 Found 882 hits of ki for UniProtKB: P43235
Found 882 hits of ki for UniProtKB: P43235 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kcal/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Cathepsin K
(Homo sapiens (Human)) | BDBM50167296
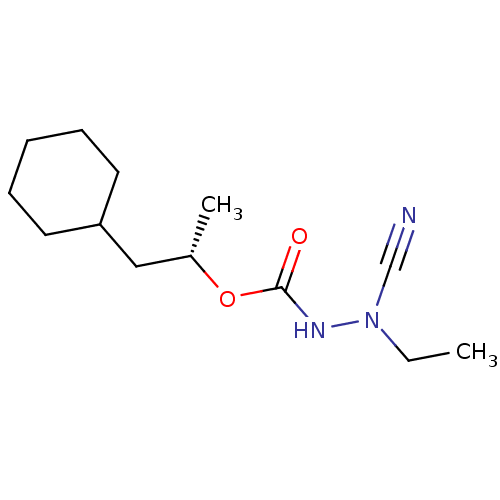
((1S)-2-cyclohexyl-1-methylethyl 2-cyano-2-ethylhyd...)Show InChI InChI=1S/C13H23N3O2/c1-3-16(10-14)15-13(17)18-11(2)9-12-7-5-4-6-8-12/h11-12H,3-9H2,1-2H3,(H,15,17)/t11-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.00300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition constant against human cathepsin K in fluorescence assay using Cbz-Phe-Arg-AMC |
Bioorg Med Chem Lett 15: 3039-43 (2005)
Article DOI: 10.1016/j.bmcl.2005.04.032
BindingDB Entry DOI: 10.7270/Q2MS3S8C |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Homo sapiens (Human)) | BDBM50167295
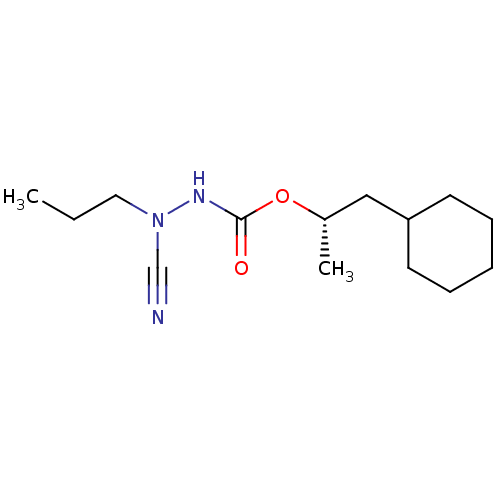
((1S)-2-cyclohexyl-1-methylethyl 2-cyano-2-propylhy...)Show InChI InChI=1S/C14H25N3O2/c1-3-9-17(11-15)16-14(18)19-12(2)10-13-7-5-4-6-8-13/h12-13H,3-10H2,1-2H3,(H,16,18)/t12-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.00400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition constant against human cathepsin K in fluorescence assay using Cbz-Phe-Arg-AMC |
Bioorg Med Chem Lett 15: 3039-43 (2005)
Article DOI: 10.1016/j.bmcl.2005.04.032
BindingDB Entry DOI: 10.7270/Q2MS3S8C |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Homo sapiens (Human)) | BDBM50167302

((1S)-2-cyclohexyl-1-methylethyl 2-cyano-2-isobutyl...)Show InChI InChI=1S/C15H27N3O2/c1-12(2)10-18(11-16)17-15(19)20-13(3)9-14-7-5-4-6-8-14/h12-14H,4-10H2,1-3H3,(H,17,19)/t13-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.00400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition constant against human cathepsin K in fluorescence assay using Cbz-Phe-Arg-AMC |
Bioorg Med Chem Lett 15: 3039-43 (2005)
Article DOI: 10.1016/j.bmcl.2005.04.032
BindingDB Entry DOI: 10.7270/Q2MS3S8C |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Homo sapiens (Human)) | BDBM50098576
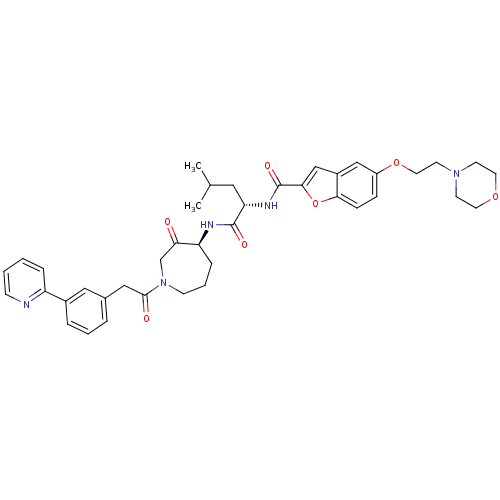
(5-(2-MORPHOLIN-4-YLETHOXY)BENZOFURAN-2-CARBOXYLIC ...)Show SMILES CC(C)C[C@H](NC(=O)c1cc2cc(OCCN3CCOCC3)ccc2o1)C(=O)N[C@H]1CCCN(CC1=O)C(=O)Cc1cccc(c1)-c1ccccn1 Show InChI InChI=1S/C40H47N5O7/c1-27(2)21-34(43-40(49)37-25-30-24-31(11-12-36(30)52-37)51-20-17-44-15-18-50-19-16-44)39(48)42-33-10-6-14-45(26-35(33)46)38(47)23-28-7-5-8-29(22-28)32-9-3-4-13-41-32/h3-5,7-9,11-13,22,24-25,27,33-34H,6,10,14-21,23,26H2,1-2H3,(H,42,48)(H,43,49)/t33-,34-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
PubMed
| 0.00480 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of Human cathepsin K |
J Med Chem 44: 1380-95 (2001)
BindingDB Entry DOI: 10.7270/Q2QR4WDC |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Cathepsin K
(Homo sapiens (Human)) | BDBM50167298
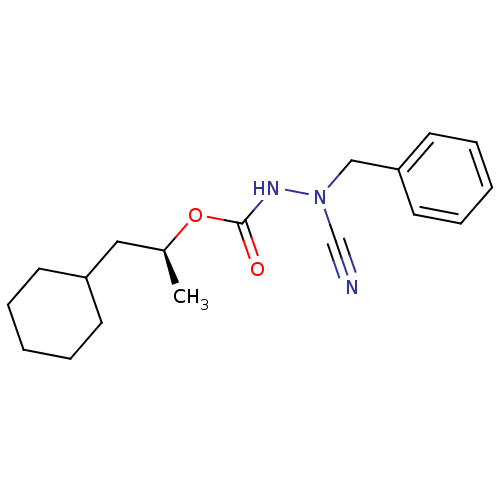
((1S)-2-cyclohexyl-1-methylethyl 2-benzyl-2-cyanohy...)Show InChI InChI=1S/C18H25N3O2/c1-15(12-16-8-4-2-5-9-16)23-18(22)20-21(14-19)13-17-10-6-3-7-11-17/h3,6-7,10-11,15-16H,2,4-5,8-9,12-13H2,1H3,(H,20,22)/t15-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 0.00600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition constant against human cathepsin K in fluorescence assay using Cbz-Phe-Arg-AMC |
Bioorg Med Chem Lett 15: 3039-43 (2005)
Article DOI: 10.1016/j.bmcl.2005.04.032
BindingDB Entry DOI: 10.7270/Q2MS3S8C |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Homo sapiens (Human)) | BDBM50167303
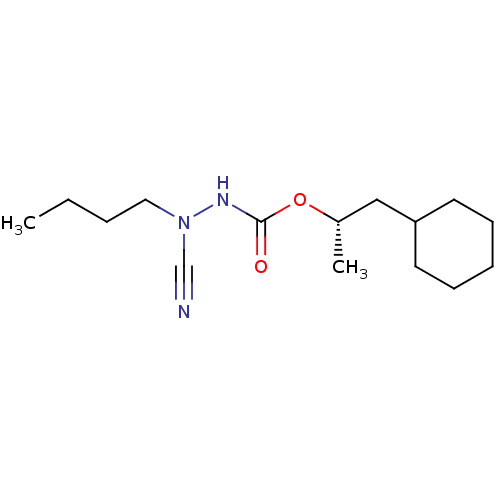
((1S)-2-cyclohexyl-1-methylethyl 2-butyl-2-cyanohyd...)Show InChI InChI=1S/C15H27N3O2/c1-3-4-10-18(12-16)17-15(19)20-13(2)11-14-8-6-5-7-9-14/h13-14H,3-11H2,1-2H3,(H,17,19)/t13-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.00700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition constant against human cathepsin K in fluorescence assay using Cbz-Phe-Arg-AMC |
Bioorg Med Chem Lett 15: 3039-43 (2005)
Article DOI: 10.1016/j.bmcl.2005.04.032
BindingDB Entry DOI: 10.7270/Q2MS3S8C |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Homo sapiens (Human)) | BDBM50167289
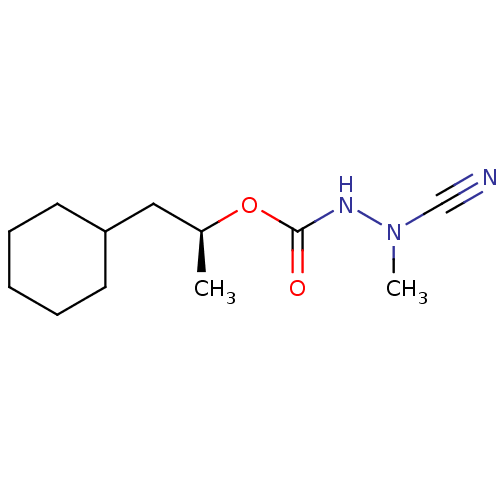
((1S)-2-cyclohexyl-1-methylethyl 2-cyano-2-methylhy...)Show InChI InChI=1S/C12H21N3O2/c1-10(8-11-6-4-3-5-7-11)17-12(16)14-15(2)9-13/h10-11H,3-8H2,1-2H3,(H,14,16)/t10-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.00900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition constant against human cathepsin K in fluorescence assay using Cbz-Phe-Arg-AMC |
Bioorg Med Chem Lett 15: 3039-43 (2005)
Article DOI: 10.1016/j.bmcl.2005.04.032
BindingDB Entry DOI: 10.7270/Q2MS3S8C |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Homo sapiens (Human)) | BDBM19770
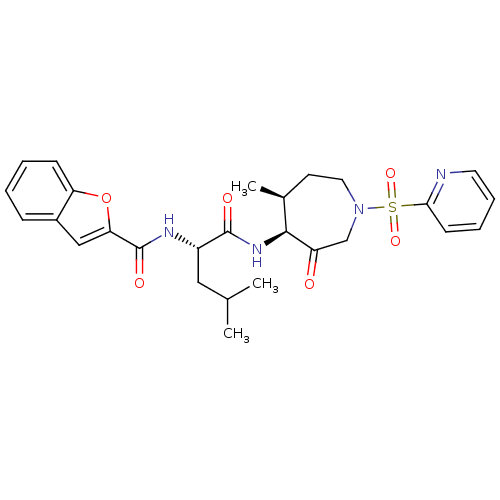
((2S)-2-(1-benzofuran-2-ylformamido)-4-methyl-N-[(4...)Show SMILES CC(C)C[C@H](NC(=O)c1cc2ccccc2o1)C(=O)N[C@H]1[C@@H](C)CCN(CC1=O)S(=O)(=O)c1ccccn1 |r| Show InChI InChI=1S/C27H32N4O6S/c1-17(2)14-20(29-27(34)23-15-19-8-4-5-9-22(19)37-23)26(33)30-25-18(3)11-13-31(16-21(25)32)38(35,36)24-10-6-7-12-28-24/h4-10,12,15,17-18,20,25H,11,13-14,16H2,1-3H3,(H,29,34)(H,30,33)/t18-,20-,25-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.00990 | -14.9 | n/a | n/a | n/a | n/a | n/a | 5.5 | 22 |
GSK
| Assay Description
Potential inhibitors were evaluated using the progress curve method. Assays were carried out in the presence of variable concentrations of test compo... |
J Med Chem 49: 1597-612 (2006)
Article DOI: 10.1021/jm050915u
BindingDB Entry DOI: 10.7270/Q2SQ8XP5 |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Cathepsin K
(Homo sapiens (Human)) | BDBM50335280

(CHEMBL1651354 | N-(Benzylcarbamoyl)-leucyl-methyla...)Show SMILES CC(C)C[C@H](NC(=O)NCc1ccccc1)C(=O)N(C)N(C)C#N |r| Show InChI InChI=1S/C17H25N5O2/c1-13(2)10-15(16(23)22(4)21(3)12-18)20-17(24)19-11-14-8-6-5-7-9-14/h5-9,13,15H,10-11H2,1-4H3,(H2,19,20,24)/t15-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 0.0110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bonn
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant cathepsin K after 30 mins by spectrophotometric assay |
J Med Chem 54: 396-400 (2011)
Article DOI: 10.1021/jm101272p
BindingDB Entry DOI: 10.7270/Q2PV6MC3 |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Homo sapiens (Human)) | BDBM50335281

(CHEMBL1651355 | N-(Phenylcarbamoyl)-leucyl-methyla...)Show SMILES CC(C)C[C@H](NC(=O)Nc1ccccc1)C(=O)N(C)N(C)C#N |r| Show InChI InChI=1S/C16H23N5O2/c1-12(2)10-14(15(22)21(4)20(3)11-17)19-16(23)18-13-8-6-5-7-9-13/h5-9,12,14H,10H2,1-4H3,(H2,18,19,23)/t14-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 0.0170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bonn
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant cathepsin K after 30 mins by spectrophotometric assay |
J Med Chem 54: 396-400 (2011)
Article DOI: 10.1021/jm101272p
BindingDB Entry DOI: 10.7270/Q2PV6MC3 |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Homo sapiens (Human)) | BDBM50167290
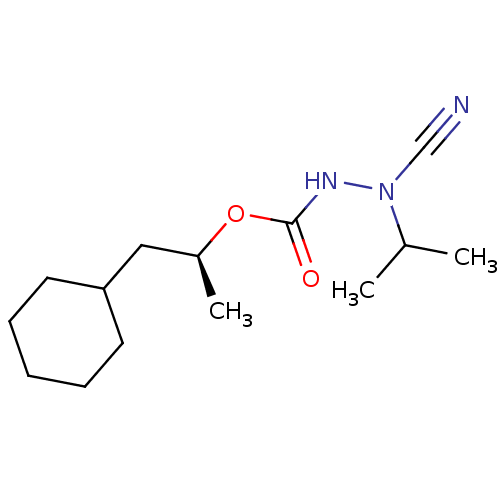
((1S)-2-cyclohexyl-1-methylethyl 2-cyano-2-isopropy...)Show InChI InChI=1S/C14H25N3O2/c1-11(2)17(10-15)16-14(18)19-12(3)9-13-7-5-4-6-8-13/h11-13H,4-9H2,1-3H3,(H,16,18)/t12-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0190 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition constant against human cathepsin K in fluorescence assay using Cbz-Phe-Arg-AMC |
Bioorg Med Chem Lett 15: 3039-43 (2005)
Article DOI: 10.1016/j.bmcl.2005.04.032
BindingDB Entry DOI: 10.7270/Q2MS3S8C |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Homo sapiens (Human)) | BDBM50335285
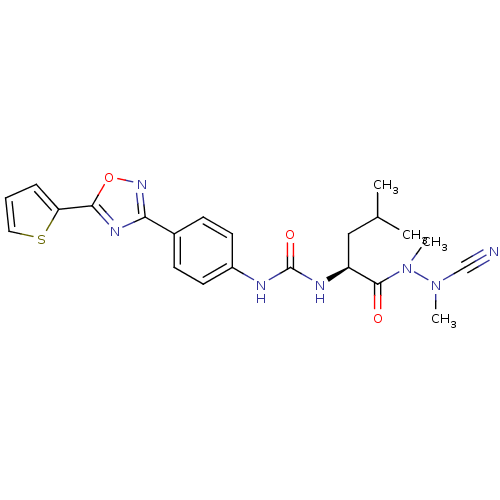
(CHEMBL1651350 | N-{4-[5-(2-Thienyl)-1,2,4-oxadiazo...)Show SMILES CC(C)C[C@H](NC(=O)Nc1ccc(cc1)-c1noc(n1)-c1cccs1)C(=O)N(C)N(C)C#N |r| Show InChI InChI=1S/C22H25N7O3S/c1-14(2)12-17(21(30)29(4)28(3)13-23)25-22(31)24-16-9-7-15(8-10-16)19-26-20(32-27-19)18-6-5-11-33-18/h5-11,14,17H,12H2,1-4H3,(H2,24,25,31)/t17-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0220 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bonn
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant cathepsin K after 30 mins by spectrophotometric assay |
J Med Chem 54: 396-400 (2011)
Article DOI: 10.1021/jm101272p
BindingDB Entry DOI: 10.7270/Q2PV6MC3 |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Homo sapiens (Human)) | BDBM50167288

((1S)-1-benzylpropyl 2-cyano-2-methylhydrazinecarbo...)Show InChI InChI=1S/C13H17N3O2/c1-3-12(9-11-7-5-4-6-8-11)18-13(17)15-16(2)10-14/h4-8,12H,3,9H2,1-2H3,(H,15,17)/t12-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 0.0320 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition constant against human cathepsin K in fluorescence assay using Cbz-Phe-Arg-AMC |
Bioorg Med Chem Lett 15: 3039-43 (2005)
Article DOI: 10.1016/j.bmcl.2005.04.032
BindingDB Entry DOI: 10.7270/Q2MS3S8C |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Homo sapiens (Human)) | BDBM50335289
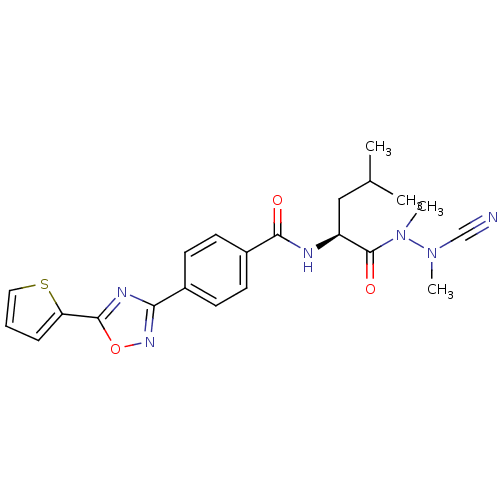
(CHEMBL1651361 | N-{4-[5-(2-Thienyl)-1,2,4-oxadiazo...)Show SMILES CC(C)C[C@H](NC(=O)c1ccc(cc1)-c1noc(n1)-c1cccs1)C(=O)N(C)N(C)C#N |r| Show InChI InChI=1S/C22H24N6O3S/c1-14(2)12-17(22(30)28(4)27(3)13-23)24-20(29)16-9-7-15(8-10-16)19-25-21(31-26-19)18-6-5-11-32-18/h5-11,14,17H,12H2,1-4H3,(H,24,29)/t17-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0320 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bonn
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant cathepsin K after 30 mins by spectrophotometric assay |
J Med Chem 54: 396-400 (2011)
Article DOI: 10.1021/jm101272p
BindingDB Entry DOI: 10.7270/Q2PV6MC3 |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Homo sapiens (Human)) | BDBM19778

((2S)-2-(1-benzofuran-2-ylformamido)-4-methyl-N-[(4...)Show SMILES CC(C)C[C@H](NC(=O)c1cc2ccccc2o1)C(=O)N[C@H]1CC[C@@H](C)N(CC1=O)S(=O)(=O)c1ccccn1 |r| Show InChI InChI=1S/C27H32N4O6S/c1-17(2)14-21(30-27(34)24-15-19-8-4-5-9-23(19)37-24)26(33)29-20-12-11-18(3)31(16-22(20)32)38(35,36)25-10-6-7-13-28-25/h4-10,13,15,17-18,20-21H,11-12,14,16H2,1-3H3,(H,29,33)(H,30,34)/t18-,20+,21+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.0410 | -14.0 | n/a | n/a | n/a | n/a | n/a | 5.5 | 22 |
GSK
| Assay Description
Potential inhibitors were evaluated using the progress curve method. Assays were carried out in the presence of variable concentrations of test compo... |
J Med Chem 49: 1597-612 (2006)
Article DOI: 10.1021/jm050915u
BindingDB Entry DOI: 10.7270/Q2SQ8XP5 |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Cathepsin K
(Homo sapiens (Human)) | BDBM50335284
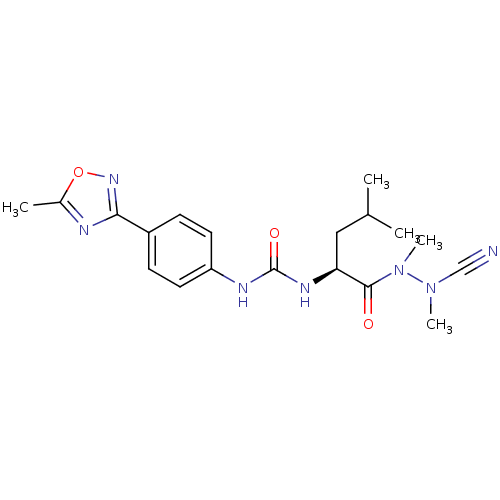
(CHEMBL1651349 | N-[4-(5-Methyl-1,2,4-oxadiazol-3-y...)Show SMILES CC(C)C[C@H](NC(=O)Nc1ccc(cc1)-c1noc(C)n1)C(=O)N(C)N(C)C#N |r| Show InChI InChI=1S/C19H25N7O3/c1-12(2)10-16(18(27)26(5)25(4)11-20)23-19(28)22-15-8-6-14(7-9-15)17-21-13(3)29-24-17/h6-9,12,16H,10H2,1-5H3,(H2,22,23,28)/t16-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 0.0450 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bonn
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant cathepsin K after 30 mins by spectrophotometric assay |
J Med Chem 54: 396-400 (2011)
Article DOI: 10.1021/jm101272p
BindingDB Entry DOI: 10.7270/Q2PV6MC3 |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Homo sapiens (Human)) | BDBM50304794

((S)-benzyl 1-(2-cyano-1,2-dimethylhydrazinyl)-4-me...)Show SMILES CC(C)C[C@H](NC(=O)OCc1ccccc1)C(=O)N(C)N(C)C#N |r| Show InChI InChI=1S/C17H24N4O3/c1-13(2)10-15(16(22)21(4)20(3)12-18)19-17(23)24-11-14-8-6-5-7-9-14/h5-9,13,15H,10-11H2,1-4H3,(H,19,23)/t15-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0640 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bonn
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant cathepsin K after 30 mins by spectrophotometric assay |
J Med Chem 54: 396-400 (2011)
Article DOI: 10.1021/jm101272p
BindingDB Entry DOI: 10.7270/Q2PV6MC3 |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Homo sapiens (Human)) | BDBM50335278

(CHEMBL1651352 | N-(Benzyloxycarbonyl)-cyclohexylal...)Show SMILES CN(C#N)N(C)C(=O)[C@H](CC1CCCCC1)NC(=O)OCc1ccccc1 |r| Show InChI InChI=1S/C20H28N4O3/c1-23(15-21)24(2)19(25)18(13-16-9-5-3-6-10-16)22-20(26)27-14-17-11-7-4-8-12-17/h4,7-8,11-12,16,18H,3,5-6,9-10,13-14H2,1-2H3,(H,22,26)/t18-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0710 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bonn
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant cathepsin K after 30 mins by spectrophotometric assay |
J Med Chem 54: 396-400 (2011)
Article DOI: 10.1021/jm101272p
BindingDB Entry DOI: 10.7270/Q2PV6MC3 |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Homo sapiens (Human)) | BDBM50335283

(CHEMBL1651357 | N-{4-[5-(2-Thienyl)-1,2,4-oxadiazo...)Show SMILES CC(C)C[C@H](NC(=O)NCc1ccc(cc1)-c1noc(n1)-c1cccs1)C(=O)N(C)N(C)C#N |r| Show InChI InChI=1S/C23H27N7O3S/c1-15(2)12-18(22(31)30(4)29(3)14-24)26-23(32)25-13-16-7-9-17(10-8-16)20-27-21(33-28-20)19-6-5-11-34-19/h5-11,15,18H,12-13H2,1-4H3,(H2,25,26,32)/t18-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0720 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bonn
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant cathepsin K after 30 mins by spectrophotometric assay |
J Med Chem 54: 396-400 (2011)
Article DOI: 10.1021/jm101272p
BindingDB Entry DOI: 10.7270/Q2PV6MC3 |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Homo sapiens (Human)) | BDBM50335282
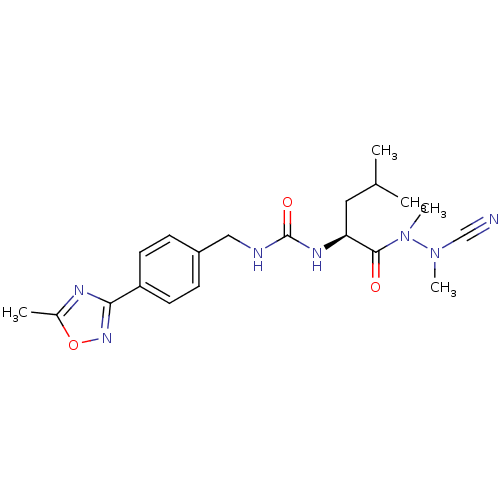
(CHEMBL1651356 | N-[4-(5-Methyl-1,2,4-oxadiazol-3-y...)Show SMILES CC(C)C[C@H](NC(=O)NCc1ccc(cc1)-c1noc(C)n1)C(=O)N(C)N(C)C#N |r| Show InChI InChI=1S/C20H27N7O3/c1-13(2)10-17(19(28)27(5)26(4)12-21)24-20(29)22-11-15-6-8-16(9-7-15)18-23-14(3)30-25-18/h6-9,13,17H,10-11H2,1-5H3,(H2,22,24,29)/t17-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 0.110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bonn
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant cathepsin K after 30 mins by spectrophotometric assay |
J Med Chem 54: 396-400 (2011)
Article DOI: 10.1021/jm101272p
BindingDB Entry DOI: 10.7270/Q2PV6MC3 |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Homo sapiens (Human)) | BDBM50304793
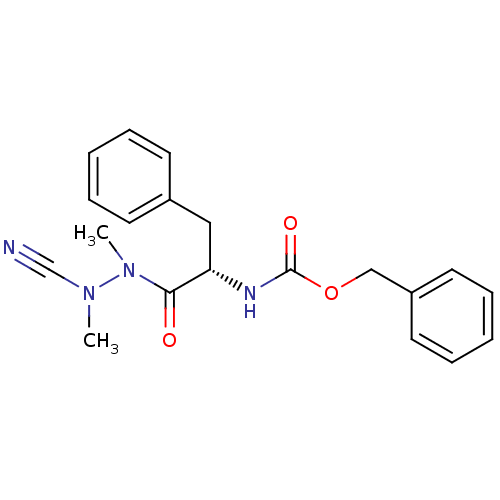
((S)-benzyl 1-(2-cyano-1,2-dimethylhydrazinyl)-1-ox...)Show SMILES CN(C#N)N(C)C(=O)[C@H](Cc1ccccc1)NC(=O)OCc1ccccc1 |r| Show InChI InChI=1S/C20H22N4O3/c1-23(15-21)24(2)19(25)18(13-16-9-5-3-6-10-16)22-20(26)27-14-17-11-7-4-8-12-17/h3-12,18H,13-14H2,1-2H3,(H,22,26)/t18-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bonn
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant cathepsin K after 30 mins by spectrophotometric assay |
J Med Chem 54: 396-400 (2011)
Article DOI: 10.1021/jm101272p
BindingDB Entry DOI: 10.7270/Q2PV6MC3 |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Homo sapiens (Human)) | BDBM19775
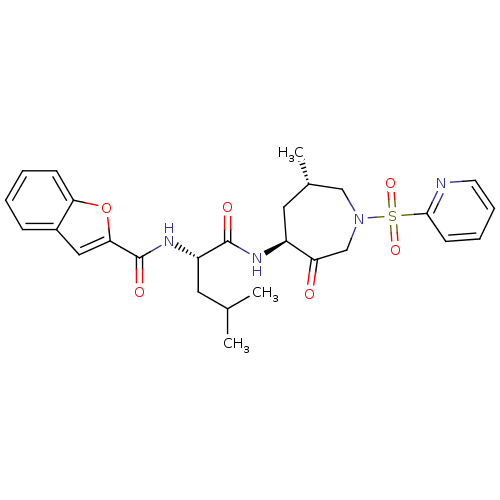
((2S)-2-(1-benzofuran-2-ylformamido)-4-methyl-N-[(4...)Show SMILES CC(C)C[C@H](NC(=O)c1cc2ccccc2o1)C(=O)N[C@H]1C[C@H](C)CN(CC1=O)S(=O)(=O)c1ccccn1 |r| Show InChI InChI=1S/C27H32N4O6S/c1-17(2)12-21(30-27(34)24-14-19-8-4-5-9-23(19)37-24)26(33)29-20-13-18(3)15-31(16-22(20)32)38(35,36)25-10-6-7-11-28-25/h4-11,14,17-18,20-21H,12-13,15-16H2,1-3H3,(H,29,33)(H,30,34)/t18-,20-,21-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.140 | -13.3 | n/a | n/a | n/a | n/a | n/a | 5.5 | 22 |
GSK
| Assay Description
Potential inhibitors were evaluated using the progress curve method. Assays were carried out in the presence of variable concentrations of test compo... |
J Med Chem 49: 1597-612 (2006)
Article DOI: 10.1021/jm050915u
BindingDB Entry DOI: 10.7270/Q2SQ8XP5 |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Cathepsin K
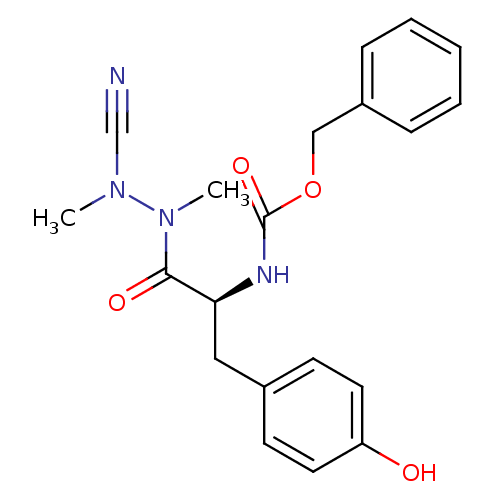
(Homo sapiens (Human)) | BDBM50335279
(CHEMBL1651353 | N-(Benzyloxycarbonyl)-tyrosyl-meth...)Show SMILES CN(C#N)N(C)C(=O)[C@H](Cc1ccc(O)cc1)NC(=O)OCc1ccccc1 |r| Show InChI InChI=1S/C20H22N4O4/c1-23(14-21)24(2)19(26)18(12-15-8-10-17(25)11-9-15)22-20(27)28-13-16-6-4-3-5-7-16/h3-11,18,25H,12-13H2,1-2H3,(H,22,27)/t18-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bonn
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant cathepsin K after 30 mins by spectrophotometric assay |
J Med Chem 54: 396-400 (2011)
Article DOI: 10.1021/jm101272p
BindingDB Entry DOI: 10.7270/Q2PV6MC3 |
More data for this
Ligand-Target Pair | |
Cathepsin K
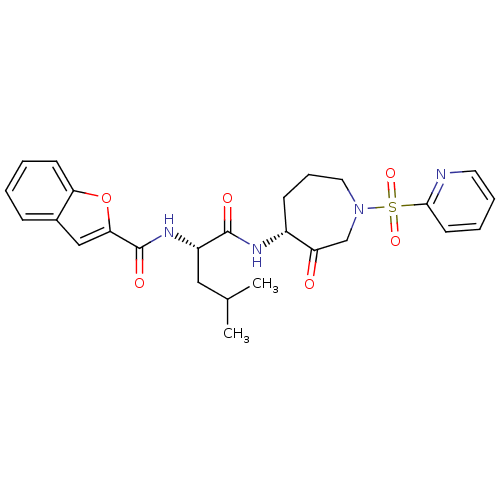
(Homo sapiens (Human)) | BDBM50098577
(Benzofuran-2-carboxylic acid {3-methyl-1-[3-oxo-1-...)Show SMILES CC(C)C[C@H](NC(=O)c1cc2ccccc2o1)C(=O)N[C@@H]1CCCN(CC1=O)S(=O)(=O)c1ccccn1 Show InChI InChI=1S/C26H30N4O6S/c1-17(2)14-20(29-26(33)23-15-18-8-3-4-10-22(18)36-23)25(32)28-19-9-7-13-30(16-21(19)31)37(34,35)24-11-5-6-12-27-24/h3-6,8,10-12,15,17,19-20H,7,9,13-14,16H2,1-2H3,(H,28,32)(H,29,33)/t19-,20+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
MSD Animal Health Innovation GmbH
Curated by ChEMBL
| Assay Description
Inhibition of human cathepsin K |
J Med Chem 56: 1478-90 (2013)
Article DOI: 10.1021/jm3013932
BindingDB Entry DOI: 10.7270/Q2W09774 |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Cathepsin K
(Homo sapiens (Human)) | BDBM19769

((2S)-2-(1-benzofuran-2-ylformamido)-4-methyl-N-[(4...)Show SMILES CC(C)C[C@H](NC(=O)c1cc2ccccc2o1)C(=O)N[C@H]1CCCN(CC1=O)S(=O)(=O)c1ccccn1 |r| Show InChI InChI=1S/C26H30N4O6S/c1-17(2)14-20(29-26(33)23-15-18-8-3-4-10-22(18)36-23)25(32)28-19-9-7-13-30(16-21(19)31)37(34,35)24-11-5-6-12-27-24/h3-6,8,10-12,15,17,19-20H,7,9,13-14,16H2,1-2H3,(H,28,32)(H,29,33)/t19-,20-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
PubMed
| 0.160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of Human cathepsin K |
J Med Chem 44: 1380-95 (2001)
BindingDB Entry DOI: 10.7270/Q2QR4WDC |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Cathepsin K
(Homo sapiens (Human)) | BDBM19769

((2S)-2-(1-benzofuran-2-ylformamido)-4-methyl-N-[(4...)Show SMILES CC(C)C[C@H](NC(=O)c1cc2ccccc2o1)C(=O)N[C@H]1CCCN(CC1=O)S(=O)(=O)c1ccccn1 |r| Show InChI InChI=1S/C26H30N4O6S/c1-17(2)14-20(29-26(33)23-15-18-8-3-4-10-22(18)36-23)25(32)28-19-9-7-13-30(16-21(19)31)37(34,35)24-11-5-6-12-27-24/h3-6,8,10-12,15,17,19-20H,7,9,13-14,16H2,1-2H3,(H,28,32)(H,29,33)/t19-,20-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 0.160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Apparent inhibitory constant against human cathepsin K |
J Med Chem 48: 6870-8 (2005)
Article DOI: 10.1021/jm0502079
BindingDB Entry DOI: 10.7270/Q2WM1CZC |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Cathepsin K
(Homo sapiens (Human)) | BDBM19769

((2S)-2-(1-benzofuran-2-ylformamido)-4-methyl-N-[(4...)Show SMILES CC(C)C[C@H](NC(=O)c1cc2ccccc2o1)C(=O)N[C@H]1CCCN(CC1=O)S(=O)(=O)c1ccccn1 |r| Show InChI InChI=1S/C26H30N4O6S/c1-17(2)14-20(29-26(33)23-15-18-8-3-4-10-22(18)36-23)25(32)28-19-9-7-13-30(16-21(19)31)37(34,35)24-11-5-6-12-27-24/h3-6,8,10-12,15,17,19-20H,7,9,13-14,16H2,1-2H3,(H,28,32)(H,29,33)/t19-,20-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 0.160 | -13.2 | n/a | n/a | n/a | n/a | n/a | 5.5 | 22 |
GSK
| Assay Description
Potential inhibitors were evaluated using the progress curve method. Assays were carried out in the presence of variable concentrations of test compo... |
J Med Chem 49: 1597-612 (2006)
Article DOI: 10.1021/jm050915u
BindingDB Entry DOI: 10.7270/Q2SQ8XP5 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Cathepsin K
(Homo sapiens (Human)) | BDBM50255753
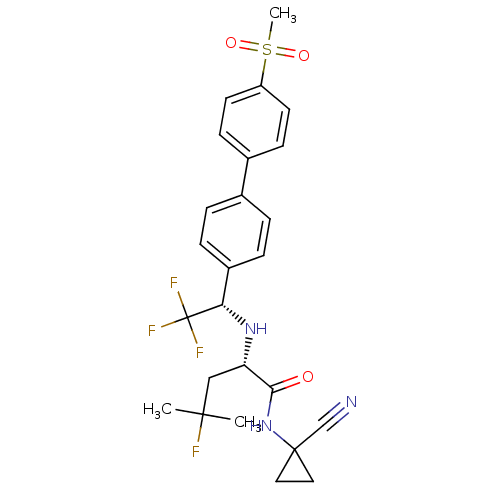
(CHEMBL481611 | MK-0822 | Odanacatib)Show SMILES CC(C)(F)C[C@H](N[C@@H](c1ccc(cc1)-c1ccc(cc1)S(C)(=O)=O)C(F)(F)F)C(=O)NC1(CC1)C#N |r| Show InChI InChI=1S/C25H27F4N3O3S/c1-23(2,26)14-20(22(33)32-24(15-30)12-13-24)31-21(25(27,28)29)18-6-4-16(5-7-18)17-8-10-19(11-9-17)36(3,34)35/h4-11,20-21,31H,12-14H2,1-3H3,(H,32,33)/t20-,21-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CNRS UPR 4301
Curated by ChEMBL
| Assay Description
Inhibition of human cathepsin K |
Eur J Med Chem 144: 201-210 (2018)
Article DOI: 10.1016/j.ejmech.2017.12.012
BindingDB Entry DOI: 10.7270/Q2MP55ZM |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Homo sapiens (Human)) | BDBM50410979
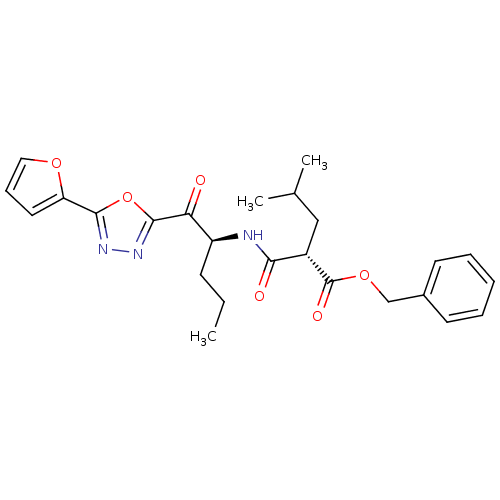
(CHEMBL207347)Show SMILES CCC[C@H](NC(=O)[C@H](CC(C)C)C(=O)OCc1ccccc1)C(=O)c1nnc(o1)-c1ccco1 Show InChI InChI=1S/C25H29N3O6/c1-4-9-19(21(29)24-28-27-23(34-24)20-12-8-13-32-20)26-22(30)18(14-16(2)3)25(31)33-15-17-10-6-5-7-11-17/h5-8,10-13,16,18-19H,4,9,14-15H2,1-3H3,(H,26,30)/t18-,19-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| <0.25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera Genomics, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human cathepsin K |
Bioorg Med Chem Lett 16: 2909-14 (2006)
Article DOI: 10.1016/j.bmcl.2006.03.001
BindingDB Entry DOI: 10.7270/Q2QN6808 |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Homo sapiens (Human)) | BDBM50410971
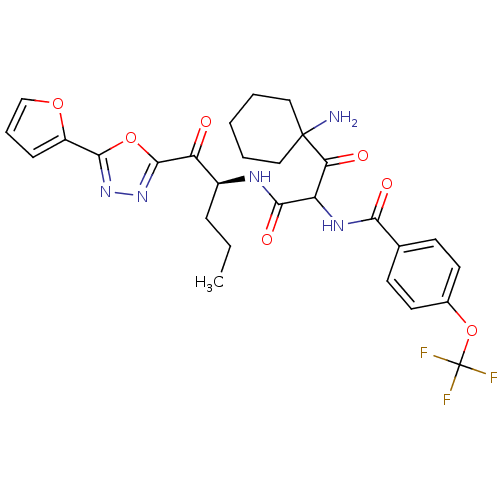
(CHEMBL378899)Show SMILES CCC[C@H](NC(=O)C(NC(=O)c1ccc(OC(F)(F)F)cc1)C(=O)C1(N)CCCCC1)C(=O)c1nnc(o1)-c1ccco1 Show InChI InChI=1S/C28H30F3N5O7/c1-2-7-18(21(37)26-36-35-25(42-26)19-8-6-15-41-19)33-24(40)20(22(38)27(32)13-4-3-5-14-27)34-23(39)16-9-11-17(12-10-16)43-28(29,30)31/h6,8-12,15,18,20H,2-5,7,13-14,32H2,1H3,(H,33,40)(H,34,39)/t18-,20?/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| <0.25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera Genomics, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human cathepsin K |
Bioorg Med Chem Lett 16: 2909-14 (2006)
Article DOI: 10.1016/j.bmcl.2006.03.001
BindingDB Entry DOI: 10.7270/Q2QN6808 |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Homo sapiens (Human)) | BDBM100291
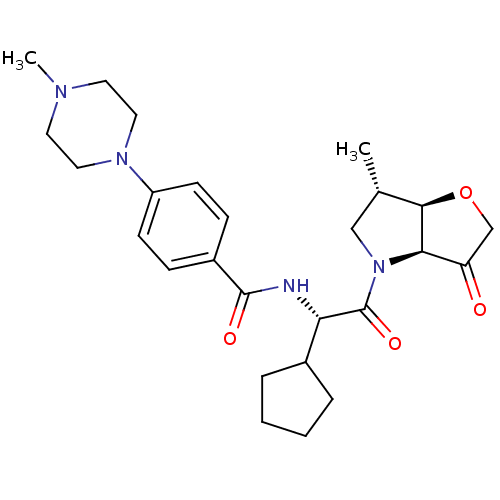
(US8501744, 7)Show SMILES C[C@H]1CN([C@H]2[C@@H]1OCC2=O)C(=O)[C@@H](NC(=O)c1ccc(cc1)N1CCN(C)CC1)C1CCCC1 |r| Show InChI InChI=1S/C26H36N4O4/c1-17-15-30(23-21(31)16-34-24(17)23)26(33)22(18-5-3-4-6-18)27-25(32)19-7-9-20(10-8-19)29-13-11-28(2)12-14-29/h7-10,17-18,22-24H,3-6,11-16H2,1-2H3,(H,27,32)/t17-,22-,23+,24+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 0.350 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amura Therapeutics, Limited
US Patent
| Assay Description
In vitro cathepsin inhibition assay. |
US Patent US8501744 (2013)
BindingDB Entry DOI: 10.7270/Q2D7992S |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Homo sapiens (Human)) | BDBM100304
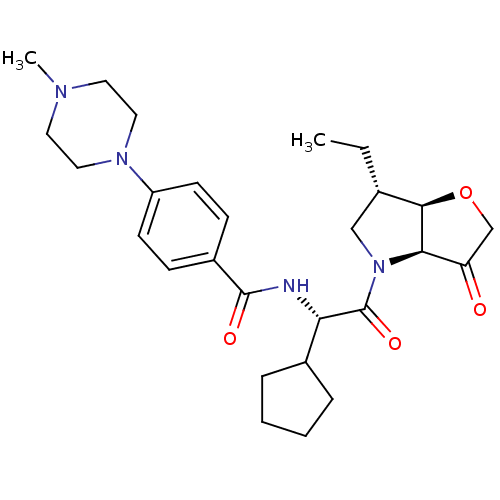
(US8501744, 29)Show SMILES CC[C@H]1CN([C@H]2[C@@H]1OCC2=O)C(=O)[C@@H](NC(=O)c1ccc(cc1)N1CCN(C)CC1)C1CCCC1 |r| Show InChI InChI=1S/C27H38N4O4/c1-3-18-16-31(24-22(32)17-35-25(18)24)27(34)23(19-6-4-5-7-19)28-26(33)20-8-10-21(11-9-20)30-14-12-29(2)13-15-30/h8-11,18-19,23-25H,3-7,12-17H2,1-2H3,(H,28,33)/t18-,23-,24+,25+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amura Therapeutics, Limited
US Patent
| Assay Description
In vitro cathepsin inhibition assay. |
US Patent US8501744 (2013)
BindingDB Entry DOI: 10.7270/Q2D7992S |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Homo sapiens (Human)) | BDBM100305
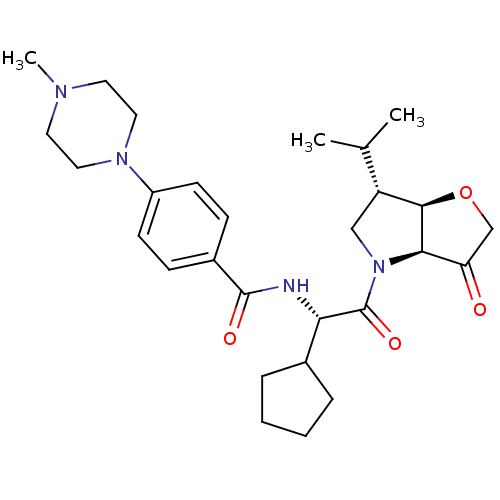
(US8501744, 30)Show SMILES CC(C)[C@H]1CN([C@H]2[C@@H]1OCC2=O)C(=O)[C@@H](NC(=O)c1ccc(cc1)N1CCN(C)CC1)C1CCCC1 |r| Show InChI InChI=1S/C28H40N4O4/c1-18(2)22-16-32(25-23(33)17-36-26(22)25)28(35)24(19-6-4-5-7-19)29-27(34)20-8-10-21(11-9-20)31-14-12-30(3)13-15-31/h8-11,18-19,22,24-26H,4-7,12-17H2,1-3H3,(H,29,34)/t22-,24+,25-,26-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 0.450 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amura Therapeutics, Limited
US Patent
| Assay Description
In vitro cathepsin inhibition assay. |
US Patent US8501744 (2013)
BindingDB Entry DOI: 10.7270/Q2D7992S |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Homo sapiens (Human)) | BDBM100296
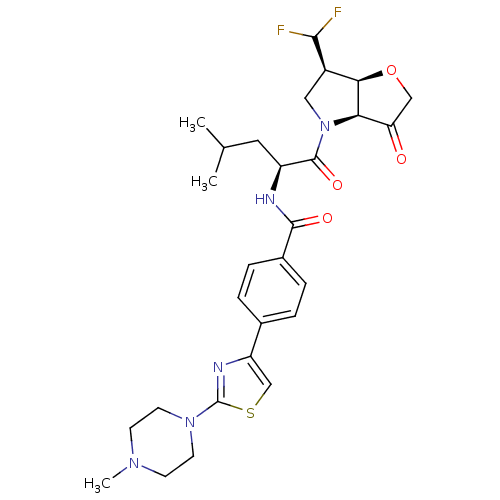
(US8501744, 17)Show SMILES CC(C)C[C@H](NC(=O)c1ccc(cc1)-c1csc(n1)N1CCN(C)CC1)C(=O)N1C[C@@H](C(F)F)[C@H]2OCC(=O)[C@@H]12 |r| Show InChI InChI=1S/C28H35F2N5O4S/c1-16(2)12-20(27(38)35-13-19(25(29)30)24-23(35)22(36)14-39-24)31-26(37)18-6-4-17(5-7-18)21-15-40-28(32-21)34-10-8-33(3)9-11-34/h4-7,15-16,19-20,23-25H,8-14H2,1-3H3,(H,31,37)/t19-,20+,23-,24-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 0.450 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amura Therapeutics, Limited
US Patent
| Assay Description
In vitro cathepsin inhibition assay. |
US Patent US8501744 (2013)
BindingDB Entry DOI: 10.7270/Q2D7992S |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Homo sapiens (Human)) | BDBM50335277

(CHEMBL1651351 | N-(Benzyloxycarbonyl)-isoleucyl-me...)Show SMILES CC[C@H](C)[C@H](NC(=O)OCc1ccccc1)C(=O)N(C)N(C)C#N |r| Show InChI InChI=1S/C17H24N4O3/c1-5-13(2)15(16(22)21(4)20(3)12-18)19-17(23)24-11-14-9-7-6-8-10-14/h6-10,13,15H,5,11H2,1-4H3,(H,19,23)/t13-,15-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.460 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bonn
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant cathepsin K after 30 mins by spectrophotometric assay |
J Med Chem 54: 396-400 (2011)
Article DOI: 10.1021/jm101272p
BindingDB Entry DOI: 10.7270/Q2PV6MC3 |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Homo sapiens (Human)) | BDBM50066647

(CHEMBL113724 | {(S)-3-Methyl-1-[3-oxo-1-(4-phenoxy...)Show SMILES CC(C)C[C@H](NC(=O)OCc1ccccc1)C(=O)NC1CCN(CC1=O)S(=O)(=O)c1ccc(Oc2ccccc2)cc1 Show InChI InChI=1S/C31H35N3O7S/c1-22(2)19-28(33-31(37)40-21-23-9-5-3-6-10-23)30(36)32-27-17-18-34(20-29(27)35)42(38,39)26-15-13-25(14-16-26)41-24-11-7-4-8-12-24/h3-16,22,27-28H,17-21H2,1-2H3,(H,32,36)(H,33,37)/t27?,28-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of cathepsin K |
J Med Chem 41: 3563-7 (1998)
Article DOI: 10.1021/jm980295f
BindingDB Entry DOI: 10.7270/Q2G15ZZB |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Homo sapiens (Human)) | BDBM194227
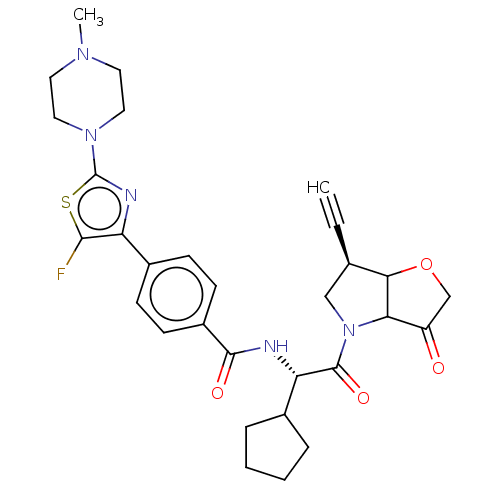
(US9200006, 5)Show SMILES CN1CCN(CC1)c1nc(c(F)s1)-c1ccc(cc1)C(=O)N[C@@H](C1CCCC1)C(=O)N1C[C@@H](C#C)C2OCC(=O)C12 |r| Show InChI InChI=1S/C30H34FN5O4S/c1-3-18-16-36(25-22(37)17-40-26(18)25)29(39)24(19-6-4-5-7-19)32-28(38)21-10-8-20(9-11-21)23-27(31)41-30(33-23)35-14-12-34(2)13-15-35/h1,8-11,18-19,24-26H,4-7,12-17H2,2H3,(H,32,38)/t18-,24+,25?,26?/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | 7.0 | n/a |
MEDIVIR AB
US Patent
| Assay Description
Standard assay conditions for the determination of kinetic constants used a fluorogenic peptide substrate, typically H-D-Ala-Leu-Lys-AMC, and were de... |
US Patent US9200006 (2015)
BindingDB Entry DOI: 10.7270/Q26W98X8 |
More data for this
Ligand-Target Pair | |
Cathepsin K
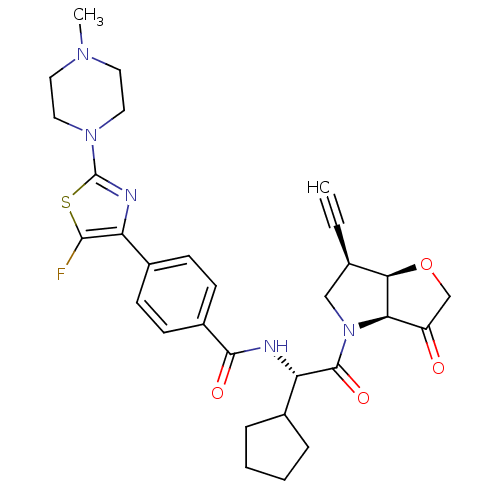
(Homo sapiens (Human)) | BDBM123101
(US11312693, Example 5 | US8735395, 5)Show SMILES CN1CCN(CC1)c1nc(c(F)s1)-c1ccc(cc1)C(=O)N[C@@H](C1CCCC1)C(=O)N1C[C@@H](C#C)[C@H]2OCC(=O)[C@@H]12 |r| Show InChI InChI=1S/C30H34FN5O4S/c1-3-18-16-36(25-22(37)17-40-26(18)25)29(39)24(19-6-4-5-7-19)32-28(38)21-10-8-20(9-11-21)23-27(31)41-30(33-23)35-14-12-34(2)13-15-35/h1,8-11,18-19,24-26H,4-7,12-17H2,2H3,(H,32,38)/t18-,24+,25-,26-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | 7.0 | n/a |
Medivir AB
US Patent
| Assay Description
Standard assay conditions for the determination of kinetic constants used a fluorogenic peptide substrate, typically H-D-Ala-Leu-Lys-AMC, and were de... |
US Patent US8735395 (2014)
BindingDB Entry DOI: 10.7270/Q2QJ7G03 |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Homo sapiens (Human)) | BDBM100295

(US8501744, 22)Show SMILES CC(C)C[C@H](NC(=O)c1ccc(cc1)-c1csc(n1)N1CCN(C)CC1)C(=O)N1C[C@H](C)[C@H]2OCC(=O)[C@@H]12 |r| Show InChI InChI=1S/C28H37N5O4S/c1-17(2)13-21(27(36)33-14-18(3)25-24(33)23(34)15-37-25)29-26(35)20-7-5-19(6-8-20)22-16-38-28(30-22)32-11-9-31(4)10-12-32/h5-8,16-18,21,24-25H,9-15H2,1-4H3,(H,29,35)/t18-,21-,24+,25+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amura Therapeutics, Limited
US Patent
| Assay Description
In vitro cathepsin inhibition assay. |
US Patent US8501744 (2013)
BindingDB Entry DOI: 10.7270/Q2D7992S |
More data for this
Ligand-Target Pair | |
Cathepsin K
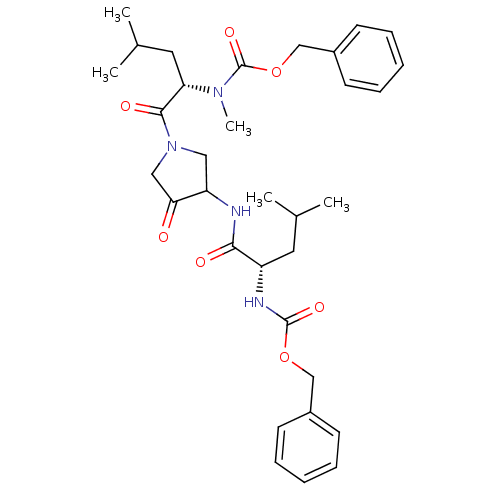
(Homo sapiens (Human)) | BDBM19808
(benzyl N-[(1S)-1-({1-[(2S)-2-{[(benzyloxy)carbonyl...)Show SMILES CC(C)C[C@H](NC(=O)OCc1ccccc1)C(=O)NC1CN(CC1=O)C(=O)[C@H](CC(C)C)N(C)C(=O)OCc1ccccc1 |r| Show InChI InChI=1S/C33H44N4O7/c1-22(2)16-26(35-32(41)43-20-24-12-8-6-9-13-24)30(39)34-27-18-37(19-29(27)38)31(40)28(17-23(3)4)36(5)33(42)44-21-25-14-10-7-11-15-25/h6-15,22-23,26-28H,16-21H2,1-5H3,(H,34,39)(H,35,41)/t26-,27?,28-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.600 | -12.4 | n/a | n/a | n/a | n/a | n/a | 5.5 | 22 |
GSK
| Assay Description
Assays were carried out in the presence of variable concentrations of test compound. Reactions were initiated by addition of enzyme to buffered solut... |
J Med Chem 44: 725-36 (2001)
Article DOI: 10.1021/jm000320t
BindingDB Entry DOI: 10.7270/Q2J67F6C |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Cathepsin K
(Homo sapiens (Human)) | BDBM456055

(US10723709, Example 5)Show SMILES CN1CCN(CC1)c1nc(c(F)s1)-c1ccc(cc1)C(=O)NC(C=O)(C1CCCC1)N1CC(C#C)C2OCC(=O)C12 Show InChI InChI=1S/C30H34FN5O4S/c1-3-19-16-36(25-23(38)17-40-26(19)25)30(18-37,22-6-4-5-7-22)33-28(39)21-10-8-20(9-11-21)24-27(31)41-29(32-24)35-14-12-34(2)13-15-35/h1,8-11,18-19,22,25-26H,4-7,12-17H2,2H3,(H,33,39) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
MEDIVIR AB
US Patent
| Assay Description
The recombinant cathepsin K can be expressed in a variety of commercially available expression systems including E coli, Pichia and Baculovirus syste... |
US Patent US10723709 (2020)
BindingDB Entry DOI: 10.7270/Q26M39W6 |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Homo sapiens (Human)) | BDBM123101

(US11312693, Example 5 | US8735395, 5)Show SMILES CN1CCN(CC1)c1nc(c(F)s1)-c1ccc(cc1)C(=O)N[C@@H](C1CCCC1)C(=O)N1C[C@@H](C#C)[C@H]2OCC(=O)[C@@H]12 |r| Show InChI InChI=1S/C30H34FN5O4S/c1-3-18-16-36(25-22(37)17-40-26(18)25)29(39)24(19-6-4-5-7-19)32-28(38)21-10-8-20(9-11-21)23-27(31)41-30(33-23)35-14-12-34(2)13-15-35/h1,8-11,18-19,24-26H,4-7,12-17H2,2H3,(H,32,38)/t18-,24+,25-,26-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Standard assay conditions for the determination of kinetic constants used a fluorogenic peptide substrate, typically H-D-Ala-Leu-Lys-AMC, and were de... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2736V47 |
More data for this
Ligand-Target Pair | |
Cathepsin K
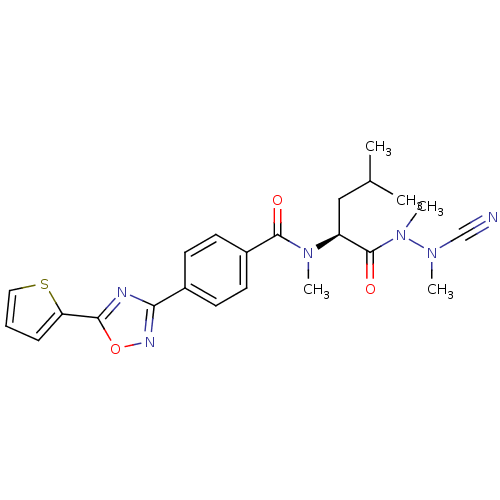
(Homo sapiens (Human)) | BDBM50335290
(CHEMBL1651362 | N-{4-[5-(2-Thienyl)-1,2,4-oxadiazo...)Show SMILES CC(C)C[C@H](N(C)C(=O)c1ccc(cc1)-c1noc(n1)-c1cccs1)C(=O)N(C)N(C)C#N |r| Show InChI InChI=1S/C23H26N6O3S/c1-15(2)13-18(23(31)29(5)27(3)14-24)28(4)22(30)17-10-8-16(9-11-17)20-25-21(32-26-20)19-7-6-12-33-19/h6-12,15,18H,13H2,1-5H3/t18-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.630 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bonn
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant cathepsin K after 30 mins by spectrophotometric assay |
J Med Chem 54: 396-400 (2011)
Article DOI: 10.1021/jm101272p
BindingDB Entry DOI: 10.7270/Q2PV6MC3 |
More data for this
Ligand-Target Pair | |
Cathepsin K
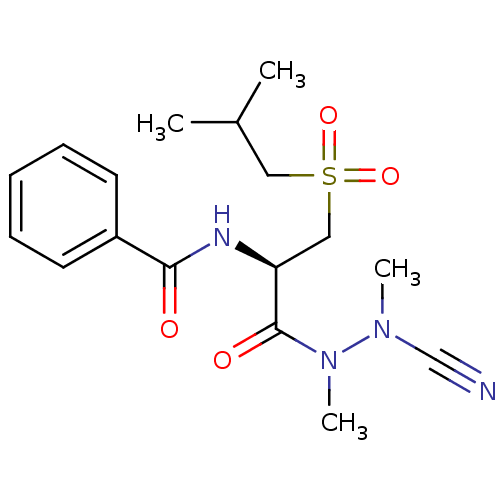
(Homo sapiens (Human)) | BDBM50392215
(CHEMBL2153161)Show SMILES CC(C)CS(=O)(=O)C[C@H](NC(=O)c1ccccc1)C(=O)N(C)N(C)C#N |r| Show InChI InChI=1S/C17H24N4O4S/c1-13(2)10-26(24,25)11-15(17(23)21(4)20(3)12-18)19-16(22)14-8-6-5-7-9-14/h5-9,13,15H,10-11H2,1-4H3,(H,19,22)/t15-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 0.660 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bonn
Curated by ChEMBL
| Assay Description
Inhibition of human cathepsin K using Z-Leu-Arg-AMC as substrate after 80 mins by fluorimetric analysis |
J Med Chem 55: 5982-6 (2012)
Article DOI: 10.1021/jm300734k
BindingDB Entry DOI: 10.7270/Q2833T40 |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Homo sapiens (Human)) | BDBM50408519
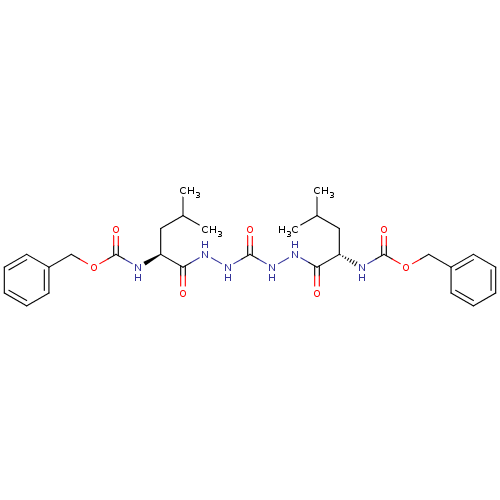
(CHEMBL115357)Show SMILES CC(C)C[C@H](NC(=O)OCc1ccccc1)C(=O)NNC(=O)NNC(=O)[C@H](CC(C)C)NC(=O)OCc1ccccc1 |r| Show InChI InChI=1S/C29H40N6O7/c1-19(2)15-23(30-28(39)41-17-21-11-7-5-8-12-21)25(36)32-34-27(38)35-33-26(37)24(16-20(3)4)31-29(40)42-18-22-13-9-6-10-14-22/h5-14,19-20,23-24H,15-18H2,1-4H3,(H,30,39)(H,31,40)(H,32,36)(H,33,37)(H2,34,35,38)/t23-,24-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
MSD Animal Health Innovation GmbH
Curated by ChEMBL
| Assay Description
Inhibition of human cathepsin K |
J Med Chem 56: 1478-90 (2013)
Article DOI: 10.1021/jm3013932
BindingDB Entry DOI: 10.7270/Q2W09774 |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Homo sapiens (Human)) | BDBM100294

(US8501744, 16)Show SMILES CC(C)C[C@H](NC(=O)c1ccc(cc1)-c1csc(n1)N1CCN(C)CC1)C(=O)N1C[C@@H](C)[C@H]2OCC(=O)[C@@H]12 |r| Show InChI InChI=1S/C28H37N5O4S/c1-17(2)13-21(27(36)33-14-18(3)25-24(33)23(34)15-37-25)29-26(35)20-7-5-19(6-8-20)22-16-38-28(30-22)32-11-9-31(4)10-12-32/h5-8,16-18,21,24-25H,9-15H2,1-4H3,(H,29,35)/t18-,21+,24-,25-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amura Therapeutics, Limited
US Patent
| Assay Description
In vitro cathepsin inhibition assay. |
US Patent US8501744 (2013)
BindingDB Entry DOI: 10.7270/Q2D7992S |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Homo sapiens (Human)) | BDBM50408519

(CHEMBL115357)Show SMILES CC(C)C[C@H](NC(=O)OCc1ccccc1)C(=O)NNC(=O)NNC(=O)[C@H](CC(C)C)NC(=O)OCc1ccccc1 |r| Show InChI InChI=1S/C29H40N6O7/c1-19(2)15-23(30-28(39)41-17-21-11-7-5-8-12-21)25(36)32-34-27(38)35-33-26(37)24(16-20(3)4)31-29(40)42-18-22-13-9-6-10-14-22/h5-14,19-20,23-24H,15-18H2,1-4H3,(H,30,39)(H,31,40)(H,32,36)(H,33,37)(H2,34,35,38)/t23-,24-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
Relative binding affinity was measured for Cathepsin K |
J Med Chem 41: 3923-7 (1998)
Article DOI: 10.1021/jm980474x
BindingDB Entry DOI: 10.7270/Q2Q81F87 |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Homo sapiens (Human)) | BDBM100297
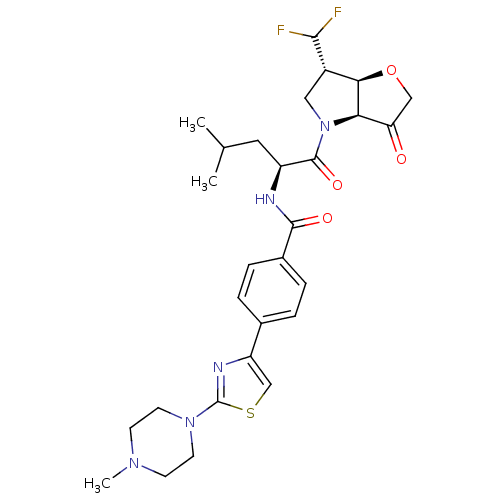
(US8501744, 18)Show SMILES CC(C)C[C@H](NC(=O)c1ccc(cc1)-c1csc(n1)N1CCN(C)CC1)C(=O)N1C[C@H](C(F)F)[C@H]2OCC(=O)[C@@H]12 |r| Show InChI InChI=1S/C28H35F2N5O4S/c1-16(2)12-20(27(38)35-13-19(25(29)30)24-23(35)22(36)14-39-24)31-26(37)18-6-4-17(5-7-18)21-15-40-28(32-21)34-10-8-33(3)9-11-34/h4-7,15-16,19-20,23-25H,8-14H2,1-3H3,(H,31,37)/t19-,20-,23+,24+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amura Therapeutics, Limited
US Patent
| Assay Description
In vitro cathepsin inhibition assay. |
US Patent US8501744 (2013)
BindingDB Entry DOI: 10.7270/Q2D7992S |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Homo sapiens (Human)) | BDBM352414
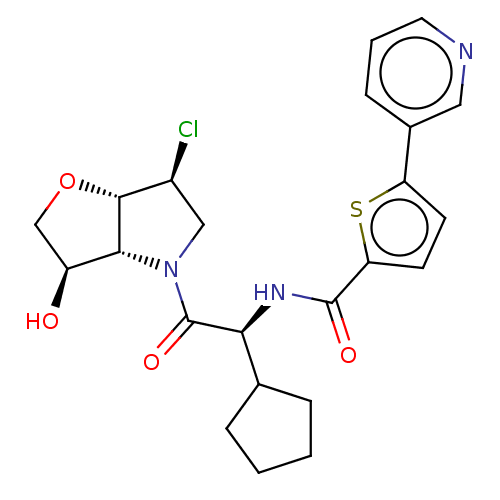
(Nó((S)-2-((3aS,6S,6aS)-6-chloro-3-oxotetrahydro-2H...)Show SMILES O[C@H]1CO[C@@H]2[C@@H](Cl)CN([C@H]12)C(=O)[C@@H](NC(=O)c1ccc(s1)-c1cccnc1)C1CCCC1 |r| Show InChI InChI=1S/C23H26ClN3O4S/c24-15-11-27(20-16(28)12-31-21(15)20)23(30)19(13-4-1-2-5-13)26-22(29)18-8-7-17(32-18)14-6-3-9-25-10-14/h3,6-10,13,15-16,19-21,28H,1-2,4-5,11-12H2,(H,26,29)/t15-,16-,19-,20+,21+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GRÜNENTHAL GMBH
US Patent
| Assay Description
Recombinant human cathepsins (CatS, CatK, CatL, CatB) were purchased from a Enzo Life Sciences. All assays were carried out in 96-well format using a... |
US Patent US9802947 (2017)
BindingDB Entry DOI: 10.7270/Q2HX1FT3 |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Homo sapiens (Human)) | BDBM50084650
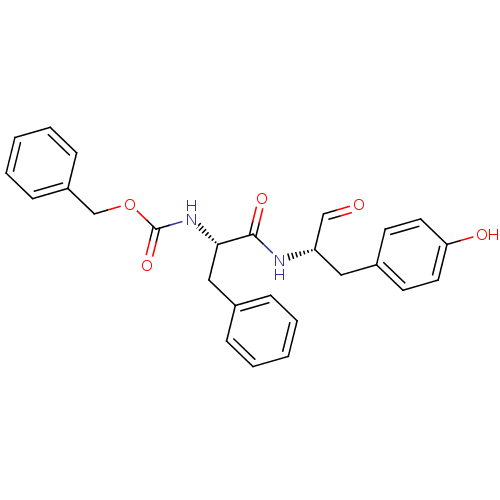
(CHEMBL177914 | {(S)-1-[(S)-1-Formyl-2-(4-hydroxy-p...)Show SMILES Oc1ccc(C[C@H](NC(=O)[C@H](Cc2ccccc2)NC(=O)OCc2ccccc2)C=O)cc1 Show InChI InChI=1S/C26H26N2O5/c29-17-22(15-20-11-13-23(30)14-12-20)27-25(31)24(16-19-7-3-1-4-8-19)28-26(32)33-18-21-9-5-2-6-10-21/h1-14,17,22,24,30H,15-16,18H2,(H,27,31)(H,28,32)/t22-,24-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.740 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Apparent inhibitory constant against human cathepsin K |
J Med Chem 48: 6870-8 (2005)
Article DOI: 10.1021/jm0502079
BindingDB Entry DOI: 10.7270/Q2WM1CZC |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data