

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Tyrosine-protein kinase Lck (Homo sapiens (Human)) | BDBM50194684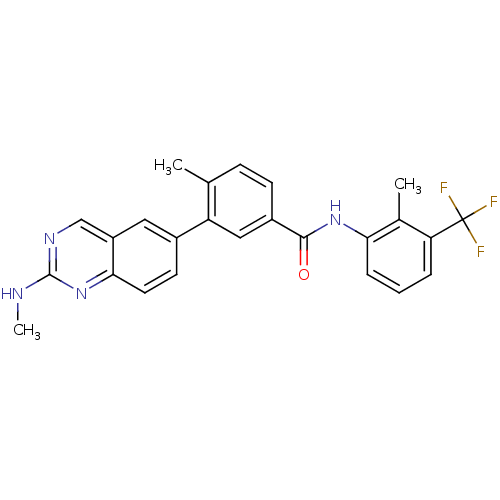 (4-methyl-N-(2-methyl-3-4-methyl-N-(2-methyl-3-(2-(...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen, Inc. Curated by ChEMBL | Assay Description Inhibition of Lck by HTRF kinase assay | J Med Chem 49: 5671-86 (2006) Article DOI: 10.1021/jm0605482 BindingDB Entry DOI: 10.7270/Q29G5MFD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Lck (Homo sapiens (Human)) | BDBM50301603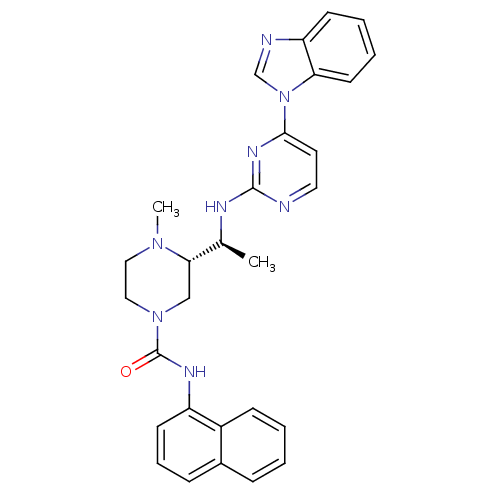 ((S)-3-((S)-1-(4-(1H-benzo[d]imidazol-1-yl)pyrimidi...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of Lck | Bioorg Med Chem Lett 19: 5440-3 (2009) Article DOI: 10.1016/j.bmcl.2009.07.102 BindingDB Entry DOI: 10.7270/Q2GX4BM0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Lck (Homo sapiens (Human)) | BDBM50194688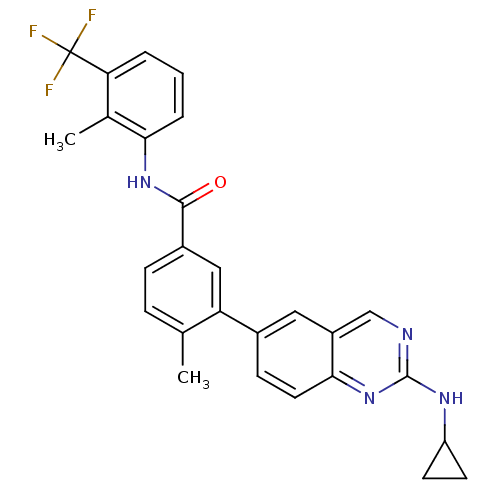 (4-methyl-N-(2-methyl-3-(trifluoromethyl)phenyl)-3-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.0700 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen, Inc. Curated by ChEMBL | Assay Description Inhibition of Lck by HTRF kinase assay | J Med Chem 49: 5671-86 (2006) Article DOI: 10.1021/jm0605482 BindingDB Entry DOI: 10.7270/Q29G5MFD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Lck (Homo sapiens (Human)) | BDBM50301619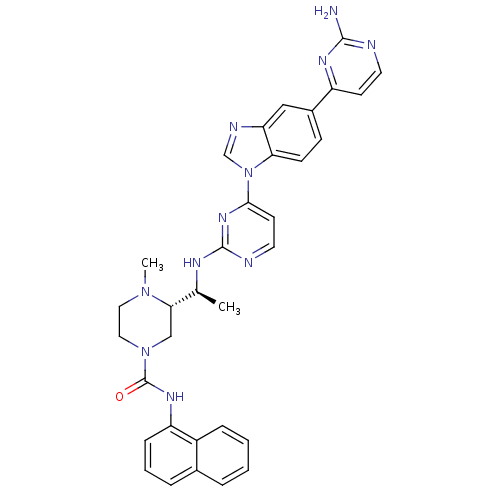 ((S)-3-((S)-1-(4-(5-(2-aminopyrimidin-4-yl)-1H-benz...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.0700 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of Lck | Bioorg Med Chem Lett 19: 5440-3 (2009) Article DOI: 10.1016/j.bmcl.2009.07.102 BindingDB Entry DOI: 10.7270/Q2GX4BM0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Lck (Homo sapiens (Human)) | BDBM50301604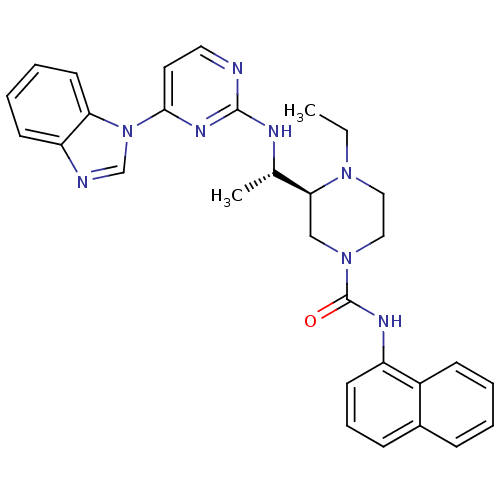 ((S)-3-((S)-1-(4-(1H-benzo[d]imidazol-1-yl)pyrimidi...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.0700 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of Lck | Bioorg Med Chem Lett 19: 5440-3 (2009) Article DOI: 10.1016/j.bmcl.2009.07.102 BindingDB Entry DOI: 10.7270/Q2GX4BM0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Lck (Homo sapiens (Human)) | BDBM50301624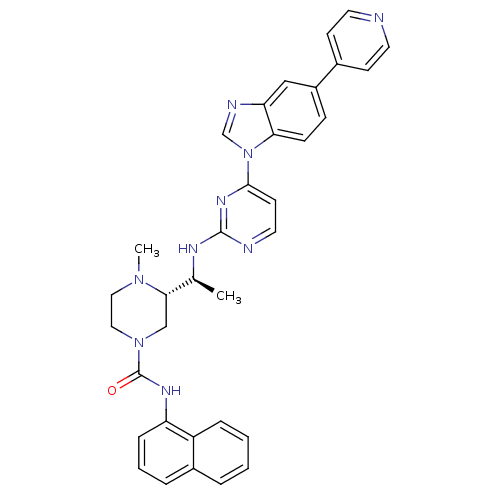 ((S)-4-methyl-N(S)-4-methyl-N-(naphthalen-1-yl)-3-(...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.120 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of Lck | Bioorg Med Chem Lett 19: 5440-3 (2009) Article DOI: 10.1016/j.bmcl.2009.07.102 BindingDB Entry DOI: 10.7270/Q2GX4BM0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Lck (Homo sapiens (Human)) | BDBM14949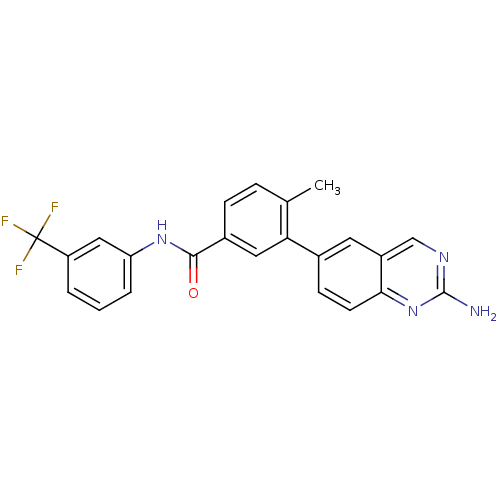 (2-aminoquinazoline 5 | 3-(2-aminoquinazolin-6-yl)-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL DrugBank MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc. Curated by ChEMBL | Assay Description Inhibition of Lck by HTRF assay | Bioorg Med Chem Lett 18: 1172-6 (2008) Article DOI: 10.1016/j.bmcl.2007.11.123 BindingDB Entry DOI: 10.7270/Q2WD41FK | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Tyrosine-protein kinase Lck (Homo sapiens (Human)) | BDBM13271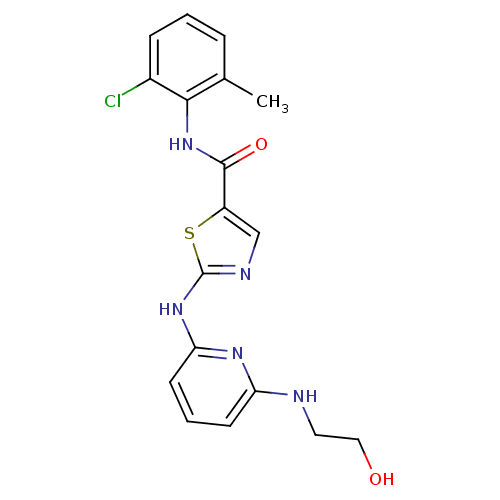 (BMS-354825 2-Heteroarylamino-thiazole Analog 12p |...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | <0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company | Assay Description IC50 is the inhibitor concentration, which inhibits 50% of kinase activity that catalyzes the transfer of the terminal phosphate from [gamma-33P] lab... | J Med Chem 49: 6819-32 (2006) Article DOI: 10.1021/jm060727j BindingDB Entry DOI: 10.7270/Q2QN6501 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Lck (Homo sapiens (Human)) | BDBM13277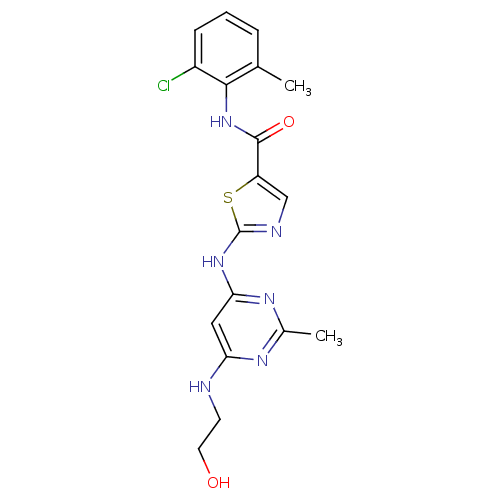 (BMS-354825 2-Heteroarylamino-thiazole Analog 12v |...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company | Assay Description IC50 is the inhibitor concentration, which inhibits 50% of kinase activity that catalyzes the transfer of the terminal phosphate from [gamma-33P] lab... | J Med Chem 49: 6819-32 (2006) Article DOI: 10.1021/jm060727j BindingDB Entry DOI: 10.7270/Q2QN6501 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Lck (Homo sapiens (Human)) | BDBM50301607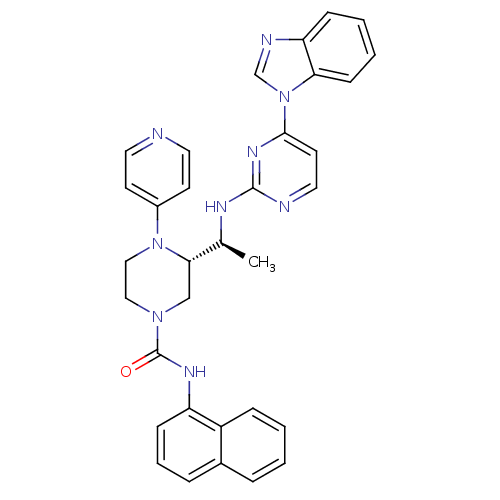 ((S)-3-((S)-1-(4-(1H-benzo[d]imidazol-1-yl)pyrimidi...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of Lck | Bioorg Med Chem Lett 19: 5440-3 (2009) Article DOI: 10.1016/j.bmcl.2009.07.102 BindingDB Entry DOI: 10.7270/Q2GX4BM0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Lck (Homo sapiens (Human)) | BDBM14949 (2-aminoquinazoline 5 | 3-(2-aminoquinazolin-6-yl)-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL DrugBank MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen, Inc. Curated by ChEMBL | Assay Description Inhibition of Lck by HTRF kinase assay | J Med Chem 49: 5671-86 (2006) Article DOI: 10.1021/jm0605482 BindingDB Entry DOI: 10.7270/Q29G5MFD | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Tyrosine-protein kinase Lck (Homo sapiens (Human)) | BDBM50194668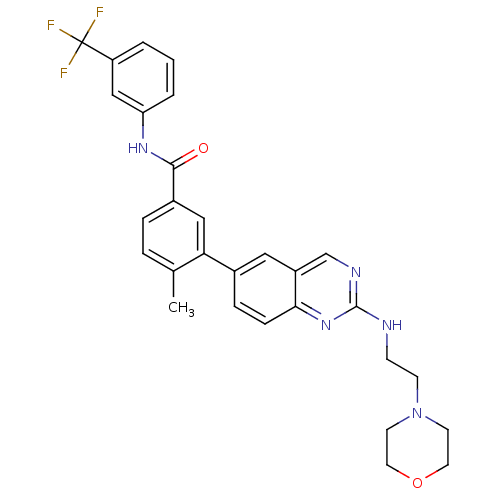 (4-methyl-3-(2-(2-morpholinoethylamino)quinazolin-6...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen, Inc. Curated by ChEMBL | Assay Description Inhibition of Lck by HTRF kinase assay | J Med Chem 49: 5671-86 (2006) Article DOI: 10.1021/jm0605482 BindingDB Entry DOI: 10.7270/Q29G5MFD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Lck (Homo sapiens (Human)) | BDBM50301605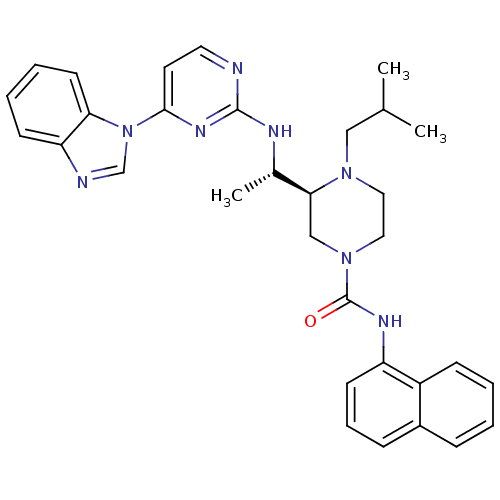 ((S)-3-((S)-1-(4-(1H-benzo[d]imidazol-1-yl)pyrimidi...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of Lck | Bioorg Med Chem Lett 19: 5440-3 (2009) Article DOI: 10.1016/j.bmcl.2009.07.102 BindingDB Entry DOI: 10.7270/Q2GX4BM0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Lck (Homo sapiens (Human)) | BDBM14949 (2-aminoquinazoline 5 | 3-(2-aminoquinazolin-6-yl)-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL DrugBank MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | 7.5 | 22 |
Amgen | Assay Description The assay involves the phosphorylation of a biotinylated substrate and the detection of this phosphorylation after the addition of a streptavidin-all... | J Med Chem 51: 1681-94 (2008) Article DOI: 10.1021/jm7010996 BindingDB Entry DOI: 10.7270/Q2Q81BCB | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Tyrosine-protein kinase Lck (Homo sapiens (Human)) | BDBM50301594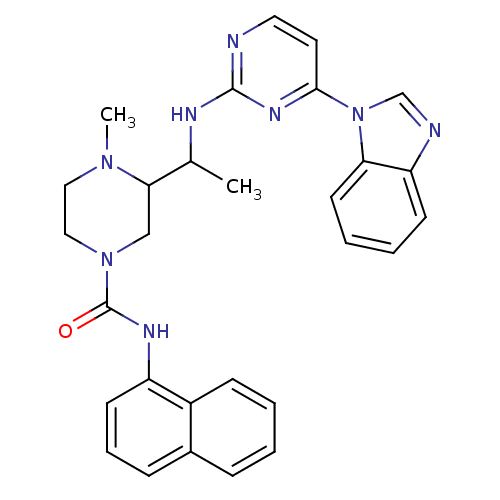 (3-(1-(4-(1H-benzo[d]imidazol-1-yl)pyrimidin-2-ylam...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.25 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of Lck | Bioorg Med Chem Lett 19: 5440-3 (2009) Article DOI: 10.1016/j.bmcl.2009.07.102 BindingDB Entry DOI: 10.7270/Q2GX4BM0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Lck (Homo sapiens (Human)) | BDBM50301594 (3-(1-(4-(1H-benzo[d]imidazol-1-yl)pyrimidin-2-ylam...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.25 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of Lck | Bioorg Med Chem Lett 19: 5440-3 (2009) Article DOI: 10.1016/j.bmcl.2009.07.102 BindingDB Entry DOI: 10.7270/Q2GX4BM0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Lck (Homo sapiens (Human)) | BDBM35317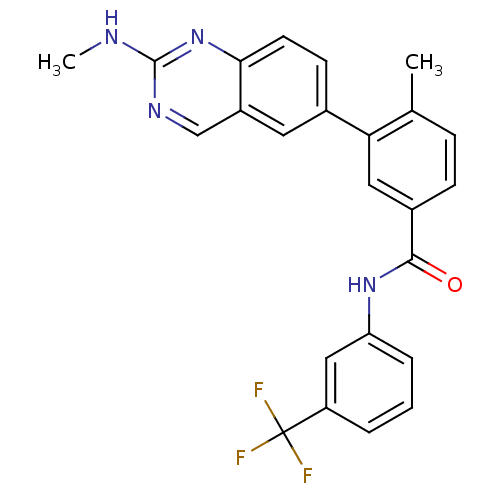 (4-Methyl-3-(2-(methylamino)quinazolin-6-yl)-N-(3-(...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.270 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen | Assay Description The assay uses purified enzyme interacting with biotinylated peptide substrate. HTRF is based on the proximity of europium cryptate (donor fluorophor... | J Med Chem 52: 6189-92 (2009) Article DOI: 10.1021/jm901081g BindingDB Entry DOI: 10.7270/Q2TH8K26 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Lck (Homo sapiens (Human)) | BDBM50322535 (3-(imidazo[1,2-b]pyridazin-3-ylethynyl)-4-methyl-N...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 0.280 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Binding affinity to human LCK using poly[Glu:Tyr] (4:1) as substrate by radiometric hotspot kinase assay | Citation and Details Article DOI: 10.1021/acsmedchemlett.8b00081 BindingDB Entry DOI: 10.7270/Q2WM1J37 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Lck (Homo sapiens (Human)) | BDBM13262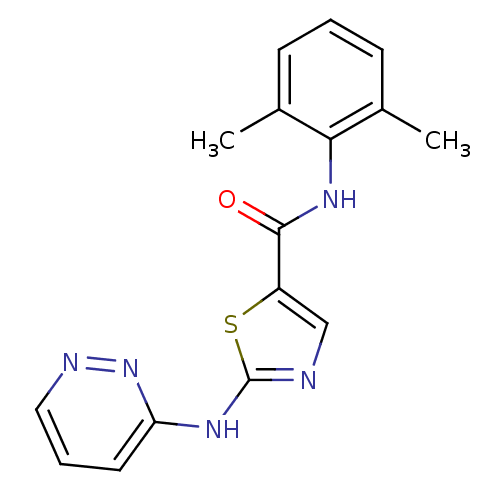 (BMS-354825 2-Heteroarylamino-thiazole Analog 12g |...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company | Assay Description IC50 is the inhibitor concentration, which inhibits 50% of kinase activity that catalyzes the transfer of the terminal phosphate from [gamma-33P] lab... | J Med Chem 49: 6819-32 (2006) Article DOI: 10.1021/jm060727j BindingDB Entry DOI: 10.7270/Q2QN6501 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Lck (Homo sapiens (Human)) | BDBM26381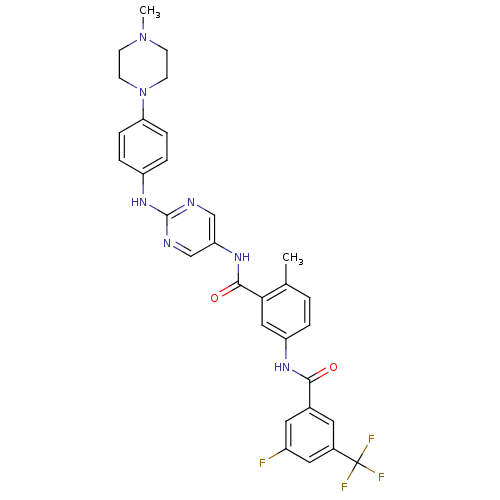 (1-N-[3-fluoro-5-(trifluoromethyl)benzene]-4-methyl...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | 7.5 | 22 |
Amgen | Assay Description The assay involves the phosphorylation of a biotinylated substrate and the detection of this phosphorylation after the addition of a streptavidin-all... | J Med Chem 51: 1681-94 (2008) Article DOI: 10.1021/jm7010996 BindingDB Entry DOI: 10.7270/Q2Q81BCB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Lck (Homo sapiens (Human)) | BDBM50305129 (6-(2-aminoethoxy)-2-(4-phenoxyphenyl)-9,10-dihydro...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Taisho Pharmaceutical Co., Ltd Curated by ChEMBL | Assay Description Inhibition of GST-tagged human LCK by scintillation counting | Bioorg Med Chem Lett 20: 112-6 (2010) Article DOI: 10.1016/j.bmcl.2009.11.013 BindingDB Entry DOI: 10.7270/Q22Z15NV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Lck (Homo sapiens (Human)) | BDBM50194694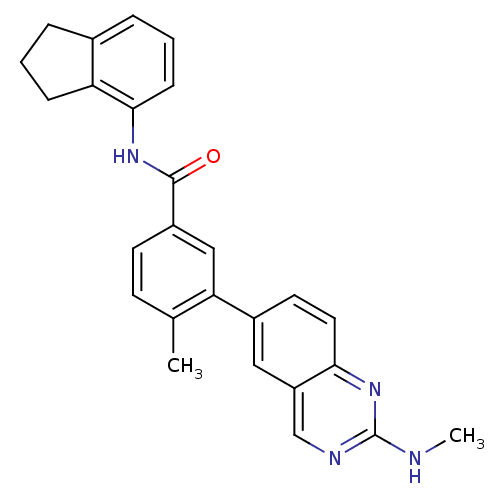 (CHEMBL427233 | N-(2,3-dihydro-1H-inden-4-yl)-4-met...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen, Inc. Curated by ChEMBL | Assay Description Inhibition of Lck by HTRF kinase assay | J Med Chem 49: 5671-86 (2006) Article DOI: 10.1021/jm0605482 BindingDB Entry DOI: 10.7270/Q29G5MFD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Lck (Homo sapiens (Human)) | BDBM50194691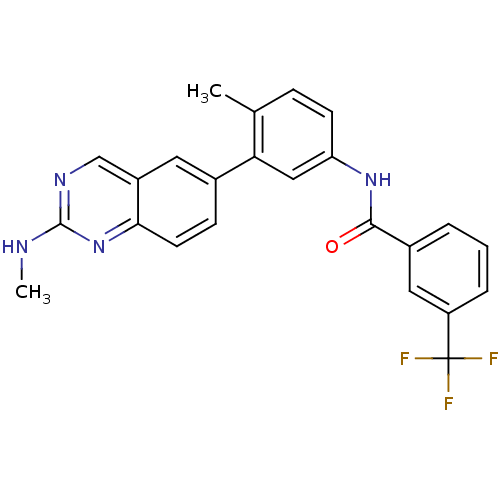 (CHEMBL212128 | N-(4-methyl-3-(2-(methylamino)quina...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen, Inc. Curated by ChEMBL | Assay Description Inhibition of Lck by HTRF kinase assay | J Med Chem 49: 5671-86 (2006) Article DOI: 10.1021/jm0605482 BindingDB Entry DOI: 10.7270/Q29G5MFD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Lck (Homo sapiens (Human)) | BDBM35317 (4-Methyl-3-(2-(methylamino)quinazolin-6-yl)-N-(3-(...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen, Inc. Curated by ChEMBL | Assay Description Inhibition of Lck by HTRF kinase assay | J Med Chem 49: 5671-86 (2006) Article DOI: 10.1021/jm0605482 BindingDB Entry DOI: 10.7270/Q29G5MFD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Lck (Homo sapiens (Human)) | BDBM13216 (BMS-354825 | CHEMBL1421 | DASATINIB | N-(2-Chloro-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00302 BindingDB Entry DOI: 10.7270/Q20Z779V | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Lck (Homo sapiens (Human)) | BDBM13216 (BMS-354825 | CHEMBL1421 | DASATINIB | N-(2-Chloro-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | 7.0 | 22 |
Bristol-Myers Squibb Company | Assay Description IC50 is the inhibitor concentration, which inhibits 50% of kinase activity that catalyzes the transfer of the terminal phosphate from [gamma-33P] lab... | J Med Chem 49: 6819-32 (2006) Article DOI: 10.1021/jm060727j BindingDB Entry DOI: 10.7270/Q2QN6501 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Lck (Homo sapiens (Human)) | BDBM26367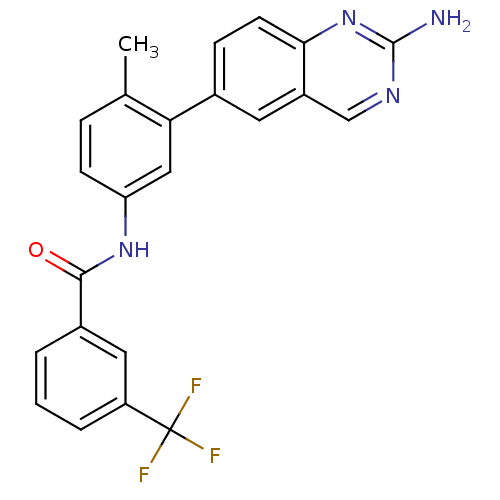 (Aminoquinazoline amide, 35 | N-[3-(2-aminoquinazol...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | 7.5 | 22 |
Amgen | Assay Description The assay involves the phosphorylation of a biotinylated substrate and the detection of this phosphorylation after the addition of a streptavidin-all... | J Med Chem 51: 1681-94 (2008) Article DOI: 10.1021/jm7010996 BindingDB Entry DOI: 10.7270/Q2Q81BCB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Lck (Homo sapiens (Human)) | BDBM13216 (BMS-354825 | CHEMBL1421 | DASATINIB | N-(2-Chloro-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of lck inase | J Med Chem 47: 6658-61 (2004) Article DOI: 10.1021/jm049486a BindingDB Entry DOI: 10.7270/Q2ZG6RRC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Lck (Homo sapiens (Human)) | BDBM50301618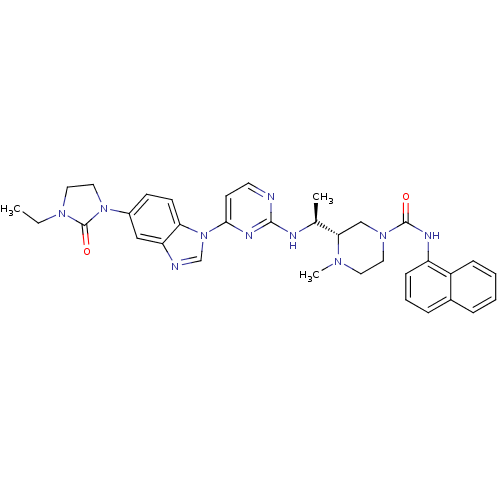 ((S)-3-((S)-1-(4-(5-(3-ethyl-2-oxoimidazolidin-1-yl...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of Lck | Bioorg Med Chem Lett 19: 5440-3 (2009) Article DOI: 10.1016/j.bmcl.2009.07.102 BindingDB Entry DOI: 10.7270/Q2GX4BM0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Lck (Homo sapiens (Human)) | BDBM50194678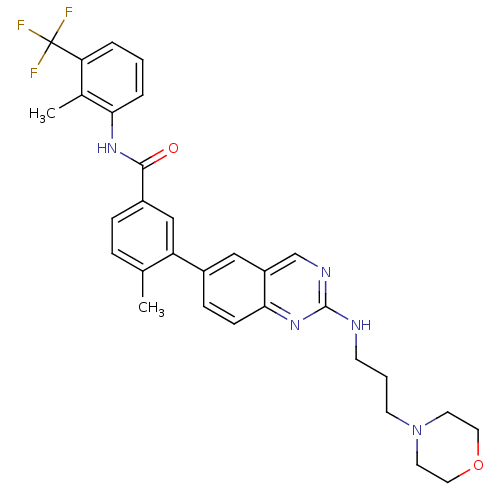 (4-methyl-N-(2-methyl-3-(trifluoromethyl)phenyl)-3-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen, Inc. Curated by ChEMBL | Assay Description Inhibition of Lck by HTRF kinase assay | J Med Chem 49: 5671-86 (2006) Article DOI: 10.1021/jm0605482 BindingDB Entry DOI: 10.7270/Q29G5MFD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Lck (Homo sapiens (Human)) | BDBM50194686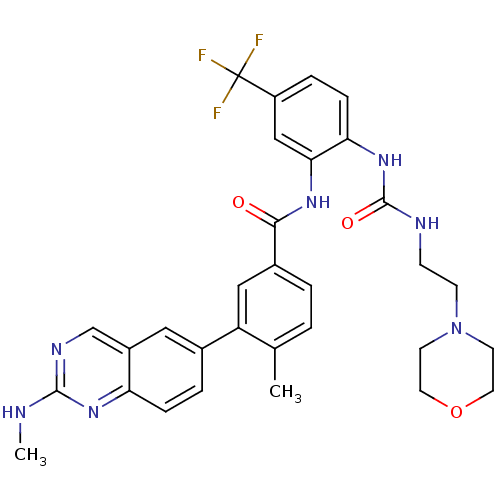 (1-(2-(4-methyl-3-(2-(methylamino)quinazolin-6-yl)b...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen, Inc. Curated by ChEMBL | Assay Description Inhibition of Lck by HTRF kinase assay | J Med Chem 49: 5671-86 (2006) Article DOI: 10.1021/jm0605482 BindingDB Entry DOI: 10.7270/Q29G5MFD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Lck (Homo sapiens (Human)) | BDBM50301588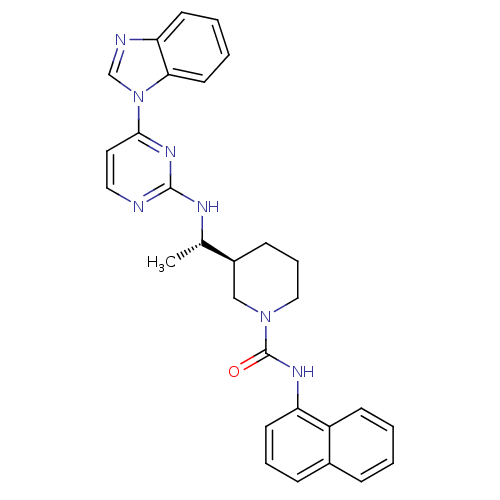 ((S)-3-((S)-1-(4-(1H-benzo[d]imidazol-1-yl)pyrimidi...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of Lck | Bioorg Med Chem Lett 19: 5440-3 (2009) Article DOI: 10.1016/j.bmcl.2009.07.102 BindingDB Entry DOI: 10.7270/Q2GX4BM0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Lck (Homo sapiens (Human)) | BDBM50194670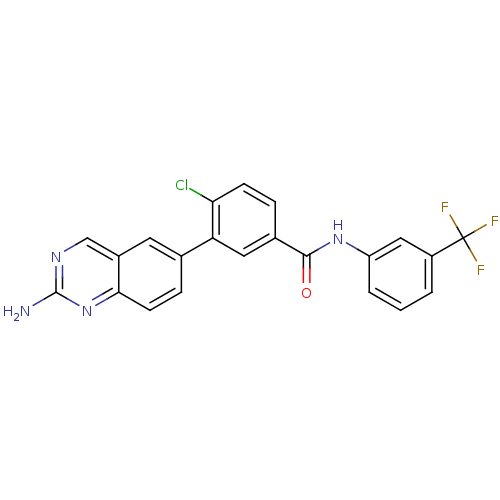 (3-(2-aminoquinazolin-6-yl)-4-chloro-N-(3-(trifluor...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen, Inc. Curated by ChEMBL | Assay Description Inhibition of Lck by HTRF kinase assay | J Med Chem 49: 5671-86 (2006) Article DOI: 10.1021/jm0605482 BindingDB Entry DOI: 10.7270/Q29G5MFD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Lck (Homo sapiens (Human)) | BDBM50305140 (7-(2-aminoethoxy)-2-(4-phenoxyphenyl)-9,10-dihydro...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.410 | n/a | n/a | n/a | n/a | n/a | n/a |
Taisho Pharmaceutical Co., Ltd Curated by ChEMBL | Assay Description Inhibition of GST-tagged human LCK by scintillation counting | Bioorg Med Chem Lett 20: 112-6 (2010) Article DOI: 10.1016/j.bmcl.2009.11.013 BindingDB Entry DOI: 10.7270/Q22Z15NV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Lck (Homo sapiens (Human)) | BDBM50305119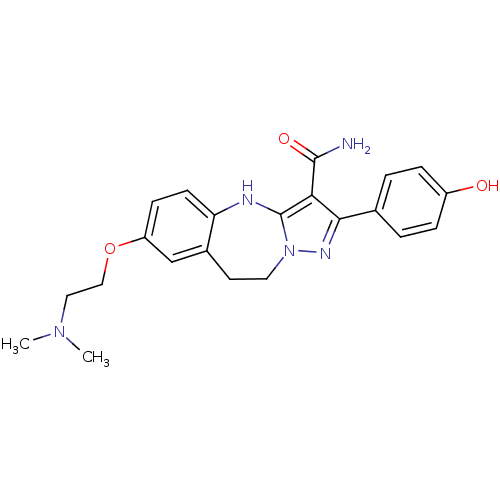 (7-(2-(dimethylamino)ethoxy)-2-(4-hydroxyphenyl)-9,...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.440 | n/a | n/a | n/a | n/a | n/a | n/a |
Taisho Pharmaceutical Co., Ltd Curated by ChEMBL | Assay Description Inhibition of GST-tagged human LCK by scintillation counting | Bioorg Med Chem Lett 20: 112-6 (2010) Article DOI: 10.1016/j.bmcl.2009.11.013 BindingDB Entry DOI: 10.7270/Q22Z15NV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Lck (Homo sapiens (Human)) | BDBM50301608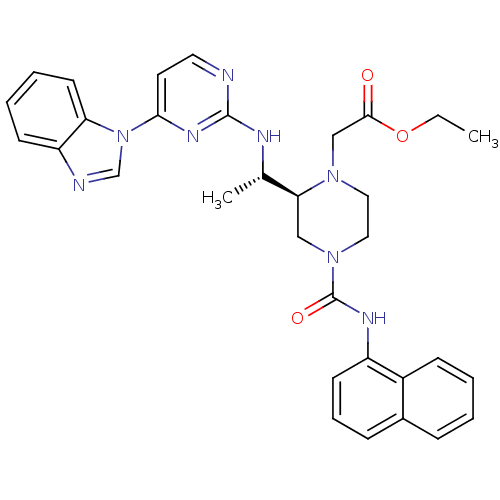 (CHEMBL566507 | ethyl 2-((S)-2-((S)-1-(4-(1H-benzo[...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.450 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of Lck | Bioorg Med Chem Lett 19: 5440-3 (2009) Article DOI: 10.1016/j.bmcl.2009.07.102 BindingDB Entry DOI: 10.7270/Q2GX4BM0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Lck (Homo sapiens (Human)) | BDBM13357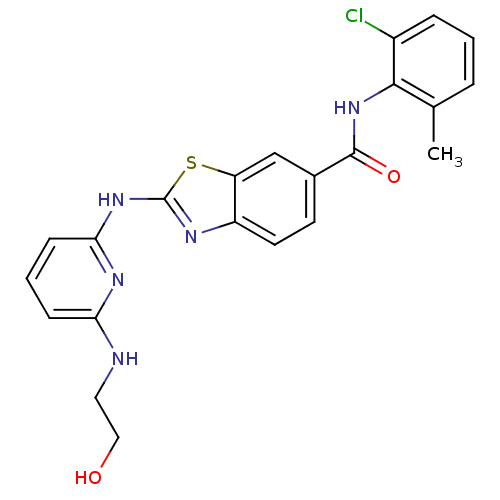 (CHEMBL312933 | N-(2-chloro-6-methylphenyl)-2-({6-[...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of p56 Lck tyrosine kinase | Bioorg Med Chem Lett 13: 2587-90 (2003) BindingDB Entry DOI: 10.7270/Q2FX78V4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Lck (Homo sapiens (Human)) | BDBM13273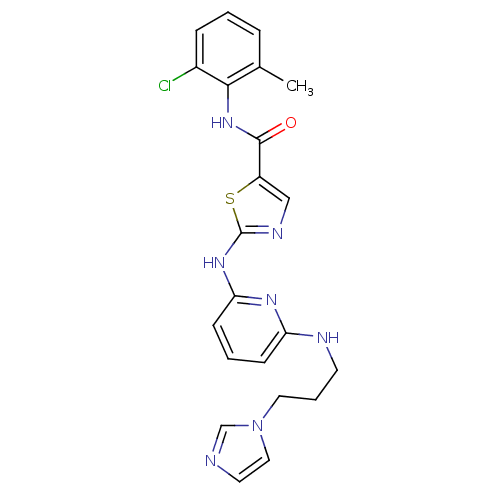 (2-(6-(3-(1H-Imidazol-1-yl)propylamino)pyridin-2-yl...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | <0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company | Assay Description IC50 is the inhibitor concentration, which inhibits 50% of kinase activity that catalyzes the transfer of the terminal phosphate from [gamma-33P] lab... | J Med Chem 49: 6819-32 (2006) Article DOI: 10.1021/jm060727j BindingDB Entry DOI: 10.7270/Q2QN6501 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Lck (Homo sapiens (Human)) | BDBM50194683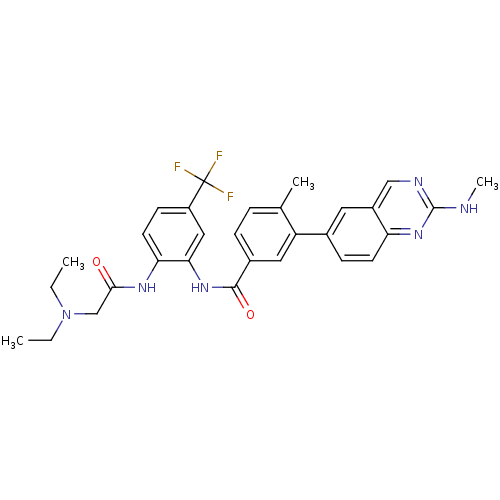 (CHEMBL212953 | N-(2-(2-(diethylamino)acetamido)-5-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen, Inc. Curated by ChEMBL | Assay Description Inhibition of Lck by HTRF kinase assay | J Med Chem 49: 5671-86 (2006) Article DOI: 10.1021/jm0605482 BindingDB Entry DOI: 10.7270/Q29G5MFD | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Tyrosine-protein kinase Lck (Homo sapiens (Human)) | BDBM50194671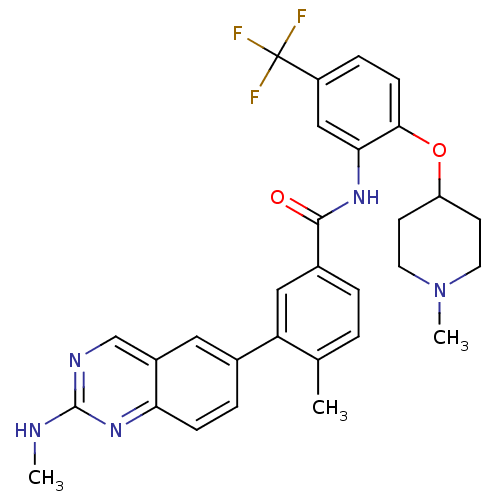 (4-methyl-3-(2-(methylamino)quinazolin-6-yl)-N-(2-(...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen, Inc. Curated by ChEMBL | Assay Description Inhibition of Lck by HTRF kinase assay | J Med Chem 49: 5671-86 (2006) Article DOI: 10.1021/jm0605482 BindingDB Entry DOI: 10.7270/Q29G5MFD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Lck (Homo sapiens (Human)) | BDBM50194681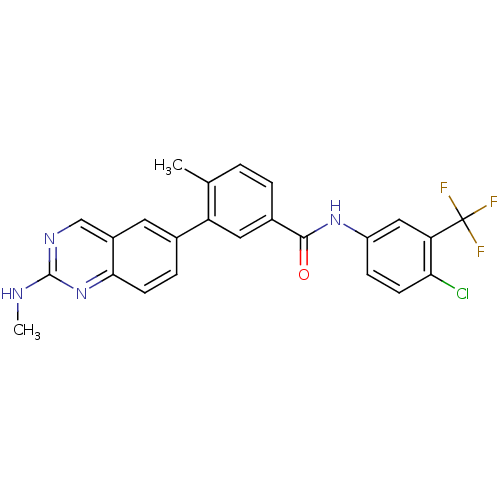 (CHEMBL215019 | N-(4-chloro-3-(trifluoromethyl)phen...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen, Inc. Curated by ChEMBL | Assay Description Inhibition of Lck by HTRF kinase assay | J Med Chem 49: 5671-86 (2006) Article DOI: 10.1021/jm0605482 BindingDB Entry DOI: 10.7270/Q29G5MFD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Lck (Homo sapiens (Human)) | BDBM13266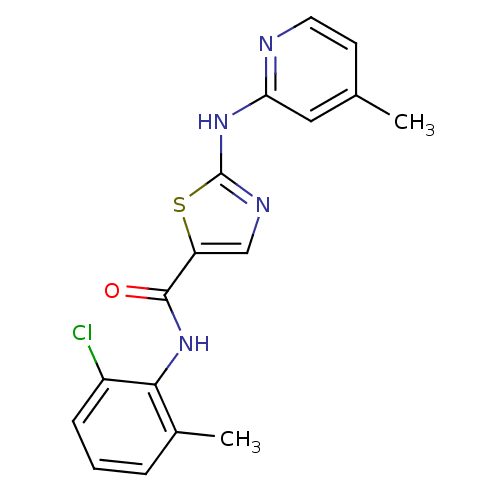 (BMS-354825 2-Heteroarylamino-thiazole Analog 12k |...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company | Assay Description IC50 is the inhibitor concentration, which inhibits 50% of kinase activity that catalyzes the transfer of the terminal phosphate from [gamma-33P] lab... | J Med Chem 49: 6819-32 (2006) Article DOI: 10.1021/jm060727j BindingDB Entry DOI: 10.7270/Q2QN6501 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Lck (Homo sapiens (Human)) | BDBM13272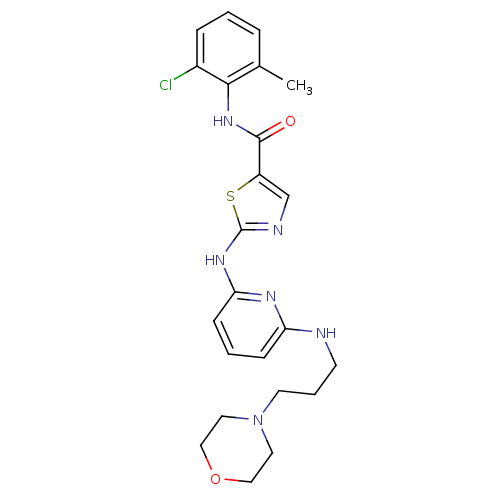 (BMS-354825 2-Heteroarylamino-thiazole Analog 12q |...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company | Assay Description IC50 is the inhibitor concentration, which inhibits 50% of kinase activity that catalyzes the transfer of the terminal phosphate from [gamma-33P] lab... | J Med Chem 49: 6819-32 (2006) Article DOI: 10.1021/jm060727j BindingDB Entry DOI: 10.7270/Q2QN6501 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Lck (Homo sapiens (Human)) | BDBM50194690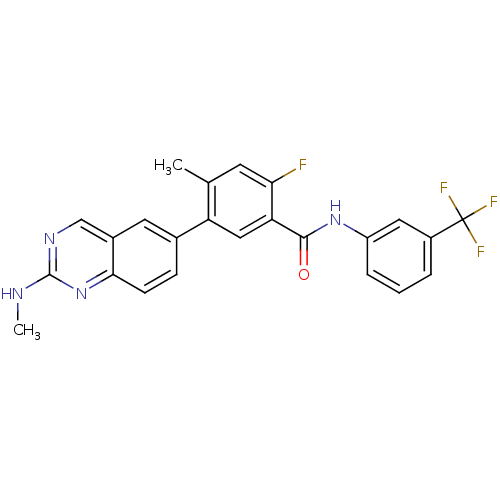 (2-fluoro-4-methyl-5-(2-(methylamino)quinazolin-6-y...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen, Inc. Curated by ChEMBL | Assay Description Inhibition of Lck by HTRF kinase assay | J Med Chem 49: 5671-86 (2006) Article DOI: 10.1021/jm0605482 BindingDB Entry DOI: 10.7270/Q29G5MFD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Lck (Homo sapiens (Human)) | BDBM13274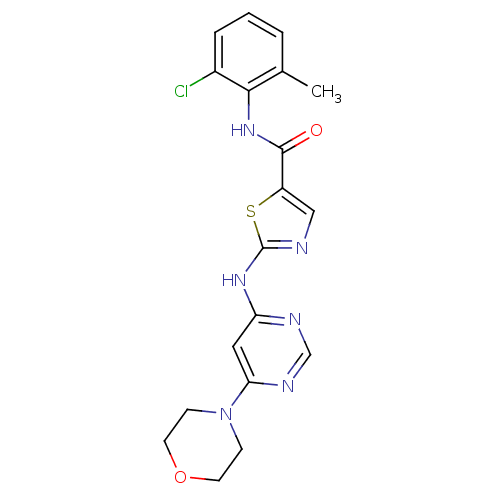 (BMS-354825 2-Heteroarylamino-thiazole Analog 12s |...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company | Assay Description IC50 is the inhibitor concentration, which inhibits 50% of kinase activity that catalyzes the transfer of the terminal phosphate from [gamma-33P] lab... | J Med Chem 49: 6819-32 (2006) Article DOI: 10.1021/jm060727j BindingDB Entry DOI: 10.7270/Q2QN6501 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Lck (Homo sapiens (Human)) | BDBM13357 (CHEMBL312933 | N-(2-chloro-6-methylphenyl)-2-({6-[...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company | Assay Description IC50 is the inhibitor concentration, which inhibits 50% of kinase activity that catalyzes the transfer of the terminal phosphate from [gamma-33P] lab... | J Med Chem 49: 6819-32 (2006) Article DOI: 10.1021/jm060727j BindingDB Entry DOI: 10.7270/Q2QN6501 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Lck (Homo sapiens (Human)) | BDBM26366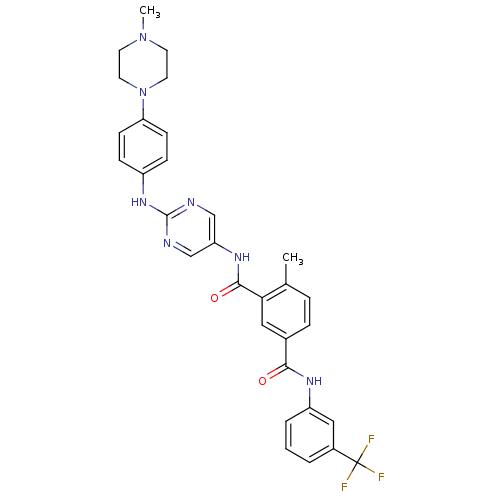 (4-methyl-3-N-(2-{[4-(4-methylpiperazin-1-yl)phenyl...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | 7.5 | 22 |
Amgen | Assay Description The assay involves the phosphorylation of a biotinylated substrate and the detection of this phosphorylation after the addition of a streptavidin-all... | J Med Chem 51: 1681-94 (2008) Article DOI: 10.1021/jm7010996 BindingDB Entry DOI: 10.7270/Q2Q81BCB | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Tyrosine-protein kinase Lck (Homo sapiens (Human)) | BDBM50126732 (2-(2,6-Dichloro-phenylamino)-7-(3-diethylamino-pro...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of p56 Lck tyrosine kinase | J Med Chem 46: 1337-49 (2003) Article DOI: 10.1021/jm020446l BindingDB Entry DOI: 10.7270/Q2DN44FW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Lck (Homo sapiens (Human)) | BDBM139540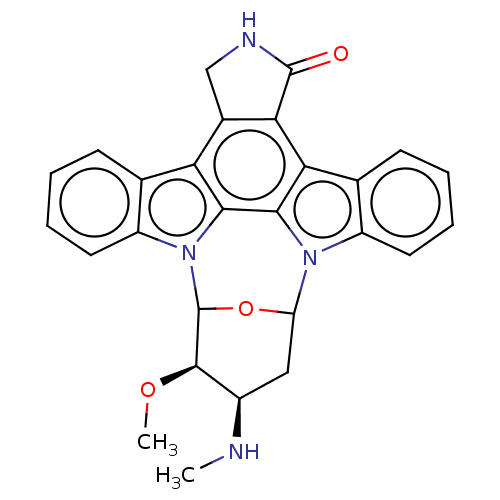 (US10189849, staurosporine | US10307427, Staurospor...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | <0.508 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Table 8-10: Compounds were tested in 10-dose IC50 mode with 3-fold serial dilution starting at 10 uM, and are relative to DMSO, the negative control.... | Citation and Details BindingDB Entry DOI: 10.7270/Q2154MW8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Lck (Homo sapiens (Human)) | BDBM538621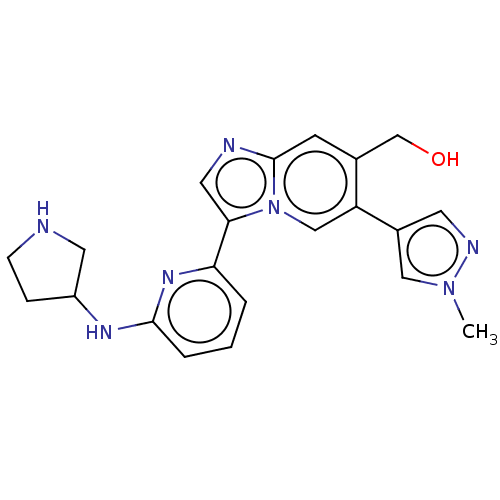 (NCGC 00371481 | US11254667, Compound I-24 | US1154...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <0.508 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Table 8-10: Compounds were tested in 10-dose IC50 mode with 3-fold serial dilution starting at 10 uM, and are relative to DMSO, the negative control.... | Citation and Details BindingDB Entry DOI: 10.7270/Q2154MW8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 2847 total ) | Next | Last >> |