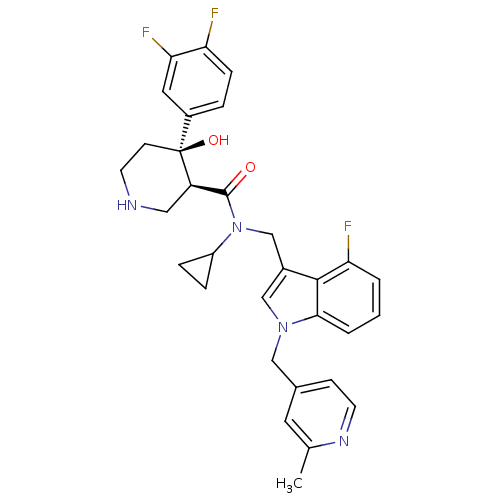 (CHEMBL1910546) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.0400 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Inhibition of human recombinant renin using DNP-Lys-His-Pro-Phe-His-Leu-Val-Ile-His-D,L-Amp as Q-FRET substrate after 3 hrs by fluorescence assay | Bioorg Med Chem Lett 21: 3976-81 (2011) Article DOI: 10.1016/j.bmcl.2011.05.014 BindingDB Entry DOI: 10.7270/Q21N81HN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50328867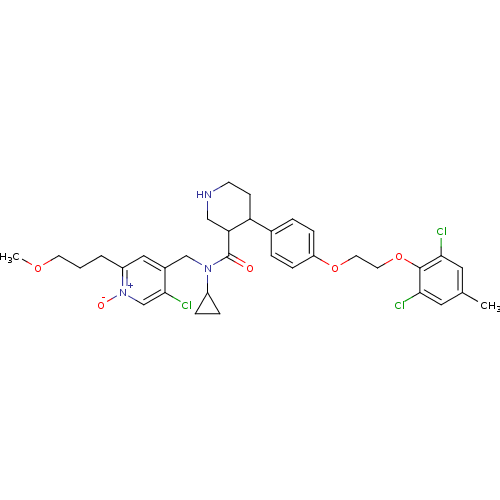 (CHEMBL1269698 | rac-5-chloro-4-((N-cyclopropyl-4-(...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.0400 | n/a | n/a | n/a | n/a | n/a | n/a |
Actelion Pharmaceuticals Ltd Curated by ChEMBL | Assay Description Inhibition of renin in buffer | Bioorg Med Chem Lett 20: 6286-90 (2010) Article DOI: 10.1016/j.bmcl.2010.08.086 BindingDB Entry DOI: 10.7270/Q2WD40T3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50340408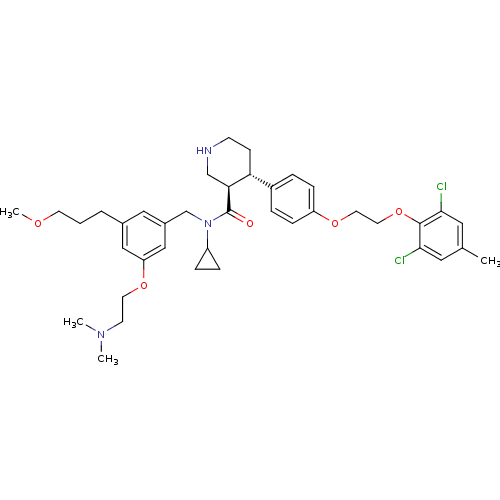 ((3R,4S)-N-cyclopropyl-4-(4-(2-(2,6-dichloro-4-meth...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.0460 | n/a | n/a | n/a | n/a | 7.4 | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Inhibition of human recombinant renin in PBS buffer using tetradecapeptide at pH 7.4 | Bioorg Med Chem Lett 21: 2430-6 (2011) Article DOI: 10.1016/j.bmcl.2011.02.067 BindingDB Entry DOI: 10.7270/Q2G16157 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50359194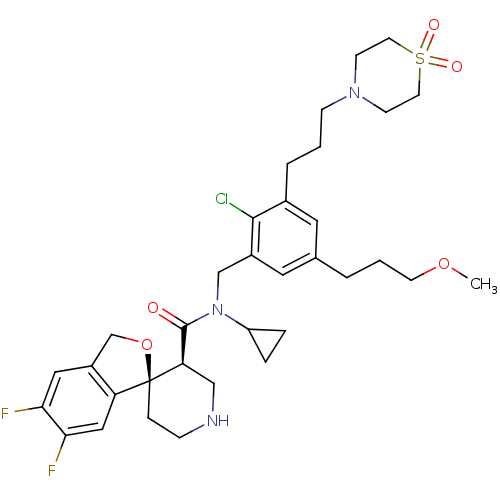 (CHEMBL1923131) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.0500 | n/a | n/a | n/a | n/a | 7.4 | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Inhibition of recombinant human renin using 9 DNP-Lys-His-Pro-Phe-His-Leu-Val-Ile-His-D,L-Amp as substrate after 3 hrs by Q-FRET assay in presence of... | Bioorg Med Chem Lett 21: 7399-404 (2011) Article DOI: 10.1016/j.bmcl.2011.10.013 BindingDB Entry DOI: 10.7270/Q2PC32SW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50365572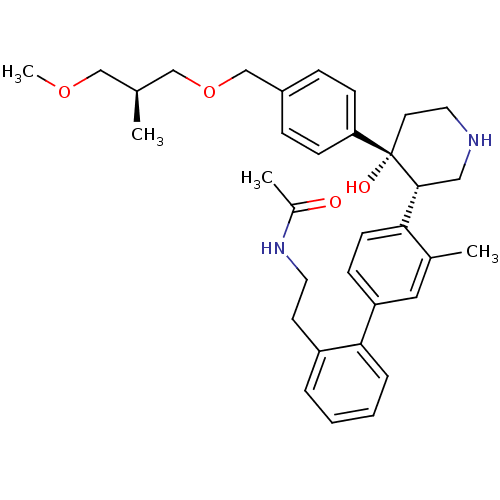 (CHEMBL1957797) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Center for Therapeutic Research Curated by ChEMBL | Assay Description Inhibition of human recombinant renin using DNP-Lys-His-Pro-Phe-His-Leu-Val-Ile-His-D,L-Amp as substrate after 3 hrs by fluorimetry | Bioorg Med Chem Lett 22: 1953-7 (2012) Article DOI: 10.1016/j.bmcl.2012.01.044 BindingDB Entry DOI: 10.7270/Q22F7NXB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50355513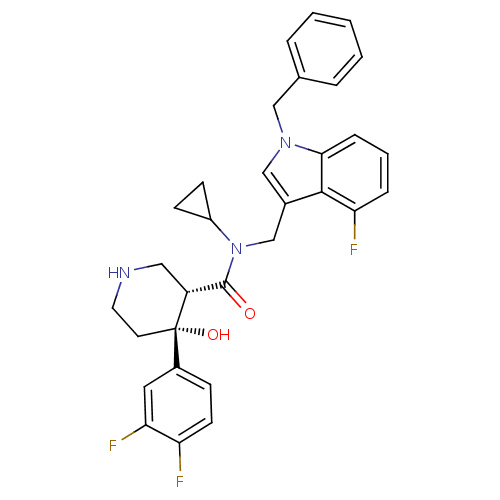 (CHEMBL1910315) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Inhibition of human recombinant renin using DNP-Lys-His-Pro-Phe-His-Leu-Val-Ile-His-D,L-Amp as Q-FRET substrate after 3 hrs by fluorescence assay | Bioorg Med Chem Lett 21: 3976-81 (2011) Article DOI: 10.1016/j.bmcl.2011.05.014 BindingDB Entry DOI: 10.7270/Q21N81HN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50346989 (CHEMBL1796075) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Inhibition of human recombinant renin using Q-FRET substrate after 3 hrs by fluorescence assay | Bioorg Med Chem Lett 21: 3970-5 (2011) Article DOI: 10.1016/j.bmcl.2011.05.013 BindingDB Entry DOI: 10.7270/Q2PN9608 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50328908 (CHEMBL1269746 | methyl 4-chloro-3-(((3R,4S)-N-cycl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a |
Actelion Pharmaceuticals Ltd Curated by ChEMBL | Assay Description Inhibition of renin in buffer | Bioorg Med Chem Lett 20: 6291-6 (2010) Article DOI: 10.1016/j.bmcl.2010.08.087 BindingDB Entry DOI: 10.7270/Q2H70G2C | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50328916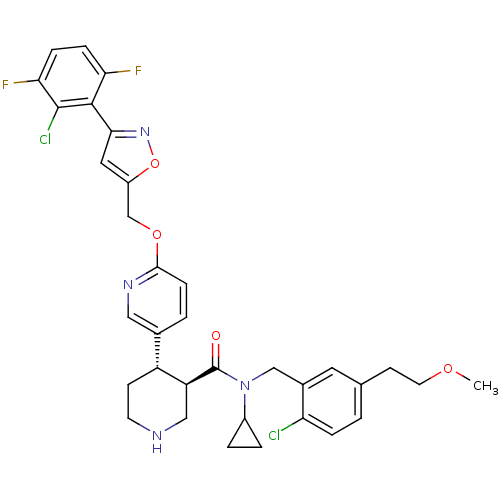 ((3R,4S)-4-(6-((3-(2-chloro-3,6-difluorophenyl)isox...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a |
Actelion Pharmaceuticals Ltd Curated by ChEMBL | Assay Description Inhibition of renin in buffer | Bioorg Med Chem Lett 20: 6291-6 (2010) Article DOI: 10.1016/j.bmcl.2010.08.087 BindingDB Entry DOI: 10.7270/Q2H70G2C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50328846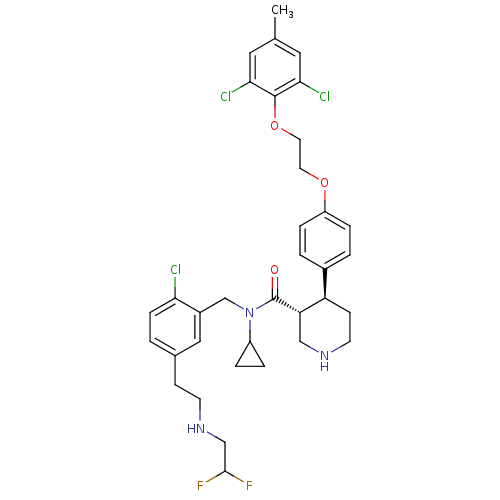 ((3R,4S)-N-(2-chloro-5-(2-(2,2-difluoroethylamino)e...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a |
Actelion Pharmaceuticals Ltd Curated by ChEMBL | Assay Description Inhibition of renin in buffer | Bioorg Med Chem Lett 20: 6286-90 (2010) Article DOI: 10.1016/j.bmcl.2010.08.086 BindingDB Entry DOI: 10.7270/Q2WD40T3 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50340420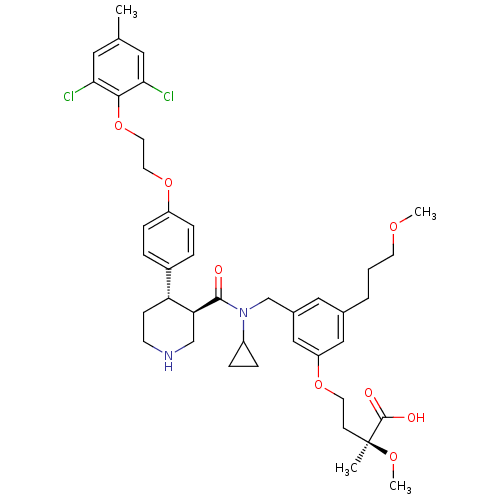 ((S)-4-(3-(((3R,4S)-N-cyclopropyl-4-(4-(2-(2,6-dich...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.0600 | n/a | n/a | n/a | n/a | 7.4 | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Inhibition of human recombinant renin in PBS buffer using tetradecapeptide at pH 7.4 | Bioorg Med Chem Lett 21: 2430-6 (2011) Article DOI: 10.1016/j.bmcl.2011.02.067 BindingDB Entry DOI: 10.7270/Q2G16157 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50346988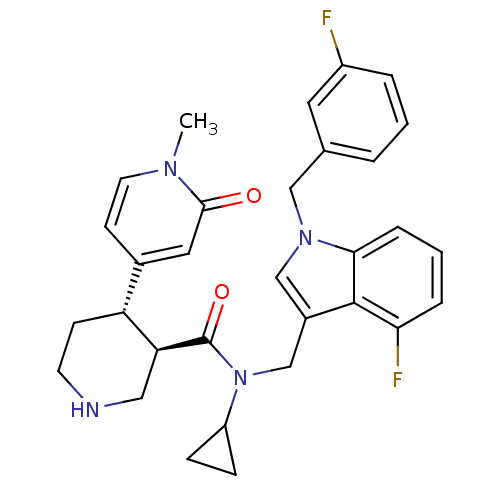 (CHEMBL1796074) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Inhibition of human recombinant renin using Q-FRET substrate after 3 hrs by fluorescence assay | Bioorg Med Chem Lett 21: 3970-5 (2011) Article DOI: 10.1016/j.bmcl.2011.05.013 BindingDB Entry DOI: 10.7270/Q2PN9608 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50077678 ((3R,4R)-3-(1,4-Dimethoxy-naphthalen-2-ylmethoxy)-4...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd Curated by ChEMBL | Assay Description In vitro inhibitory activity against purified recombinant human renin | Bioorg Med Chem Lett 9: 1397-402 (1999) BindingDB Entry DOI: 10.7270/Q24F1PWN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50330350 ((R)-3-((3S,4R,5R)-4-(4-(3-(2-methoxybenzyloxy)prop...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharmaceuticals Corp Curated by ChEMBL | Assay Description Inhibition of human recombinant renin in buffer | J Med Chem 53: 7490-520 (2010) Article DOI: 10.1021/jm901885s BindingDB Entry DOI: 10.7270/Q2S75GKG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50355506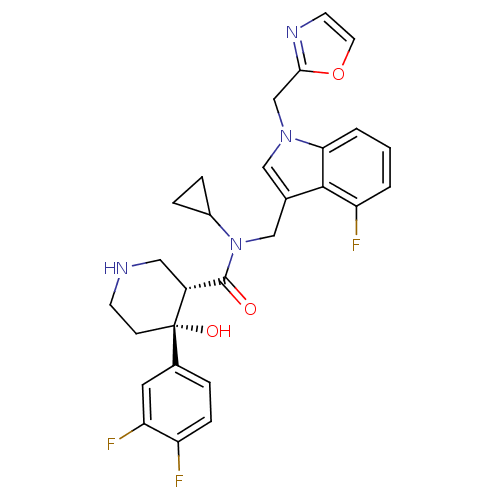 (CHEMBL1910308) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Inhibition of human recombinant renin using DNP-Lys-His-Pro-Phe-His-Leu-Val-Ile-His-D,L-Amp as Q-FRET substrate after 3 hrs by fluorescence assay | Bioorg Med Chem Lett 21: 3976-81 (2011) Article DOI: 10.1016/j.bmcl.2011.05.014 BindingDB Entry DOI: 10.7270/Q21N81HN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50380979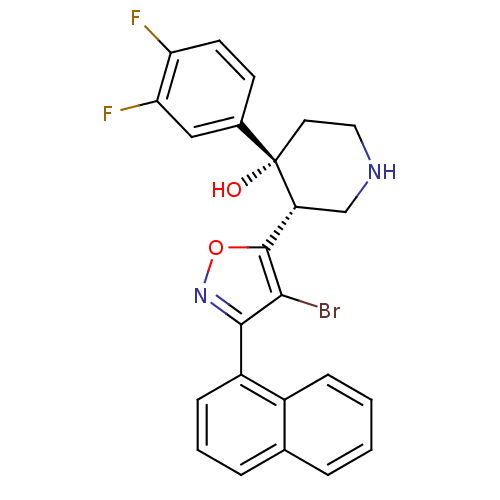 (CHEMBL2017100) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Inhibition of human recombinant renin using DNP-Lys-His-Pro-Phe-His-Leu-Val-Ile-His-D,L-Amp as substrate after 3 hrs by fluorescence analysis | Bioorg Med Chem Lett 22: 2670-4 (2012) Article DOI: 10.1016/j.bmcl.2012.03.014 BindingDB Entry DOI: 10.7270/Q2MW2J5S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50340415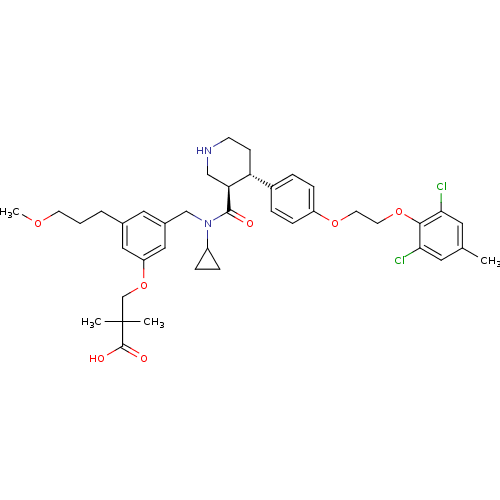 (3-(3-(((3R,4S)-N-cyclopropyl-4-(4-(2-(2,6-dichloro...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.0600 | n/a | n/a | n/a | n/a | 7.4 | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Inhibition of human recombinant renin in PBS buffer using tetradecapeptide at pH 7.4 | Bioorg Med Chem Lett 21: 2430-6 (2011) Article DOI: 10.1016/j.bmcl.2011.02.067 BindingDB Entry DOI: 10.7270/Q2G16157 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50340412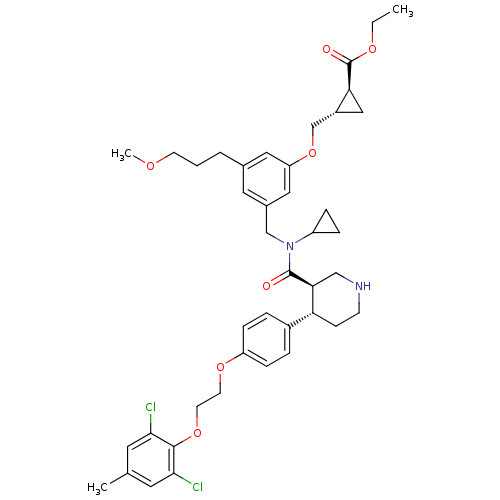 ((1S,2S)-ethyl 2-((3-(((3R,4S)-N-cyclopropyl-4-(4-(...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.0640 | n/a | n/a | n/a | n/a | 7.4 | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Inhibition of human recombinant renin in PBS buffer using tetradecapeptide at pH 7.4 | Bioorg Med Chem Lett 21: 2430-6 (2011) Article DOI: 10.1016/j.bmcl.2011.02.067 BindingDB Entry DOI: 10.7270/Q2G16157 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM18012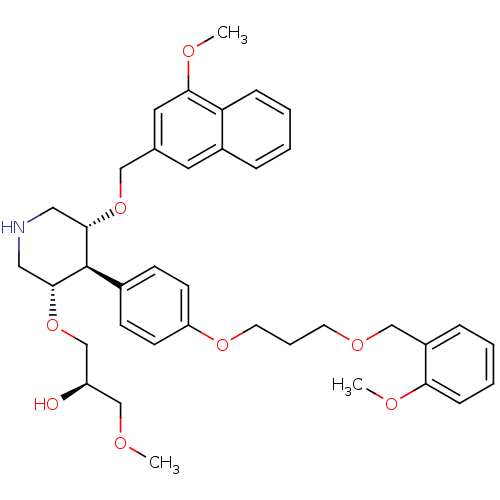 (trans,trans-4-arylpiperidine-based compound, 1) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.0670 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharmaceuticals Corp Curated by ChEMBL | Assay Description Inhibition of human recombinant renin in buffer | J Med Chem 53: 7490-520 (2010) Article DOI: 10.1021/jm901885s BindingDB Entry DOI: 10.7270/Q2S75GKG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM18012 (trans,trans-4-arylpiperidine-based compound, 1) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.0670 | n/a | n/a | n/a | n/a | 7.4 | 22 |
Pfizer | Assay Description The renin assay utilized a tandem Green Flourescent Protein (tGFP) substrate that was hydrolyzed by human rennin. The tGFP substrate contained a nine... | Bioorg Med Chem Lett 17: 3575-80 (2007) Article DOI: 10.1016/j.bmcl.2007.04.052 BindingDB Entry DOI: 10.7270/Q2B56H0X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM18012 (trans,trans-4-arylpiperidine-based compound, 1) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.0670 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1021/acsmedchemlett.2c00280 BindingDB Entry DOI: 10.7270/Q28K7F4S | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM18012 (trans,trans-4-arylpiperidine-based compound, 1) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.0670 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1021/acs.jmedchem.2c00834 BindingDB Entry DOI: 10.7270/Q2JS9VH0 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50355534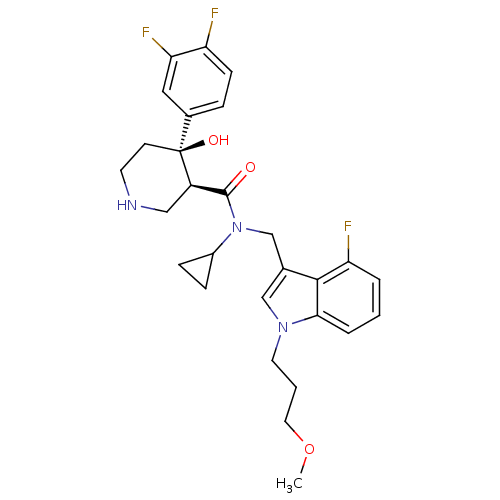 (CHEMBL1910300) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.0700 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Inhibition of human recombinant renin using DNP-Lys-His-Pro-Phe-His-Leu-Val-Ile-His-D,L-Amp as Q-FRET substrate after 3 hrs by fluorescence assay | Bioorg Med Chem Lett 21: 3976-81 (2011) Article DOI: 10.1016/j.bmcl.2011.05.014 BindingDB Entry DOI: 10.7270/Q21N81HN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50347018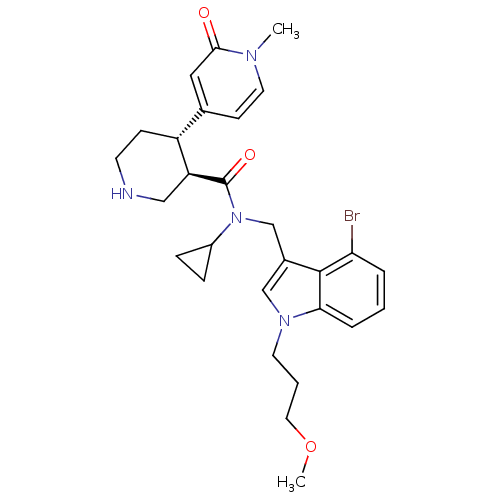 (CHEMBL1796071) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.0700 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Inhibition of human recombinant renin using Q-FRET substrate after 3 hrs by fluorescence assay | Bioorg Med Chem Lett 21: 3970-5 (2011) Article DOI: 10.1016/j.bmcl.2011.05.013 BindingDB Entry DOI: 10.7270/Q2PN9608 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50328905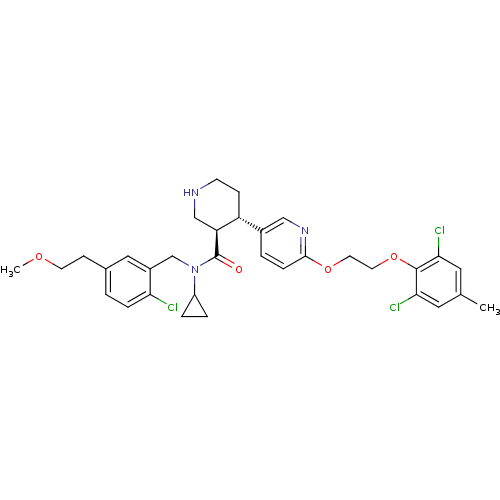 ((3R,4S)-N-(2-chloro-5-(2-methoxyethyl)benzyl)-N-cy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.0700 | n/a | n/a | n/a | n/a | n/a | n/a |
Actelion Pharmaceuticals Ltd Curated by ChEMBL | Assay Description Inhibition of renin in buffer | Bioorg Med Chem Lett 20: 6291-6 (2010) Article DOI: 10.1016/j.bmcl.2010.08.087 BindingDB Entry DOI: 10.7270/Q2H70G2C | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50359190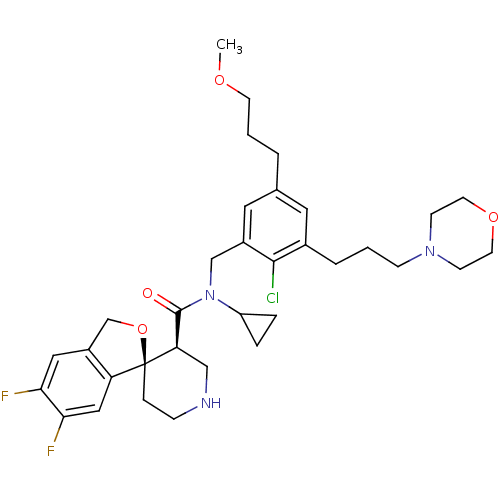 (CHEMBL1923127) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.0700 | n/a | n/a | n/a | n/a | 7.4 | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Inhibition of recombinant human renin using 9 DNP-Lys-His-Pro-Phe-His-Leu-Val-Ile-His-D,L-Amp as substrate after 3 hrs by Q-FRET assay in presence of... | Bioorg Med Chem Lett 21: 7399-404 (2011) Article DOI: 10.1016/j.bmcl.2011.10.013 BindingDB Entry DOI: 10.7270/Q2PC32SW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 4419 total ) | Next | Last >> |