

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM10597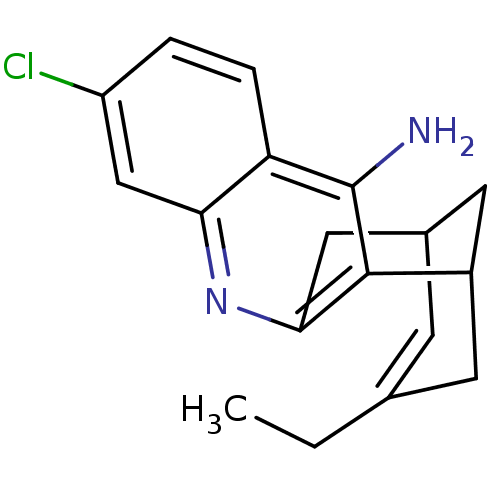 ((1S)-7-chloro-15-ethyl-10-azatetracyclo[11.3.1.0^{...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 0.0260 | -14.4 | n/a | n/a | n/a | n/a | n/a | 8.0 | 25 |
Universita di Siena | Assay Description The cholinesterase assays were performed using colorimetric method reported by E llman. Inhibition of enzyme activity was measured over a substrate c... | J Med Chem 49: 3421-5 (2006) Article DOI: 10.1021/jm060257t BindingDB Entry DOI: 10.7270/Q2WW7FWB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM8965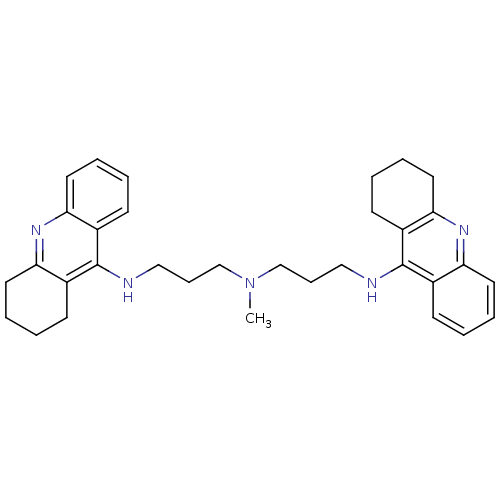 (CHEMBL338755 | Tacrine Dimer 4a | methylbis[3-(1,2...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0600 | -13.9 | n/a | n/a | n/a | n/a | n/a | 8.0 | 25 |
Universita di Siena | Assay Description Inhibition of enzyme activity was measured over a substrate concentration range of 0.01-30 mM and at least six inhibitor concentrations to determine ... | J Med Chem 48: 1919-29 (2005) Article DOI: 10.1021/jm049510k BindingDB Entry DOI: 10.7270/Q27P8WMJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM8968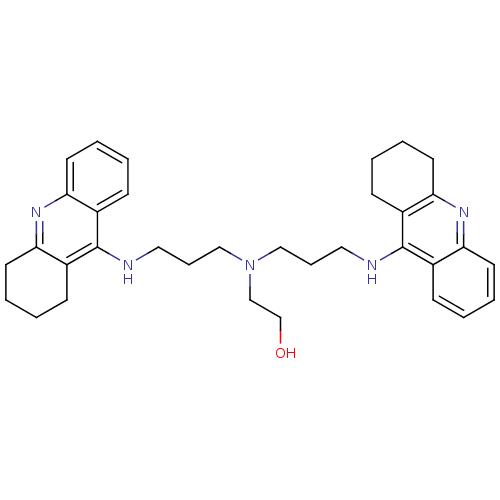 (2-{bis[3-(1,2,3,4-tetrahydroacridin-9-ylamino)prop...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.650 | -12.5 | n/a | n/a | n/a | n/a | n/a | 8.0 | 25 |
Universita di Siena | Assay Description Inhibition of enzyme activity was measured over a substrate concentration range of 0.01-30 mM and at least six inhibitor concentrations to determine ... | J Med Chem 48: 1919-29 (2005) Article DOI: 10.1021/jm049510k BindingDB Entry DOI: 10.7270/Q27P8WMJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Tetronarce californica (Pacific electric ray) (Tor...) | BDBM10439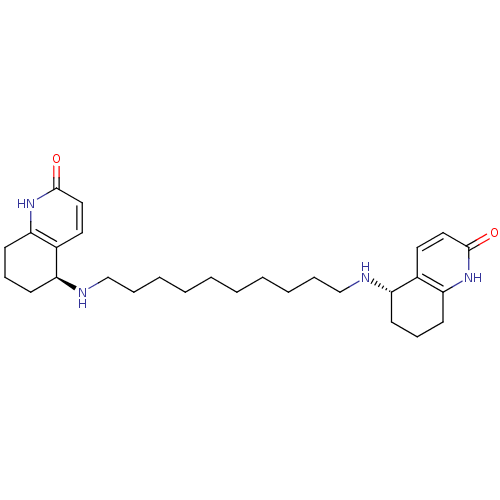 ((5S)-5-[(10-{[(5S)-2-oxo-1,2,5,6,7,8-hexahydroquin...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | MMDB PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 0.800 | -12.3 | 2.40 | n/a | n/a | n/a | n/a | 7.0 | 22 |
Weizmann Institute of Science | Assay Description The cholinesterase assays were performed using colorimetric method reported by Ellman. Enzyme activity was determined by measuring the absorbance at ... | J Am Chem Soc 125: 363-73 (2003) Article DOI: 10.1021/ja021111w BindingDB Entry DOI: 10.7270/Q26D5R69 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM10516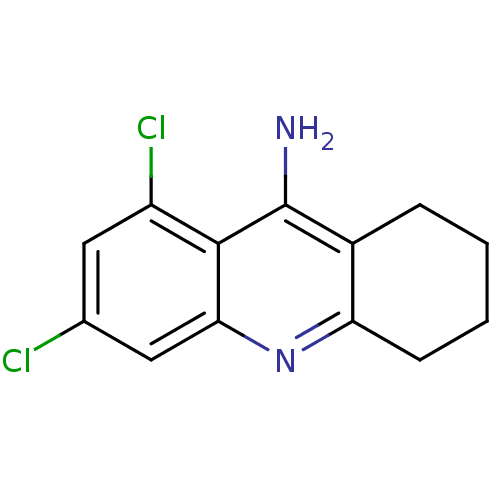 (6,8-dichloro-1,2,3,4-tetrahydroacridin-9-amine | C...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1 | -12.3 | n/a | n/a | n/a | n/a | n/a | 8.0 | 25 |
Universita degli Studi di Siena | Assay Description Inhibition of enzyme activity was measured over a substrate concentration range of 0.01-30 mM and at least six inhibitor concentrations to determine ... | Bioorg Med Chem Lett 11: 1779-82 (2001) Article DOI: 10.1016/s0960-894x(01)00294-3 BindingDB Entry DOI: 10.7270/Q2DZ06JN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM8963 (CHEMBL32823 | Homodimeric Tacrine Analog 3b | N-[7...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | 1.30 | -12.1 | n/a | n/a | n/a | n/a | n/a | 8.0 | 25 |
Universita degli Studi di Siena | Assay Description Inhibition of enzyme activity was measured over a substrate concentration range of 0.01-30 mM and at least six inhibitor concentrations to determine ... | Bioorg Med Chem Lett 11: 1779-82 (2001) Article DOI: 10.1016/s0960-894x(01)00294-3 BindingDB Entry DOI: 10.7270/Q2DZ06JN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM8963 (CHEMBL32823 | Homodimeric Tacrine Analog 3b | N-[7...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | 1.30 | -12.1 | n/a | n/a | n/a | n/a | n/a | 8.0 | 25 |
Universita di Siena | Assay Description Inhibition of enzyme activity was measured over a substrate concentration range of 0.01-30 mM and at least six inhibitor concentrations to determine ... | J Med Chem 48: 1919-29 (2005) Article DOI: 10.1021/jm049510k BindingDB Entry DOI: 10.7270/Q27P8WMJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM8967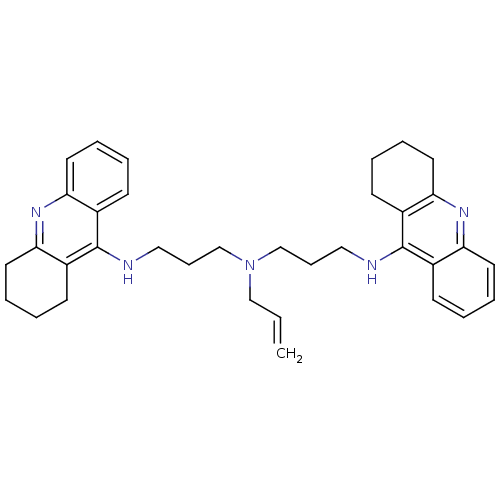 (N,N-Bis[3-[(1,2,3,4-tetrahydroacridin-9-yl)amino]p...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 1.60 | -12.0 | n/a | n/a | n/a | n/a | n/a | 8.0 | 25 |
Universita di Siena | Assay Description Inhibition of enzyme activity was measured over a substrate concentration range of 0.01-30 mM and at least six inhibitor concentrations to determine ... | J Med Chem 48: 1919-29 (2005) Article DOI: 10.1021/jm049510k BindingDB Entry DOI: 10.7270/Q27P8WMJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM8964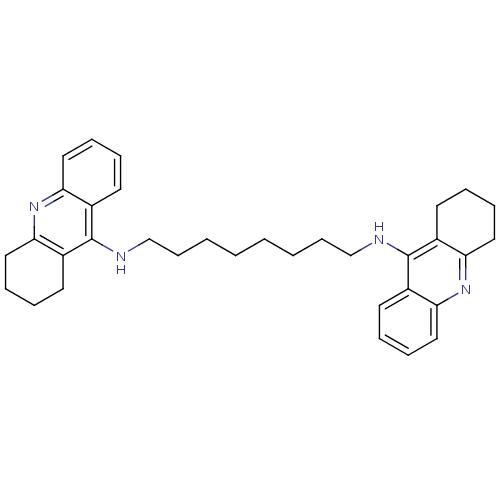 (CHEMBL75274 | Homodimeric Tacrine Analog 3c | N-[8...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.90 | -11.9 | n/a | n/a | n/a | n/a | n/a | 8.0 | 25 |
Universita di Siena | Assay Description Inhibition of enzyme activity was measured over a substrate concentration range of 0.01-30 mM and at least six inhibitor concentrations to determine ... | J Med Chem 48: 1919-29 (2005) Article DOI: 10.1021/jm049510k BindingDB Entry DOI: 10.7270/Q27P8WMJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM8966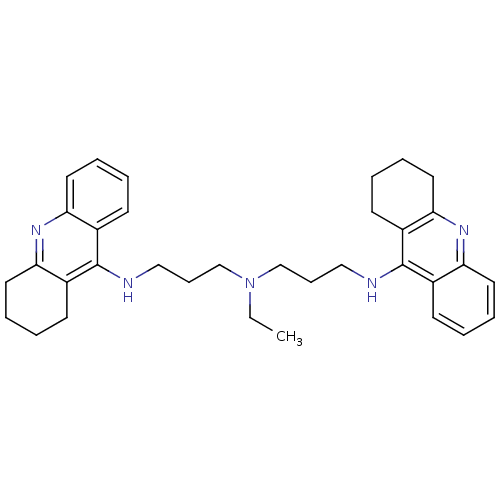 (N,N-Bis[3-[(1,2,3,4-tetrahydroacridin-9-yl)amino]p...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 2.80 | -11.7 | n/a | n/a | n/a | n/a | n/a | 8.0 | 25 |
Universita di Siena | Assay Description Inhibition of enzyme activity was measured over a substrate concentration range of 0.01-30 mM and at least six inhibitor concentrations to determine ... | J Med Chem 48: 1919-29 (2005) Article DOI: 10.1021/jm049510k BindingDB Entry DOI: 10.7270/Q27P8WMJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM8960 ((+/-)-2-[(1-benzylpiperidin-4-yl)methyl]-5,6-dimet...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | DrugBank PDB Article PubMed | 2.90 | -11.6 | n/a | n/a | n/a | n/a | n/a | 8.0 | 25 |
Universita di Siena | Assay Description Inhibition of enzyme activity was measured over a substrate concentration range of 0.01-30 mM and at least six inhibitor concentrations to determine ... | J Med Chem 48: 1919-29 (2005) Article DOI: 10.1021/jm049510k BindingDB Entry DOI: 10.7270/Q27P8WMJ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM13543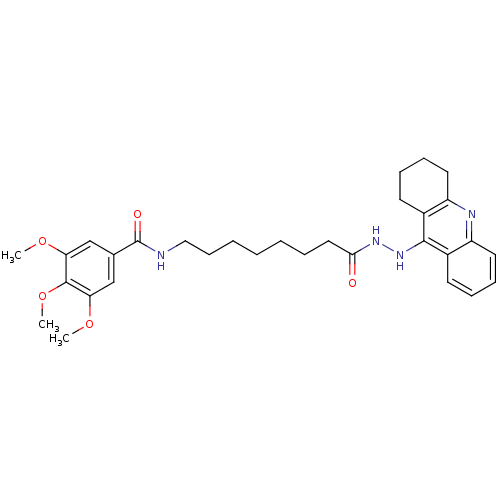 (3,4,5-Trimethoxy-N-(8-oxo-8-(2-(1,2,3,4-tetrahydro...) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 3.23 | n/a | 3.25 | n/a | n/a | n/a | n/a | n/a | n/a |
Rheinische Friedrich-Wilhelms-Universitat Bonn | Assay Description The cholinesterase assays were performed using colorimetric method reported by Ellman. The absorbance changed at 412 nm and was monitored with spectr... | J Med Chem 49: 7540-4 (2006) Article DOI: 10.1021/jm060742o BindingDB Entry DOI: 10.7270/Q21Z42NF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Tetronarce californica (Pacific electric ray) (Tor...) | BDBM10440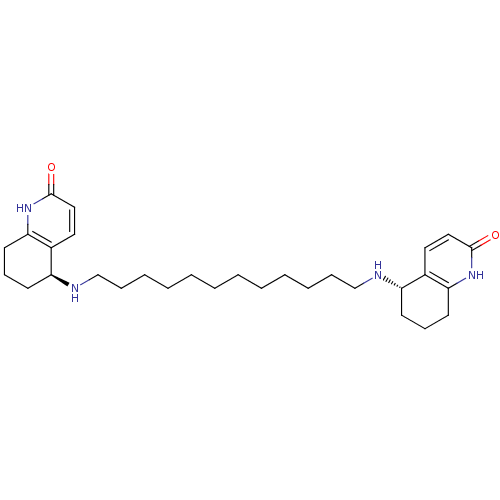 ((5S)-5-[(12-{[(5S)-2-oxo-1,2,5,6,7,8-hexahydroquin...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | MMDB PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 4.5 | -11.3 | 16 | n/a | n/a | n/a | n/a | 7.0 | 22 |
Weizmann Institute of Science | Assay Description The cholinesterase assays were performed using colorimetric method reported by Ellman. Enzyme activity was determined by measuring the absorbance at ... | J Am Chem Soc 125: 363-73 (2003) Article DOI: 10.1021/ja021111w BindingDB Entry DOI: 10.7270/Q26D5R69 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM10519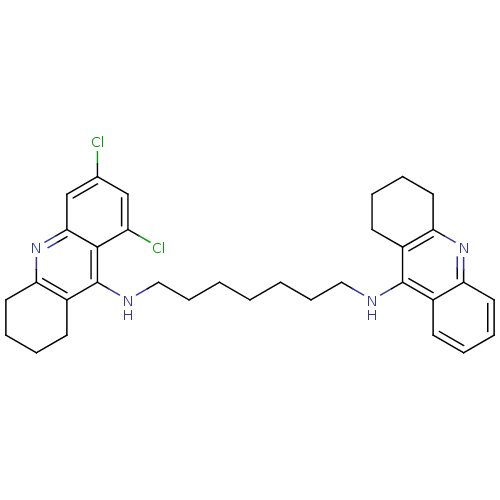 (CHEMBL51350 | N-{7-[(6,8-dichloro-1,2,3,4-tetrahyd...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 6 | -11.2 | n/a | n/a | n/a | n/a | n/a | 8.0 | 25 |
Universita degli Studi di Siena | Assay Description Inhibition of enzyme activity was measured over a substrate concentration range of 0.01-30 mM and at least six inhibitor concentrations to determine ... | Bioorg Med Chem Lett 11: 1779-82 (2001) Article DOI: 10.1016/s0960-894x(01)00294-3 BindingDB Entry DOI: 10.7270/Q2DZ06JN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM10595 ((9E)-7-(7-(1,2,3,4-Tetrahydroacridin-9-ylamino)hep...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 6.40 | -11.2 | n/a | n/a | n/a | n/a | n/a | 8.0 | 25 |
Universita di Siena | Assay Description The cholinesterase assays were performed using colorimetric method reported by E llman. Inhibition of enzyme activity was measured over a substrate c... | J Med Chem 49: 3421-5 (2006) Article DOI: 10.1021/jm060257t BindingDB Entry DOI: 10.7270/Q2WW7FWB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM8961 (1,2,3,4-tetrahydro-9-acridinamine | 1,2,3,4-tetrah...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 6.90 | -11.0 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
University of North Carolina at Chapel Hill | Assay Description The cholinesterase assays were performed using colorimetric method reported by Ellman. Estimates of the competitive inhibition constants (Ki) were ob... | Chem Biol 10: 341-9 (2003) Article DOI: 10.1016/s1074-5521(03)00071-1 BindingDB Entry DOI: 10.7270/Q2N014T9 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM8974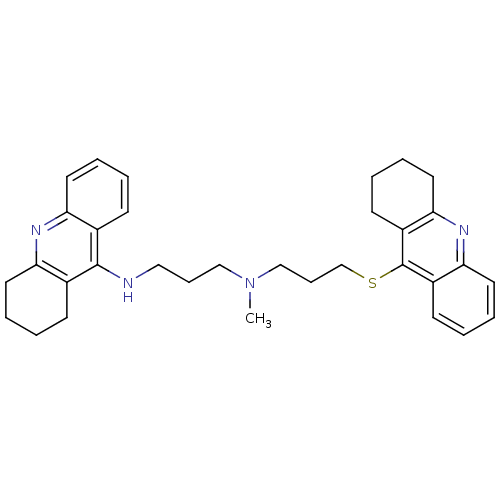 (CHEMBL367067 | N-Methyl-N-(1,2,3,4-tetrahydroacrid...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 9.10 | -11.0 | n/a | n/a | n/a | n/a | n/a | 8.0 | 25 |
Universita di Siena | Assay Description Inhibition of enzyme activity was measured over a substrate concentration range of 0.01-30 mM and at least six inhibitor concentrations to determine ... | J Med Chem 48: 1919-29 (2005) Article DOI: 10.1021/jm049510k BindingDB Entry DOI: 10.7270/Q27P8WMJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM10594 ((9E)-N1-(7-(1,2,3,4-Tetrahydroacridin-9-ylamino)he...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 15.7 | -10.6 | n/a | n/a | n/a | n/a | n/a | 8.0 | 25 |
Universita di Siena | Assay Description The cholinesterase assays were performed using colorimetric method reported by E llman. Inhibition of enzyme activity was measured over a substrate c... | J Med Chem 49: 3421-5 (2006) Article DOI: 10.1021/jm060257t BindingDB Entry DOI: 10.7270/Q2WW7FWB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM10593 ((9E)-N1-(7-(1,2,3,4-Tetrahydroacridin-9-ylamino)he...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 16.5 | -10.6 | n/a | n/a | n/a | n/a | n/a | 8.0 | 25 |
Universita di Siena | Assay Description The cholinesterase assays were performed using colorimetric method reported by E llman. Inhibition of enzyme activity was measured over a substrate c... | J Med Chem 49: 3421-5 (2006) Article DOI: 10.1021/jm060257t BindingDB Entry DOI: 10.7270/Q2WW7FWB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Rattus norvegicus (rat)) | BDBM10440 ((5S)-5-[(12-{[(5S)-2-oxo-1,2,5,6,7,8-hexahydroquin...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | 19.6 | -10.4 | 52 | n/a | n/a | n/a | n/a | 7.0 | 22 |
Weizmann Institute of Science | Assay Description The cholinesterase assays were performed using colorimetric method reported by Ellman. Enzyme activity was determined by measuring the absorbance at ... | J Am Chem Soc 125: 363-73 (2003) Article DOI: 10.1021/ja021111w BindingDB Entry DOI: 10.7270/Q26D5R69 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM8960 ((+/-)-2-[(1-benzylpiperidin-4-yl)methyl]-5,6-dimet...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | DrugBank PDB Article PubMed | 20.5 | -10.9 | 23.1 | n/a | n/a | n/a | n/a | 8.0 | 37 |
University of Bologna | Assay Description The cholinesterase assays were performed using colorimetric method reported by Ellman. Estimates of the competitive inhibition constants (Ki) were ob... | J Med Chem 46: 2279-82 (2003) Article DOI: 10.1021/jm0340602 BindingDB Entry DOI: 10.7270/Q29Z9332 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM10949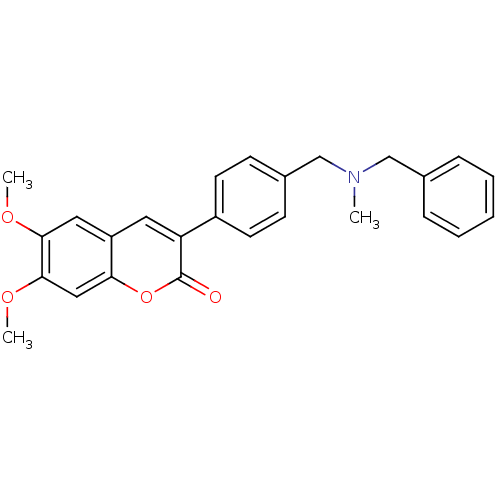 (3-(4-{[Benzyl(methyl)amino]methyl}-phenyl)-6,7-dim...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 21.7 | -10.9 | 44.5 | n/a | n/a | n/a | n/a | 8.0 | 37 |
University of Bologna | Assay Description The cholinesterase assays were performed using colorimetric method reported by Ellman. Estimates of the competitive inhibition constants (Ki) were ob... | J Med Chem 46: 2279-82 (2003) Article DOI: 10.1021/jm0340602 BindingDB Entry DOI: 10.7270/Q29Z9332 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM8973 (N-(1,2,3,4-Tetrahydroacridin-9-yl)-N-[8-(1,2,3,4-t...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 30 | -10.3 | n/a | n/a | n/a | n/a | n/a | 8.0 | 25 |
Universita di Siena | Assay Description Inhibition of enzyme activity was measured over a substrate concentration range of 0.01-30 mM and at least six inhibitor concentrations to determine ... | J Med Chem 48: 1919-29 (2005) Article DOI: 10.1021/jm049510k BindingDB Entry DOI: 10.7270/Q27P8WMJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM8961 (1,2,3,4-tetrahydro-9-acridinamine | 1,2,3,4-tetrah...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 40 | -10.1 | n/a | n/a | n/a | n/a | n/a | 8.0 | 25 |
Universita di Siena | Assay Description Inhibition of enzyme activity was measured over a substrate concentration range of 0.01-30 mM and at least six inhibitor concentrations to determine ... | J Med Chem 48: 1919-29 (2005) Article DOI: 10.1021/jm049510k BindingDB Entry DOI: 10.7270/Q27P8WMJ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM8961 (1,2,3,4-tetrahydro-9-acridinamine | 1,2,3,4-tetrah...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 40 | -10.1 | n/a | n/a | n/a | n/a | n/a | 8.0 | 25 |
Universita degli Studi di Siena | Assay Description Inhibition of enzyme activity was measured over a substrate concentration range of 0.01-30 mM and at least six inhibitor concentrations to determine ... | Bioorg Med Chem Lett 11: 1779-82 (2001) Article DOI: 10.1016/s0960-894x(01)00294-3 BindingDB Entry DOI: 10.7270/Q2DZ06JN | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM10523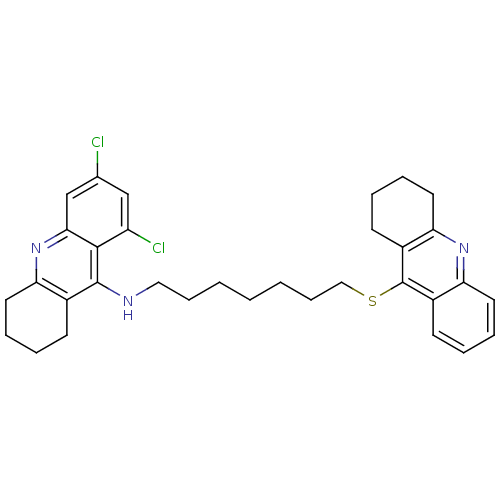 (6,8-dichloro-N-[7-(1,2,3,4-tetrahydroacridin-9-yls...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 41 | -10.1 | n/a | n/a | n/a | n/a | n/a | 8.0 | 25 |
Universita degli Studi di Siena | Assay Description Inhibition of enzyme activity was measured over a substrate concentration range of 0.01-30 mM and at least six inhibitor concentrations to determine ... | Bioorg Med Chem Lett 11: 1779-82 (2001) Article DOI: 10.1016/s0960-894x(01)00294-3 BindingDB Entry DOI: 10.7270/Q2DZ06JN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM8977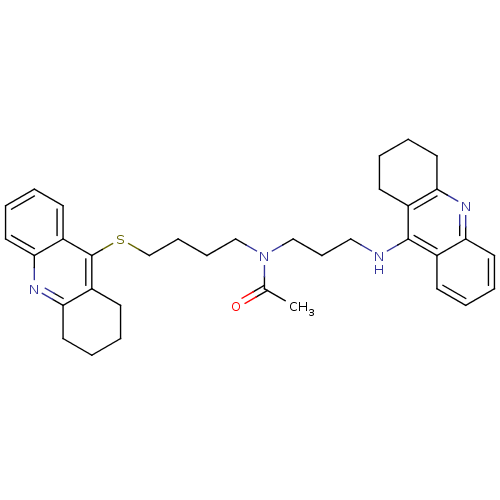 (CHEMBL175949 | N-[3-(1,2,3,4-Tetrahydroacridin-9-y...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 47 | -9.99 | n/a | n/a | n/a | n/a | n/a | 8.0 | 25 |
Universita di Siena | Assay Description Inhibition of enzyme activity was measured over a substrate concentration range of 0.01-30 mM and at least six inhibitor concentrations to determine ... | J Med Chem 48: 1919-29 (2005) Article DOI: 10.1021/jm049510k BindingDB Entry DOI: 10.7270/Q27P8WMJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM10441 ((+)-Huperzine A | (+/-)Huperzine A | (-)-Huperzine...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE PC cid PC sid PDB UniChem Similars | Article PubMed | 47 | -9.99 | n/a | n/a | n/a | n/a | n/a | 8.0 | 25 |
Universita di Siena | Assay Description The cholinesterase assays were performed using colorimetric method reported by E llman. Inhibition of enzyme activity was measured over a substrate c... | J Med Chem 49: 3421-5 (2006) Article DOI: 10.1021/jm060257t BindingDB Entry DOI: 10.7270/Q2WW7FWB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Rattus norvegicus (rat)) | BDBM10441 ((+)-Huperzine A | (+/-)Huperzine A | (-)-Huperzine...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase DrugBank MCE PC cid PC sid PDB UniChem Similars | Article PubMed | 47.1 | -9.89 | 114 | n/a | n/a | n/a | n/a | 7.0 | 22 |
Weizmann Institute of Science | Assay Description The cholinesterase assays were performed using colorimetric method reported by Ellman. Enzyme activity was determined by measuring the absorbance at ... | J Am Chem Soc 125: 363-73 (2003) Article DOI: 10.1021/ja021111w BindingDB Entry DOI: 10.7270/Q26D5R69 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM24710 (6-chloro-2-methoxyacridin-9-amine | 9-Amino-6-Chlo...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Patents | Article PubMed | 49 | -9.86 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
University of North Carolina at Chapel Hill | Assay Description The cholinesterase assays were performed using colorimetric method reported by Ellman. Estimates of the competitive inhibition constants (Ki) were ob... | Chem Biol 10: 341-9 (2003) Article DOI: 10.1016/s1074-5521(03)00071-1 BindingDB Entry DOI: 10.7270/Q2N014T9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM8975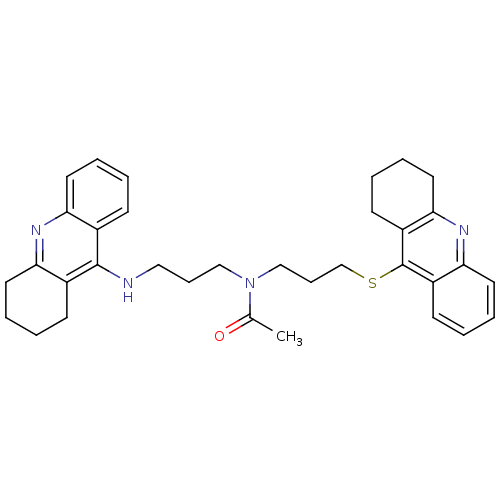 (CHEMBL179192 | N-[3-(1,2,3,4-Tetrahydroacridin-9-y...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 50 | -9.95 | n/a | n/a | n/a | n/a | n/a | 8.0 | 25 |
Universita di Siena | Assay Description Inhibition of enzyme activity was measured over a substrate concentration range of 0.01-30 mM and at least six inhibitor concentrations to determine ... | J Med Chem 48: 1919-29 (2005) Article DOI: 10.1021/jm049510k BindingDB Entry DOI: 10.7270/Q27P8WMJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM10525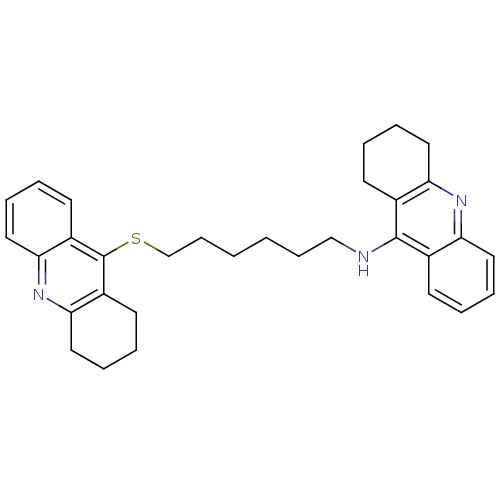 (N-[6-(1,2,3,4-tetrahydroacridin-9-ylsulfanyl)hexyl...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 100 | -9.54 | n/a | n/a | n/a | n/a | n/a | 8.0 | 25 |
Universita degli Studi di Siena | Assay Description Inhibition of enzyme activity was measured over a substrate concentration range of 0.01-30 mM and at least six inhibitor concentrations to determine ... | Bioorg Med Chem Lett 11: 1779-82 (2001) Article DOI: 10.1016/s0960-894x(01)00294-3 BindingDB Entry DOI: 10.7270/Q2DZ06JN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM10524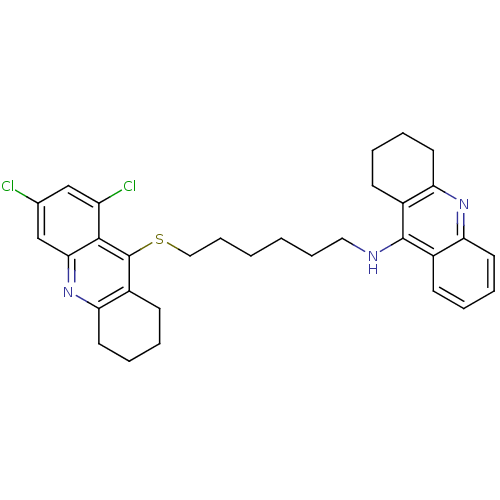 (N-{6-[(6,8-dichloro-1,2,3,4-tetrahydroacridin-9-yl...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 120 | -9.43 | n/a | n/a | n/a | n/a | n/a | 8.0 | 25 |
Universita degli Studi di Siena | Assay Description Inhibition of enzyme activity was measured over a substrate concentration range of 0.01-30 mM and at least six inhibitor concentrations to determine ... | Bioorg Med Chem Lett 11: 1779-82 (2001) Article DOI: 10.1016/s0960-894x(01)00294-3 BindingDB Entry DOI: 10.7270/Q2DZ06JN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM8976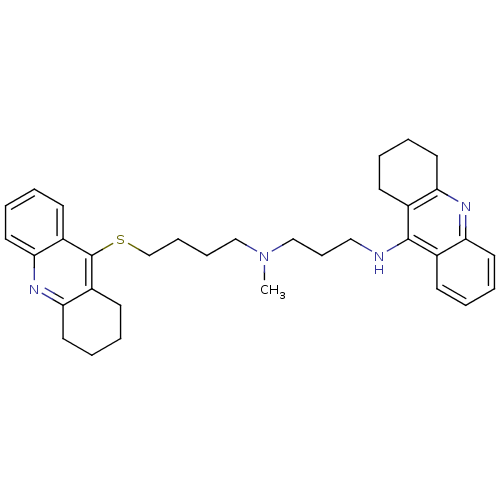 (CHEMBL175555 | N-Methyl-N-(1,2,3,4-tetrahydroacrid...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 130 | -9.39 | n/a | n/a | n/a | n/a | n/a | 8.0 | 25 |
Universita di Siena | Assay Description Inhibition of enzyme activity was measured over a substrate concentration range of 0.01-30 mM and at least six inhibitor concentrations to determine ... | J Med Chem 48: 1919-29 (2005) Article DOI: 10.1021/jm049510k BindingDB Entry DOI: 10.7270/Q27P8WMJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM8961 (1,2,3,4-tetrahydro-9-acridinamine | 1,2,3,4-tetrah...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 137 | -9.36 | n/a | n/a | n/a | n/a | n/a | 8.0 | 25 |
Universita di Siena | Assay Description The cholinesterase assays were performed using colorimetric method reported by E llman. Inhibition of enzyme activity was measured over a substrate c... | J Med Chem 49: 3421-5 (2006) Article DOI: 10.1021/jm060257t BindingDB Entry DOI: 10.7270/Q2WW7FWB | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM10518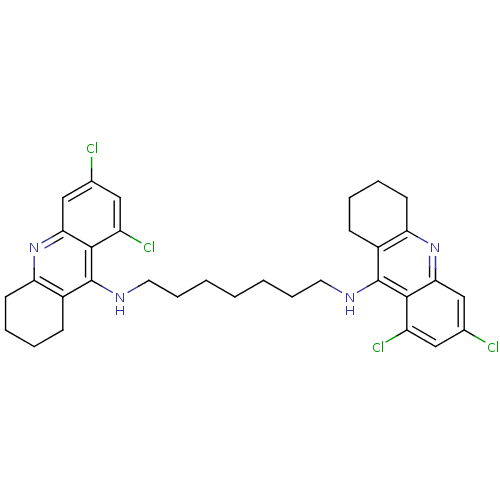 (6,8-dichloro-N-{7-[(6,8-dichloro-1,2,3,4-tetrahydr...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 150 | -9.30 | n/a | n/a | n/a | n/a | n/a | 8.0 | 25 |
Universita degli Studi di Siena | Assay Description Inhibition of enzyme activity was measured over a substrate concentration range of 0.01-30 mM and at least six inhibitor concentrations to determine ... | Bioorg Med Chem Lett 11: 1779-82 (2001) Article DOI: 10.1016/s0960-894x(01)00294-3 BindingDB Entry DOI: 10.7270/Q2DZ06JN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM8961 (1,2,3,4-tetrahydro-9-acridinamine | 1,2,3,4-tetrah...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 151 | n/a | 250 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bologna | Assay Description The cholinesterase assays were performed using colorimetric method reported by Ellman. The absorbance changes at 412 nm were recorded for 5 min with ... | Bioorg Med Chem 8: 497-506 (2000) Article DOI: 10.1016/s0968-0896(99)00306-5 BindingDB Entry DOI: 10.7270/Q2BG2M6F | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM10512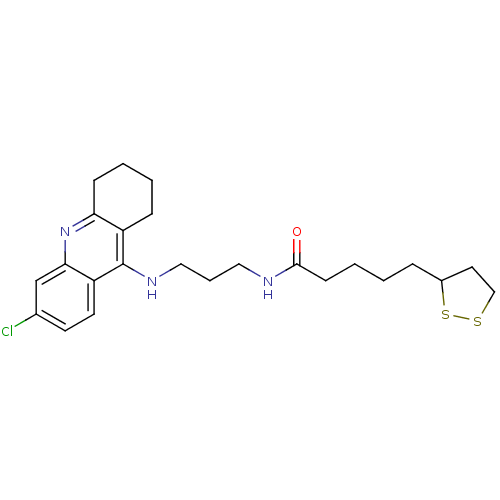 (CHEMBL194823 | N-{3-[(6-chloro-1,2,3,4-tetrahydroa...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | 155 | -9.66 | 0.253 | n/a | n/a | n/a | n/a | 8.0 | 37 |
University of Bologna | Assay Description The cholinesterase assays were performed using colorimetric method reported by Ellman. Enzyme activity was determined by measuring the absorbance at... | J Med Chem 48: 360-3 (2005) Article DOI: 10.1021/jm049112h BindingDB Entry DOI: 10.7270/Q2JQ0Z7J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM22782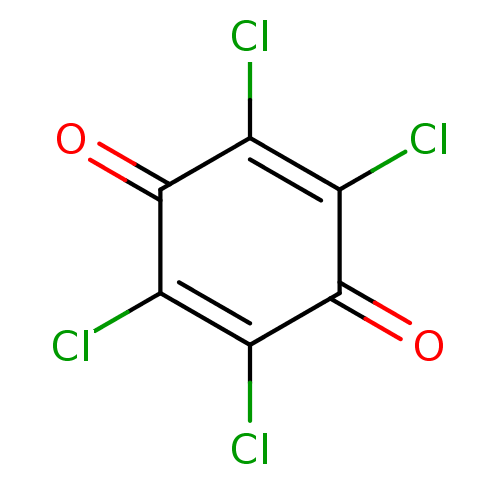 (2,3,5,6-tetrachlorocyclohexa-2,5-diene-1,4-dione |...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE KEGG PC cid PC sid UniChem Patents | Article PubMed | 163 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Mississippi | Assay Description The cholinesterase assays were performed using colorimetric method reported by Ellman. Estimates of the competitive inhibition constants (Ki) were ob... | J Med Chem 48: 2906-15 (2005) Article DOI: 10.1021/jm049011j BindingDB Entry DOI: 10.7270/Q2ZP44DR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Tetronarce californica (Pacific electric ray) (Tor...) | BDBM10441 ((+)-Huperzine A | (+/-)Huperzine A | (-)-Huperzine...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase DrugBank MCE PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 175 | -9.12 | 414 | n/a | n/a | n/a | n/a | 7.0 | 22 |
Weizmann Institute of Science | Assay Description The cholinesterase assays were performed using colorimetric method reported by Ellman. Enzyme activity was determined by measuring the absorbance at ... | J Am Chem Soc 125: 363-73 (2003) Article DOI: 10.1021/ja021111w BindingDB Entry DOI: 10.7270/Q26D5R69 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Acetylcholinesterase (Tetronarce californica (Pacific electric ray) (Tor...) | BDBM10441 ((+)-Huperzine A | (+/-)Huperzine A | (-)-Huperzine...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase DrugBank MCE PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 175 | -9.21 | n/a | n/a | n/a | n/a | n/a | 7.0 | 25 |
Weizmann Institute of Science | Assay Description The cholinesterase assays were performed using colorimetric method reported by Ellman. Enzyme activity was determined by measuring the absorbance at ... | Biochemistry 41: 10810-8 (2002) Article DOI: 10.1021/bi020151+ BindingDB Entry DOI: 10.7270/Q2HQ3X52 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM8972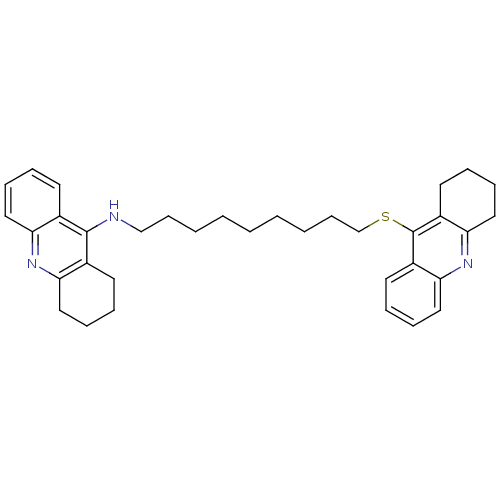 (N-(1,2,3,4-Tetrahydroacridin-9-yl)-9-[(1,2,3,4-tet...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 195 | -9.15 | n/a | n/a | n/a | n/a | n/a | 8.0 | 25 |
Universita di Siena | Assay Description Inhibition of enzyme activity was measured over a substrate concentration range of 0.01-30 mM and at least six inhibitor concentrations to determine ... | J Med Chem 48: 1919-29 (2005) Article DOI: 10.1021/jm049510k BindingDB Entry DOI: 10.7270/Q27P8WMJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM9074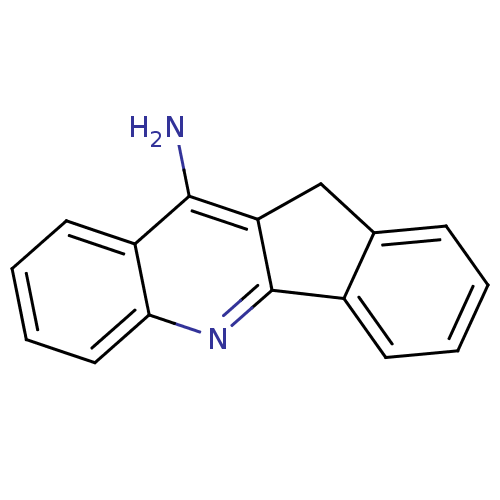 (10H-indeno[1,2-b]quinolin-11-amine | 11H-indeno-[1...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 210 | n/a | 680 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bologna | Assay Description The cholinesterase assays were performed using colorimetric method reported by Ellman. The absorbance changes at 412 nm were recorded for 5 min with ... | Bioorg Med Chem 8: 497-506 (2000) Article DOI: 10.1016/s0968-0896(99)00306-5 BindingDB Entry DOI: 10.7270/Q2BG2M6F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM8962 (Bis-THA inhibitor 4 | CHEMBL179732 | N-[5-(1,2,3,4...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | 210 | -9.10 | n/a | n/a | n/a | n/a | n/a | 8.0 | 25 |
Universita di Siena | Assay Description Inhibition of enzyme activity was measured over a substrate concentration range of 0.01-30 mM and at least six inhibitor concentrations to determine ... | J Med Chem 48: 1919-29 (2005) Article DOI: 10.1021/jm049510k BindingDB Entry DOI: 10.7270/Q27P8WMJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM8971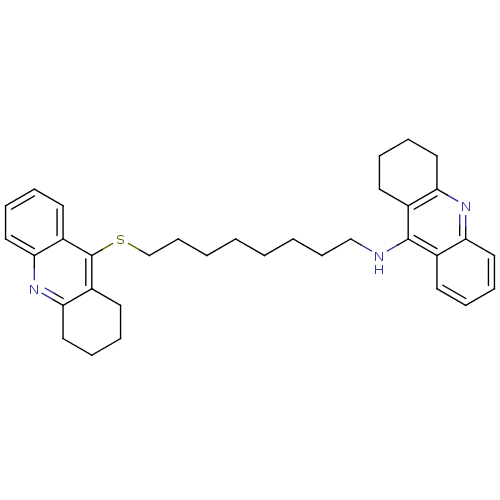 (CHEMBL129108 | N-[8-(1,2,3,4-tetrahydroacridin-9-y...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | 250 | -9.00 | n/a | n/a | n/a | n/a | n/a | 8.0 | 25 |
Universita di Siena | Assay Description Inhibition of enzyme activity was measured over a substrate concentration range of 0.01-30 mM and at least six inhibitor concentrations to determine ... | J Med Chem 48: 1919-29 (2005) Article DOI: 10.1021/jm049510k BindingDB Entry DOI: 10.7270/Q27P8WMJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM10522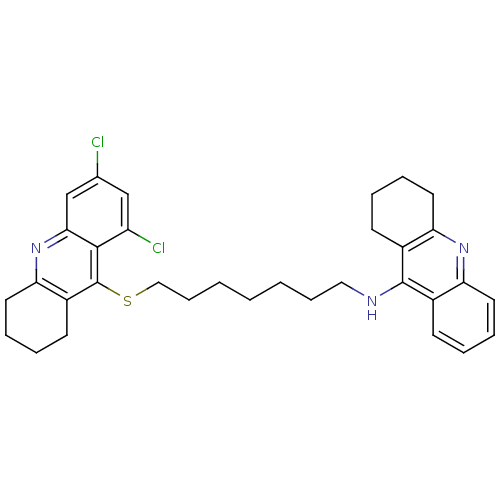 (N-{7-[(6,8-dichloro-1,2,3,4-tetrahydroacridin-9-yl...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 290 | -8.91 | n/a | n/a | n/a | n/a | n/a | 8.0 | 25 |
Universita degli Studi di Siena | Assay Description Inhibition of enzyme activity was measured over a substrate concentration range of 0.01-30 mM and at least six inhibitor concentrations to determine ... | Bioorg Med Chem Lett 11: 1779-82 (2001) Article DOI: 10.1016/s0960-894x(01)00294-3 BindingDB Entry DOI: 10.7270/Q2DZ06JN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM22851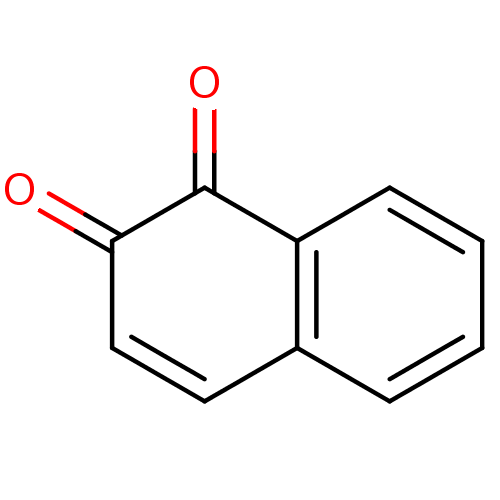 (1,2-Dione-Based Compound, 8 | 1,2-dihydronaphthale...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | 320 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
St. Jude Research Hospital | Assay Description The cholinesterase assays were performed using colorimetric method reported by Ellman. Estimates of the competitive inhibition constants (Ki) were ob... | J Med Chem 50: 5727-34 (2007) Article DOI: 10.1021/jm0706867 BindingDB Entry DOI: 10.7270/Q2Q52MWQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Tetronarce californica (Pacific electric ray) (Tor...) | BDBM10632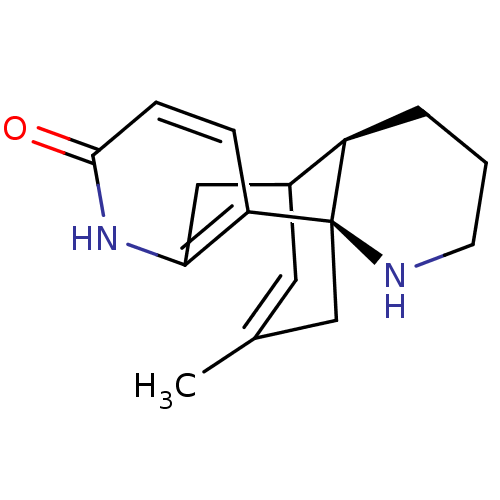 ((-)-Huperzine B | (1R,10R)-16-methyl-6,14-diazatet...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 334 | -8.83 | n/a | n/a | n/a | n/a | n/a | 7.0 | 25 |
Weizmann Institute of Science | Assay Description The cholinesterase assays were performed using colorimetric method reported by Ellman. Enzyme activity was determined by measuring the absorbance at ... | Biochemistry 41: 10810-8 (2002) Article DOI: 10.1021/bi020151+ BindingDB Entry DOI: 10.7270/Q2HQ3X52 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM8970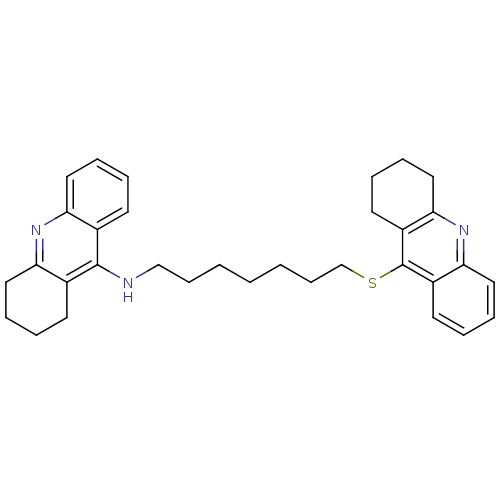 (CHEMBL51197 | N-[7-(1,2,3,4-tetrahydroacridin-9-yl...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 340 | -8.82 | n/a | n/a | n/a | n/a | n/a | 8.0 | 25 |
Universita di Siena | Assay Description Inhibition of enzyme activity was measured over a substrate concentration range of 0.01-30 mM and at least six inhibitor concentrations to determine ... | J Med Chem 48: 1919-29 (2005) Article DOI: 10.1021/jm049510k BindingDB Entry DOI: 10.7270/Q27P8WMJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM8970 (CHEMBL51197 | N-[7-(1,2,3,4-tetrahydroacridin-9-yl...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 340 | -8.82 | n/a | n/a | n/a | n/a | n/a | 8.0 | 25 |
Universita degli Studi di Siena | Assay Description Inhibition of enzyme activity was measured over a substrate concentration range of 0.01-30 mM and at least six inhibitor concentrations to determine ... | Bioorg Med Chem Lett 11: 1779-82 (2001) Article DOI: 10.1016/s0960-894x(01)00294-3 BindingDB Entry DOI: 10.7270/Q2DZ06JN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 893 total ) | Next | Last >> |