 Found 205 hits of ki for UniProtKB: P20231
Found 205 hits of ki for UniProtKB: P20231 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kcal/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Tryptase beta-2
(Homo sapiens (Human)) | BDBM50093142
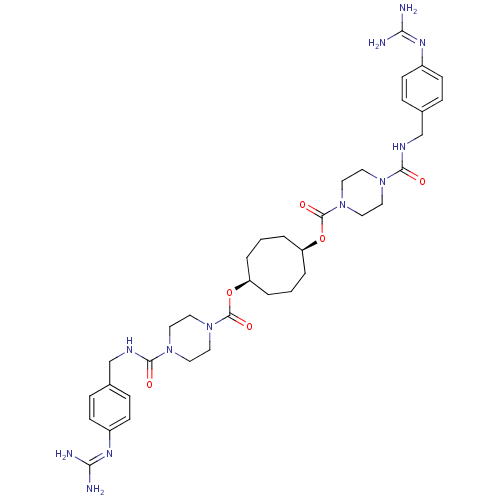
(1,5-di{4-[4-amino(imino)methylaminobenzylcarbamoyl...)Show SMILES [#7]\[#6](-[#7])=[#7]/c1ccc(-[#6]-[#7]-[#6](=O)-[#7]-2-[#6]-[#6]-[#7](-[#6]-[#6]-2)-[#6](=O)-[#8]-[#6@H]-2-[#6]-[#6]-[#6]-[#6@H](-[#6]-[#6]-[#6]-2)-[#8]-[#6](=O)-[#7]-2-[#6]-[#6]-[#7](-[#6]-[#6]-2)-[#6](=O)-[#7]-[#6]-c2ccc(cc2)\[#7]=[#6](/[#7])-[#7])cc1 Show InChI InChI=1S/C36H52N12O6/c37-31(38)43-27-11-7-25(8-12-27)23-41-33(49)45-15-19-47(20-16-45)35(51)53-29-3-1-4-30(6-2-5-29)54-36(52)48-21-17-46(18-22-48)34(50)42-24-26-9-13-28(14-10-26)44-32(39)40/h7-14,29-30H,1-6,15-24H2,(H,41,49)(H,42,50)(H4,37,38,43)(H4,39,40,44)/t29-,30+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 0.0700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Axys Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity against human tryptase enzyme |
Bioorg Med Chem Lett 11: 2325-30 (2001)
BindingDB Entry DOI: 10.7270/Q21835S6 |
More data for this
Ligand-Target Pair | |
Tryptase beta-2
(Homo sapiens (Human)) | BDBM50101018
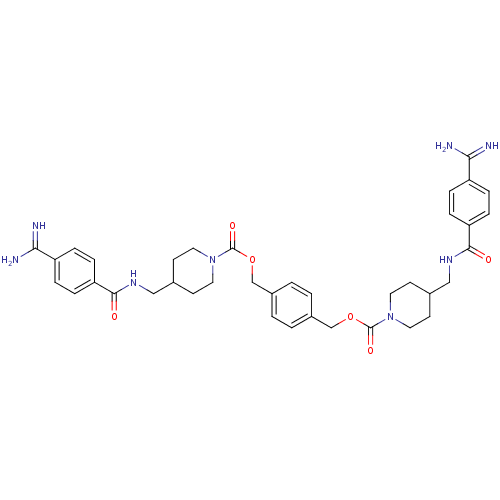
(1,4-di{4-[4-amino(imino)methylphenylcarboxamidomet...)Show SMILES NC(=N)c1ccc(cc1)C(=O)NCC1CCN(CC1)C(=O)OCc1ccc(COC(=O)N2CCC(CNC(=O)c3ccc(cc3)C(N)=N)CC2)cc1 Show InChI InChI=1S/C38H46N8O6/c39-33(40)29-5-9-31(10-6-29)35(47)43-21-25-13-17-45(18-14-25)37(49)51-23-27-1-2-28(4-3-27)24-52-38(50)46-19-15-26(16-20-46)22-44-36(48)32-11-7-30(8-12-32)34(41)42/h1-12,25-26H,13-24H2,(H3,39,40)(H3,41,42)(H,43,47)(H,44,48) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Axys Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity against human tryptase enzyme |
Bioorg Med Chem Lett 11: 2325-30 (2001)
BindingDB Entry DOI: 10.7270/Q21835S6 |
More data for this
Ligand-Target Pair | |
Tryptase beta-2
(Homo sapiens (Human)) | BDBM50084616
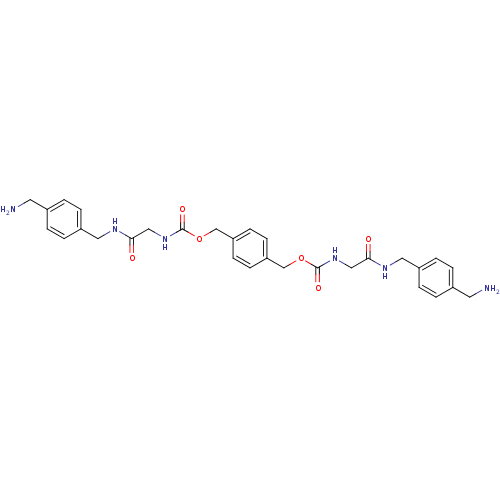
(CHEMBL310290 | [(4-Aminomethyl-benzylcarbamoyl)-me...)Show SMILES NCc1ccc(CNC(=O)CNC(=O)OCc2ccc(COC(=O)NCC(=O)NCc3ccc(CN)cc3)cc2)cc1 Show InChI InChI=1S/C30H36N6O6/c31-13-21-1-5-23(6-2-21)15-33-27(37)17-35-29(39)41-19-25-9-11-26(12-10-25)20-42-30(40)36-18-28(38)34-16-24-7-3-22(14-32)4-8-24/h1-12H,13-20,31-32H2,(H,33,37)(H,34,38)(H,35,39)(H,36,40) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Axys Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibitory potency against tryptase |
Bioorg Med Chem Lett 10: 2357-60 (2001)
BindingDB Entry DOI: 10.7270/Q2NP23NX |
More data for this
Ligand-Target Pair | |
Tryptase beta-2
(Homo sapiens (Human)) | BDBM50156461
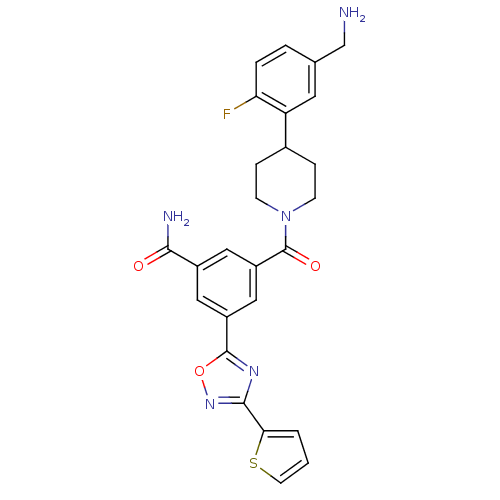
(3-[4-(5-Aminomethyl-2-fluoro-phenyl)-piperidine-1-...)Show SMILES NCc1ccc(F)c(c1)C1CCN(CC1)C(=O)c1cc(cc(c1)-c1nc(no1)-c1cccs1)C(N)=O Show InChI InChI=1S/C26H24FN5O3S/c27-21-4-3-15(14-28)10-20(21)16-5-7-32(8-6-16)26(34)19-12-17(23(29)33)11-18(13-19)25-30-24(31-35-25)22-2-1-9-36-22/h1-4,9-13,16H,5-8,14,28H2,(H2,29,33) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aventis Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibitory activity against human mast cell tryptase beta |
Bioorg Med Chem Lett 14: 6053-6 (2004)
Article DOI: 10.1016/j.bmcl.2004.09.065
BindingDB Entry DOI: 10.7270/Q2KH0MST |
More data for this
Ligand-Target Pair | |
Tryptase beta-2
(Homo sapiens (Human)) | BDBM50156460
(3-[4-(3-Aminomethyl-phenyl)-piperidine-1-carbonyl]...)Show SMILES NCc1cccc(c1)C1CCN(CC1)C(=O)c1cc(cc(c1)-c1nc(no1)-c1cccs1)C(N)=O Show InChI InChI=1S/C26H25N5O3S/c27-15-16-3-1-4-18(11-16)17-6-8-31(9-7-17)26(33)21-13-19(23(28)32)12-20(14-21)25-29-24(30-34-25)22-5-2-10-35-22/h1-5,10-14,17H,6-9,15,27H2,(H2,28,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aventis Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibitory activity against human mast cell tryptase beta |
Bioorg Med Chem Lett 14: 6053-6 (2004)
Article DOI: 10.1016/j.bmcl.2004.09.065
BindingDB Entry DOI: 10.7270/Q2KH0MST |
More data for this
Ligand-Target Pair | |
Tryptase beta-2
(Homo sapiens (Human)) | BDBM50093132
(CHEMBL75749 | [3-(4-Guanidino-benzyl)-ureido]-acet...)Show SMILES [#7]\[#6](-[#7])=[#7]/c1ccc(-[#6]-[#7]-[#6](=O)-[#7]-[#6]-[#6](=O)-[#8]-[#6@H]-2-[#6]-[#6]-[#6]-[#6@H](-[#6]-[#6]-[#6]-2)-[#8]-[#6](=O)-[#6]-[#7]-[#6](=O)-[#7]-[#6]-c2ccc(cc2)\[#7]=[#6](\[#7])-[#7])cc1 Show InChI InChI=1S/C30H42N10O6/c31-27(32)39-21-11-7-19(8-12-21)15-35-29(43)37-17-25(41)45-23-3-1-4-24(6-2-5-23)46-26(42)18-38-30(44)36-16-20-9-13-22(14-10-20)40-28(33)34/h7-14,23-24H,1-6,15-18H2,(H4,31,32,39)(H4,33,34,40)(H2,35,37,43)(H2,36,38,44)/t23-,24+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Axys Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibitory potency against tryptase |
Bioorg Med Chem Lett 10: 2357-60 (2001)
BindingDB Entry DOI: 10.7270/Q2NP23NX |
More data for this
Ligand-Target Pair | |
Tryptase beta-2
(Homo sapiens (Human)) | BDBM50411015
(CHEMBL539086)Show SMILES NCCCC[C@H](NC(=O)c1ccco1)C(=O)c1noc(Cc2ccc(OCCc3ccc(Cl)c(Cl)c3)cc2)n1 |r| Show InChI InChI=1S/C28H28Cl2N4O5/c29-21-11-8-19(16-22(21)30)12-15-37-20-9-6-18(7-10-20)17-25-33-27(34-39-25)26(35)23(4-1-2-13-31)32-28(36)24-5-3-14-38-24/h3,5-11,14,16,23H,1-2,4,12-13,15,17,31H2,(H,32,36)/t23-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera Genomics
Curated by ChEMBL
| Assay Description
Inhibition of human B tryptase |
Bioorg Med Chem Lett 16: 3434-9 (2006)
Article DOI: 10.1016/j.bmcl.2006.04.013
BindingDB Entry DOI: 10.7270/Q2G44RHP |
More data for this
Ligand-Target Pair | |
Tryptase beta-2
(Homo sapiens (Human)) | BDBM50411006
(CHEMBL539088)Show SMILES NCCCC[C@H](NC(=O)c1ccc(F)cc1)C(=O)c1noc(Cc2ccc(OCCc3ccc(Cl)c(Cl)c3)cc2)n1 |r| Show InChI InChI=1S/C30H29Cl2FN4O4/c31-24-13-6-20(17-25(24)32)14-16-40-23-11-4-19(5-12-23)18-27-36-29(37-41-27)28(38)26(3-1-2-15-34)35-30(39)21-7-9-22(33)10-8-21/h4-13,17,26H,1-3,14-16,18,34H2,(H,35,39)/t26-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera Genomics
Curated by ChEMBL
| Assay Description
Inhibition of human B tryptase |
Bioorg Med Chem Lett 16: 3434-9 (2006)
Article DOI: 10.1016/j.bmcl.2006.04.013
BindingDB Entry DOI: 10.7270/Q2G44RHP |
More data for this
Ligand-Target Pair | |
Tryptase beta-2
(Homo sapiens (Human)) | BDBM50411011
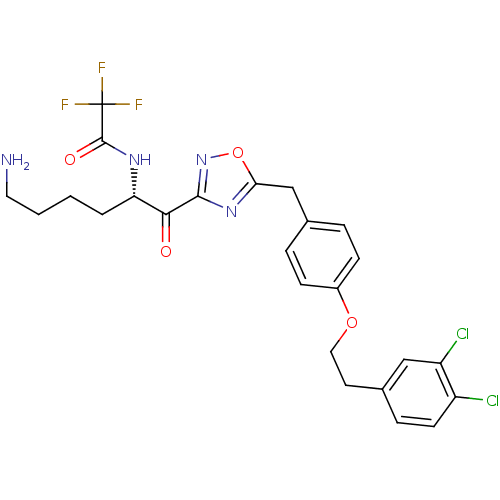
(CHEMBL537868)Show SMILES NCCCC[C@H](NC(=O)C(F)(F)F)C(=O)c1noc(Cc2ccc(OCCc3ccc(Cl)c(Cl)c3)cc2)n1 |r| Show InChI InChI=1S/C25H25Cl2F3N4O4/c26-18-9-6-16(13-19(18)27)10-12-37-17-7-4-15(5-8-17)14-21-33-23(34-38-21)22(35)20(3-1-2-11-31)32-24(36)25(28,29)30/h4-9,13,20H,1-3,10-12,14,31H2,(H,32,36)/t20-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera Genomics
Curated by ChEMBL
| Assay Description
Inhibition of human B tryptase |
Bioorg Med Chem Lett 16: 3434-9 (2006)
Article DOI: 10.1016/j.bmcl.2006.04.013
BindingDB Entry DOI: 10.7270/Q2G44RHP |
More data for this
Ligand-Target Pair | |
Tryptase beta-2
(Homo sapiens (Human)) | BDBM50093143
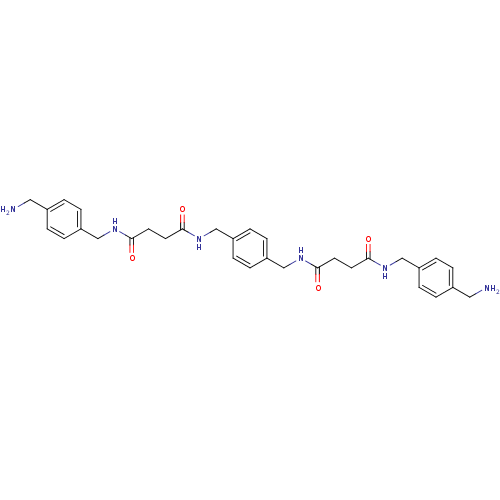
(CHEMBL76733 | N-(4-Aminomethyl-benzyl)-N'-(4-{[3-(...)Show SMILES NCc1ccc(CNC(=O)CCC(=O)NCc2ccc(CNC(=O)CCC(=O)NCc3ccc(CN)cc3)cc2)cc1 Show InChI InChI=1S/C32H40N6O4/c33-17-23-1-5-25(6-2-23)19-35-29(39)13-15-31(41)37-21-27-9-11-28(12-10-27)22-38-32(42)16-14-30(40)36-20-26-7-3-24(18-34)4-8-26/h1-12H,13-22,33-34H2,(H,35,39)(H,36,40)(H,37,41)(H,38,42) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Axys Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibitory potency against tryptase |
Bioorg Med Chem Lett 10: 2357-60 (2001)
BindingDB Entry DOI: 10.7270/Q2NP23NX |
More data for this
Ligand-Target Pair | |
Tryptase beta-2
(Homo sapiens (Human)) | BDBM14312
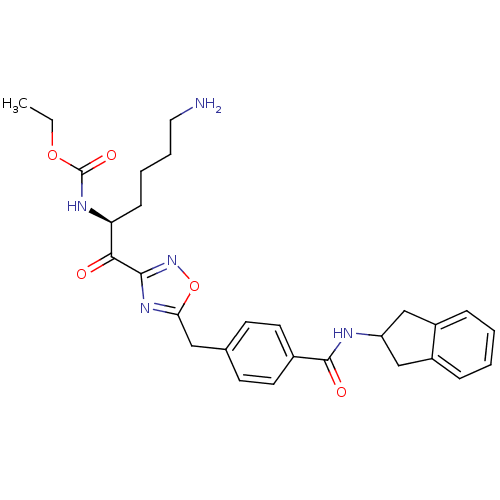
(CHEMBL214368 | CRA23 | ethyl N-[(2S)-6-amino-1-(5-...)Show SMILES CCOC(=O)N[C@@H](CCCCN)C(=O)c1noc(Cc2ccc(cc2)C(=O)NC2Cc3ccccc3C2)n1 |r| Show InChI InChI=1S/C28H33N5O5/c1-2-37-28(36)31-23(9-5-6-14-29)25(34)26-32-24(38-33-26)15-18-10-12-19(13-11-18)27(35)30-22-16-20-7-3-4-8-21(20)17-22/h3-4,7-8,10-13,22-23H,2,5-6,9,14-17,29H2,1H3,(H,30,35)(H,31,36)/t23-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera
| Assay Description
Each enzyme was assayed with a set of different concentrations of each inhibitor. After addition of the appropriate substrate, the rate of hydrolysis... |
Biochemistry 45: 5964-73 (2006)
Article DOI: 10.1021/bi060173m
BindingDB Entry DOI: 10.7270/Q2W09450 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Tryptase beta-2
(Homo sapiens (Human)) | BDBM50411017
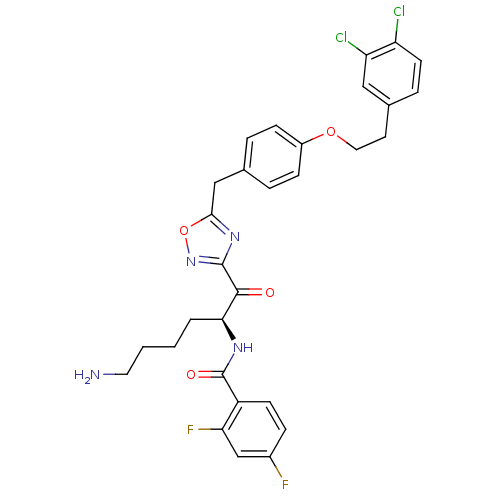
(CHEMBL539603)Show SMILES NCCCC[C@H](NC(=O)c1ccc(F)cc1F)C(=O)c1noc(Cc2ccc(OCCc3ccc(Cl)c(Cl)c3)cc2)n1 |r| Show InChI InChI=1S/C30H28Cl2F2N4O4/c31-23-11-6-19(15-24(23)32)12-14-41-21-8-4-18(5-9-21)16-27-37-29(38-42-27)28(39)26(3-1-2-13-35)36-30(40)22-10-7-20(33)17-25(22)34/h4-11,15,17,26H,1-3,12-14,16,35H2,(H,36,40)/t26-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera Genomics
Curated by ChEMBL
| Assay Description
Inhibition of human B tryptase |
Bioorg Med Chem Lett 16: 3434-9 (2006)
Article DOI: 10.1016/j.bmcl.2006.04.013
BindingDB Entry DOI: 10.7270/Q2G44RHP |
More data for this
Ligand-Target Pair | |
Tryptase beta-2
(Homo sapiens (Human)) | BDBM50411009
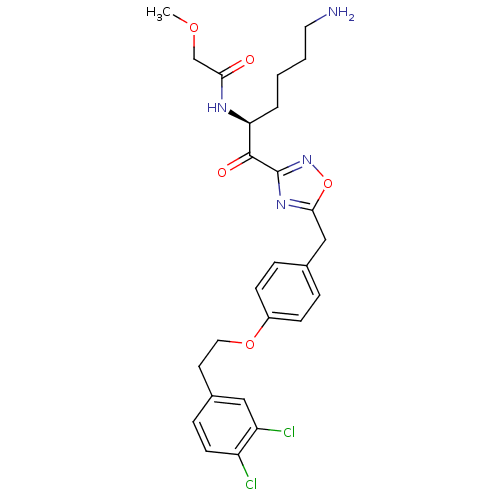
(CHEMBL538574)Show SMILES COCC(=O)N[C@@H](CCCCN)C(=O)c1noc(Cc2ccc(OCCc3ccc(Cl)c(Cl)c3)cc2)n1 |r| Show InChI InChI=1S/C26H30Cl2N4O5/c1-35-16-23(33)30-22(4-2-3-12-29)25(34)26-31-24(37-32-26)15-17-5-8-19(9-6-17)36-13-11-18-7-10-20(27)21(28)14-18/h5-10,14,22H,2-4,11-13,15-16,29H2,1H3,(H,30,33)/t22-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera Genomics
Curated by ChEMBL
| Assay Description
Inhibition of human B tryptase |
Bioorg Med Chem Lett 16: 3434-9 (2006)
Article DOI: 10.1016/j.bmcl.2006.04.013
BindingDB Entry DOI: 10.7270/Q2G44RHP |
More data for this
Ligand-Target Pair | |
Tryptase beta-2
(Homo sapiens (Human)) | BDBM50411002
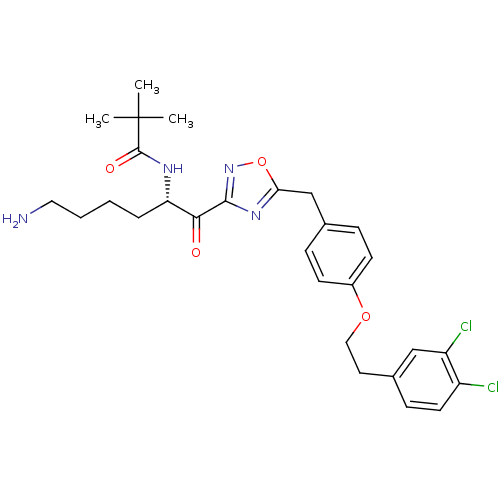
(CHEMBL556994)Show SMILES CC(C)(C)C(=O)N[C@@H](CCCCN)C(=O)c1noc(Cc2ccc(OCCc3ccc(Cl)c(Cl)c3)cc2)n1 |r| Show InChI InChI=1S/C28H34Cl2N4O4/c1-28(2,3)27(36)32-23(6-4-5-14-31)25(35)26-33-24(38-34-26)17-18-7-10-20(11-8-18)37-15-13-19-9-12-21(29)22(30)16-19/h7-12,16,23H,4-6,13-15,17,31H2,1-3H3,(H,32,36)/t23-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.75 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera Genomics
Curated by ChEMBL
| Assay Description
Inhibition of human B tryptase |
Bioorg Med Chem Lett 16: 3434-9 (2006)
Article DOI: 10.1016/j.bmcl.2006.04.013
BindingDB Entry DOI: 10.7270/Q2G44RHP |
More data for this
Ligand-Target Pair | |
Tryptase beta-2
(Homo sapiens (Human)) | BDBM14311
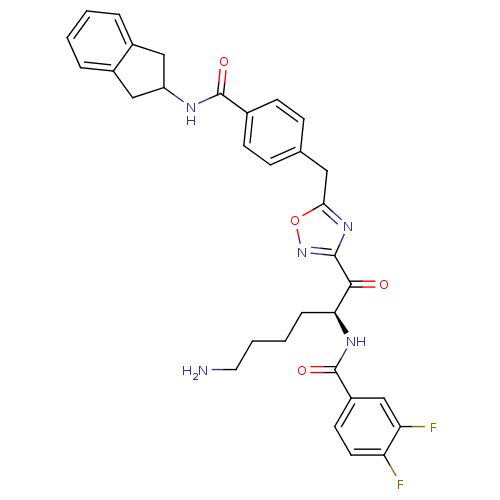
(CRA22 | N-[(2S)-6-amino-1-(5-{[4-(2,3-dihydro-1H-i...)Show SMILES NCCCC[C@H](NC(=O)c1ccc(F)c(F)c1)C(=O)c1noc(Cc2ccc(cc2)C(=O)NC2Cc3ccccc3C2)n1 |r| Show InChI InChI=1S/C32H31F2N5O4/c33-25-13-12-23(18-26(25)34)32(42)37-27(7-3-4-14-35)29(40)30-38-28(43-39-30)15-19-8-10-20(11-9-19)31(41)36-24-16-21-5-1-2-6-22(21)17-24/h1-2,5-6,8-13,18,24,27H,3-4,7,14-17,35H2,(H,36,41)(H,37,42)/t27-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera
| Assay Description
Each enzyme was assayed with a set of different concentrations of each inhibitor. After addition of the appropriate substrate, the rate of hydrolysis... |
Biochemistry 45: 5964-73 (2006)
Article DOI: 10.1021/bi060173m
BindingDB Entry DOI: 10.7270/Q2W09450 |
More data for this
Ligand-Target Pair | |
Tryptase beta-2
(Homo sapiens (Human)) | BDBM50156457
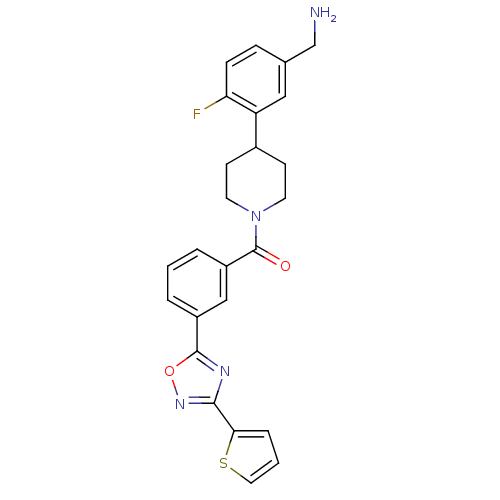
(CHEMBL186730 | [4-(5-Aminomethyl-2-fluoro-phenyl)-...)Show SMILES NCc1ccc(F)c(c1)C1CCN(CC1)C(=O)c1cccc(c1)-c1nc(no1)-c1cccs1 Show InChI InChI=1S/C25H23FN4O2S/c26-21-7-6-16(15-27)13-20(21)17-8-10-30(11-9-17)25(31)19-4-1-3-18(14-19)24-28-23(29-32-24)22-5-2-12-33-22/h1-7,12-14,17H,8-11,15,27H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aventis Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibitory activity against human mast cell tryptase beta |
Bioorg Med Chem Lett 14: 6053-6 (2004)
Article DOI: 10.1016/j.bmcl.2004.09.065
BindingDB Entry DOI: 10.7270/Q2KH0MST |
More data for this
Ligand-Target Pair | |
Tryptase beta-2
(Homo sapiens (Human)) | BDBM50411013
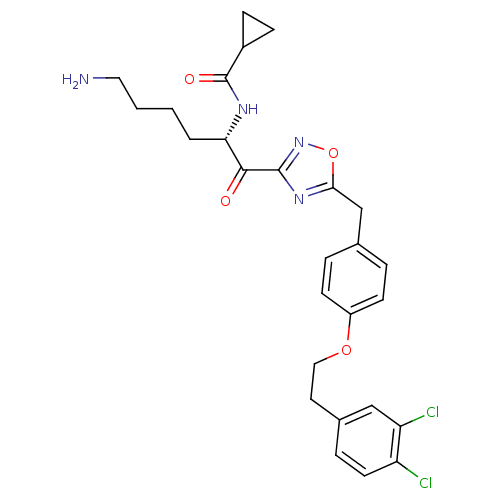
(CHEMBL534493)Show SMILES NCCCC[C@H](NC(=O)C1CC1)C(=O)c1noc(Cc2ccc(OCCc3ccc(Cl)c(Cl)c3)cc2)n1 |r| Show InChI InChI=1S/C27H30Cl2N4O4/c28-21-11-6-18(15-22(21)29)12-14-36-20-9-4-17(5-10-20)16-24-32-26(33-37-24)25(34)23(3-1-2-13-30)31-27(35)19-7-8-19/h4-6,9-11,15,19,23H,1-3,7-8,12-14,16,30H2,(H,31,35)/t23-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera Genomics
Curated by ChEMBL
| Assay Description
Inhibition of human B tryptase |
Bioorg Med Chem Lett 16: 3434-9 (2006)
Article DOI: 10.1016/j.bmcl.2006.04.013
BindingDB Entry DOI: 10.7270/Q2G44RHP |
More data for this
Ligand-Target Pair | |
Tryptase beta-2
(Homo sapiens (Human)) | BDBM50410996
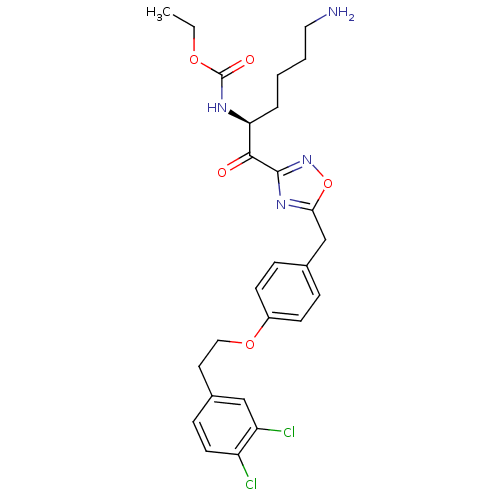
(CHEMBL537647)Show SMILES CCOC(=O)N[C@@H](CCCCN)C(=O)c1noc(Cc2ccc(OCCc3ccc(Cl)c(Cl)c3)cc2)n1 |r| Show InChI InChI=1S/C26H30Cl2N4O5/c1-2-35-26(34)30-22(5-3-4-13-29)24(33)25-31-23(37-32-25)16-17-6-9-19(10-7-17)36-14-12-18-8-11-20(27)21(28)15-18/h6-11,15,22H,2-5,12-14,16,29H2,1H3,(H,30,34)/t22-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera Genomics
Curated by ChEMBL
| Assay Description
Inhibition of human B tryptase |
Bioorg Med Chem Lett 16: 3434-9 (2006)
Article DOI: 10.1016/j.bmcl.2006.04.013
BindingDB Entry DOI: 10.7270/Q2G44RHP |
More data for this
Ligand-Target Pair | |
Tryptase beta-2
(Homo sapiens (Human)) | BDBM50093131
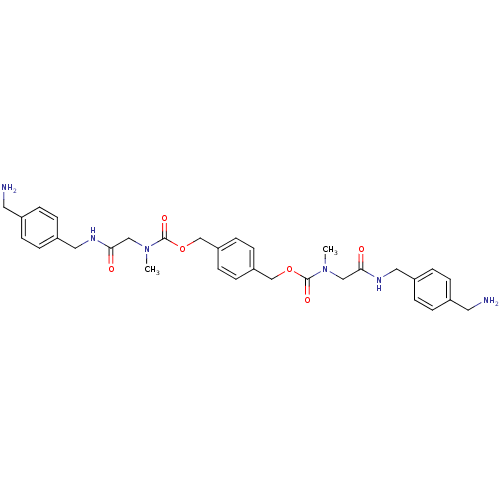
(CHEMBL80210 | [(4-Aminomethyl-benzylcarbamoyl)-met...)Show SMILES CN(CC(=O)NCc1ccc(CN)cc1)C(=O)OCc1ccc(COC(=O)N(C)CC(=O)NCc2ccc(CN)cc2)cc1 Show InChI InChI=1S/C32H40N6O6/c1-37(19-29(39)35-17-25-7-3-23(15-33)4-8-25)31(41)43-21-27-11-13-28(14-12-27)22-44-32(42)38(2)20-30(40)36-18-26-9-5-24(16-34)6-10-26/h3-14H,15-22,33-34H2,1-2H3,(H,35,39)(H,36,40) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Axys Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibitory potency against tryptase |
Bioorg Med Chem Lett 10: 2357-60 (2001)
BindingDB Entry DOI: 10.7270/Q2NP23NX |
More data for this
Ligand-Target Pair | |
Tryptase beta-2
(Homo sapiens (Human)) | BDBM50093133
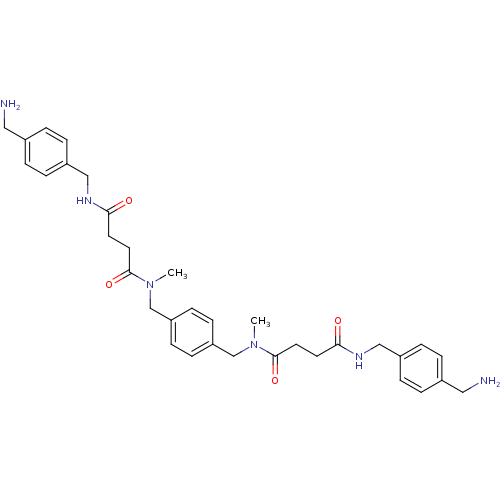
(CHEMBL79822 | N-(4-Aminomethyl-benzyl)-N'-[4-({[3-...)Show SMILES CN(Cc1ccc(CN(C)C(=O)CCC(=O)NCc2ccc(CN)cc2)cc1)C(=O)CCC(=O)NCc1ccc(CN)cc1 Show InChI InChI=1S/C34H44N6O4/c1-39(33(43)17-15-31(41)37-21-27-7-3-25(19-35)4-8-27)23-29-11-13-30(14-12-29)24-40(2)34(44)18-16-32(42)38-22-28-9-5-26(20-36)6-10-28/h3-14H,15-24,35-36H2,1-2H3,(H,37,41)(H,38,42) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Axys Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibitory potency against tryptase |
Bioorg Med Chem Lett 10: 2357-60 (2001)
BindingDB Entry DOI: 10.7270/Q2NP23NX |
More data for this
Ligand-Target Pair | |
Tryptase beta-2
(Homo sapiens (Human)) | BDBM16127
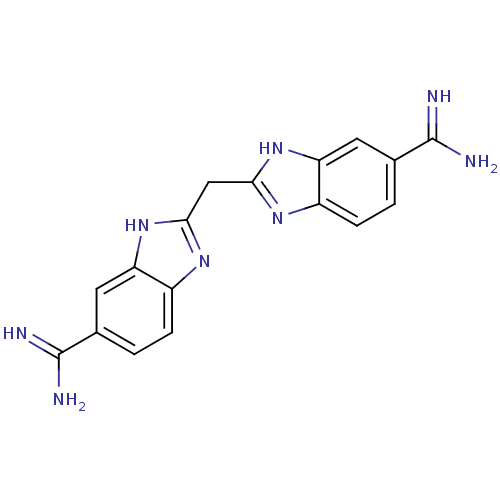
(2,2 -methanediylbis(1H-benzimidazole-6-carboximida...)Show SMILES NC(=N)c1ccc2nc(Cc3nc4ccc(cc4[nH]3)C(N)=N)[nH]c2c1 Show InChI InChI=1S/C17H16N8/c18-16(19)8-1-3-10-12(5-8)24-14(22-10)7-15-23-11-4-2-9(17(20)21)6-13(11)25-15/h1-6H,7H2,(H3,18,19)(H3,20,21)(H,22,24)(H,23,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 5 | -11.2 | n/a | n/a | n/a | n/a | n/a | 8.2 | 22 |
Arris
| Assay Description
Enzymes were incubated with inhibitors at eight inhibitor concentrations bracketing the Ki, prepared by serial dilution along with control lacking th... |
Nature 391: 608-12 (1998)
Article DOI: 10.1038/35422
BindingDB Entry DOI: 10.7270/Q29P2ZW9 |
More data for this
Ligand-Target Pair | |
Tryptase beta-2
(Homo sapiens (Human)) | BDBM50411019
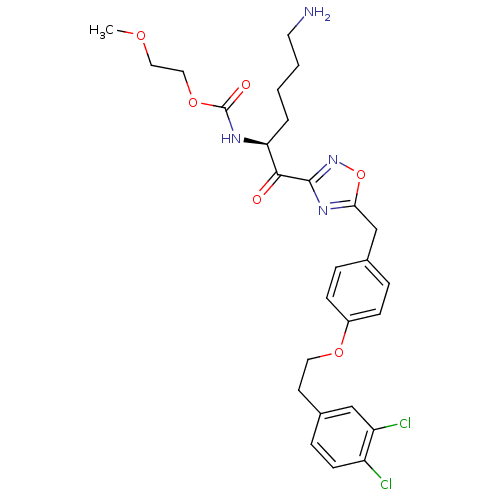
(CHEMBL558199)Show SMILES COCCOC(=O)N[C@@H](CCCCN)C(=O)c1noc(Cc2ccc(OCCc3ccc(Cl)c(Cl)c3)cc2)n1 |r| Show InChI InChI=1S/C27H32Cl2N4O6/c1-36-14-15-38-27(35)31-23(4-2-3-12-30)25(34)26-32-24(39-33-26)17-18-5-8-20(9-6-18)37-13-11-19-7-10-21(28)22(29)16-19/h5-10,16,23H,2-4,11-15,17,30H2,1H3,(H,31,35)/t23-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera Genomics
Curated by ChEMBL
| Assay Description
Inhibition of human B tryptase |
Bioorg Med Chem Lett 16: 3434-9 (2006)
Article DOI: 10.1016/j.bmcl.2006.04.013
BindingDB Entry DOI: 10.7270/Q2G44RHP |
More data for this
Ligand-Target Pair | |
Tryptase beta-2
(Homo sapiens (Human)) | BDBM50411005
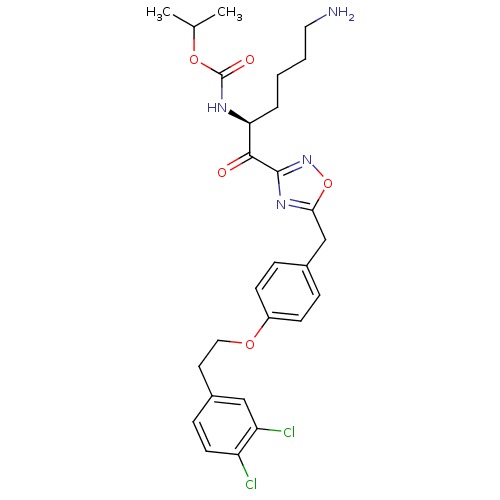
(CHEMBL537646)Show SMILES CC(C)OC(=O)N[C@@H](CCCCN)C(=O)c1noc(Cc2ccc(OCCc3ccc(Cl)c(Cl)c3)cc2)n1 |r| Show InChI InChI=1S/C27H32Cl2N4O5/c1-17(2)37-27(35)31-23(5-3-4-13-30)25(34)26-32-24(38-33-26)16-18-6-9-20(10-7-18)36-14-12-19-8-11-21(28)22(29)15-19/h6-11,15,17,23H,3-5,12-14,16,30H2,1-2H3,(H,31,35)/t23-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera Genomics
Curated by ChEMBL
| Assay Description
Inhibition of human B tryptase |
Bioorg Med Chem Lett 16: 3434-9 (2006)
Article DOI: 10.1016/j.bmcl.2006.04.013
BindingDB Entry DOI: 10.7270/Q2G44RHP |
More data for this
Ligand-Target Pair | |
Tryptase beta-2
(Homo sapiens (Human)) | BDBM50187800

((S)-ethyl 1-(5-(4-phenethoxybenzyl)-1,2,4-oxadiazo...)Show SMILES CCOC(=O)N[C@@H](CCCCN)C(=O)c1noc(Cc2ccc(OCCc3ccccc3)cc2)n1 Show InChI InChI=1S/C26H32N4O5/c1-2-33-26(32)28-22(10-6-7-16-27)24(31)25-29-23(35-30-25)18-20-11-13-21(14-12-20)34-17-15-19-8-4-3-5-9-19/h3-5,8-9,11-14,22H,2,6-7,10,15-18,27H2,1H3,(H,28,32)/t22-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera Genomics
Curated by ChEMBL
| Assay Description
Binding affinity to human tryptase beta2 |
Bioorg Med Chem Lett 16: 4085-9 (2006)
Article DOI: 10.1016/j.bmcl.2006.04.088
BindingDB Entry DOI: 10.7270/Q2X929W7 |
More data for this
Ligand-Target Pair | |
Tryptase beta-2
(Homo sapiens (Human)) | BDBM50187800

((S)-ethyl 1-(5-(4-phenethoxybenzyl)-1,2,4-oxadiazo...)Show SMILES CCOC(=O)N[C@@H](CCCCN)C(=O)c1noc(Cc2ccc(OCCc3ccccc3)cc2)n1 Show InChI InChI=1S/C26H32N4O5/c1-2-33-26(32)28-22(10-6-7-16-27)24(31)25-29-23(35-30-25)18-20-11-13-21(14-12-20)34-17-15-19-8-4-3-5-9-19/h3-5,8-9,11-14,22H,2,6-7,10,15-18,27H2,1H3,(H,28,32)/t22-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera Genomics
Curated by ChEMBL
| Assay Description
Inhibition of human B tryptase |
Bioorg Med Chem Lett 16: 3434-9 (2006)
Article DOI: 10.1016/j.bmcl.2006.04.013
BindingDB Entry DOI: 10.7270/Q2G44RHP |
More data for this
Ligand-Target Pair | |
Tryptase beta-2
(Homo sapiens (Human)) | BDBM50411001
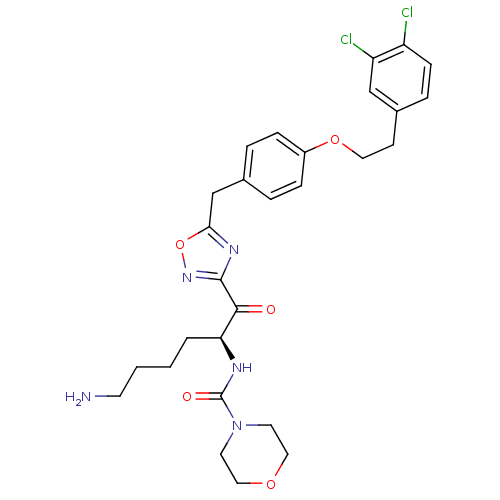
(CHEMBL558763)Show SMILES NCCCC[C@H](NC(=O)N1CCOCC1)C(=O)c1noc(Cc2ccc(OCCc3ccc(Cl)c(Cl)c3)cc2)n1 |r| Show InChI InChI=1S/C28H33Cl2N5O5/c29-22-9-6-20(17-23(22)30)10-14-39-21-7-4-19(5-8-21)18-25-33-27(34-40-25)26(36)24(3-1-2-11-31)32-28(37)35-12-15-38-16-13-35/h4-9,17,24H,1-3,10-16,18,31H2,(H,32,37)/t24-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera Genomics
Curated by ChEMBL
| Assay Description
Inhibition of human B tryptase |
Bioorg Med Chem Lett 16: 3434-9 (2006)
Article DOI: 10.1016/j.bmcl.2006.04.013
BindingDB Entry DOI: 10.7270/Q2G44RHP |
More data for this
Ligand-Target Pair | |
Tryptase beta-2
(Homo sapiens (Human)) | BDBM50411008
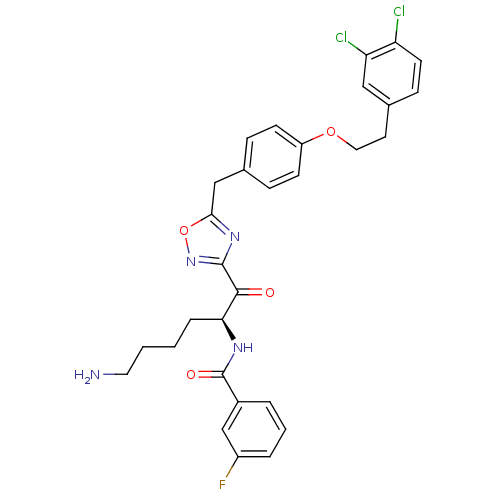
(CHEMBL535836)Show SMILES NCCCC[C@H](NC(=O)c1cccc(F)c1)C(=O)c1noc(Cc2ccc(OCCc3ccc(Cl)c(Cl)c3)cc2)n1 |r| Show InChI InChI=1S/C30H29Cl2FN4O4/c31-24-12-9-20(16-25(24)32)13-15-40-23-10-7-19(8-11-23)17-27-36-29(37-41-27)28(38)26(6-1-2-14-34)35-30(39)21-4-3-5-22(33)18-21/h3-5,7-12,16,18,26H,1-2,6,13-15,17,34H2,(H,35,39)/t26-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera Genomics
Curated by ChEMBL
| Assay Description
Inhibition of human B tryptase |
Bioorg Med Chem Lett 16: 3434-9 (2006)
Article DOI: 10.1016/j.bmcl.2006.04.013
BindingDB Entry DOI: 10.7270/Q2G44RHP |
More data for this
Ligand-Target Pair | |
Tryptase beta-2
(Homo sapiens (Human)) | BDBM14316
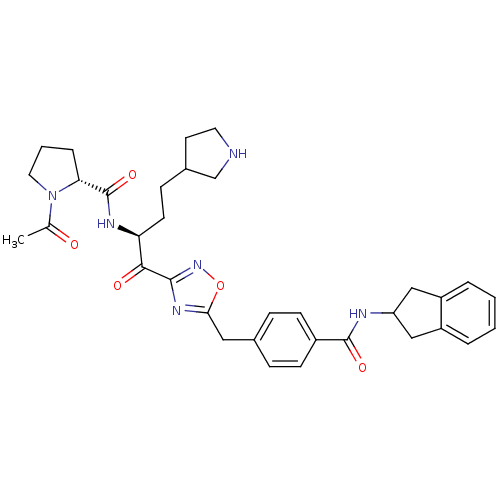
((2R)-N-[(2S)-1-(5-{[4-(2,3-dihydro-1H-inden-2-ylca...)Show SMILES CC(=O)N1CCC[C@@H]1C(=O)N[C@@H](CCC1CCNC1)C(=O)c1noc(Cc2ccc(cc2)C(=O)NC2Cc3ccccc3C2)n1 |r| Show InChI InChI=1S/C34H40N6O5/c1-21(41)40-16-4-7-29(40)34(44)37-28(13-10-23-14-15-35-20-23)31(42)32-38-30(45-39-32)17-22-8-11-24(12-9-22)33(43)36-27-18-25-5-2-3-6-26(25)19-27/h2-3,5-6,8-9,11-12,23,27-29,35H,4,7,10,13-20H2,1H3,(H,36,43)(H,37,44)/t23?,28-,29+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera
| Assay Description
Each enzyme was assayed with a set of different concentrations of each inhibitor. After addition of the appropriate substrate, the rate of hydrolysis... |
Biochemistry 45: 5964-73 (2006)
Article DOI: 10.1021/bi060173m
BindingDB Entry DOI: 10.7270/Q2W09450 |
More data for this
Ligand-Target Pair | |
Tryptase beta-2
(Homo sapiens (Human)) | BDBM50411016
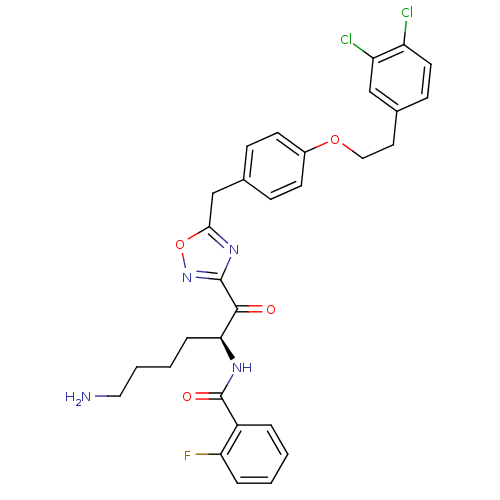
(CHEMBL559161)Show SMILES NCCCC[C@H](NC(=O)c1ccccc1F)C(=O)c1noc(Cc2ccc(OCCc3ccc(Cl)c(Cl)c3)cc2)n1 |r| Show InChI InChI=1S/C30H29Cl2FN4O4/c31-23-13-10-20(17-24(23)32)14-16-40-21-11-8-19(9-12-21)18-27-36-29(37-41-27)28(38)26(7-3-4-15-34)35-30(39)22-5-1-2-6-25(22)33/h1-2,5-6,8-13,17,26H,3-4,7,14-16,18,34H2,(H,35,39)/t26-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 6.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera Genomics
Curated by ChEMBL
| Assay Description
Inhibition of human B tryptase |
Bioorg Med Chem Lett 16: 3434-9 (2006)
Article DOI: 10.1016/j.bmcl.2006.04.013
BindingDB Entry DOI: 10.7270/Q2G44RHP |
More data for this
Ligand-Target Pair | |
Tryptase beta-2
(Homo sapiens (Human)) | BDBM50411007
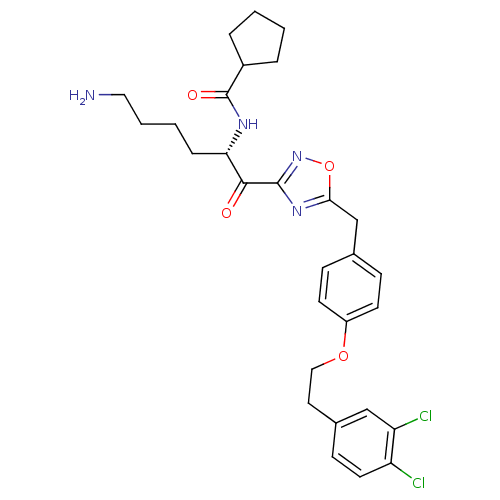
(CHEMBL534492)Show SMILES NCCCC[C@H](NC(=O)C1CCCC1)C(=O)c1noc(Cc2ccc(OCCc3ccc(Cl)c(Cl)c3)cc2)n1 |r| Show InChI InChI=1S/C29H34Cl2N4O4/c30-23-13-10-20(17-24(23)31)14-16-38-22-11-8-19(9-12-22)18-26-34-28(35-39-26)27(36)25(7-3-4-15-32)33-29(37)21-5-1-2-6-21/h8-13,17,21,25H,1-7,14-16,18,32H2,(H,33,37)/t25-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 6.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera Genomics
Curated by ChEMBL
| Assay Description
Inhibition of human B tryptase |
Bioorg Med Chem Lett 16: 3434-9 (2006)
Article DOI: 10.1016/j.bmcl.2006.04.013
BindingDB Entry DOI: 10.7270/Q2G44RHP |
More data for this
Ligand-Target Pair | |
Tryptase beta-2
(Homo sapiens (Human)) | BDBM50131975

(1-(2-Methylamino-3-phenyl-propionyl)-pyrrolidine-2...)Show SMILES CN[C@H](Cc1ccccc1)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CCCNC(N)=N)C(=O)c1nc2ccccc2s1 Show InChI InChI=1S/C28H35N7O3S/c1-31-21(17-18-9-3-2-4-10-18)27(38)35-16-8-13-22(35)25(37)33-20(12-7-15-32-28(29)30)24(36)26-34-19-11-5-6-14-23(19)39-26/h2-6,9-11,14,20-22,31H,7-8,12-13,15-17H2,1H3,(H,33,37)(H4,29,30,32)/t20-,21+,22-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 6.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development
Curated by ChEMBL
| Assay Description
In vitro inhibition of human Tryptase beta. |
J Med Chem 46: 3865-76 (2003)
Article DOI: 10.1021/jm030050p
BindingDB Entry DOI: 10.7270/Q23T9J0V |
More data for this
Ligand-Target Pair | |
Tryptase beta-2
(Homo sapiens (Human)) | BDBM50411018
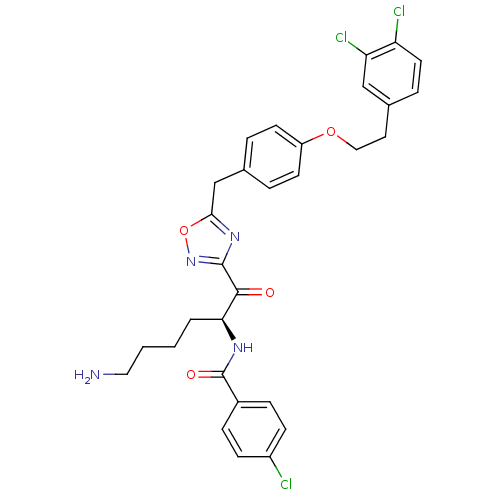
(CHEMBL559184)Show SMILES NCCCC[C@H](NC(=O)c1ccc(Cl)cc1)C(=O)c1noc(Cc2ccc(OCCc3ccc(Cl)c(Cl)c3)cc2)n1 |r| Show InChI InChI=1S/C30H29Cl3N4O4/c31-22-9-7-21(8-10-22)30(39)35-26(3-1-2-15-34)28(38)29-36-27(41-37-29)18-19-4-11-23(12-5-19)40-16-14-20-6-13-24(32)25(33)17-20/h4-13,17,26H,1-3,14-16,18,34H2,(H,35,39)/t26-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 6.55 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera Genomics
Curated by ChEMBL
| Assay Description
Inhibition of human B tryptase |
Bioorg Med Chem Lett 16: 3434-9 (2006)
Article DOI: 10.1016/j.bmcl.2006.04.013
BindingDB Entry DOI: 10.7270/Q2G44RHP |
More data for this
Ligand-Target Pair | |
Tryptase beta-2
(Homo sapiens (Human)) | BDBM50411010

(CHEMBL535163)Show SMILES CC(C)C(=O)N[C@@H](CCCCN)C(=O)c1noc(Cc2ccc(OCCc3ccc(Cl)c(Cl)c3)cc2)n1 |r| Show InChI InChI=1S/C27H32Cl2N4O4/c1-17(2)27(35)31-23(5-3-4-13-30)25(34)26-32-24(37-33-26)16-18-6-9-20(10-7-18)36-14-12-19-8-11-21(28)22(29)15-19/h6-11,15,17,23H,3-5,12-14,16,30H2,1-2H3,(H,31,35)/t23-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 6.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera Genomics
Curated by ChEMBL
| Assay Description
Inhibition of human B tryptase |
Bioorg Med Chem Lett 16: 3434-9 (2006)
Article DOI: 10.1016/j.bmcl.2006.04.013
BindingDB Entry DOI: 10.7270/Q2G44RHP |
More data for this
Ligand-Target Pair | |
Tryptase beta-2
(Homo sapiens (Human)) | BDBM50410995
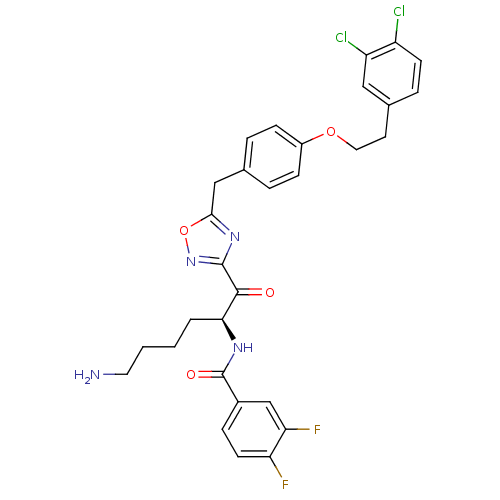
(CHEMBL536972)Show SMILES NCCCC[C@H](NC(=O)c1ccc(F)c(F)c1)C(=O)c1noc(Cc2ccc(OCCc3ccc(Cl)c(Cl)c3)cc2)n1 |r| Show InChI InChI=1S/C30H28Cl2F2N4O4/c31-22-10-6-19(15-23(22)32)12-14-41-21-8-4-18(5-9-21)16-27-37-29(38-42-27)28(39)26(3-1-2-13-35)36-30(40)20-7-11-24(33)25(34)17-20/h4-11,15,17,26H,1-3,12-14,16,35H2,(H,36,40)/t26-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 7.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera Genomics
Curated by ChEMBL
| Assay Description
Inhibition of human B tryptase |
Bioorg Med Chem Lett 16: 3434-9 (2006)
Article DOI: 10.1016/j.bmcl.2006.04.013
BindingDB Entry DOI: 10.7270/Q2G44RHP |
More data for this
Ligand-Target Pair | |
Tryptase beta-2
(Homo sapiens (Human)) | BDBM50093141
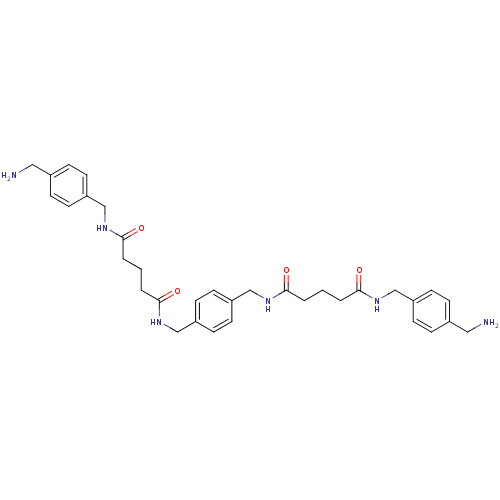
(CHEMBL306537 | Pentanedioic acid 4-aminomethyl-ben...)Show SMILES NCc1ccc(CNC(=O)CCCC(=O)NCc2ccc(CNC(=O)CCCC(=O)NCc3ccc(CN)cc3)cc2)cc1 Show InChI InChI=1S/C34H44N6O4/c35-19-25-7-11-27(12-8-25)21-37-31(41)3-1-5-33(43)39-23-29-15-17-30(18-16-29)24-40-34(44)6-2-4-32(42)38-22-28-13-9-26(20-36)10-14-28/h7-18H,1-6,19-24,35-36H2,(H,37,41)(H,38,42)(H,39,43)(H,40,44) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Axys Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibitory potency against tryptase |
Bioorg Med Chem Lett 10: 2357-60 (2001)
BindingDB Entry DOI: 10.7270/Q2NP23NX |
More data for this
Ligand-Target Pair | |
Tryptase beta-2
(Homo sapiens (Human)) | BDBM50410998
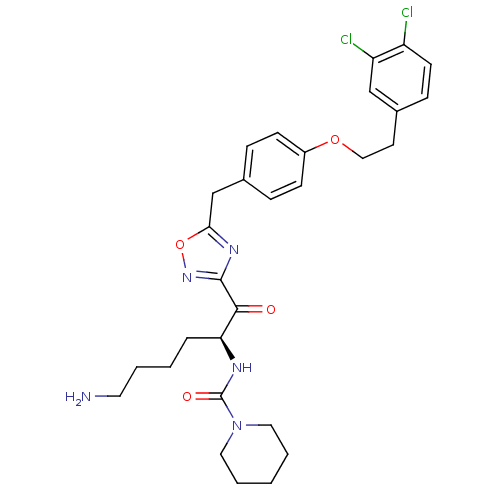
(CHEMBL535617)Show SMILES NCCCC[C@H](NC(=O)N1CCCCC1)C(=O)c1noc(Cc2ccc(OCCc3ccc(Cl)c(Cl)c3)cc2)n1 |r| Show InChI InChI=1S/C29H35Cl2N5O4/c30-23-12-9-21(18-24(23)31)13-17-39-22-10-7-20(8-11-22)19-26-34-28(35-40-26)27(37)25(6-2-3-14-32)33-29(38)36-15-4-1-5-16-36/h7-12,18,25H,1-6,13-17,19,32H2,(H,33,38)/t25-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 9.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera Genomics
Curated by ChEMBL
| Assay Description
Inhibition of human B tryptase |
Bioorg Med Chem Lett 16: 3434-9 (2006)
Article DOI: 10.1016/j.bmcl.2006.04.013
BindingDB Entry DOI: 10.7270/Q2G44RHP |
More data for this
Ligand-Target Pair | |
Tryptase beta-2
(Homo sapiens (Human)) | BDBM50411004
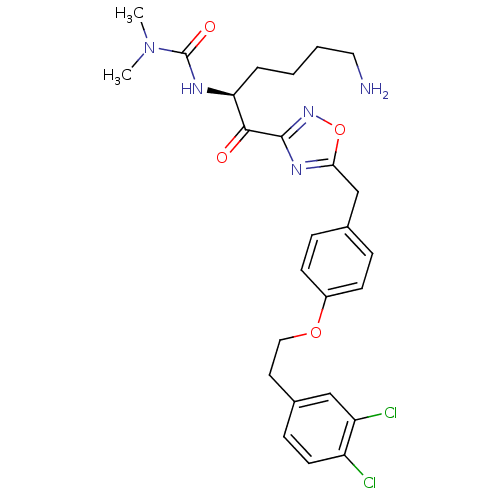
(CHEMBL535386)Show SMILES CN(C)C(=O)N[C@@H](CCCCN)C(=O)c1noc(Cc2ccc(OCCc3ccc(Cl)c(Cl)c3)cc2)n1 |r| Show InChI InChI=1S/C26H31Cl2N5O4/c1-33(2)26(35)30-22(5-3-4-13-29)24(34)25-31-23(37-32-25)16-17-6-9-19(10-7-17)36-14-12-18-8-11-20(27)21(28)15-18/h6-11,15,22H,3-5,12-14,16,29H2,1-2H3,(H,30,35)/t22-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 9.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera Genomics
Curated by ChEMBL
| Assay Description
Inhibition of human B tryptase |
Bioorg Med Chem Lett 16: 3434-9 (2006)
Article DOI: 10.1016/j.bmcl.2006.04.013
BindingDB Entry DOI: 10.7270/Q2G44RHP |
More data for this
Ligand-Target Pair | |
Tryptase beta-2
(Homo sapiens (Human)) | BDBM50411000
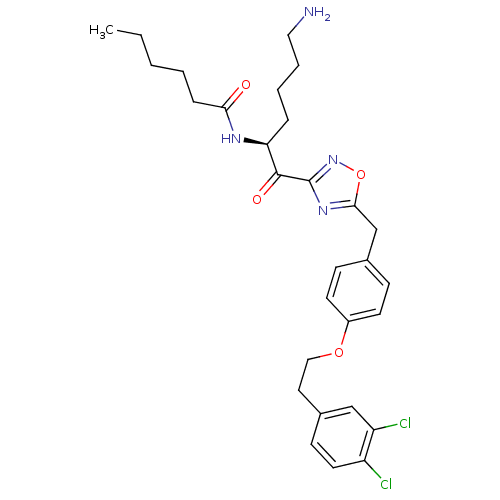
(CHEMBL558178)Show SMILES CCCCCC(=O)N[C@@H](CCCCN)C(=O)c1noc(Cc2ccc(OCCc3ccc(Cl)c(Cl)c3)cc2)n1 |r| Show InChI InChI=1S/C29H36Cl2N4O4/c1-2-3-4-8-26(36)33-25(7-5-6-16-32)28(37)29-34-27(39-35-29)19-20-9-12-22(13-10-20)38-17-15-21-11-14-23(30)24(31)18-21/h9-14,18,25H,2-8,15-17,19,32H2,1H3,(H,33,36)/t25-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 9.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera Genomics
Curated by ChEMBL
| Assay Description
Inhibition of human B tryptase |
Bioorg Med Chem Lett 16: 3434-9 (2006)
Article DOI: 10.1016/j.bmcl.2006.04.013
BindingDB Entry DOI: 10.7270/Q2G44RHP |
More data for this
Ligand-Target Pair | |
Tryptase beta-2
(Homo sapiens (Human)) | BDBM50112064
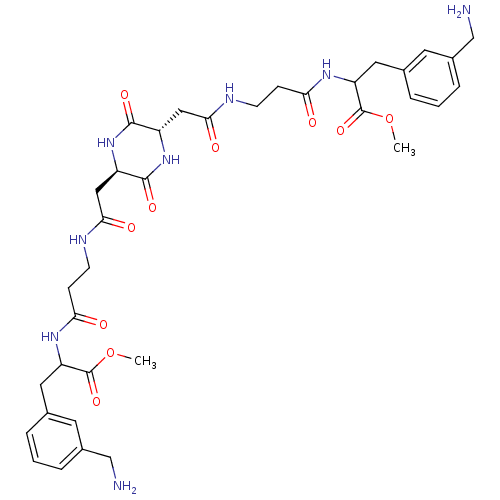
(3-(3-Aminomethyl-phenyl)-2-(3-{2-[(2R,5S)-5-({2-[2...)Show SMILES COC(=O)C(Cc1cccc(CN)c1)NC(=O)CCNC(=O)C[C@H]1NC(=O)[C@H](CC(=O)NCCC(=O)NC(Cc2cccc(CN)c2)C(=O)OC)NC1=O Show InChI InChI=1S/C36H48N8O10/c1-53-35(51)27(15-21-5-3-7-23(13-21)19-37)41-29(45)9-11-39-31(47)17-25-33(49)44-26(34(50)43-25)18-32(48)40-12-10-30(46)42-28(36(52)54-2)16-22-6-4-8-24(14-22)20-38/h3-8,13-14,25-28H,9-12,15-20,37-38H2,1-2H3,(H,39,47)(H,40,48)(H,41,45)(H,42,46)(H,43,50)(H,44,49)/t25-,26+,27?,28? | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institut für Biochemie
Curated by ChEMBL
| Assay Description
Inhibitory activity against human Tryptase beta was evaluated |
Bioorg Med Chem Lett 12: 985-8 (2002)
BindingDB Entry DOI: 10.7270/Q2MP52MW |
More data for this
Ligand-Target Pair | |
Tryptase beta-2
(Homo sapiens (Human)) | BDBM50093135
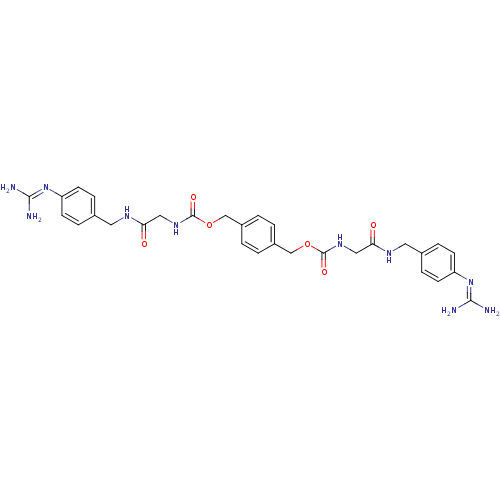
(CHEMBL77911 | [(4-Guanidino-benzylcarbamoyl)-methy...)Show SMILES [#7]\[#6](-[#7])=[#7]/c1ccc(-[#6]-[#7]-[#6](=O)-[#6]-[#7]-[#6](=O)-[#8]-[#6]-c2ccc(-[#6]-[#8]-[#6](=O)-[#7]-[#6]-[#6](=O)-[#7]-[#6]-c3ccc(cc3)\[#7]=[#6](/[#7])-[#7])cc2)cc1 Show InChI InChI=1S/C30H36N10O6/c31-27(32)39-23-9-5-19(6-10-23)13-35-25(41)15-37-29(43)45-17-21-1-2-22(4-3-21)18-46-30(44)38-16-26(42)36-14-20-7-11-24(12-8-20)40-28(33)34/h1-12H,13-18H2,(H,35,41)(H,36,42)(H,37,43)(H,38,44)(H4,31,32,39)(H4,33,34,40) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Axys Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibitory potency against tryptase |
Bioorg Med Chem Lett 10: 2357-60 (2001)
BindingDB Entry DOI: 10.7270/Q2NP23NX |
More data for this
Ligand-Target Pair | |
Tryptase beta-2
(Homo sapiens (Human)) | BDBM50410999

(CHEMBL557979)Show SMILES CC(C)(C)COC(=O)N[C@@H](CCCCN)C(=O)c1noc(Cc2ccc(OCCc3ccc(Cl)c(Cl)c3)cc2)n1 |r| Show InChI InChI=1S/C29H36Cl2N4O5/c1-29(2,3)18-39-28(37)33-24(6-4-5-14-32)26(36)27-34-25(40-35-27)17-19-7-10-21(11-8-19)38-15-13-20-9-12-22(30)23(31)16-20/h7-12,16,24H,4-6,13-15,17-18,32H2,1-3H3,(H,33,37)/t24-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera Genomics
Curated by ChEMBL
| Assay Description
Inhibition of human B tryptase |
Bioorg Med Chem Lett 16: 3434-9 (2006)
Article DOI: 10.1016/j.bmcl.2006.04.013
BindingDB Entry DOI: 10.7270/Q2G44RHP |
More data for this
Ligand-Target Pair | |
Tryptase beta-2
(Homo sapiens (Human)) | BDBM50410997
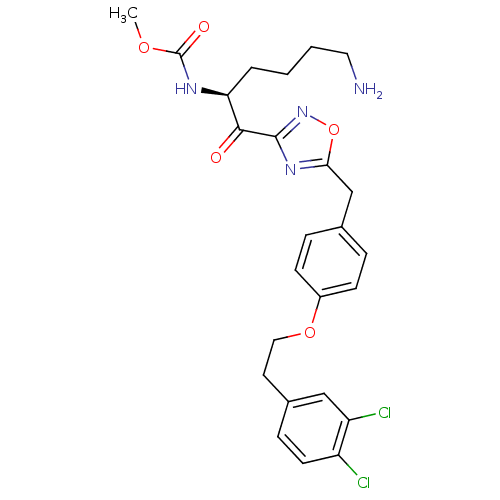
(CHEMBL557987)Show SMILES COC(=O)N[C@@H](CCCCN)C(=O)c1noc(Cc2ccc(OCCc3ccc(Cl)c(Cl)c3)cc2)n1 |r| Show InChI InChI=1S/C25H28Cl2N4O5/c1-34-25(33)29-21(4-2-3-12-28)23(32)24-30-22(36-31-24)15-16-5-8-18(9-6-16)35-13-11-17-7-10-19(26)20(27)14-17/h5-10,14,21H,2-4,11-13,15,28H2,1H3,(H,29,33)/t21-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 17 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera Genomics
Curated by ChEMBL
| Assay Description
Inhibition of human B tryptase |
Bioorg Med Chem Lett 16: 3434-9 (2006)
Article DOI: 10.1016/j.bmcl.2006.04.013
BindingDB Entry DOI: 10.7270/Q2G44RHP |
More data for this
Ligand-Target Pair | |
Tryptase beta-2
(Homo sapiens (Human)) | BDBM50112065
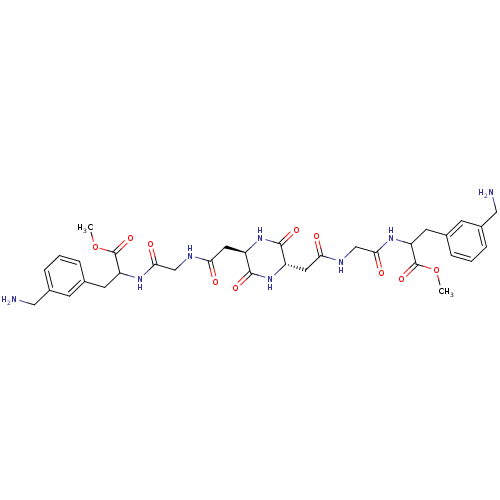
(3-(3-Aminomethyl-phenyl)-2-[2-(2-{(2S,5R)-5-[({[2-...)Show SMILES COC(=O)C(Cc1cccc(CN)c1)NC(=O)CNC(=O)C[C@H]1NC(=O)[C@H](CC(=O)NCC(=O)NC(Cc2cccc(CN)c2)C(=O)OC)NC1=O Show InChI InChI=1S/C34H44N8O10/c1-51-33(49)25(11-19-5-3-7-21(9-19)15-35)39-29(45)17-37-27(43)13-23-31(47)42-24(32(48)41-23)14-28(44)38-18-30(46)40-26(34(50)52-2)12-20-6-4-8-22(10-20)16-36/h3-10,23-26H,11-18,35-36H2,1-2H3,(H,37,43)(H,38,44)(H,39,45)(H,40,46)(H,41,48)(H,42,47)/t23-,24+,25?,26? | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 18 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institut für Biochemie
Curated by ChEMBL
| Assay Description
Inhibitory activity against human Tryptase beta was evaluated |
Bioorg Med Chem Lett 12: 985-8 (2002)
BindingDB Entry DOI: 10.7270/Q2MP52MW |
More data for this
Ligand-Target Pair | |
Tryptase beta-2
(Homo sapiens (Human)) | BDBM50410994
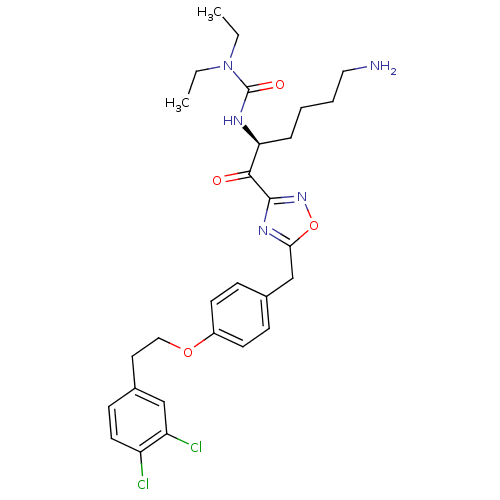
(CHEMBL536290)Show SMILES CCN(CC)C(=O)N[C@@H](CCCCN)C(=O)c1noc(Cc2ccc(OCCc3ccc(Cl)c(Cl)c3)cc2)n1 |r| Show InChI InChI=1S/C28H35Cl2N5O4/c1-3-35(4-2)28(37)32-24(7-5-6-15-31)26(36)27-33-25(39-34-27)18-19-8-11-21(12-9-19)38-16-14-20-10-13-22(29)23(30)17-20/h8-13,17,24H,3-7,14-16,18,31H2,1-2H3,(H,32,37)/t24-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 18 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera Genomics
Curated by ChEMBL
| Assay Description
Inhibition of human B tryptase |
Bioorg Med Chem Lett 16: 3434-9 (2006)
Article DOI: 10.1016/j.bmcl.2006.04.013
BindingDB Entry DOI: 10.7270/Q2G44RHP |
More data for this
Ligand-Target Pair | |
Tryptase beta-2
(Homo sapiens (Human)) | BDBM50131980

(1-Acetyl-pyrrolidine-2-carboxylic acid [1-(benzoth...)Show SMILES CC(=O)N1CCC[C@H]1C(=O)N[C@@H](CCCNC(N)=N)C(=O)c1nc2ccccc2s1 Show InChI InChI=1S/C20H26N6O3S/c1-12(27)26-11-5-8-15(26)18(29)24-14(7-4-10-23-20(21)22)17(28)19-25-13-6-2-3-9-16(13)30-19/h2-3,6,9,14-15H,4-5,7-8,10-11H2,1H3,(H,24,29)(H4,21,22,23)/t14-,15-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 19 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development
Curated by ChEMBL
| Assay Description
In vitro inhibition of human Tryptase beta. |
J Med Chem 46: 3865-76 (2003)
Article DOI: 10.1021/jm030050p
BindingDB Entry DOI: 10.7270/Q23T9J0V |
More data for this
Ligand-Target Pair | |
Tryptase beta-2
(Homo sapiens (Human)) | BDBM50131984
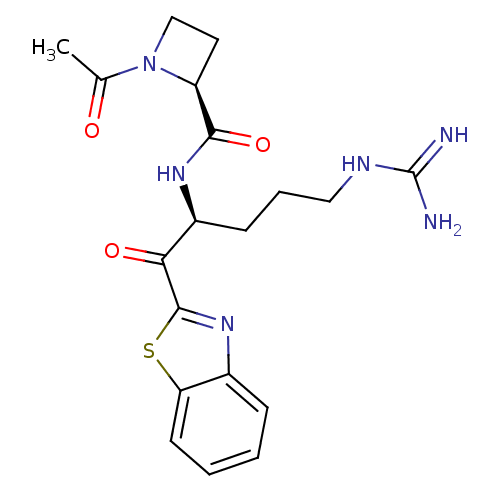
(1-Acetyl-azetidine-2-carboxylic acid [1-(benzothia...)Show SMILES CC(=O)N1CC[C@H]1C(=O)N[C@@H](CCCNC(N)=N)C(=O)c1nc2ccccc2s1 Show InChI InChI=1S/C19H24N6O3S/c1-11(26)25-10-8-14(25)17(28)23-13(6-4-9-22-19(20)21)16(27)18-24-12-5-2-3-7-15(12)29-18/h2-3,5,7,13-14H,4,6,8-10H2,1H3,(H,23,28)(H4,20,21,22)/t13-,14-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development
Curated by ChEMBL
| Assay Description
In vitro inhibition of human Tryptase beta. |
J Med Chem 46: 3865-76 (2003)
Article DOI: 10.1021/jm030050p
BindingDB Entry DOI: 10.7270/Q23T9J0V |
More data for this
Ligand-Target Pair | |
Tryptase beta-2
(Homo sapiens (Human)) | BDBM14317
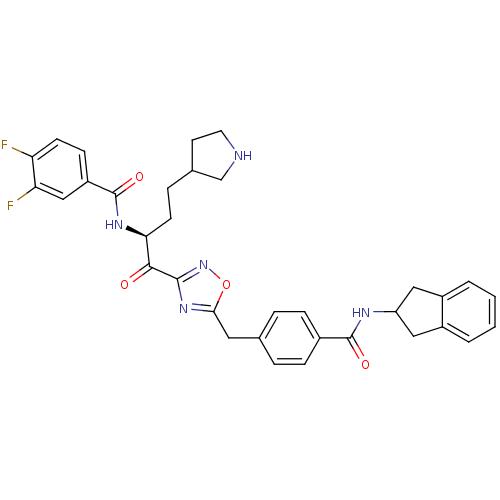
(CRA28 | N-[(2S)-1-(5-{[4-(2,3-dihydro-1H-inden-2-y...)Show SMILES Fc1ccc(cc1F)C(=O)N[C@@H](CCC1CCNC1)C(=O)c1noc(Cc2ccc(cc2)C(=O)NC2Cc3ccccc3C2)n1 |r| Show InChI InChI=1S/C34H33F2N5O4/c35-27-11-10-25(18-28(27)36)34(44)39-29(12-7-21-13-14-37-19-21)31(42)32-40-30(45-41-32)15-20-5-8-22(9-6-20)33(43)38-26-16-23-3-1-2-4-24(23)17-26/h1-6,8-11,18,21,26,29,37H,7,12-17,19H2,(H,38,43)(H,39,44)/t21?,29-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 21 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera
| Assay Description
Each enzyme was assayed with a set of different concentrations of each inhibitor. After addition of the appropriate substrate, the rate of hydrolysis... |
Biochemistry 45: 5964-73 (2006)
Article DOI: 10.1021/bi060173m
BindingDB Entry DOI: 10.7270/Q2W09450 |
More data for this
Ligand-Target Pair | |
Tryptase beta-2
(Homo sapiens (Human)) | BDBM50131977

(1-Acetyl-4-hydroxy-pyrrolidine-2-carboxylic acid [...)Show SMILES CC(=O)N1C[C@H](O)C[C@H]1C(=O)N[C@@H](CCCNC(N)=N)C(=O)c1nc2ccccc2s1 Show InChI InChI=1S/C20H26N6O4S/c1-11(27)26-10-12(28)9-15(26)18(30)24-14(6-4-8-23-20(21)22)17(29)19-25-13-5-2-3-7-16(13)31-19/h2-3,5,7,12,14-15,28H,4,6,8-10H2,1H3,(H,24,30)(H4,21,22,23)/t12-,14+,15+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 22 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development
Curated by ChEMBL
| Assay Description
In vitro inhibition of human Tryptase beta. |
J Med Chem 46: 3865-76 (2003)
Article DOI: 10.1021/jm030050p
BindingDB Entry DOI: 10.7270/Q23T9J0V |
More data for this
Ligand-Target Pair | |
Tryptase beta-2
(Homo sapiens (Human)) | BDBM50411003
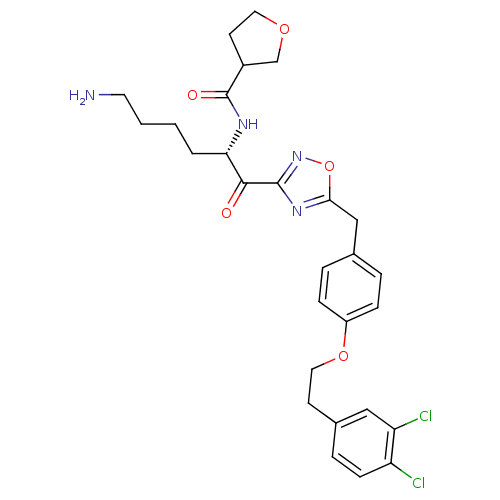
(CHEMBL536973)Show SMILES NCCCC[C@H](NC(=O)C1CCOC1)C(=O)c1noc(Cc2ccc(OCCc3ccc(Cl)c(Cl)c3)cc2)n1 |r| Show InChI InChI=1S/C28H32Cl2N4O5/c29-22-9-6-19(15-23(22)30)10-14-38-21-7-4-18(5-8-21)16-25-33-27(34-39-25)26(35)24(3-1-2-12-31)32-28(36)20-11-13-37-17-20/h4-9,15,20,24H,1-3,10-14,16-17,31H2,(H,32,36)/t20?,24-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 22 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera Genomics
Curated by ChEMBL
| Assay Description
Inhibition of human B tryptase |
Bioorg Med Chem Lett 16: 3434-9 (2006)
Article DOI: 10.1016/j.bmcl.2006.04.013
BindingDB Entry DOI: 10.7270/Q2G44RHP |
More data for this
Ligand-Target Pair | |
Tryptase beta-2
(Homo sapiens (Human)) | BDBM14307
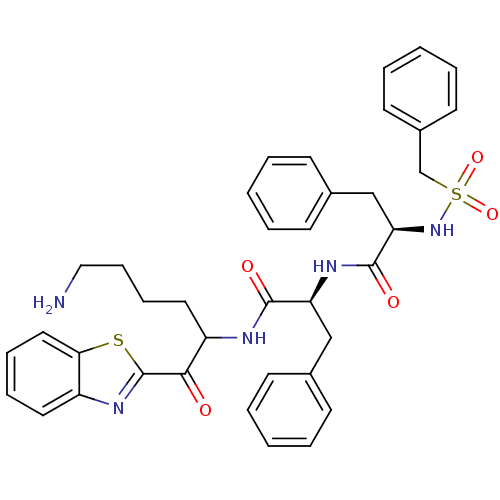
((2R)-N-[(1S)-1-{[6-amino-1-(1,3-benzothiazol-2-yl)...)Show SMILES NCCCCC(NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@@H](Cc1ccccc1)NS(=O)(=O)Cc1ccccc1)C(=O)c1nc2ccccc2s1 |r| Show InChI InChI=1S/C38H41N5O5S2/c39-23-13-12-21-31(35(44)38-42-30-20-10-11-22-34(30)49-38)40-36(45)32(24-27-14-4-1-5-15-27)41-37(46)33(25-28-16-6-2-7-17-28)43-50(47,48)26-29-18-8-3-9-19-29/h1-11,14-20,22,31-33,43H,12-13,21,23-26,39H2,(H,40,45)(H,41,46)/t31?,32-,33+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 23 | -10.3 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
Celera
| Assay Description
Each enzyme was assayed with a set of different concentrations of each inhibitor. After addition of the appropriate substrate, the rate of hydrolysis... |
Biochemistry 45: 5964-73 (2006)
Article DOI: 10.1021/bi060173m
BindingDB Entry DOI: 10.7270/Q2W09450 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data