

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cholecystokinin receptor type A (RAT) | BDBM50089269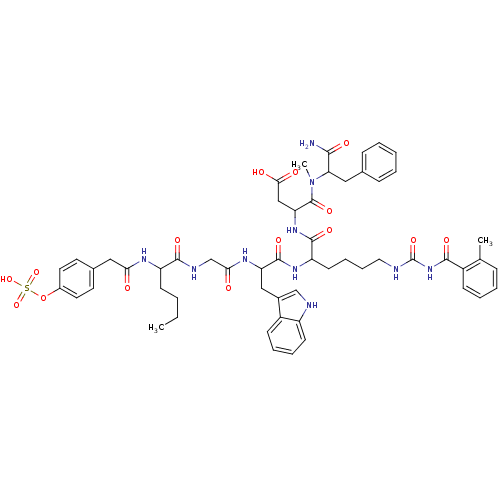 (CHEMBL276192 | N-(1-Carbamoyl-2-phenyl-ethyl)-3-{2...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca R&D Boston Curated by ChEMBL | Assay Description Inhibition of [125I]BH-CCK-8 binding to Cholecystokinin type A receptor of rat pancreatic tissue | J Med Chem 43: 2350-5 (2000) BindingDB Entry DOI: 10.7270/Q2FX7B4J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholecystokinin receptor type A (RAT) | BDBM50061828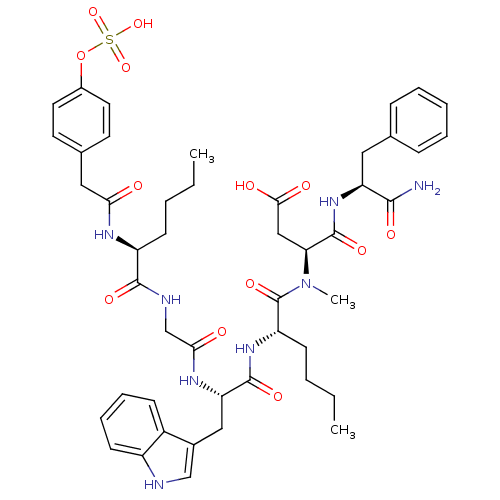 (CHEMBL76248 | CID44356929 | N-(1-Carbamoyl-2-pheny...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rochester Curated by ChEMBL | Assay Description Binding affinity for Cholecystokinin type A receptor using [125I]BH-CCK-8 in rat pancreatic tissue | J Med Chem 40: 4302-7 (1998) Article DOI: 10.1021/jm970477u BindingDB Entry DOI: 10.7270/Q2B858TF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholecystokinin receptor type A (RAT) | BDBM50061829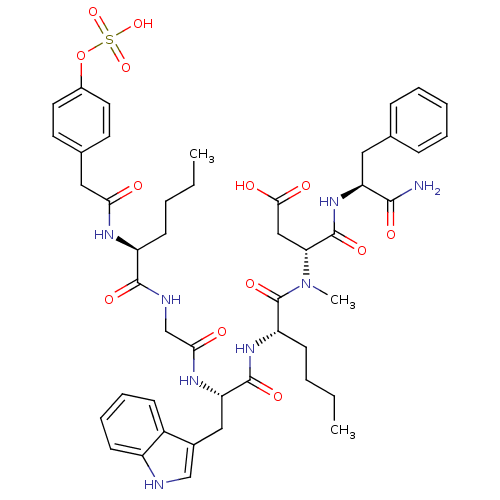 ((R)-N-((S)-1-Carbamoyl-2-phenyl-ethyl)-3-({(S)-2-[...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rochester Curated by ChEMBL | Assay Description Binding affinity for Cholecystokinin type A receptor using [125I]BH-CCK-8 in rat pancreatic tissue | J Med Chem 40: 4302-7 (1998) Article DOI: 10.1021/jm970477u BindingDB Entry DOI: 10.7270/Q2B858TF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholecystokinin receptor type A (RAT) | BDBM50061828 (CHEMBL76248 | CID44356929 | N-(1-Carbamoyl-2-pheny...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca R&D Boston Curated by ChEMBL | Assay Description Inhibition of [125I]BH-CCK-8 binding to Cholecystokinin type A receptor of rat pancreatic tissue | J Med Chem 43: 2350-5 (2000) BindingDB Entry DOI: 10.7270/Q2FX7B4J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholecystokinin receptor type A (RAT) | BDBM50089268 (CHEMBL267861 | N-(1-Carbamoyl-2-phenyl-ethyl)-3-{2...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca R&D Boston Curated by ChEMBL | Assay Description Inhibition of [125I]BH-CCK-8 binding to Cholecystokinin type A receptor of rat pancreatic tissue | J Med Chem 43: 2350-5 (2000) BindingDB Entry DOI: 10.7270/Q2FX7B4J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholecystokinin receptor type A (RAT) | BDBM50061832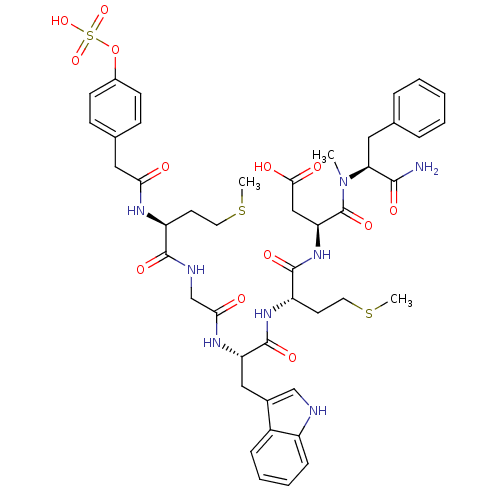 ((S)-N-((S)-1-Carbamoyl-2-phenyl-ethyl)-3-{(S)-2-[(...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | 0.0700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rochester Curated by ChEMBL | Assay Description Binding affinity for Cholecystokinin type A receptor using [125I]BH-CCK-8 in rat pancreatic tissue | J Med Chem 40: 4302-7 (1998) Article DOI: 10.1021/jm970477u BindingDB Entry DOI: 10.7270/Q2B858TF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholecystokinin receptor type A (RAT) | BDBM21147 ((3S)-3-[(2S)-2-[(2S)-2-{2-[(2S)-2-[(2S)-2-[(3S)-3-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid UniChem Similars | Article PubMed | 0.0900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rochester Curated by ChEMBL | Assay Description Binding affinity for Cholecystokinin type A receptor using [125I]BH-CCK-8 in rat pancreatic tissue | J Med Chem 40: 4302-7 (1998) Article DOI: 10.1021/jm970477u BindingDB Entry DOI: 10.7270/Q2B858TF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholecystokinin receptor type A (RAT) | BDBM21147 ((3S)-3-[(2S)-2-[(2S)-2-{2-[(2S)-2-[(2S)-2-[(3S)-3-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid UniChem Similars | PubMed | 0.0900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca R&D Boston Curated by ChEMBL | Assay Description Inhibition of [125I]BH-CCK-8 binding to Cholecystokinin type A receptor of rat pancreatic tissue | J Med Chem 43: 2350-5 (2000) BindingDB Entry DOI: 10.7270/Q2FX7B4J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholecystokinin receptor type A (RAT) | BDBM81962 (S-L-365,260) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | 0.148 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Medicines Research Centre Curated by ChEMBL | Assay Description Inhibition by displacing [3H]CCK-8S against Cholecystokinin type A receptor of rat pancreatic membranes | J Med Chem 43: 3596-613 (2000) Article DOI: 10.1021/jm990967h BindingDB Entry DOI: 10.7270/Q26H4M5V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholecystokinin receptor type A (RAT) | BDBM50449787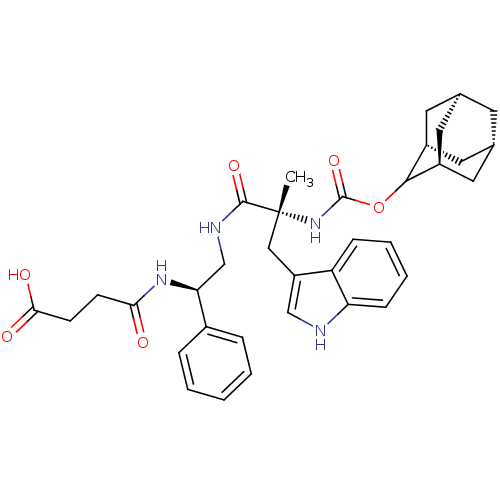 (CHEMBL2062154 | PD-134308) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.148 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Medicines Research Centre Curated by ChEMBL | Assay Description Inhibition by displacing [3H]CCK-8S against Cholecystokinin type A receptor of rat pancreatic membranes | J Med Chem 43: 3596-613 (2000) Article DOI: 10.1021/jm990967h BindingDB Entry DOI: 10.7270/Q26H4M5V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholecystokinin receptor type A (RAT) | BDBM50005463 ((R)-1H-Indole-2-carboxylic acid (1-methyl-2-oxo-5-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 0.148 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Medicines Research Centre Curated by ChEMBL | Assay Description Inhibition by displacing [3H]CCK-8S against Cholecystokinin type A receptor of rat pancreatic membranes | J Med Chem 43: 3596-613 (2000) Article DOI: 10.1021/jm990967h BindingDB Entry DOI: 10.7270/Q26H4M5V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholecystokinin receptor type A (RAT) | BDBM50061833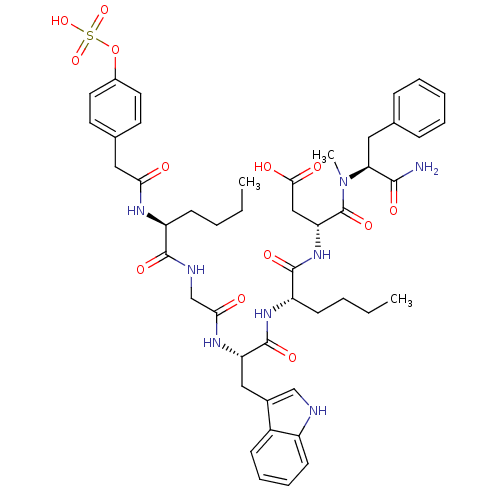 ((R)-N-((S)-1-Carbamoyl-2-phenyl-ethyl)-3-{(S)-2-[(...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rochester Curated by ChEMBL | Assay Description Binding affinity for Cholecystokinin type A receptor using [125I]BH-CCK-8 in rat pancreatic tissue | J Med Chem 40: 4302-7 (1998) Article DOI: 10.1021/jm970477u BindingDB Entry DOI: 10.7270/Q2B858TF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholecystokinin receptor type A (RAT) | BDBM50369326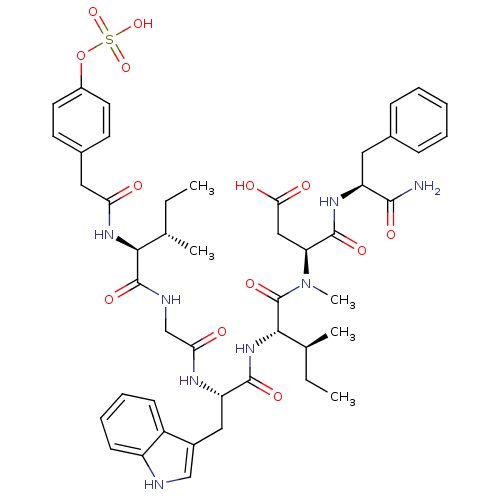 (CHEMBL1791002) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | 0.240 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rochester Curated by ChEMBL | Assay Description Binding affinity for Cholecystokinin type A receptor using [125I]BH-CCK-8 in rat pancreatic tissue | J Med Chem 40: 4302-7 (1998) Article DOI: 10.1021/jm970477u BindingDB Entry DOI: 10.7270/Q2B858TF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholecystokinin receptor type A (RAT) | BDBM50060321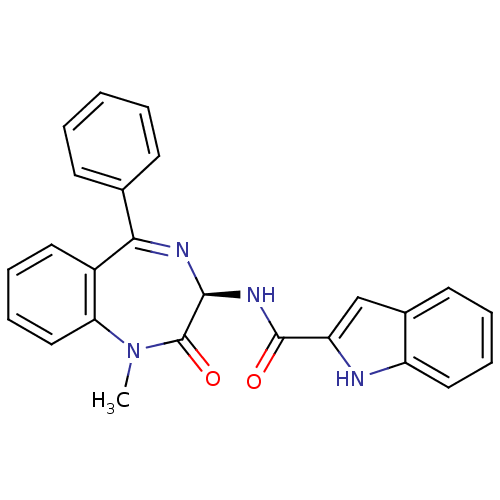 (1H-Indole-2-carboxylic acid ((R)-1-methyl-2-oxo-5-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Química Médica (CSIC) Curated by ChEMBL | Assay Description Inhibition of [3H]-pCCK-8 specific binding to Cholecystokinin type A receptor of rat pancreas | J Med Chem 40: 3402-7 (1997) Article DOI: 10.1021/jm9703247 BindingDB Entry DOI: 10.7270/Q2B56HV4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholecystokinin receptor type A (RAT) | BDBM21147 ((3S)-3-[(2S)-2-[(2S)-2-{2-[(2S)-2-[(2S)-2-[(3S)-3-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid UniChem Similars | Article PubMed | 0.520 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Química Médica (CSIC) Curated by ChEMBL | Assay Description Inhibition of [3H]-pCCK-8 specific binding to Cholecystokinin type A receptor of rat pancreas | J Med Chem 40: 3402-7 (1997) Article DOI: 10.1021/jm9703247 BindingDB Entry DOI: 10.7270/Q2B56HV4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholecystokinin receptor type A (RAT) | BDBM50369325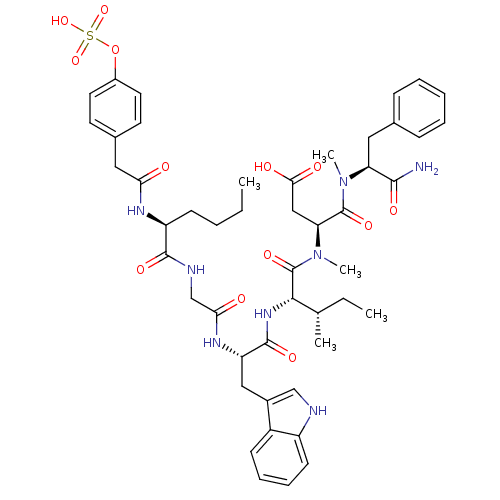 (CHEMBL1791005) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | 0.530 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rochester Curated by ChEMBL | Assay Description Binding affinity for Cholecystokinin type A receptor using [125I]BH-CCK-8 in rat pancreatic tissue | J Med Chem 40: 4302-7 (1998) Article DOI: 10.1021/jm970477u BindingDB Entry DOI: 10.7270/Q2B858TF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholecystokinin receptor type A (RAT) | BDBM50060322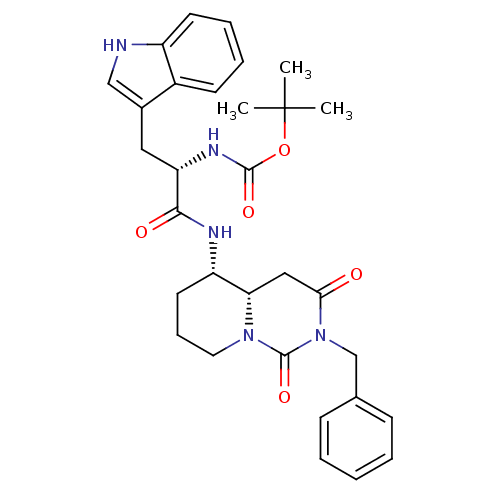 (CHEMBL332261 | [(S)-1-((4aS,5S)-2-Benzyl-1,3-dioxo...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.620 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Química Médica (CSIC) Curated by ChEMBL | Assay Description Inhibition of [3H]-pCCK-8 specific binding to Cholecystokinin type A receptor of rat pancreas | J Med Chem 40: 3402-7 (1997) Article DOI: 10.1021/jm9703247 BindingDB Entry DOI: 10.7270/Q2B56HV4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholecystokinin receptor type A (RAT) | BDBM50005463 ((R)-1H-Indole-2-carboxylic acid (1-methyl-2-oxo-5-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid PDB UniChem Patents Similars | PubMed | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Center for Bio-Pharmaceutical Sciences Curated by ChEMBL | Assay Description Inhibition of [3H]-L-364,718 binding to cholecystokinin type A receptor in rat pancreas membranes | J Med Chem 35: 1042-9 (1992) BindingDB Entry DOI: 10.7270/Q2HT2N8R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholecystokinin receptor type A (RAT) | BDBM50089276 (CHEMBL430608 | N-(1-Carbamoyl-2-phenyl-ethyl)-3-{2...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | 0.980 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca R&D Boston Curated by ChEMBL | Assay Description Inhibition of [125I]BH-CCK-8 binding to Cholecystokinin type A receptor of rat pancreatic tissue | J Med Chem 43: 2350-5 (2000) BindingDB Entry DOI: 10.7270/Q2FX7B4J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholecystokinin receptor type A (RAT) | BDBM50061834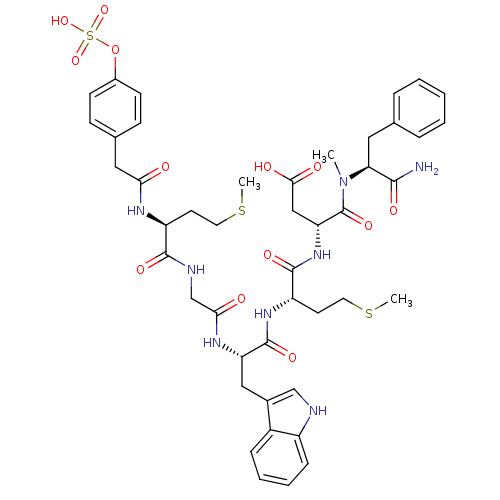 ((R)-N-((S)-1-Carbamoyl-2-phenyl-ethyl)-3-{(S)-2-[(...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | 0.980 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rochester Curated by ChEMBL | Assay Description Binding affinity for Cholecystokinin type A receptor using [125I]BH-CCK-8 in rat pancreatic tissue | J Med Chem 40: 4302-7 (1998) Article DOI: 10.1021/jm970477u BindingDB Entry DOI: 10.7270/Q2B858TF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholecystokinin receptor type A (RAT) | BDBM50211670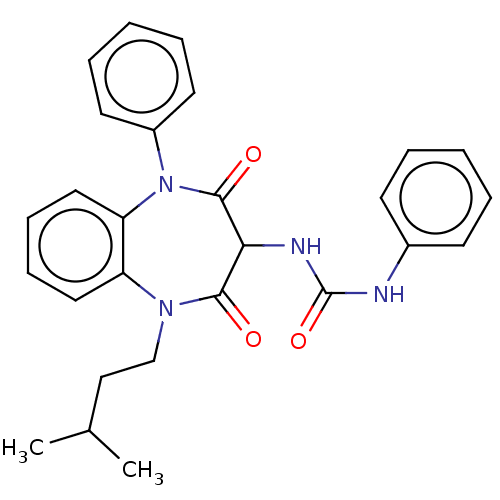 (CHEMBL103750) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Assay Description Binding affinity was measured by its ability to displace [3H]CCK-8S from CCK-A receptor in rat pancreas | Citation and Details BindingDB Entry DOI: 10.7270/Q2CV4KX9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholecystokinin receptor type A (RAT) | BDBM50089275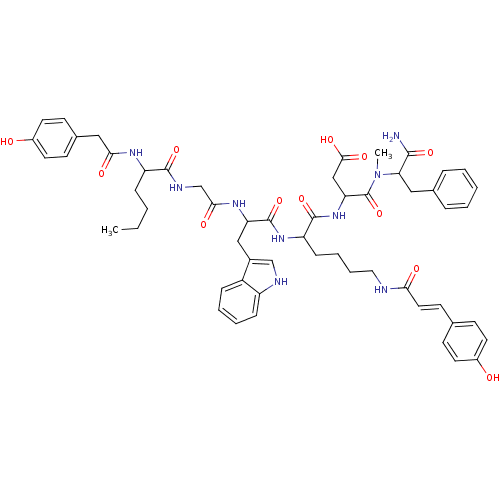 (CHEMBL384303 | N-(1-Carbamoyl-2-phenyl-ethyl)-3-{2...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 3.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca R&D Boston Curated by ChEMBL | Assay Description Inhibition of [125I]BH-CCK-8 binding to Cholecystokinin type A receptor of rat pancreatic tissue | J Med Chem 43: 2350-5 (2000) BindingDB Entry DOI: 10.7270/Q2FX7B4J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholecystokinin receptor type A (RAT) | BDBM50089277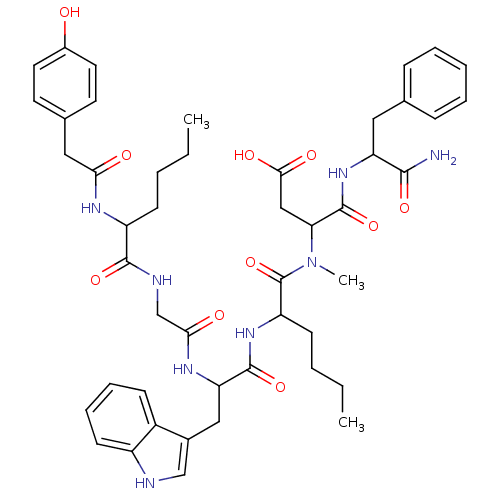 (CHEMBL311187 | N-(1-Carbamoyl-2-phenyl-ethyl)-3-({...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 4.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca R&D Boston Curated by ChEMBL | Assay Description Inhibition of [125I]BH-CCK-8 binding to Cholecystokinin type A receptor of rat pancreatic tissue | J Med Chem 43: 2350-5 (2000) BindingDB Entry DOI: 10.7270/Q2FX7B4J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholecystokinin receptor type A (RAT) | BDBM50016425 ((S)-3-{(S)-2-[(S)-2-(2-{(S)-2-[(S)-2-tert-Butoxyca...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | 4.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Evaluated for binding affinity measured by inhibiting [3H]Boc[Nle28,31]CCK27-33 specific binding to Cholecystokinin receptor in rat pancreas membrane... | J Med Chem 30: 962-8 (1987) BindingDB Entry DOI: 10.7270/Q21J98R1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholecystokinin receptor type A (RAT) | BDBM50060156 (CHEMBL319795) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | 4.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Assay Description Binding affinity was measured by its ability to displace [3H]CCK-8S from CCK-A receptor in rat pancreas | Citation and Details BindingDB Entry DOI: 10.7270/Q2CV4KX9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholecystokinin receptor type A (RAT) | BDBM50211668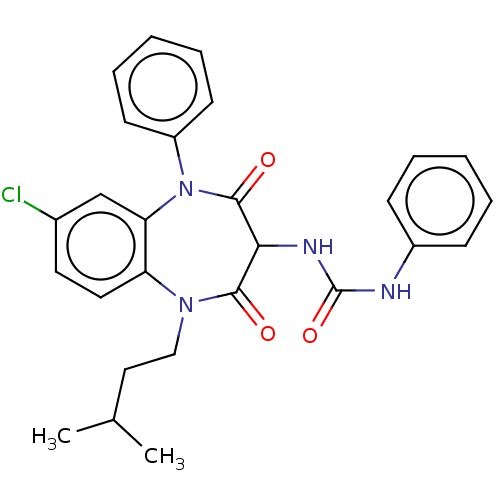 (CHEMBL103968) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | 4.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Assay Description Binding affinity was measured by its ability to displace [3H]CCK-8S from CCK-A receptor in rat pancreas | Citation and Details BindingDB Entry DOI: 10.7270/Q2CV4KX9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholecystokinin receptor type A (RAT) | BDBM50472887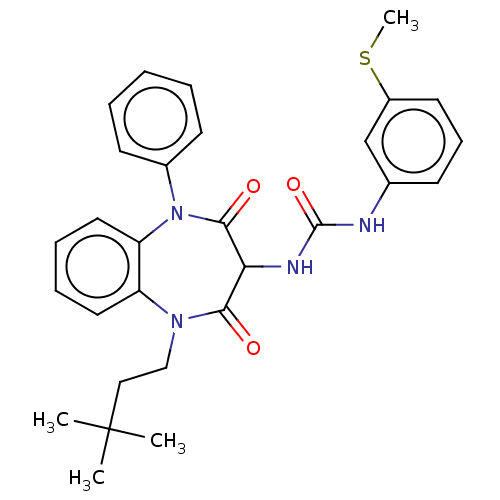 (CHEMBL330773) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 7.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Medicines Research Centre Curated by ChEMBL | Assay Description In vitro inhibition of binding of [3H]pCCK-8 against Cholecystokinin type A receptor of rat pancreatic membranes | J Med Chem 43: 3596-613 (2000) Article DOI: 10.1021/jm990967h BindingDB Entry DOI: 10.7270/Q26H4M5V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholecystokinin receptor type A (RAT) | BDBM21147 ((3S)-3-[(2S)-2-[(2S)-2-{2-[(2S)-2-[(2S)-2-[(3S)-3-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid UniChem Similars | PubMed | 8.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Evaluated for binding affinity measured by inhibiting [3H]Boc[Nle28,31]CCK27-33 specific binding to Cholecystokinin receptor in rat pancreas membrane... | J Med Chem 30: 962-8 (1987) BindingDB Entry DOI: 10.7270/Q21J98R1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholecystokinin receptor type A (RAT) | BDBM50212321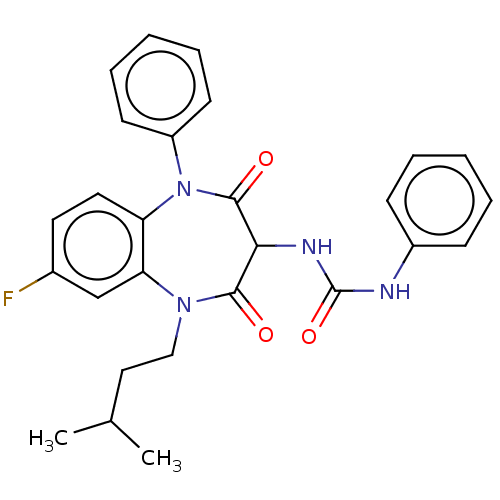 (CHEMBL317081) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | 8.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Assay Description Binding affinity was measured by its ability to displace [3H]CCK-8S from CCK-A receptor in rat pancreas | Citation and Details BindingDB Entry DOI: 10.7270/Q2CV4KX9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholecystokinin receptor type A (RAT) | BDBM50212326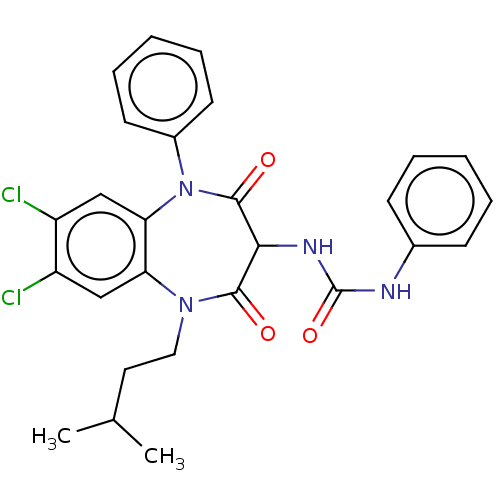 (CHEMBL102867) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | 9.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Assay Description Binding affinity was measured by its ability to displace [3H]CCK-8S from CCK-A receptor in rat pancreas | Citation and Details BindingDB Entry DOI: 10.7270/Q2CV4KX9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholecystokinin receptor type A (RAT) | BDBM50060323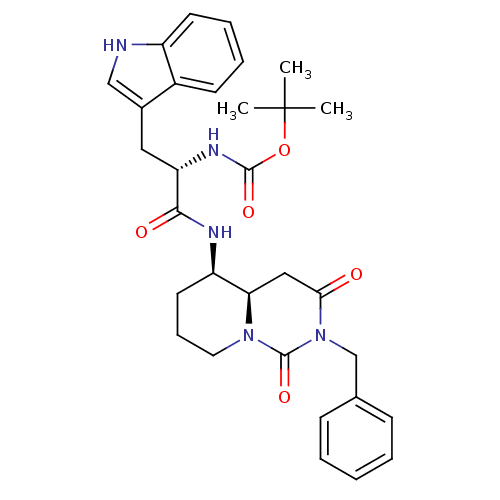 (CHEMBL431858 | [(S)-1-((4aR,5R)-2-Benzyl-1,3-dioxo...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 10.6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Química Médica (CSIC) Curated by ChEMBL | Assay Description Inhibition of [3H]-pCCK-8 specific binding to Cholecystokinin type A receptor of rat pancreas | J Med Chem 40: 3402-7 (1997) Article DOI: 10.1021/jm9703247 BindingDB Entry DOI: 10.7270/Q2B56HV4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholecystokinin receptor type A (RAT) | BDBM50472882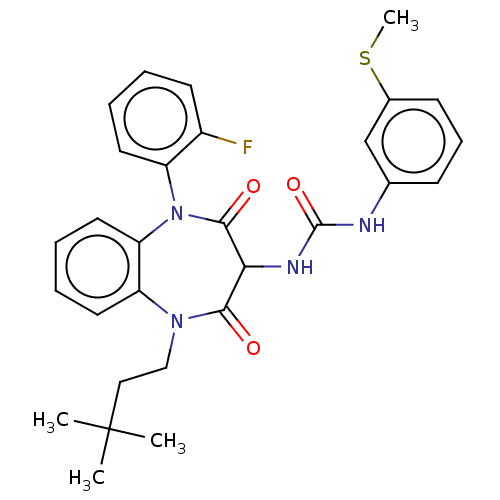 (CHEMBL332403) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Medicines Research Centre Curated by ChEMBL | Assay Description In vitro inhibition of binding of [3H]pCCK-8 against Cholecystokinin type A receptor of rat pancreatic membranes | J Med Chem 43: 3596-613 (2000) Article DOI: 10.1021/jm990967h BindingDB Entry DOI: 10.7270/Q26H4M5V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholecystokinin receptor type A (RAT) | BDBM50472876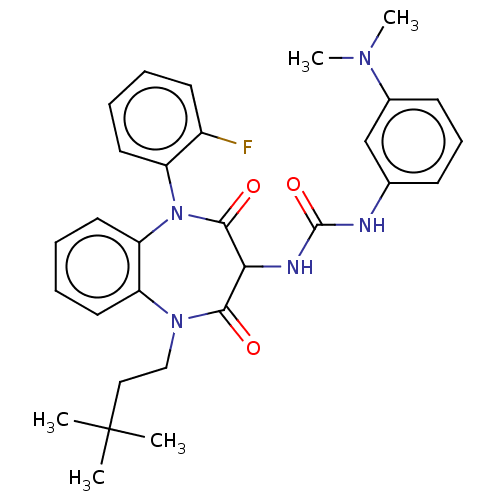 (CHEMBL332568) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 19 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Medicines Research Centre Curated by ChEMBL | Assay Description In vitro inhibition of binding of [3H]pCCK-8 against Cholecystokinin type A receptor of rat pancreatic membranes | J Med Chem 43: 3596-613 (2000) Article DOI: 10.1021/jm990967h BindingDB Entry DOI: 10.7270/Q26H4M5V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholecystokinin receptor type A (RAT) | BDBM50061830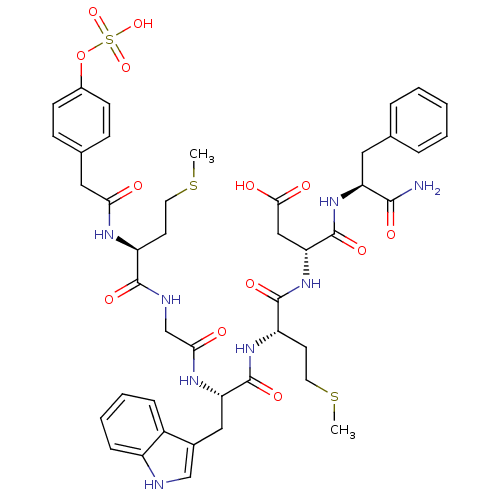 ((R)-N-((S)-1-Carbamoyl-2-phenyl-ethyl)-3-{(S)-2-[(...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | 19 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rochester Curated by ChEMBL | Assay Description Binding affinity for Cholecystokinin type A receptor using [125I]BH-CCK-8 in rat pancreatic tissue | J Med Chem 40: 4302-7 (1998) Article DOI: 10.1021/jm970477u BindingDB Entry DOI: 10.7270/Q2B858TF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholecystokinin receptor type A (RAT) | BDBM50089272 (CHEMBL312359 | N-(1-Carbamoyl-2-phenyl-ethyl)-3-{2...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | 19 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca R&D Boston Curated by ChEMBL | Assay Description Inhibition of [125I]BH-CCK-8 binding to Cholecystokinin type A receptor of rat pancreatic tissue | J Med Chem 43: 2350-5 (2000) BindingDB Entry DOI: 10.7270/Q2FX7B4J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholecystokinin receptor type A (RAT) | BDBM50472870 (CHEMBL331975) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 22 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Medicines Research Centre Curated by ChEMBL | Assay Description In vitro inhibition of binding of [3H]pCCK-8 against Cholecystokinin type A receptor of rat pancreatic membranes | J Med Chem 43: 3596-613 (2000) Article DOI: 10.1021/jm990967h BindingDB Entry DOI: 10.7270/Q26H4M5V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholecystokinin receptor type A (RAT) | BDBM50472847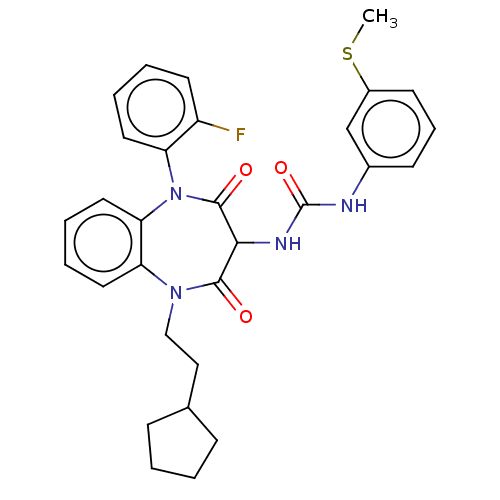 (CHEMBL121331) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 22 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Medicines Research Centre Curated by ChEMBL | Assay Description In vitro inhibition of binding of [3H]pCCK-8 against Cholecystokinin type A receptor of rat pancreatic membranes | J Med Chem 43: 3596-613 (2000) Article DOI: 10.1021/jm990967h BindingDB Entry DOI: 10.7270/Q26H4M5V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholecystokinin receptor type A (RAT) | BDBM50472898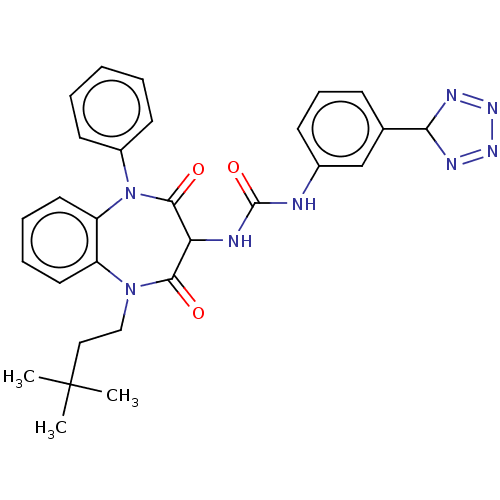 (CHEMBL332902) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 23 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Medicines Research Centre Curated by ChEMBL | Assay Description In vitro inhibition of binding of [3H]pCCK-8 against Cholecystokinin type A receptor of rat pancreatic membranes | J Med Chem 43: 3596-613 (2000) Article DOI: 10.1021/jm990967h BindingDB Entry DOI: 10.7270/Q26H4M5V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholecystokinin receptor type A (RAT) | BDBM50472884 (CHEMBL121021) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 36 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Medicines Research Centre Curated by ChEMBL | Assay Description In vitro inhibition of binding of [3H]pCCK-8 against Cholecystokinin type A receptor of rat pancreatic membranes | J Med Chem 43: 3596-613 (2000) Article DOI: 10.1021/jm990967h BindingDB Entry DOI: 10.7270/Q26H4M5V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholecystokinin receptor type A (RAT) | BDBM50212329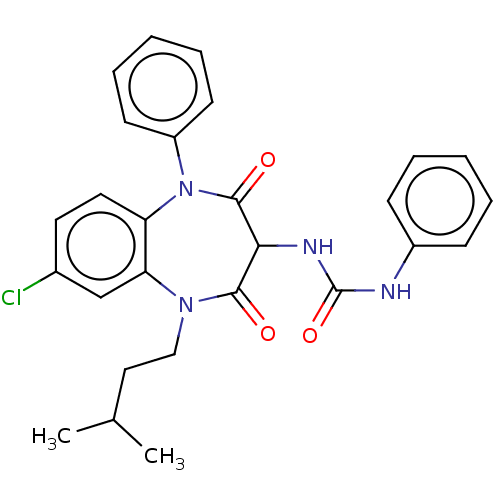 (CHEMBL101125) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | 42 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Assay Description Binding affinity was measured by its ability to displace [3H]CCK-8S from CCK-A receptor in rat pancreas | Citation and Details BindingDB Entry DOI: 10.7270/Q2CV4KX9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholecystokinin receptor type A (RAT) | BDBM50472892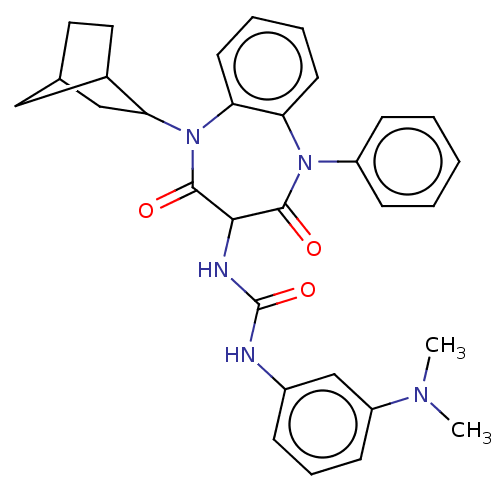 (CHEMBL331262) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 45 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Medicines Research Centre Curated by ChEMBL | Assay Description In vitro inhibition of binding of [3H]pCCK-8 against Cholecystokinin type A receptor of rat pancreatic membranes | J Med Chem 43: 3596-613 (2000) Article DOI: 10.1021/jm990967h BindingDB Entry DOI: 10.7270/Q26H4M5V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholecystokinin receptor type A (RAT) | BDBM50049805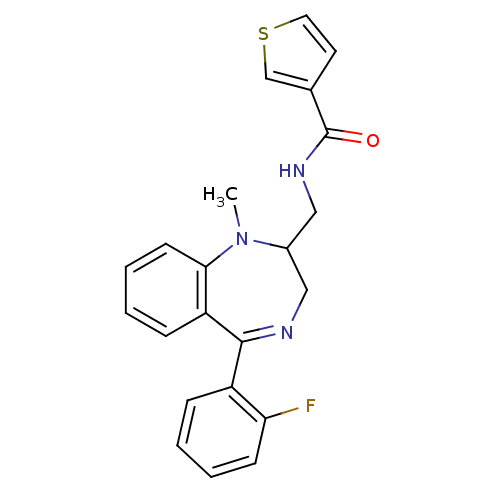 (CHEMBL169703 | Thiophene-3-carboxylic acid [5-(2-f...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | 47 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universitá di Siena Curated by ChEMBL | Assay Description Binding affinity against Cholecystokinin type A receptor using [125I]-(BH)-CCK-8 as radioligand in rat pancreas. | J Med Chem 39: 860-72 (1996) Article DOI: 10.1021/jm950423p BindingDB Entry DOI: 10.7270/Q2222SVD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholecystokinin receptor type A (RAT) | BDBM50472880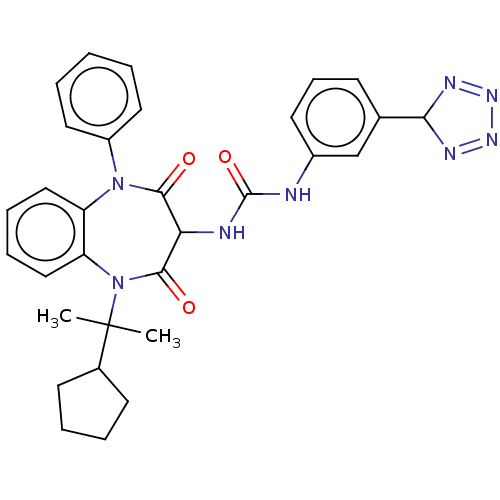 (CHEMBL338278) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 47 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Medicines Research Centre Curated by ChEMBL | Assay Description In vitro inhibition of binding of [3H]pCCK-8 against Cholecystokinin type A receptor of rat pancreatic membranes | J Med Chem 43: 3596-613 (2000) Article DOI: 10.1021/jm990967h BindingDB Entry DOI: 10.7270/Q26H4M5V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholecystokinin receptor type A (RAT) | BDBM50472886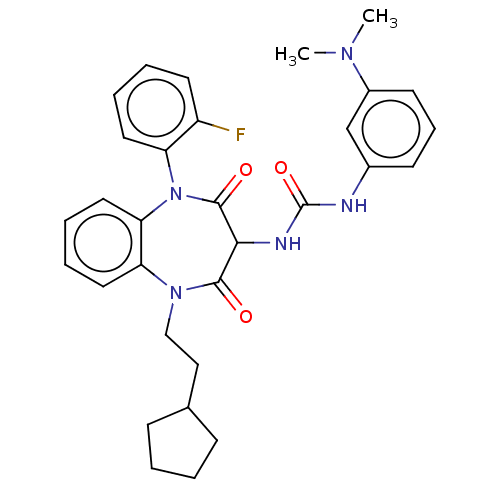 (CHEMBL421271) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 48 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Medicines Research Centre Curated by ChEMBL | Assay Description In vitro inhibition of binding of [3H]pCCK-8 against Cholecystokinin type A receptor of rat pancreatic membranes | J Med Chem 43: 3596-613 (2000) Article DOI: 10.1021/jm990967h BindingDB Entry DOI: 10.7270/Q26H4M5V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholecystokinin receptor type A (RAT) | BDBM50472861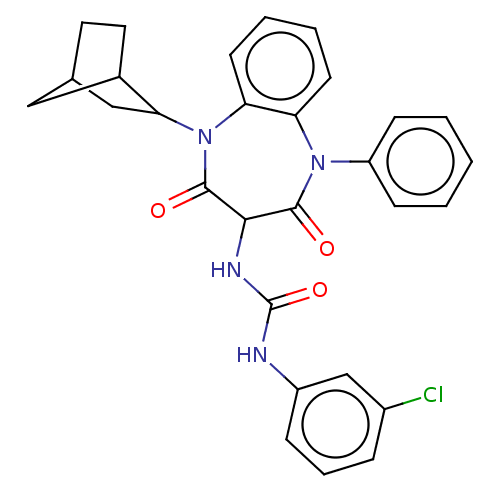 (CHEMBL121267) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 48 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Medicines Research Centre Curated by ChEMBL | Assay Description In vitro inhibition of binding of [3H]pCCK-8 against Cholecystokinin type A receptor of rat pancreatic membranes | J Med Chem 43: 3596-613 (2000) Article DOI: 10.1021/jm990967h BindingDB Entry DOI: 10.7270/Q26H4M5V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholecystokinin receptor type A (RAT) | BDBM50005459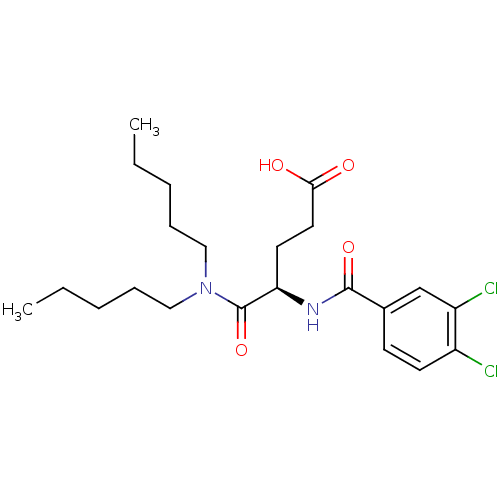 (4-(3,4-Dichloro-benzoylamino)-4-dipentylcarbamoyl-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 49 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Center for Bio-Pharmaceutical Sciences Curated by ChEMBL | Assay Description Inhibition of [3H]-L-364,718 binding to cholecystokinin type A receptor in rat pancreas membranes | J Med Chem 35: 1042-9 (1992) BindingDB Entry DOI: 10.7270/Q2HT2N8R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholecystokinin receptor type A (RAT) | BDBM50472868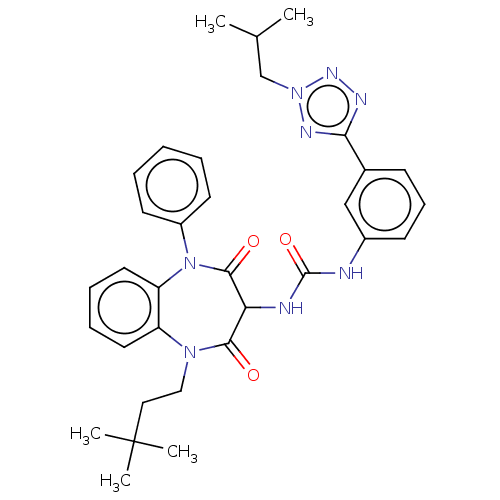 (CHEMBL120168) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 49 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Medicines Research Centre Curated by ChEMBL | Assay Description In vitro inhibition of binding of [3H]pCCK-8 against Cholecystokinin type A receptor of rat pancreatic membranes | J Med Chem 43: 3596-613 (2000) Article DOI: 10.1021/jm990967h BindingDB Entry DOI: 10.7270/Q26H4M5V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholecystokinin receptor type A (RAT) | BDBM50089270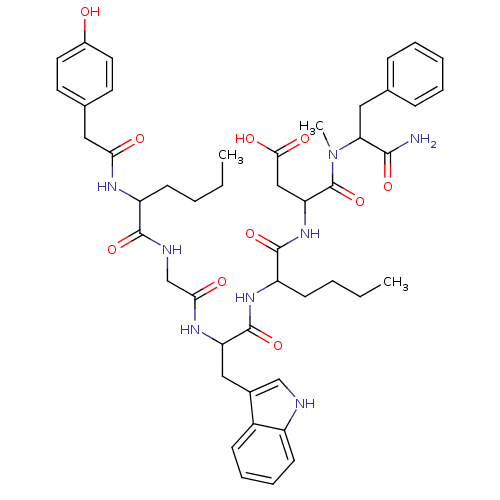 (CHEMBL308622 | N-(1-Carbamoyl-2-phenyl-ethyl)-3-{2...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 51 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca R&D Boston Curated by ChEMBL | Assay Description Inhibition of [125I]BH-CCK-8 binding to Cholecystokinin type A receptor of rat pancreatic tissue | J Med Chem 43: 2350-5 (2000) BindingDB Entry DOI: 10.7270/Q2FX7B4J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholecystokinin receptor type A (RAT) | BDBM50214389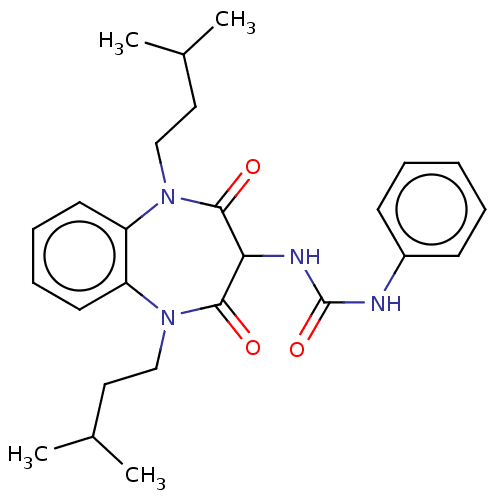 (CHEMBL101063) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | 62 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Assay Description Evaluated in vitro for Cholecystokinin type A receptor affinity by measuring its ability to displace tritiated CCK-8S bound on rat pancreas Cholecyst... | Citation and Details BindingDB Entry DOI: 10.7270/Q27W6FCB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholecystokinin receptor type A (RAT) | BDBM50472875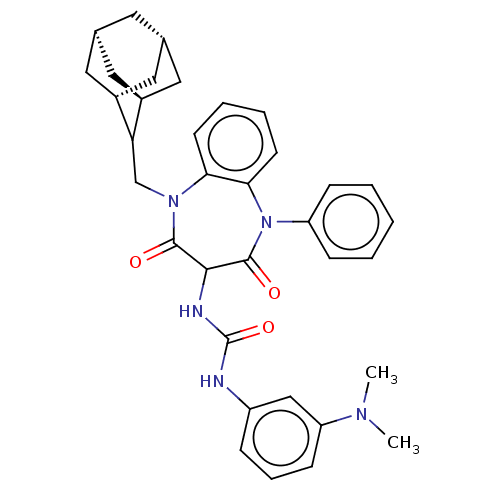 (CHEMBL333930) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 63 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Medicines Research Centre Curated by ChEMBL | Assay Description In vitro inhibition of binding of [3H]pCCK-8 against Cholecystokinin type A receptor of rat pancreatic membranes | J Med Chem 43: 3596-613 (2000) Article DOI: 10.1021/jm990967h BindingDB Entry DOI: 10.7270/Q26H4M5V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 200 total ) | Next | Last >> |