

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Plasminogen (Homo sapiens (Human)) | BDBM50120225 (2-{[2-(4-Guanidino-butyrylamino)-3-(4-nitro-phenyl...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.230 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Concentration of the compound required to inhibit Plasmin was determined | Bioorg Med Chem Lett 12: 3183-6 (2002) BindingDB Entry DOI: 10.7270/Q20P0ZBK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasminogen (Homo sapiens (Human)) | BDBM50514073 (CHEMBL4576519) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.320 | n/a | n/a | n/a | n/a | n/a | n/a |
Philipps University Marburg Curated by ChEMBL | Assay Description Inhibition of human plasmin preincubated for 15 mins followed by MeOSuc-Ala-Phe-Lys-AMC substrate addition and measured after 30 mins by fluorescence... | J Med Chem 63: 1445-1472 (2020) Article DOI: 10.1021/acs.jmedchem.9b01060 BindingDB Entry DOI: 10.7270/Q2VQ361P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasminogen (Homo sapiens (Human)) | BDBM50514076 (CHEMBL4456691) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Philipps University Marburg Curated by ChEMBL | Assay Description Inhibition of human plasmin preincubated for 15 mins followed by MeOSuc-Ala-Phe-Lys-AMC substrate addition and measured after 30 mins by fluorescence... | J Med Chem 63: 1445-1472 (2020) Article DOI: 10.1021/acs.jmedchem.9b01060 BindingDB Entry DOI: 10.7270/Q2VQ361P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasminogen (Homo sapiens (Human)) | BDBM50514062 (CHEMBL4436082) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Philipps University Marburg Curated by ChEMBL | Assay Description Inhibition of human plasmin preincubated for 15 mins followed by MeOSuc-Ala-Phe-Lys-AMC substrate addition and measured after 30 mins by fluorescence... | J Med Chem 63: 1445-1472 (2020) Article DOI: 10.1021/acs.jmedchem.9b01060 BindingDB Entry DOI: 10.7270/Q2VQ361P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasminogen (Homo sapiens (Human)) | BDBM50514063 (CHEMBL4447001) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.420 | n/a | n/a | n/a | n/a | n/a | n/a |
Philipps University Marburg Curated by ChEMBL | Assay Description Inhibition of human plasmin preincubated for 15 mins followed by MeOSuc-Ala-Phe-Lys-AMC substrate addition and measured after 30 mins by fluorescence... | J Med Chem 63: 1445-1472 (2020) Article DOI: 10.1021/acs.jmedchem.9b01060 BindingDB Entry DOI: 10.7270/Q2VQ361P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasminogen (Homo sapiens (Human)) | BDBM50514065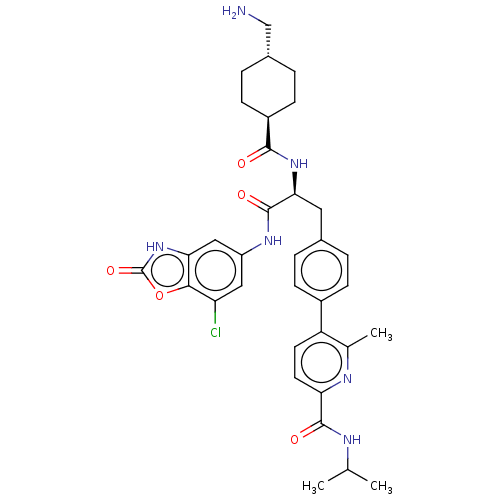 (CHEMBL4570952) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
Philipps University Marburg Curated by ChEMBL | Assay Description Inhibition of human plasmin preincubated for 15 mins followed by MeOSuc-Ala-Phe-Lys-AMC substrate addition and measured after 30 mins by fluorescence... | J Med Chem 63: 1445-1472 (2020) Article DOI: 10.1021/acs.jmedchem.9b01060 BindingDB Entry DOI: 10.7270/Q2VQ361P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasminogen (Homo sapiens (Human)) | BDBM50514079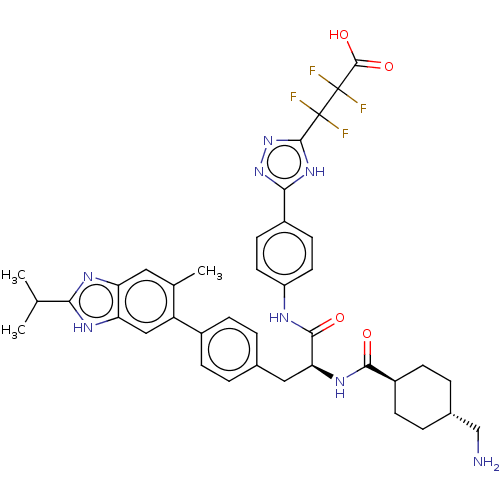 (CHEMBL4449958) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.870 | n/a | n/a | n/a | n/a | n/a | n/a |
Philipps University Marburg Curated by ChEMBL | Assay Description Inhibition of human plasmin preincubated for 15 mins followed by MeOSuc-Ala-Phe-Lys-AMC substrate addition and measured after 30 mins by fluorescence... | J Med Chem 63: 1445-1472 (2020) Article DOI: 10.1021/acs.jmedchem.9b01060 BindingDB Entry DOI: 10.7270/Q2VQ361P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasminogen (Homo sapiens (Human)) | BDBM50514077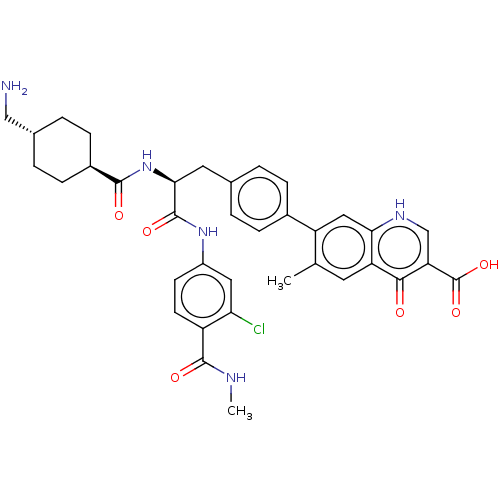 (CHEMBL4475211) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Philipps University Marburg Curated by ChEMBL | Assay Description Inhibition of human plasmin preincubated for 15 mins followed by MeOSuc-Ala-Phe-Lys-AMC substrate addition and measured after 30 mins by fluorescence... | J Med Chem 63: 1445-1472 (2020) Article DOI: 10.1021/acs.jmedchem.9b01060 BindingDB Entry DOI: 10.7270/Q2VQ361P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasminogen (Homo sapiens (Human)) | BDBM50514078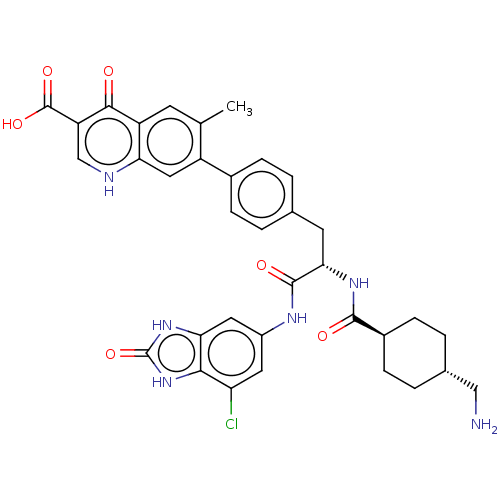 (CHEMBL4582882) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Philipps University Marburg Curated by ChEMBL | Assay Description Inhibition of human plasmin preincubated for 15 mins followed by MeOSuc-Ala-Phe-Lys-AMC substrate addition and measured after 30 mins by fluorescence... | J Med Chem 63: 1445-1472 (2020) Article DOI: 10.1021/acs.jmedchem.9b01060 BindingDB Entry DOI: 10.7270/Q2VQ361P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasminogen (Homo sapiens (Human)) | BDBM652813 (3-chloro-N-((2-((1-methylpiperidin-4-yl)methoxy)py...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | UniChem | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasminogen (Homo sapiens (Human)) | BDBM652497 (N-((6-(((1-methylpiperidin-4-yl)methyl)amino)pyrid...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | UniChem | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasminogen (Homo sapiens (Human)) | BDBM652723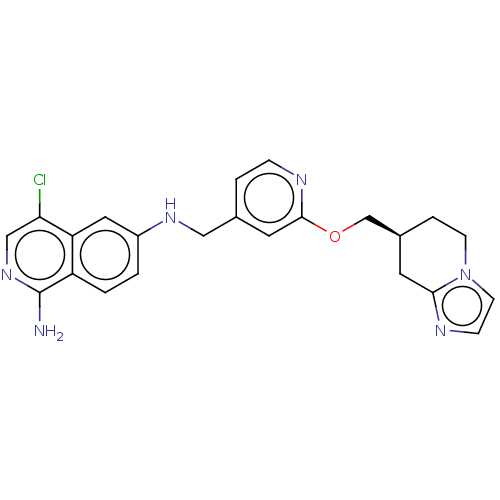 ((S*)-4-chloro-N6-((2-((5,6,7,8-tetrahydroimidazo[1...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | UniChem | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasminogen (Homo sapiens (Human)) | BDBM652421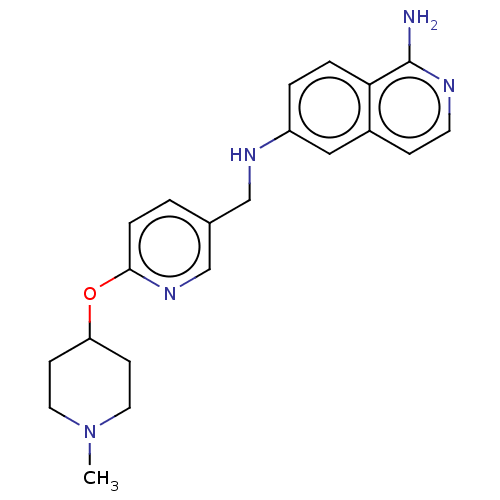 (6-N-({6-[(1-methylpiperidin-4-yl)oxy]pyridin-3-yl}...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | UniChem | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasminogen (Homo sapiens (Human)) | BDBM652422 (5-N-({4-[(1-methylpiperidin-4-yl)oxy]phenyl}methyl...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | UniChem | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasminogen (Homo sapiens (Human)) | BDBM652473 (5-N-[(6-{5H,6H,8H-imidazo[1,2-a]piperazin-7-yl}pyr...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | UniChem | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasminogen (Homo sapiens (Human)) | BDBM652731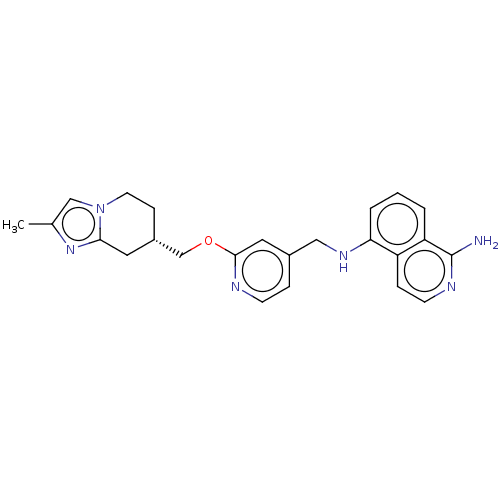 ((S*)-N5-((2-((2-methyl-5,6,7,8-tetrahydroimidazo[1...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | UniChem | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasminogen (Homo sapiens (Human)) | BDBM652632 (5-N-[(2-fluoro-4-{2-[(15,4S)-5-isopropyl-2,5-diaza...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | UniChem | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasminogen (Homo sapiens (Human)) | BDBM652645 (N6-((2-((1-methylpiperidin-4-yl)oxy)pyridin-4-yl)m...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | UniChem | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasminogen (Homo sapiens (Human)) | BDBM652648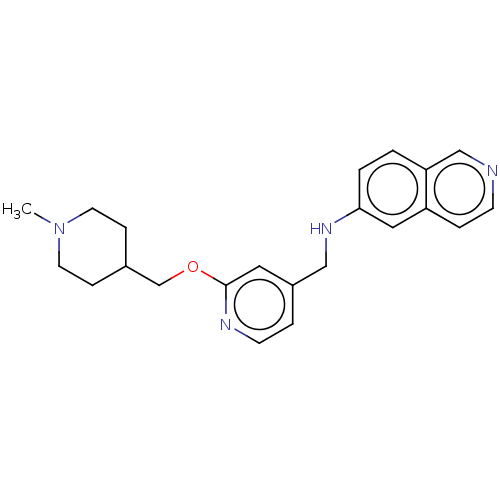 (N-({2-[(1-methylpiperidin-4-yl)methoxy]pyridin-4-y...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | UniChem | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasminogen (Homo sapiens (Human)) | BDBM652654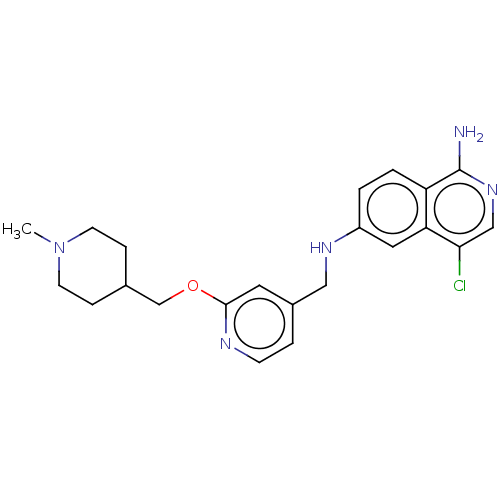 (4-chloro-6-N-({2-[(1-methylpiperidin-4-yl)methoxy]...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | UniChem | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasminogen (Homo sapiens (Human)) | BDBM652657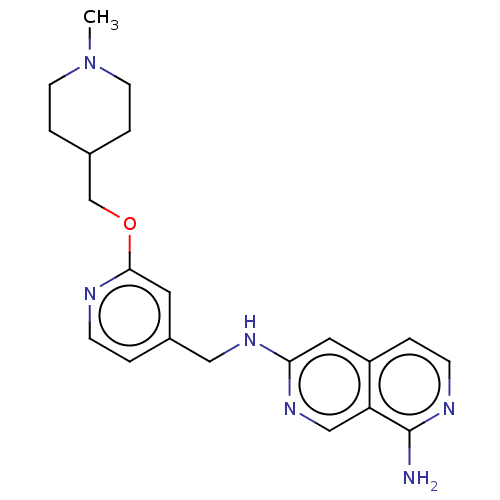 (6-N-({2-[(1-methylpiperidin-4-yl)methoxy]pyridin-4...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | UniChem | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasminogen (Homo sapiens (Human)) | BDBM652689 (4-fluoro-5-N-[(2-{5H,6H,7H,8H-imidazo[1,2-a]pyridi...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | UniChem | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasminogen (Homo sapiens (Human)) | BDBM652722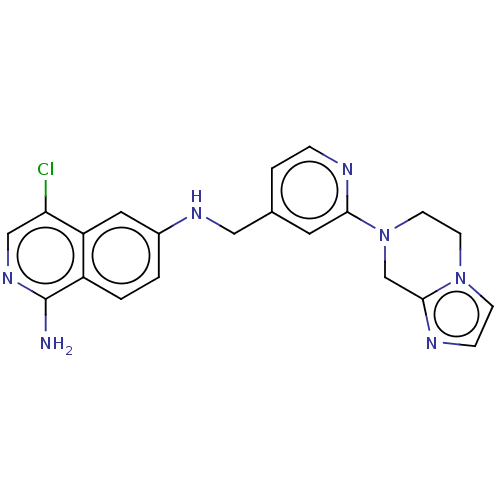 (4-chloro-6-N-[(2-{5H,6H,8H-imidazo[1,2-a]pyrazin-7...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | UniChem | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasminogen (Homo sapiens (Human)) | BDBM50514075 (CHEMBL4541084) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 8.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Philipps University Marburg Curated by ChEMBL | Assay Description Inhibition of human plasmin preincubated for 15 mins followed by MeOSuc-Ala-Phe-Lys-AMC substrate addition and measured after 30 mins by fluorescence... | J Med Chem 63: 1445-1472 (2020) Article DOI: 10.1021/acs.jmedchem.9b01060 BindingDB Entry DOI: 10.7270/Q2VQ361P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasminogen (Homo sapiens (Human)) | BDBM50029498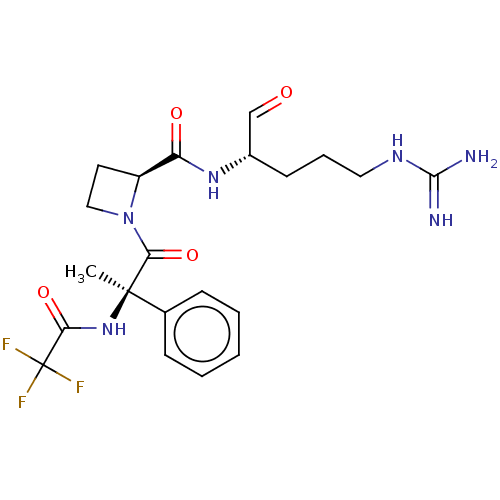 (1N-[2-{2-[4-amino(imino)methylamino-1-formyl-(1S)-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 9.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description In vitro Enzyme Inhibitory activity measured against Plasmin | J Med Chem 38: 4446-53 (1995) BindingDB Entry DOI: 10.7270/Q2RB73MV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasminogen (Homo sapiens (Human)) | BDBM50591422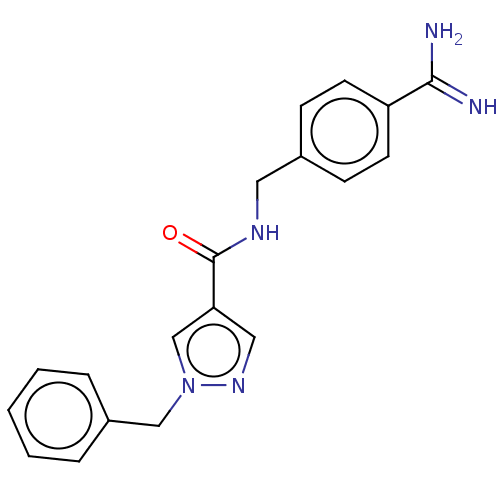 (CHEMBL5175689) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | n/a | n/a | >10 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasminogen [101-181] (Homo sapiens (Human)) | BDBM290292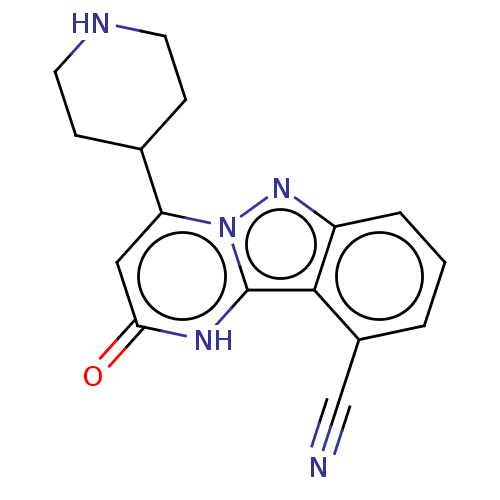 (2-Oxo-4-(piperidin-4-yl)-1,2-dihydropyrimido[1,2-b...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
BAYER PHARMA AKTIENGESELLSCHAFT US Patent | Assay Description The clot-lysis test system configures the kinetics of clot formation and degradation in vitro and allows quantifying modulation of the process by sel... | US Patent US10668071 (2020) BindingDB Entry DOI: 10.7270/Q2KP8554 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasminogen (Homo sapiens (Human)) | BDBM50124990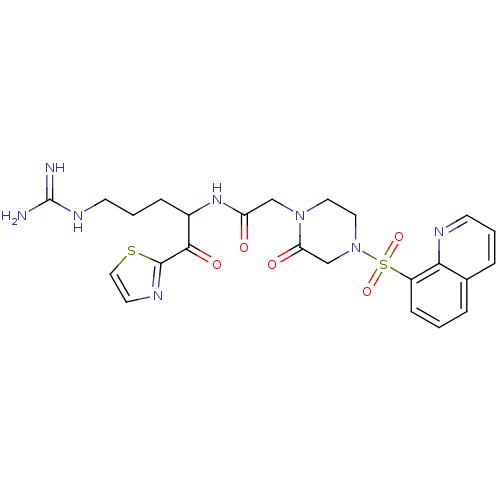 (CHEMBL355376 | N-[4-Guanidino-1-(thiazole-2-carbon...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals Inc. Curated by ChEMBL | Assay Description Inhibitory activity against Plasmin | Bioorg Med Chem Lett 13: 723-8 (2003) BindingDB Entry DOI: 10.7270/Q2Z037JH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasminogen [101-181] (Homo sapiens (Human)) | BDBM290292 (2-Oxo-4-(piperidin-4-yl)-1,2-dihydropyrimido[1,2-b...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 10 | n/a | n/a | n/a | n/a | 7.0 | n/a |
BAYER PHARMA AKTIENGESELLSCHAFT US Patent | Assay Description The test compounds were dissolved in 1% acetic acid and further complemented with an equal volume of DMSO. The resulting stock solutions were seriall... | US Patent US10098883 (2018) BindingDB Entry DOI: 10.7270/Q2G73GSM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasminogen [101-181] (Homo sapiens (Human)) | BDBM290336 (10-Chloro-8-methyl-4-(piperidin-4-yl)pyrimido[1,2-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 11 | n/a | n/a | n/a | n/a | 7.0 | n/a |
BAYER PHARMA AKTIENGESELLSCHAFT US Patent | Assay Description The test compounds were dissolved in 1% acetic acid and further complemented with an equal volume of DMSO. The resulting stock solutions were seriall... | US Patent US10098883 (2018) BindingDB Entry DOI: 10.7270/Q2G73GSM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasminogen [101-181] (Homo sapiens (Human)) | BDBM290336 (10-Chloro-8-methyl-4-(piperidin-4-yl)pyrimido[1,2-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
BAYER PHARMA AKTIENGESELLSCHAFT US Patent | Assay Description The clot-lysis test system configures the kinetics of clot formation and degradation in vitro and allows quantifying modulation of the process by sel... | US Patent US10668071 (2020) BindingDB Entry DOI: 10.7270/Q2KP8554 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasminogen [101-181] (Homo sapiens (Human)) | BDBM290334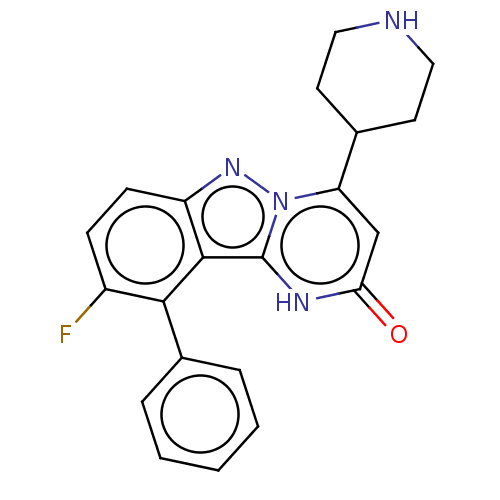 (9-Fluoro-10-phenyl-4-(piperidin-4-yl)pyrimido[1,2-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
BAYER PHARMA AKTIENGESELLSCHAFT US Patent | Assay Description The clot-lysis test system configures the kinetics of clot formation and degradation in vitro and allows quantifying modulation of the process by sel... | US Patent US10668071 (2020) BindingDB Entry DOI: 10.7270/Q2KP8554 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasminogen [101-181] (Homo sapiens (Human)) | BDBM290334 (9-Fluoro-10-phenyl-4-(piperidin-4-yl)pyrimido[1,2-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 11 | n/a | n/a | n/a | n/a | 7.0 | n/a |
BAYER PHARMA AKTIENGESELLSCHAFT US Patent | Assay Description The test compounds were dissolved in 1% acetic acid and further complemented with an equal volume of DMSO. The resulting stock solutions were seriall... | US Patent US10098883 (2018) BindingDB Entry DOI: 10.7270/Q2G73GSM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasminogen [101-181] (Homo sapiens (Human)) | BDBM290324 (4-(Piperidin-4-yl)-10-[2-(trifluoromethyl)phenyl]p...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
BAYER PHARMA AKTIENGESELLSCHAFT US Patent | Assay Description The clot-lysis test system configures the kinetics of clot formation and degradation in vitro and allows quantifying modulation of the process by sel... | US Patent US10668071 (2020) BindingDB Entry DOI: 10.7270/Q2KP8554 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasminogen [101-181] (Homo sapiens (Human)) | BDBM290314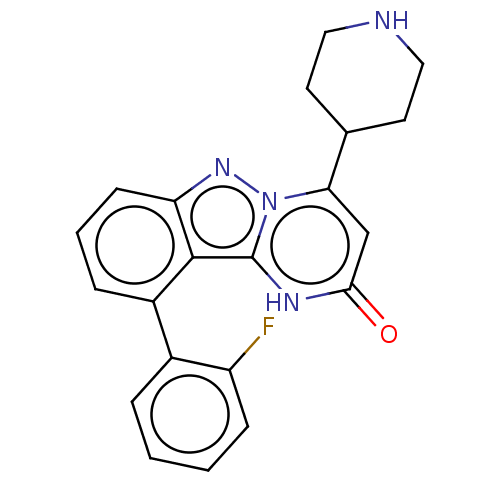 (10-(2-Fluorophenyl)-4-(piperidin-4-yl)pyrimido[1,2...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 13 | n/a | n/a | n/a | n/a | 7.0 | n/a |
BAYER PHARMA AKTIENGESELLSCHAFT US Patent | Assay Description The test compounds were dissolved in 1% acetic acid and further complemented with an equal volume of DMSO. The resulting stock solutions were seriall... | US Patent US10098883 (2018) BindingDB Entry DOI: 10.7270/Q2G73GSM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasminogen [101-181] (Homo sapiens (Human)) | BDBM290314 (10-(2-Fluorophenyl)-4-(piperidin-4-yl)pyrimido[1,2...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
BAYER PHARMA AKTIENGESELLSCHAFT US Patent | Assay Description The clot-lysis test system configures the kinetics of clot formation and degradation in vitro and allows quantifying modulation of the process by sel... | US Patent US10668071 (2020) BindingDB Entry DOI: 10.7270/Q2KP8554 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasminogen [101-181] (Homo sapiens (Human)) | BDBM290309 ((−)-trans-10-Ethoxy-4-(2-methylpiperidin-4-y...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 13 | n/a | n/a | n/a | n/a | 7.0 | n/a |
BAYER PHARMA AKTIENGESELLSCHAFT US Patent | Assay Description The test compounds were dissolved in 1% acetic acid and further complemented with an equal volume of DMSO. The resulting stock solutions were seriall... | US Patent US10098883 (2018) BindingDB Entry DOI: 10.7270/Q2G73GSM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasminogen [101-181] (Homo sapiens (Human)) | BDBM290286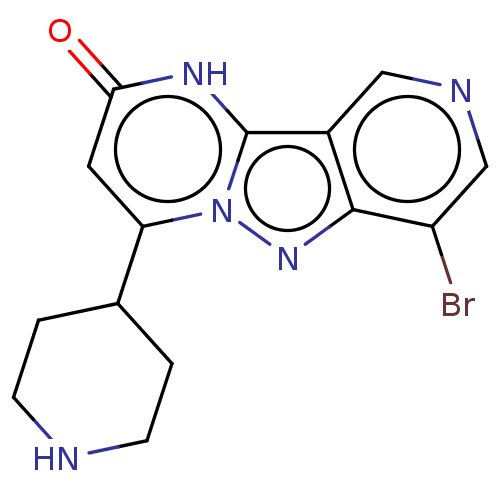 (7-Bromo-4-(piperidin-4-yl)pyrido[4′,3′...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
BAYER PHARMA AKTIENGESELLSCHAFT US Patent | Assay Description The clot-lysis test system configures the kinetics of clot formation and degradation in vitro and allows quantifying modulation of the process by sel... | US Patent US10668071 (2020) BindingDB Entry DOI: 10.7270/Q2KP8554 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasminogen [101-181] (Homo sapiens (Human)) | BDBM290320 (10-Cyclopentyl-4-(piperidin-4-yl)pyrimido[1,2-b]in...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
BAYER PHARMA AKTIENGESELLSCHAFT US Patent | Assay Description The clot-lysis test system configures the kinetics of clot formation and degradation in vitro and allows quantifying modulation of the process by sel... | US Patent US10668071 (2020) BindingDB Entry DOI: 10.7270/Q2KP8554 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasminogen [101-181] (Homo sapiens (Human)) | BDBM290324 (4-(Piperidin-4-yl)-10-[2-(trifluoromethyl)phenyl]p...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 13 | n/a | n/a | n/a | n/a | 7.0 | n/a |
BAYER PHARMA AKTIENGESELLSCHAFT US Patent | Assay Description The test compounds were dissolved in 1% acetic acid and further complemented with an equal volume of DMSO. The resulting stock solutions were seriall... | US Patent US10098883 (2018) BindingDB Entry DOI: 10.7270/Q2G73GSM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasminogen [101-181] (Homo sapiens (Human)) | BDBM290286 (7-Bromo-4-(piperidin-4-yl)pyrido[4′,3′...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 13 | n/a | n/a | n/a | n/a | 7.0 | n/a |
BAYER PHARMA AKTIENGESELLSCHAFT US Patent | Assay Description The test compounds were dissolved in 1% acetic acid and further complemented with an equal volume of DMSO. The resulting stock solutions were seriall... | US Patent US10098883 (2018) BindingDB Entry DOI: 10.7270/Q2G73GSM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasminogen [101-181] (Homo sapiens (Human)) | BDBM290309 ((−)-trans-10-Ethoxy-4-(2-methylpiperidin-4-y...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
BAYER PHARMA AKTIENGESELLSCHAFT US Patent | Assay Description The clot-lysis test system configures the kinetics of clot formation and degradation in vitro and allows quantifying modulation of the process by sel... | US Patent US10668071 (2020) BindingDB Entry DOI: 10.7270/Q2KP8554 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasminogen [101-181] (Homo sapiens (Human)) | BDBM290320 (10-Cyclopentyl-4-(piperidin-4-yl)pyrimido[1,2-b]in...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 13 | n/a | n/a | n/a | n/a | 7.0 | n/a |
BAYER PHARMA AKTIENGESELLSCHAFT US Patent | Assay Description The test compounds were dissolved in 1% acetic acid and further complemented with an equal volume of DMSO. The resulting stock solutions were seriall... | US Patent US10098883 (2018) BindingDB Entry DOI: 10.7270/Q2G73GSM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasminogen [101-181] (Homo sapiens (Human)) | BDBM290307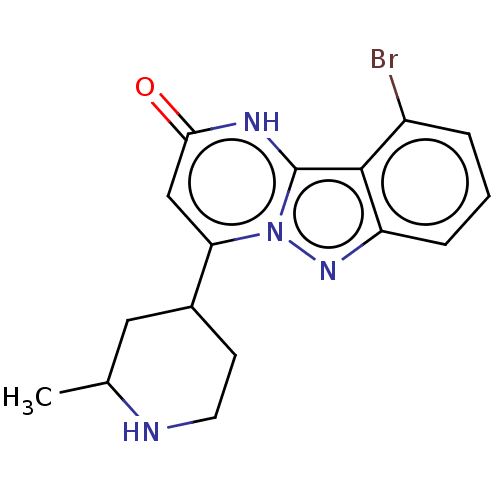 ((−)-trans-10-Bromo-4-(2-methylpiperidin-4-yl...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
BAYER PHARMA AKTIENGESELLSCHAFT US Patent | Assay Description The clot-lysis test system configures the kinetics of clot formation and degradation in vitro and allows quantifying modulation of the process by sel... | US Patent US10668071 (2020) BindingDB Entry DOI: 10.7270/Q2KP8554 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasminogen [101-181] (Homo sapiens (Human)) | BDBM290329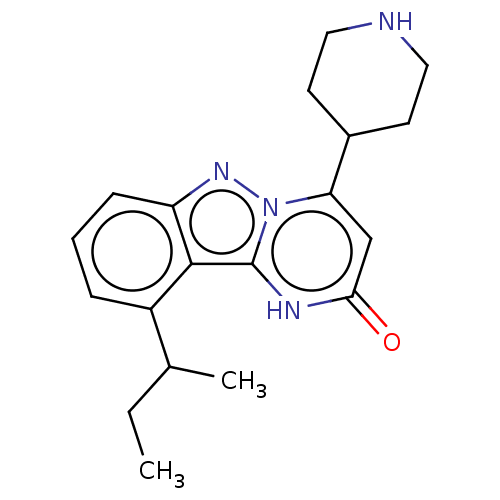 (10-sec-Butyl-4-(piperidin-4-yl)pyrimido[1,2-b]inda...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 14 | n/a | n/a | n/a | n/a | 7.0 | n/a |
BAYER PHARMA AKTIENGESELLSCHAFT US Patent | Assay Description The test compounds were dissolved in 1% acetic acid and further complemented with an equal volume of DMSO. The resulting stock solutions were seriall... | US Patent US10098883 (2018) BindingDB Entry DOI: 10.7270/Q2G73GSM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasminogen [101-181] (Homo sapiens (Human)) | BDBM290269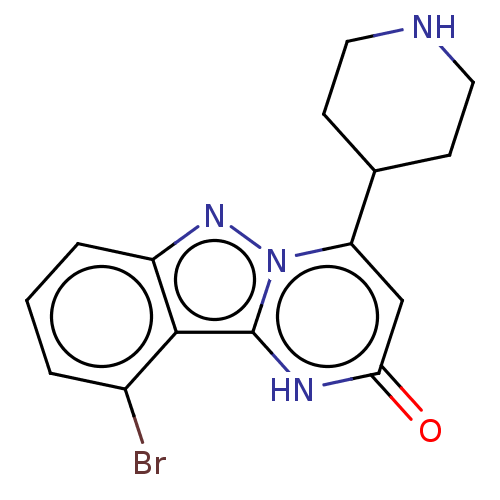 (10-Bromo-4-(piperidin-4-yl)pyrimido[1,2-b]indazol-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 14 | n/a | n/a | n/a | n/a | 7.0 | n/a |
BAYER PHARMA AKTIENGESELLSCHAFT US Patent | Assay Description The test compounds were dissolved in 1% acetic acid and further complemented with an equal volume of DMSO. The resulting stock solutions were seriall... | US Patent US10098883 (2018) BindingDB Entry DOI: 10.7270/Q2G73GSM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasminogen [101-181] (Homo sapiens (Human)) | BDBM290313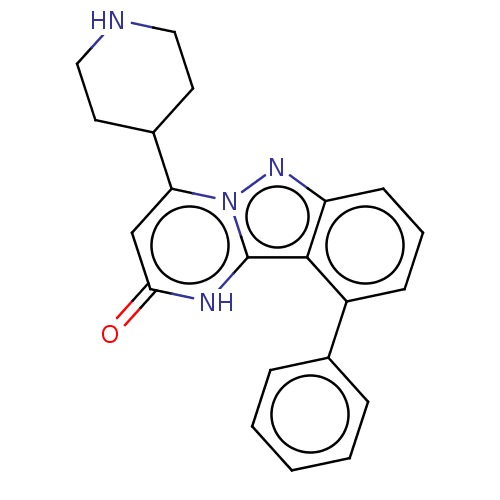 (10-Phenyl-4-(piperidin-4-yl)pyrimido[1,2-b]indazol...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 14 | n/a | n/a | n/a | n/a | 7.0 | n/a |
BAYER PHARMA AKTIENGESELLSCHAFT US Patent | Assay Description The test compounds were dissolved in 1% acetic acid and further complemented with an equal volume of DMSO. The resulting stock solutions were seriall... | US Patent US10098883 (2018) BindingDB Entry DOI: 10.7270/Q2G73GSM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasminogen [101-181] (Homo sapiens (Human)) | BDBM290312 (4-(Piperidin-4-yl)-10-[4-(trifluoromethyl)phenyl]p...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 14 | n/a | n/a | n/a | n/a | 7.0 | n/a |
BAYER PHARMA AKTIENGESELLSCHAFT US Patent | Assay Description The test compounds were dissolved in 1% acetic acid and further complemented with an equal volume of DMSO. The resulting stock solutions were seriall... | US Patent US10098883 (2018) BindingDB Entry DOI: 10.7270/Q2G73GSM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasminogen [101-181] (Homo sapiens (Human)) | BDBM290307 ((−)-trans-10-Bromo-4-(2-methylpiperidin-4-yl...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 14 | n/a | n/a | n/a | n/a | 7.0 | n/a |
BAYER PHARMA AKTIENGESELLSCHAFT US Patent | Assay Description The test compounds were dissolved in 1% acetic acid and further complemented with an equal volume of DMSO. The resulting stock solutions were seriall... | US Patent US10098883 (2018) BindingDB Entry DOI: 10.7270/Q2G73GSM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasminogen [101-181] (Homo sapiens (Human)) | BDBM290329 (10-sec-Butyl-4-(piperidin-4-yl)pyrimido[1,2-b]inda...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
BAYER PHARMA AKTIENGESELLSCHAFT US Patent | Assay Description The clot-lysis test system configures the kinetics of clot formation and degradation in vitro and allows quantifying modulation of the process by sel... | US Patent US10668071 (2020) BindingDB Entry DOI: 10.7270/Q2KP8554 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 966 total ) | Next | Last >> |