 Found 784 hits of ic50 for UniProtKB: P50052
Found 784 hits of ic50 for UniProtKB: P50052 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kcal/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Type-2 angiotensin II receptor
(Homo sapiens (Human)) | BDBM50236697

(5-L-isoleucineangiotensin II | 5-isoleucine-angiot...)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@@H](NC(=O)[C@H](CCCN=C(N)N)NC(=O)[C@@H](N)CC(O)=O)C(C)C)C(=O)N[C@@H](Cc1cnc[nH]1)C(=O)N1CCC[C@H]1C(=O)N[C@@H](Cc1ccccc1)C(O)=O |wU:4.4,47.47,60.63,35.36,20.21,wD:2.2,24.32,8.17,64.66,(24.13,1.24,;24.1,-.31,;25.42,-1.09,;26.77,-.35,;25.4,-2.63,;24.05,-3.38,;22.73,-2.59,;22.75,-1.05,;21.38,-3.34,;21.36,-4.88,;22.68,-5.66,;24.01,-4.93,;25.33,-5.71,;25.32,-7.24,;26.64,-8.05,;23.97,-8.01,;22.65,-7.22,;20.06,-2.55,;18.71,-3.29,;18.68,-4.83,;17.38,-2.5,;16.04,-3.25,;14.72,-2.46,;14.74,-.92,;13.37,-3.2,;13.34,-4.74,;14.66,-5.53,;14.64,-7.08,;15.96,-7.87,;15.93,-9.4,;14.59,-10.16,;17.25,-10.21,;12.05,-2.42,;10.69,-3.15,;10.67,-4.69,;9.37,-2.36,;8.03,-3.1,;9.4,-.82,;10.75,-.07,;12.07,-.88,;10.78,1.47,;17.41,-.96,;18.76,-.21,;16.1,-.17,;26.72,-3.43,;26.69,-4.97,;28.07,-2.68,;29.38,-3.48,;30.73,-2.73,;30.75,-1.19,;29.53,-.27,;30.03,1.18,;31.57,1.17,;32.02,-.31,;29.35,-5.02,;28.01,-5.76,;30.54,-5.79,;31.99,-5.25,;32.95,-6.46,;32.1,-7.74,;30.62,-7.33,;29.33,-8.36,;27.89,-7.82,;29.58,-9.88,;28.39,-10.86,;26.95,-10.32,;25.76,-11.3,;26.02,-12.81,;24.84,-13.79,;23.4,-13.26,;23.14,-11.74,;24.32,-10.75,;28.64,-12.38,;27.45,-13.36,;30.08,-12.92,)| Show InChI InChI=1S/C50H71N13O12/c1-5-28(4)41(47(72)59-36(23-31-25-54-26-56-31)48(73)63-20-10-14-38(63)45(70)60-37(49(74)75)22-29-11-7-6-8-12-29)62-44(69)35(21-30-15-17-32(64)18-16-30)58-46(71)40(27(2)3)61-43(68)34(13-9-19-55-50(52)53)57-42(67)33(51)24-39(65)66/h6-8,11-12,15-18,25-28,33-38,40-41,64H,5,9-10,13-14,19-24,51H2,1-4H3,(H,54,56)(H,57,67)(H,58,71)(H,59,72)(H,60,70)(H,61,68)(H,62,69)(H,65,66)(H,74,75)(H4,52,53,55)/t28-,33-,34-,35-,36-,37-,38-,40-,41-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MMDB
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a |
Jagiellonian University Medical College
Curated by ChEMBL
| Assay Description
Displacement of [125I]CGP 42112A from human recombinant AT2 receptor expressed in HEK293 cells measured after 4 hrs by scintillation counting method |
Bioorg Med Chem 25: 471-482 (2017)
Article DOI: 10.1016/j.bmc.2016.11.014
BindingDB Entry DOI: 10.7270/Q2CF9S3S |
More data for this
Ligand-Target Pair | |
Type-2 angiotensin II receptor
(Homo sapiens (Human)) | BDBM50155247
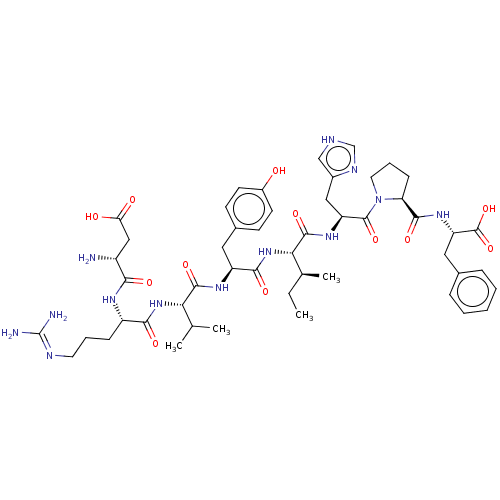
(CHEMBL385433)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@@H](NC(=O)[C@H](CCCN=C(N)N)NC(=O)[C@H](N)CC(O)=O)C(C)C)C(=O)N[C@@H](Cc1c[nH]cn1)C(=O)N1CCC[C@H]1C(=O)N[C@@H](Cc1ccccc1)C(O)=O |wU:20.21,64.66,8.8,35.36,2.2,wD:60.63,4.4,47.47,24.25,(3.98,-9.71,;3.98,-8.48,;5.32,-7.71,;6.38,-8.33,;5.33,-6.17,;3.99,-5.4,;4,-3.86,;5.07,-3.24,;2.66,-3.08,;2.67,-1.54,;1.33,-.77,;,-1.54,;-1.34,-.77,;-1.34,.77,;-2.4,1.39,;,1.54,;1.33,.77,;1.33,-3.86,;1.33,-5.4,;2.4,-6.01,;-.01,-6.17,;-.01,-7.71,;-1.34,-8.48,;-2.41,-7.86,;-1.34,-10.02,;-.01,-10.79,;-.01,-12.34,;1.32,-13.11,;1.32,-14.65,;2.65,-15.42,;2.65,-16.65,;3.72,-14.81,;-2.68,-10.79,;-2.68,-12.33,;-1.61,-12.95,;-4.01,-13.1,;-5.08,-12.49,;-4.01,-14.64,;-5.34,-15.42,;-6.41,-14.8,;-5.35,-16.65,;-1.34,-5.4,;-2.41,-6.02,;-1.34,-4.17,;6.66,-5.4,;6.67,-4.17,;8,-6.18,;9.33,-5.41,;10.67,-6.18,;10.67,-7.72,;11.91,-8.61,;11.43,-10.08,;9.89,-10.07,;9.42,-8.61,;9.34,-3.87,;8.28,-3.25,;10.68,-3.1,;12.06,-3.75,;13.1,-2.61,;12.34,-1.27,;10.83,-1.58,;9.68,-.56,;9.93,.65,;8.22,-1.04,;7.07,-.01,;5.61,-.49,;4.45,.53,;2.99,.06,;1.84,1.08,;2.16,2.59,;3.62,3.07,;4.77,2.04,;7.38,1.5,;8.55,1.88,;6.46,2.32,)| Show InChI InChI=1S/C50H71N13O12/c1-5-28(4)41(47(72)59-36(23-31-25-54-26-56-31)48(73)63-20-10-14-38(63)45(70)60-37(49(74)75)22-29-11-7-6-8-12-29)62-44(69)35(21-30-15-17-32(64)18-16-30)58-46(71)40(27(2)3)61-43(68)34(13-9-19-55-50(52)53)57-42(67)33(51)24-39(65)66/h6-8,11-12,15-18,25-28,33-38,40-41,64H,5,9-10,13-14,19-24,51H2,1-4H3,(H,54,56)(H,57,67)(H,58,71)(H,59,72)(H,60,70)(H,61,68)(H,62,69)(H,65,66)(H,74,75)(H4,52,53,55)/t28-,33+,34-,35-,36-,37-,38-,40-,41-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a |
Jagiellonian University Medical College
Curated by ChEMBL
| Assay Description
Displacement of [125I]CGP42112A from human recombinant AT2 receptor expressed in HEK293 cells |
Bioorg Med Chem 24: 1793-810 (2016)
Article DOI: 10.1016/j.bmc.2016.03.006
BindingDB Entry DOI: 10.7270/Q2J67JS7 |
More data for this
Ligand-Target Pair | |
Type-2 angiotensin II receptor
(Homo sapiens (Human)) | BDBM50030711

(CHEMBL338027 | L-163958 | Pentanoic acid {4-bromo-...)Show SMILES CCCCC(=O)Nc1ccc(Br)c(c1)-n1nc(CC)n(Cc2ccc(cc2F)-c2ccccc2S(=O)(=O)NC(=O)OC(C)(C)C)c1=O Show InChI InChI=1S/C33H37BrFN5O6S/c1-6-8-13-30(41)36-23-16-17-25(34)27(19-23)40-32(43)39(29(7-2)37-40)20-22-15-14-21(18-26(22)35)24-11-9-10-12-28(24)47(44,45)38-31(42)46-33(3,4)5/h9-12,14-19H,6-8,13,20H2,1-5H3,(H,36,41)(H,38,42) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.120 | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Merck Pharmaceutical Company
Curated by ChEMBL
| Assay Description
Inhibition of Angiotensin II receptor, type 2 |
J Med Chem 39: 625-56 (1996)
Article DOI: 10.1021/jm9504722
BindingDB Entry DOI: 10.7270/Q29P3299 |
More data for this
Ligand-Target Pair | |
Type-2 angiotensin II receptor
(Homo sapiens (Human)) | BDBM50009718
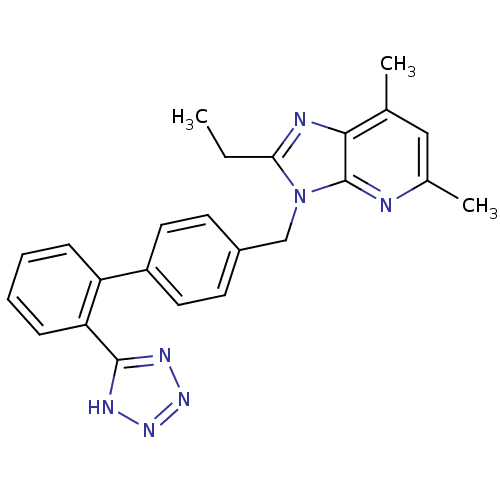
(2-Ethyl-5,7-dimethyl-3-[2'-(1H-tetrazol-5-yl)-biph...)Show SMILES CCc1nc2c(C)cc(C)nc2n1Cc1ccc(cc1)-c1ccccc1-c1nnn[nH]1 Show InChI InChI=1S/C24H23N7/c1-4-21-26-22-15(2)13-16(3)25-24(22)31(21)14-17-9-11-18(12-10-17)19-7-5-6-8-20(19)23-27-29-30-28-23/h5-13H,4,14H2,1-3H3,(H,27,28,29,30) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
The M. S. University of Baroda
Curated by ChEMBL
| Assay Description
Binding affinity to angiotensin AT2 receptor |
Bioorg Med Chem 18: 8418-56 (2010)
Article DOI: 10.1016/j.bmc.2010.10.043
BindingDB Entry DOI: 10.7270/Q2H70G3T |
More data for this
Ligand-Target Pair | |
Type-2 angiotensin II receptor
(Homo sapiens (Human)) | BDBM50043280

(4'-((1,4'-dimethyl-2'-propyl(2,6'-bi-1H-benzimidaz...)Show SMILES CCCc1nc2c(C)cc(cc2n1Cc1ccc(cc1)-c1ccccc1C(O)=O)-c1nc2ccccc2n1C Show InChI InChI=1S/C33H30N4O2/c1-4-9-30-35-31-21(2)18-24(32-34-27-12-7-8-13-28(27)36(32)3)19-29(31)37(30)20-22-14-16-23(17-15-22)25-10-5-6-11-26(25)33(38)39/h5-8,10-19H,4,9,20H2,1-3H3,(H,38,39) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.330 | n/a | n/a | n/a | n/a | n/a | n/a |
Beijing Institute of Technology
Curated by ChEMBL
| Assay Description
Displacement of [125I]Sar1 Ile8-Ang 2 from angiotensin 2 AT2 receptor after 180 mins by gamma counting |
Eur J Med Chem 49: 183-90 (2012)
Article DOI: 10.1016/j.ejmech.2012.01.009
BindingDB Entry DOI: 10.7270/Q29K4BPW |
More data for this
Ligand-Target Pair | |
Type-2 angiotensin II receptor
(Homo sapiens (Human)) | BDBM50048114
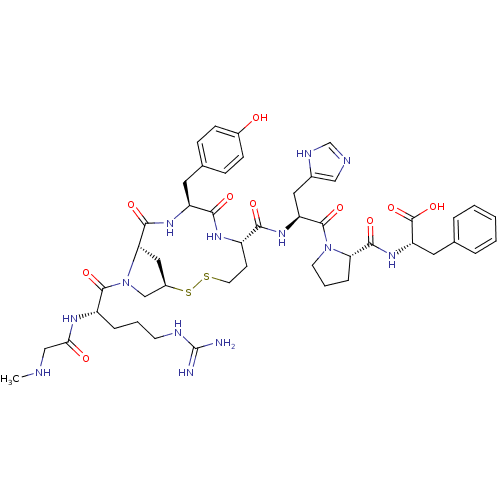
(CHEMBL384352 | c[Sar1-Arg2-Mpt3-Tyr4-Hcy5-His6-Pro...)Show SMILES CNCC(=O)N[C@@H](CCCNC(N)=N)C(=O)N1C[C@H]2C[C@H]1C(=O)N[C@@H](Cc1ccc(O)cc1)C(=O)N[C@@H](CCSS2)C(=O)N[C@@H](Cc1cnc[nH]1)C(=O)N1CCC[C@H]1C(=O)N[C@@H](Cc1ccccc1)C(O)=O Show InChI InChI=1S/C47H63N13O10S2/c1-50-24-39(62)54-33(9-5-16-52-47(48)49)44(67)60-25-31-22-38(60)43(66)56-34(19-28-11-13-30(61)14-12-28)41(64)55-32(15-18-71-72-31)40(63)57-35(21-29-23-51-26-53-29)45(68)59-17-6-10-37(59)42(65)58-36(46(69)70)20-27-7-3-2-4-8-27/h2-4,7-8,11-14,23,26,31-38,50,61H,5-6,9-10,15-22,24-25H2,1H3,(H,51,53)(H,54,62)(H,55,64)(H,56,66)(H,57,63)(H,58,65)(H,69,70)(H4,48,49,52)/t31-,32+,33+,34+,35+,36+,37+,38+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.470 | n/a | n/a | n/a | n/a | n/a | n/a |
Washington University School of Medicine
Curated by ChEMBL
| Assay Description
In vitro binding affinity at angiotensin II (type 2) receptor in rabbit uterus. |
J Med Chem 36: 1902-13 (1993)
BindingDB Entry DOI: 10.7270/Q2N58KF4 |
More data for this
Ligand-Target Pair | |
Type-2 angiotensin II receptor
(Homo sapiens (Human)) | BDBM50283763
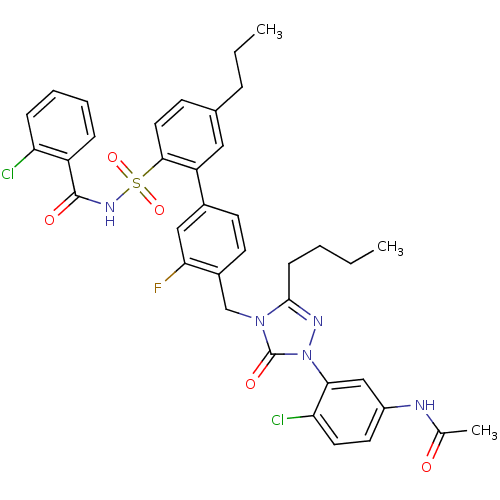
(CHEMBL321531 | N-(3-{3-Butyl-4-[2'-(2-chloro-benzo...)Show SMILES CCCCc1nn(-c2cc(NC(C)=O)ccc2Cl)c(=O)n1Cc1ccc(cc1F)-c1cc(CCC)ccc1S(=O)(=O)NC(=O)c1ccccc1Cl Show InChI InChI=1S/C37H36Cl2FN5O5S/c1-4-6-12-35-42-45(33-21-27(41-23(3)46)16-17-31(33)39)37(48)44(35)22-26-15-14-25(20-32(26)40)29-19-24(9-5-2)13-18-34(29)51(49,50)43-36(47)28-10-7-8-11-30(28)38/h7-8,10-11,13-21H,4-6,9,12,22H2,1-3H3,(H,41,46)(H,43,47) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
| n/a | n/a | 0.480 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory concentration of the compound against cloned human AT1 receptor |
Bioorg Med Chem Lett 4: 2787-2792 (1994)
Article DOI: 10.1016/S0960-894X(01)80595-3
BindingDB Entry DOI: 10.7270/Q2WS8T68 |
More data for this
Ligand-Target Pair | |
Type-2 angiotensin II receptor
(Homo sapiens (Human)) | BDBM50009338
((S)-2-((S)-1-((S)-2-((S)-2-((S)-2-((S)-2-((S)-5-(d...)Show SMILES CNCC(=O)N[C@@H](CCCN=C(N)N)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](Cc1ccc(O)cc1)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](Cc1cnc[nH]1)C(=O)N1CCC[C@H]1C(=O)N[C@@H](C)C(O)=O |r,wU:60.63,wD:24.23,36.36,43.43,56.59,17.16,6.5,(6.84,-31.4,;5.3,-31.4,;4.53,-30.07,;2.99,-30.07,;2.22,-31.4,;2.22,-28.73,;2.99,-27.4,;4.53,-27.4,;5.3,-26.07,;4.53,-24.73,;5.3,-23.4,;6.84,-23.4,;7.61,-24.73,;7.61,-22.07,;2.22,-26.07,;2.99,-24.73,;.68,-26.07,;-.09,-24.73,;.68,-23.4,;2.22,-23.4,;-.09,-22.07,;-1.63,-24.73,;-2.4,-23.4,;-2.4,-26.07,;-3.94,-26.07,;-4.71,-24.73,;-6.25,-24.73,;-7.02,-23.4,;-8.56,-23.4,;-9.33,-24.73,;-10.87,-24.73,;-8.56,-26.07,;-7.02,-26.07,;-4.71,-27.4,;-6.25,-27.4,;-3.94,-28.73,;-4.71,-30.07,;-6.25,-30.07,;-7.02,-28.73,;-7.02,-31.4,;-3.94,-31.4,;-2.4,-31.4,;-4.71,-32.73,;-3.94,-34.07,;-4.71,-35.4,;-6.25,-35.4,;-7.15,-34.16,;-8.62,-34.63,;-8.62,-36.17,;-7.15,-36.65,;-2.4,-34.07,;-1.63,-32.73,;-1.63,-35.4,;-2.26,-36.81,;-1.12,-37.84,;.22,-37.07,;-.1,-35.56,;.93,-34.42,;.45,-32.95,;2.42,-34.82,;3.5,-33.73,;3.11,-32.24,;5,-34.13,;6.09,-33.04,;5.39,-35.61,)| Show InChI InChI=1S/C42H65N13O10/c1-22(2)33(53-35(58)28(50-32(57)20-45-6)9-7-15-47-42(43)44)38(61)51-29(17-25-11-13-27(56)14-12-25)36(59)54-34(23(3)4)39(62)52-30(18-26-19-46-21-48-26)40(63)55-16-8-10-31(55)37(60)49-24(5)41(64)65/h11-14,19,21-24,28-31,33-34,45,56H,7-10,15-18,20H2,1-6H3,(H,46,48)(H,49,60)(H,50,57)(H,51,61)(H,52,62)(H,53,58)(H,54,59)(H,64,65)(H4,43,44,47)/t24-,28-,29-,30-,31-,33-,34-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.530 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human angiotensin II AT2 receptor |
Bioorg Med Chem 18: 7675-99 (2010)
Article DOI: 10.1016/j.bmc.2010.07.034
BindingDB Entry DOI: 10.7270/Q2DF6S69 |
More data for this
Ligand-Target Pair | |
Type-2 angiotensin II receptor
(Homo sapiens (Human)) | BDBM50282401
(CHEMBL157510 | PD-126055 | Potassium; (S)-5-benzyl...)Show SMILES COc1ccc2CN([C@@H](Cc2c1OCc1ccccc1)C([O-])=O)C(=O)C(c1ccccc1)c1ccccc1 Show InChI InChI=1S/C32H29NO5/c1-37-28-18-17-25-20-33(31(34)29(23-13-7-3-8-14-23)24-15-9-4-10-16-24)27(32(35)36)19-26(25)30(28)38-21-22-11-5-2-6-12-22/h2-18,27,29H,19-21H2,1H3,(H,35,36)/p-1/t27-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 0.580 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity against angiotensin II type 2 (AT2) receptor in rabbit uterine membranes. |
Bioorg Med Chem Lett 4: 57-62 (1994)
Article DOI: 10.1016/S0960-894X(01)81122-7
BindingDB Entry DOI: 10.7270/Q2SJ1KJV |
More data for this
Ligand-Target Pair | |
Type-2 angiotensin II receptor
(Homo sapiens (Human)) | BDBM50049182

((S)-5-Benzyloxy-2-diphenylacetyl-6-methoxy-1,2,3,4...)Show SMILES COc1ccc2CN([C@@H](Cc2c1OCc1ccccc1)C(O)=O)C(=O)C(c1ccccc1)c1ccccc1 Show InChI InChI=1S/C32H29NO5/c1-37-28-18-17-25-20-33(31(34)29(23-13-7-3-8-14-23)24-15-9-4-10-16-24)27(32(35)36)19-26(25)30(28)38-21-22-11-5-2-6-12-22/h2-18,27,29H,19-21H2,1H3,(H,35,36)/t27-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
| n/a | n/a | 0.580 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro binding affinity of compound was measured against angiotensin II (AT2) receptor |
Bioorg Med Chem Lett 4: 1479-1484 (1994)
Article DOI: 10.1016/S0960-894X(01)80517-5
BindingDB Entry DOI: 10.7270/Q2BC401G |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Type-2 angiotensin II receptor
(Homo sapiens (Human)) | BDBM50049182

((S)-5-Benzyloxy-2-diphenylacetyl-6-methoxy-1,2,3,4...)Show SMILES COc1ccc2CN([C@@H](Cc2c1OCc1ccccc1)C(O)=O)C(=O)C(c1ccccc1)c1ccccc1 Show InChI InChI=1S/C32H29NO5/c1-37-28-18-17-25-20-33(31(34)29(23-13-7-3-8-14-23)24-15-9-4-10-16-24)27(32(35)36)19-26(25)30(28)38-21-22-11-5-2-6-12-22/h2-18,27,29H,19-21H2,1H3,(H,35,36)/t27-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Merck Pharmaceutical Company
Curated by ChEMBL
| Assay Description
Inhibitory activity was evaluated against Angiotensin II receptor, type 2 |
J Med Chem 39: 625-56 (1996)
Article DOI: 10.1021/jm9504722
BindingDB Entry DOI: 10.7270/Q29P3299 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Type-2 angiotensin II receptor
(Homo sapiens (Human)) | BDBM50228195

(Angiotensin Ii | CHEBI:2719)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@@H](NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@@H](N)CC(O)=O)C(C)C)C(=O)N[C@@H](Cc1cnc[nH]1)C(=O)N1CCC[C@H]1C(=O)N[C@@H](Cc1ccccc1)C(O)=O |r| Show InChI InChI=1S/C50H71N13O12/c1-5-28(4)41(47(72)59-36(23-31-25-54-26-56-31)48(73)63-20-10-14-38(63)45(70)60-37(49(74)75)22-29-11-7-6-8-12-29)62-44(69)35(21-30-15-17-32(64)18-16-30)58-46(71)40(27(2)3)61-43(68)34(13-9-19-55-50(52)53)57-42(67)33(51)24-39(65)66/h6-8,11-12,15-18,25-28,33-38,40-41,64H,5,9-10,13-14,19-24,51H2,1-4H3,(H,54,56)(H,57,67)(H,58,71)(H,59,72)(H,60,70)(H,61,68)(H,62,69)(H,65,66)(H,74,75)(H4,52,53,55)/t28-,33-,34-,35-,36-,37-,38-,40-,41-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.617 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [125-I]-[Sar1, Ile8] from recombinant human AT2R transfected in human HEK293 cells incubated for 2 hrs by liquid scintillation counte... |
Citation and Details
Article DOI: 10.1016/j.bmcl.2021.128086
BindingDB Entry DOI: 10.7270/Q28S4TPF |
More data for this
Ligand-Target Pair | |
Type-2 angiotensin II receptor
(Homo sapiens (Human)) | BDBM50030707
(CHEMBL122273 | Pentanoic acid (4-bromo-3-{3-ethyl-...)Show SMILES CCCCC(=O)Nc1ccc(Br)c(c1)-n1nc(CC)n(Cc2ccc(cc2F)-c2ccccc2S(=O)(=O)NC(=O)c2ccccc2F)c1=O Show InChI InChI=1S/C35H32BrF2N5O5S/c1-3-5-14-33(44)39-24-17-18-27(36)30(20-24)43-35(46)42(32(4-2)40-43)21-23-16-15-22(19-29(23)38)25-10-7-9-13-31(25)49(47,48)41-34(45)26-11-6-8-12-28(26)37/h6-13,15-20H,3-5,14,21H2,1-2H3,(H,39,44)(H,41,45) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.640 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against Angiotensin II receptor type 2 in human adrenal membrane preparations. |
J Med Chem 38: 3741-58 (1995)
BindingDB Entry DOI: 10.7270/Q2Q81C35 |
More data for this
Ligand-Target Pair | |
Type-2 angiotensin II receptor
(Homo sapiens (Human)) | BDBM50048119
(CHEMBL385283 | c[Sar1-Arg2-Hcy3-Tyr4-MPt5-His6-Pro...)Show SMILES CNCC(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@H]1CCSS[C@@H]2C[C@H](N(C2)C(=O)[C@H](Cc2ccc(O)cc2)NC1=O)C(=O)N[C@@H](Cc1cnc[nH]1)C(=O)N1CCC[C@H]1C(=O)N[C@@H](Cc1ccccc1)C(O)=O Show InChI InChI=1S/C47H63N13O10S2/c1-50-24-39(62)54-32(9-5-16-52-47(48)49)40(63)55-33-15-18-71-72-31-22-38(60(25-31)45(68)34(56-41(33)64)19-28-11-13-30(61)14-12-28)43(66)57-35(21-29-23-51-26-53-29)44(67)59-17-6-10-37(59)42(65)58-36(46(69)70)20-27-7-3-2-4-8-27/h2-4,7-8,11-14,23,26,31-38,50,61H,5-6,9-10,15-22,24-25H2,1H3,(H,51,53)(H,54,62)(H,55,63)(H,56,64)(H,57,66)(H,58,65)(H,69,70)(H4,48,49,52)/t31-,32+,33+,34+,35+,36+,37+,38+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.650 | n/a | n/a | n/a | n/a | n/a | n/a |
Washington University School of Medicine
Curated by ChEMBL
| Assay Description
In vitro binding affinity at angiotensin II (type 2) receptor in rabbit uterus. |
J Med Chem 36: 1902-13 (1993)
BindingDB Entry DOI: 10.7270/Q2N58KF4 |
More data for this
Ligand-Target Pair | |
Type-2 angiotensin II receptor
(Homo sapiens (Human)) | BDBM50283244

(Biphenylsulfonylcarbamate compound | CHEMBL71125)Show SMILES CCCc1nc(CC)c(C(=O)OCc2ccccc2C(=O)c2ccccc2)n1Cc1ccc(cc1F)-c1ccccc1S(=O)(=O)NC(=O)OCCc1ccccc1 Show InChI InChI=1S/C45H42FN3O7S/c1-3-15-41-47-39(4-2)42(44(51)56-30-35-20-11-12-22-37(35)43(50)32-18-9-6-10-19-32)49(41)29-34-25-24-33(28-38(34)46)36-21-13-14-23-40(36)57(53,54)48-45(52)55-27-26-31-16-7-5-8-17-31/h5-14,16-25,28H,3-4,15,26-27,29-30H2,1-2H3,(H,48,52) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
| n/a | n/a | 0.690 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity for angiotensin II AT-2 receptor |
Bioorg Med Chem Lett 4: 2235-2240 (1994)
Article DOI: 10.1016/S0960-894X(00)80077-3
BindingDB Entry DOI: 10.7270/Q2DZ088W |
More data for this
Ligand-Target Pair | |
Type-2 angiotensin II receptor
(Homo sapiens (Human)) | BDBM50049206
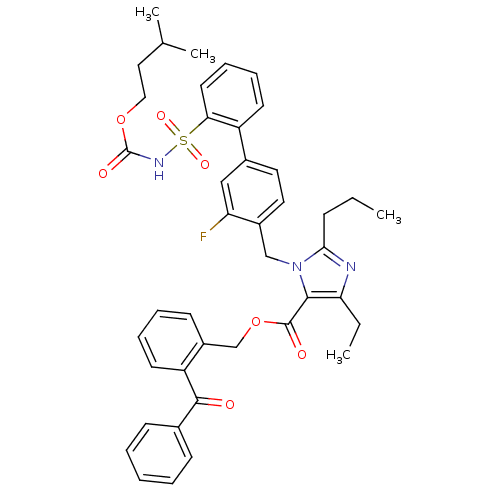
(5-Ethyl-3-(3-fluoro-2'-isopentyloxycarbaronylsulfa...)Show SMILES CCCc1nc(CC)c(C(=O)OCc2ccccc2C(=O)c2ccccc2)n1Cc1ccc(cc1F)-c1ccccc1S(=O)(=O)NC(=O)OCCC(C)C Show InChI InChI=1S/C42H44FN3O7S/c1-5-14-38-44-36(6-2)39(41(48)53-27-32-17-10-11-19-34(32)40(47)29-15-8-7-9-16-29)46(38)26-31-22-21-30(25-35(31)43)33-18-12-13-20-37(33)54(50,51)45-42(49)52-24-23-28(3)4/h7-13,15-22,25,28H,5-6,14,23-24,26-27H2,1-4H3,(H,45,49) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.690 | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Merck Pharmaceutical Company
Curated by ChEMBL
| Assay Description
Inhibition of Angiotensin II receptor, type 2 |
J Med Chem 39: 625-56 (1996)
Article DOI: 10.1021/jm9504722
BindingDB Entry DOI: 10.7270/Q29P3299 |
More data for this
Ligand-Target Pair | |
Type-2 angiotensin II receptor
(Homo sapiens (Human)) | BDBM50049206

(5-Ethyl-3-(3-fluoro-2'-isopentyloxycarbaronylsulfa...)Show SMILES CCCc1nc(CC)c(C(=O)OCc2ccccc2C(=O)c2ccccc2)n1Cc1ccc(cc1F)-c1ccccc1S(=O)(=O)NC(=O)OCCC(C)C Show InChI InChI=1S/C42H44FN3O7S/c1-5-14-38-44-36(6-2)39(41(48)53-27-32-17-10-11-19-34(32)40(47)29-15-8-7-9-16-29)46(38)26-31-22-21-30(25-35(31)43)33-18-12-13-20-37(33)54(50,51)45-42(49)52-24-23-28(3)4/h7-13,15-22,25,28H,5-6,14,23-24,26-27H2,1-4H3,(H,45,49) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
| n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity for angiotensin II AT-2 receptor |
Bioorg Med Chem Lett 4: 2235-2240 (1994)
Article DOI: 10.1016/S0960-894X(00)80077-3
BindingDB Entry DOI: 10.7270/Q2DZ088W |
More data for this
Ligand-Target Pair | |
Type-2 angiotensin II receptor
(Homo sapiens (Human)) | BDBM50141059
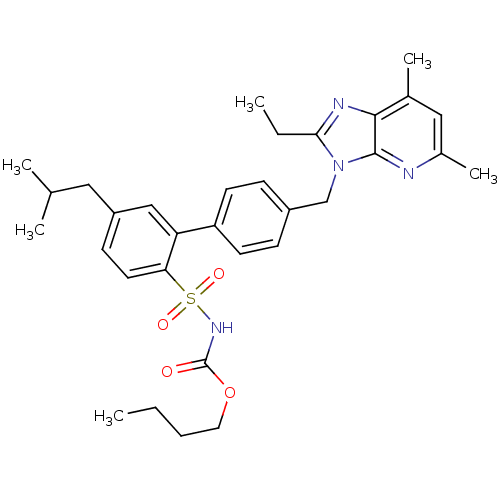
(CHEMBL289614 | L-162782 | N-Butyloxycarbonyl-4'-(2...)Show SMILES CCCCOC(=O)NS(=O)(=O)c1ccc(CC(C)C)cc1-c1ccc(Cn2c(CC)nc3c(C)cc(C)nc23)cc1 Show InChI InChI=1S/C32H40N4O4S/c1-7-9-16-40-32(37)35-41(38,39)28-15-12-25(17-21(3)4)19-27(28)26-13-10-24(11-14-26)20-36-29(8-2)34-30-22(5)18-23(6)33-31(30)36/h10-15,18-19,21H,7-9,16-17,20H2,1-6H3,(H,35,37) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University
Curated by ChEMBL
| Assay Description
Binding affinity to type-2 angiotensin-2 receptor (unknown origin) |
Bioorg Med Chem Lett 26: 1355-9 (2016)
Article DOI: 10.1016/j.bmcl.2015.10.084
BindingDB Entry DOI: 10.7270/Q2SN0BT6 |
More data for this
Ligand-Target Pair | |
Type-2 angiotensin II receptor
(Homo sapiens (Human)) | BDBM50141059

(CHEMBL289614 | L-162782 | N-Butyloxycarbonyl-4'-(2...)Show SMILES CCCCOC(=O)NS(=O)(=O)c1ccc(CC(C)C)cc1-c1ccc(Cn2c(CC)nc3c(C)cc(C)nc23)cc1 Show InChI InChI=1S/C32H40N4O4S/c1-7-9-16-40-32(37)35-41(38,39)28-15-12-25(17-21(3)4)19-27(28)26-13-10-24(11-14-26)20-36-29(8-2)34-30-22(5)18-23(6)33-31(30)36/h10-15,18-19,21H,7-9,16-17,20H2,1-6H3,(H,35,37) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University
Curated by ChEMBL
| Assay Description
Binding affinity to AT2 receptor (unknown origin) |
ACS Med Chem Lett 6: 178-82 (2015)
Article DOI: 10.1021/ml500427r
BindingDB Entry DOI: 10.7270/Q2HD7X98 |
More data for this
Ligand-Target Pair | |
Type-2 angiotensin II receptor
(Homo sapiens (Human)) | BDBM50141059

(CHEMBL289614 | L-162782 | N-Butyloxycarbonyl-4'-(2...)Show SMILES CCCCOC(=O)NS(=O)(=O)c1ccc(CC(C)C)cc1-c1ccc(Cn2c(CC)nc3c(C)cc(C)nc23)cc1 Show InChI InChI=1S/C32H40N4O4S/c1-7-9-16-40-32(37)35-41(38,39)28-15-12-25(17-21(3)4)19-27(28)26-13-10-24(11-14-26)20-36-29(8-2)34-30-22(5)18-23(6)33-31(30)36/h10-15,18-19,21H,7-9,16-17,20H2,1-6H3,(H,35,37) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University
Curated by ChEMBL
| Assay Description
Binding affinity of the compound towards Angiotensin II receptor, type 2 was measured through displacement of [125I]-Ang II in pig uterus myometrial ... |
J Med Chem 47: 1536-46 (2004)
Article DOI: 10.1021/jm031031i
BindingDB Entry DOI: 10.7270/Q2NS0TBV |
More data for this
Ligand-Target Pair | |
Type-2 angiotensin II receptor
(Homo sapiens (Human)) | BDBM82433
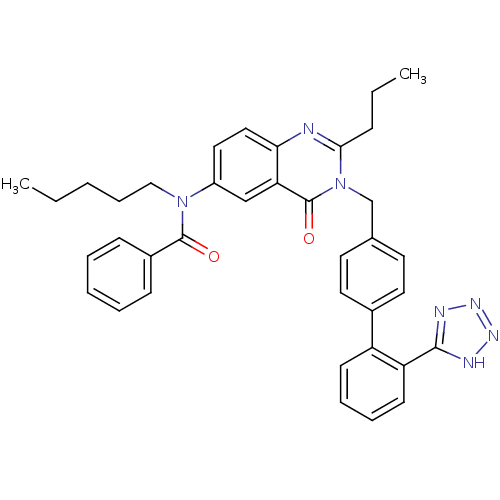
(CHEMBL302102 | L-159,689 | L-159689 | N-Pentyl-N-[...)Show SMILES CCCCCN(C(=O)c1ccccc1)c1ccc2nc(CCC)n(Cc3ccc(cc3)-c3ccccc3-c3nnn[nH]3)c(=O)c2c1 Show InChI InChI=1S/C37H37N7O2/c1-3-5-11-23-43(36(45)28-13-7-6-8-14-28)29-21-22-33-32(24-29)37(46)44(34(38-33)12-4-2)25-26-17-19-27(20-18-26)30-15-9-10-16-31(30)35-39-41-42-40-35/h6-10,13-22,24H,3-5,11-12,23,25H2,1-2H3,(H,39,40,41,42) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
Organon Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory concentration against angiotensin II receptor, type 2 |
J Med Chem 48: 6523-43 (2005)
Article DOI: 10.1021/jm058225d
BindingDB Entry DOI: 10.7270/Q2SF2WZ9 |
More data for this
Ligand-Target Pair | |
Type-2 angiotensin II receptor
(Homo sapiens (Human)) | BDBM50048113
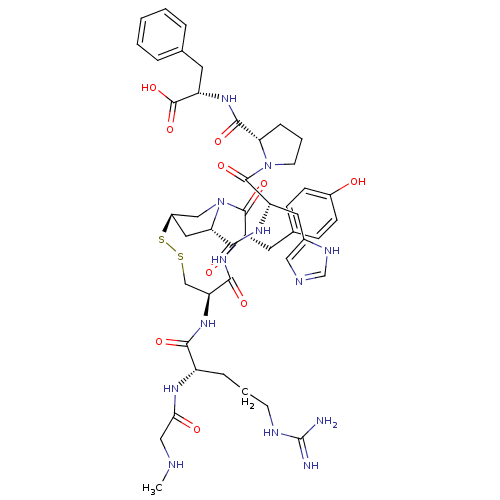
(CHEMBL414533 | c[Sar1-Arg2-Cys3-Tyr4-MPt5-His6-Pro...)Show SMILES CNCC(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@H]1CSS[C@@H]2C[C@H](N(C2)C(=O)[C@H](Cc2ccc(O)cc2)NC1=O)C(=O)N[C@@H](Cc1cnc[nH]1)C(=O)N1CCC[C@H]1C(=O)N[C@@H](Cc1ccccc1)C(O)=O Show InChI InChI=1S/C46H61N13O10S2/c1-49-22-38(61)53-31(9-5-15-51-46(47)48)39(62)57-35-24-70-71-30-20-37(59(23-30)44(67)32(54-40(35)63)17-27-11-13-29(60)14-12-27)42(65)55-33(19-28-21-50-25-52-28)43(66)58-16-6-10-36(58)41(64)56-34(45(68)69)18-26-7-3-2-4-8-26/h2-4,7-8,11-14,21,25,30-37,49,60H,5-6,9-10,15-20,22-24H2,1H3,(H,50,52)(H,53,61)(H,54,63)(H,55,65)(H,56,64)(H,57,62)(H,68,69)(H4,47,48,51)/t30-,31+,32+,33+,34+,35+,36+,37+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.820 | n/a | n/a | n/a | n/a | n/a | n/a |
Washington University School of Medicine
Curated by ChEMBL
| Assay Description
In vitro binding affinity at angiotensin II (type 2) receptor in rabbit uterus. |
J Med Chem 36: 1902-13 (1993)
BindingDB Entry DOI: 10.7270/Q2N58KF4 |
More data for this
Ligand-Target Pair | |
Type-2 angiotensin II receptor
(Homo sapiens (Human)) | BDBM50030732
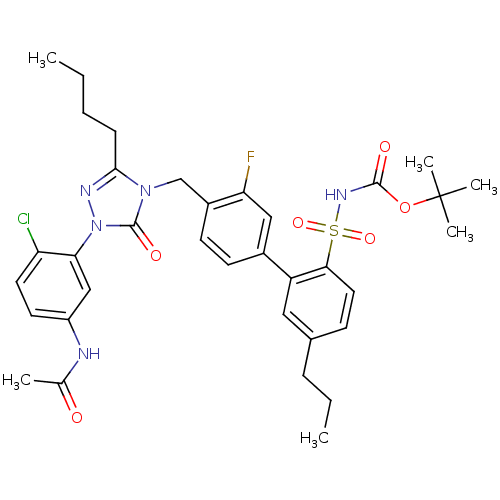
(CHEMBL323506 | N-{3-[3-Butyl-4-(3-fluoro-2'(tert-b...)Show SMILES CCCCc1nn(-c2cc(NC(C)=O)ccc2Cl)c(=O)n1Cc1ccc(cc1F)-c1cc(CCC)ccc1S(=O)(=O)NC(=O)OC(C)(C)C Show InChI InChI=1S/C35H41ClFN5O6S/c1-7-9-11-32-39-42(30-20-26(38-22(3)43)15-16-28(30)36)34(45)41(32)21-25-14-13-24(19-29(25)37)27-18-23(10-8-2)12-17-31(27)49(46,47)40-33(44)48-35(4,5)6/h12-20H,7-11,21H2,1-6H3,(H,38,43)(H,40,44) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
| n/a | n/a | 0.820 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory concentration of the compound against cloned human AT1 receptor |
Bioorg Med Chem Lett 4: 2787-2792 (1994)
Article DOI: 10.1016/S0960-894X(01)80595-3
BindingDB Entry DOI: 10.7270/Q2WS8T68 |
More data for this
Ligand-Target Pair | |
Type-2 angiotensin II receptor
(Homo sapiens (Human)) | BDBM50049182

((S)-5-Benzyloxy-2-diphenylacetyl-6-methoxy-1,2,3,4...)Show SMILES COc1ccc2CN([C@@H](Cc2c1OCc1ccccc1)C(O)=O)C(=O)C(c1ccccc1)c1ccccc1 Show InChI InChI=1S/C32H29NO5/c1-37-28-18-17-25-20-33(31(34)29(23-13-7-3-8-14-23)24-15-9-4-10-16-24)27(32(35)36)19-26(25)30(28)38-21-22-11-5-2-6-12-22/h2-18,27,29H,19-21H2,1H3,(H,35,36)/t27-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
| n/a | n/a | 0.840 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity against angiotensin II type 2 (AT2) receptor in rabbit uterine membranes. |
Bioorg Med Chem Lett 4: 57-62 (1994)
Article DOI: 10.1016/S0960-894X(01)81122-7
BindingDB Entry DOI: 10.7270/Q2SJ1KJV |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Type-2 angiotensin II receptor
(Homo sapiens (Human)) | BDBM50049182

((S)-5-Benzyloxy-2-diphenylacetyl-6-methoxy-1,2,3,4...)Show SMILES COc1ccc2CN([C@@H](Cc2c1OCc1ccccc1)C(O)=O)C(=O)C(c1ccccc1)c1ccccc1 Show InChI InChI=1S/C32H29NO5/c1-37-28-18-17-25-20-33(31(34)29(23-13-7-3-8-14-23)24-15-9-4-10-16-24)27(32(35)36)19-26(25)30(28)38-21-22-11-5-2-6-12-22/h2-18,27,29H,19-21H2,1H3,(H,35,36)/t27-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
| n/a | n/a | 0.840 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity against angiotensin II type 2 (AT2) receptor in rabbit uterine membranes. |
Bioorg Med Chem Lett 4: 57-62 (1994)
Article DOI: 10.1016/S0960-894X(01)81122-7
BindingDB Entry DOI: 10.7270/Q2SJ1KJV |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Type-2 angiotensin II receptor
(Homo sapiens (Human)) | BDBM50030709

(CHEMBL338434 | Pentanoic acid {3-[3-butyl-4-(3-flu...)Show SMILES CCCCC(=O)Nc1ccc(c(c1)-n1nc(CCCC)n(Cc2ccc(cc2F)-c2ccccc2S(=O)(=O)NC(=O)OC(C)(C)C)c1=O)C(F)(F)F Show InChI InChI=1S/C36H41F4N5O6S/c1-6-8-14-31-42-45(29-21-25(41-32(46)15-9-7-2)18-19-27(29)36(38,39)40)34(48)44(31)22-24-17-16-23(20-28(24)37)26-12-10-11-13-30(26)52(49,50)43-33(47)51-35(3,4)5/h10-13,16-21H,6-9,14-15,22H2,1-5H3,(H,41,46)(H,43,47) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 0.880 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against Angiotensin II receptor, type 2 in human adrenal membrane preparations. For this assay, only 0.02%BSA was presen... |
J Med Chem 38: 3741-58 (1995)
BindingDB Entry DOI: 10.7270/Q2Q81C35 |
More data for this
Ligand-Target Pair | |
Type-2 angiotensin II receptor
(Homo sapiens (Human)) | BDBM50280907
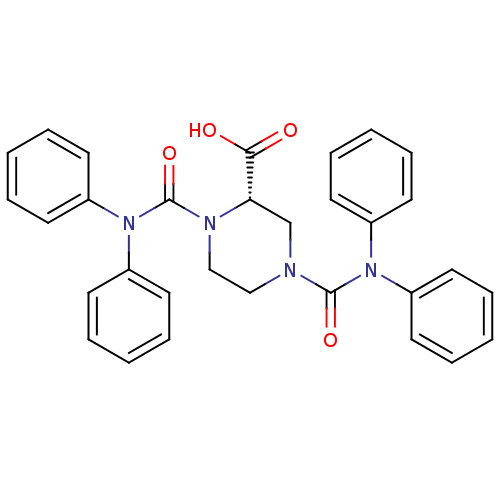
((S)-1,4-Bis-diphenylcarbamoyl-piperazine-2-carboxy...)Show SMILES OC(=O)[C@@H]1CN(CCN1C(=O)N(c1ccccc1)c1ccccc1)C(=O)N(c1ccccc1)c1ccccc1 Show InChI InChI=1S/C31H28N4O4/c36-29(37)28-23-32(30(38)34(24-13-5-1-6-14-24)25-15-7-2-8-16-25)21-22-33(28)31(39)35(26-17-9-3-10-18-26)27-19-11-4-12-20-27/h1-20,28H,21-23H2,(H,36,37)/t28-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 0.890 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity against angiotensin II type 2 (AT2) receptor in rabbit uterine membranes. |
Bioorg Med Chem Lett 4: 57-62 (1994)
Article DOI: 10.1016/S0960-894X(01)81122-7
BindingDB Entry DOI: 10.7270/Q2SJ1KJV |
More data for this
Ligand-Target Pair | |
Type-2 angiotensin II receptor
(Homo sapiens (Human)) | BDBM50283200
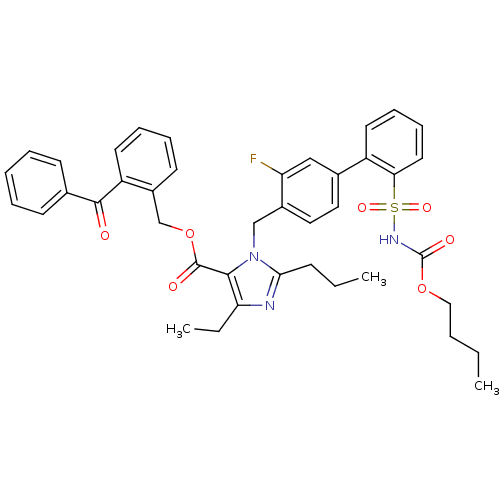
(Biphenylsulfonylcarbamate compound | CHEMBL71172)Show SMILES CCCCOC(=O)NS(=O)(=O)c1ccccc1-c1ccc(Cn2c(CCC)nc(CC)c2C(=O)OCc2ccccc2C(=O)c2ccccc2)c(F)c1 Show InChI InChI=1S/C41H42FN3O7S/c1-4-7-24-51-41(48)44-53(49,50)36-21-14-13-19-32(36)29-22-23-30(34(42)25-29)26-45-37(15-5-2)43-35(6-3)38(45)40(47)52-27-31-18-11-12-20-33(31)39(46)28-16-9-8-10-17-28/h8-14,16-23,25H,4-7,15,24,26-27H2,1-3H3,(H,44,48) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
| n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity for angiotensin II AT-2 receptor |
Bioorg Med Chem Lett 4: 2235-2240 (1994)
Article DOI: 10.1016/S0960-894X(00)80077-3
BindingDB Entry DOI: 10.7270/Q2DZ088W |
More data for this
Ligand-Target Pair | |
Type-2 angiotensin II receptor
(Homo sapiens (Human)) | BDBM50283199
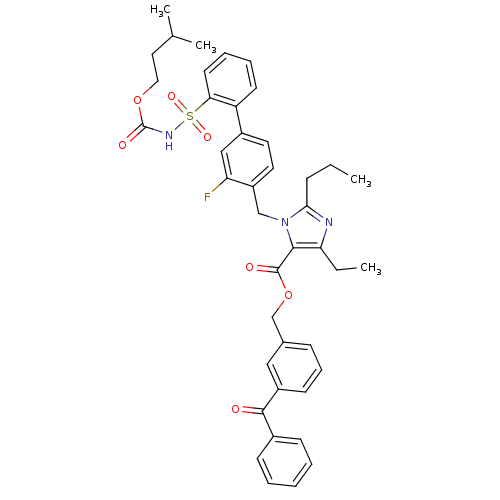
(Biphenylsulfonylcarbamate compound | CHEMBL71220)Show SMILES CCCc1nc(CC)c(C(=O)OCc2cccc(c2)C(=O)c2ccccc2)n1Cc1ccc(cc1F)-c1ccccc1S(=O)(=O)NC(=O)OCCC(C)C Show InChI InChI=1S/C42H44FN3O7S/c1-5-13-38-44-36(6-2)39(41(48)53-27-29-14-12-17-32(24-29)40(47)30-15-8-7-9-16-30)46(38)26-33-21-20-31(25-35(33)43)34-18-10-11-19-37(34)54(50,51)45-42(49)52-23-22-28(3)4/h7-12,14-21,24-25,28H,5-6,13,22-23,26-27H2,1-4H3,(H,45,49) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity for angiotensin II AT-2 receptor |
Bioorg Med Chem Lett 4: 2235-2240 (1994)
Article DOI: 10.1016/S0960-894X(00)80077-3
BindingDB Entry DOI: 10.7270/Q2DZ088W |
More data for this
Ligand-Target Pair | |
Type-2 angiotensin II receptor
(Homo sapiens (Human)) | BDBM50285762
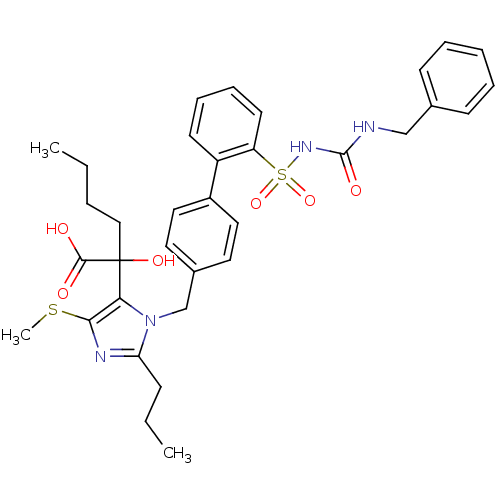
(5-alpha-hydroxyacid imidazolyl biphenyl sulfonyl u...)Show SMILES CCCCC(O)(C(O)=O)c1c(SC)nc(CCC)n1Cc1ccc(cc1)-c1ccccc1S(=O)(=O)NC(=O)NCc1ccccc1 Show InChI InChI=1S/C34H40N4O6S2/c1-4-6-21-34(42,32(39)40)30-31(45-3)36-29(12-5-2)38(30)23-25-17-19-26(20-18-25)27-15-10-11-16-28(27)46(43,44)37-33(41)35-22-24-13-8-7-9-14-24/h7-11,13-20,42H,4-6,12,21-23H2,1-3H3,(H,39,40)(H2,35,37,41) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro binding affinity at Angiotensin II Type 2 receptor in rabbit uterus membrane by [125I]-AII displacement. |
Bioorg Med Chem Lett 5: 2611-2616 (1995)
Article DOI: 10.1016/0960-894X(95)00479-D
BindingDB Entry DOI: 10.7270/Q2GT5N57 |
More data for this
Ligand-Target Pair | |
Type-2 angiotensin II receptor
(Homo sapiens (Human)) | BDBM50283245
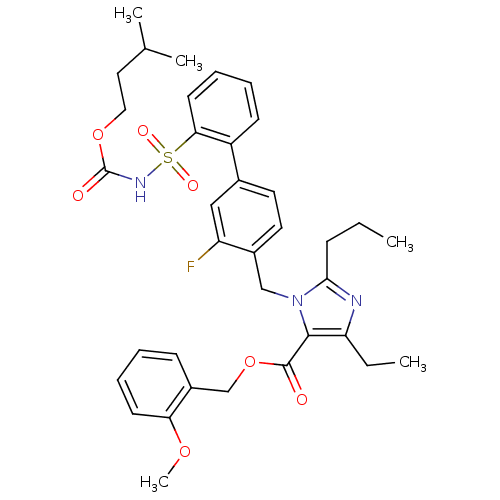
(Biphenylsulfonylcarbamate compound | CHEMBL70789)Show SMILES CCCc1nc(CC)c(C(=O)OCc2ccccc2OC)n1Cc1ccc(cc1F)-c1ccccc1S(=O)(=O)NC(=O)OCCC(C)C Show InChI InChI=1S/C36H42FN3O7S/c1-6-12-33-38-30(7-2)34(35(41)47-23-27-13-8-10-15-31(27)45-5)40(33)22-26-18-17-25(21-29(26)37)28-14-9-11-16-32(28)48(43,44)39-36(42)46-20-19-24(3)4/h8-11,13-18,21,24H,6-7,12,19-20,22-23H2,1-5H3,(H,39,42) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity for angiotensin II AT-2 receptor |
Bioorg Med Chem Lett 4: 2235-2240 (1994)
Article DOI: 10.1016/S0960-894X(00)80077-3
BindingDB Entry DOI: 10.7270/Q2DZ088W |
More data for this
Ligand-Target Pair | |
Type-2 angiotensin II receptor
(Homo sapiens (Human)) | BDBM50283234
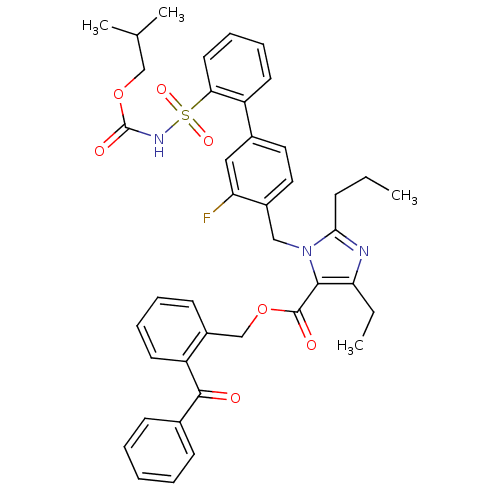
(Biphenylsulfonylcarbamate compound | CHEMBL72595)Show SMILES CCCc1nc(CC)c(C(=O)OCc2ccccc2C(=O)c2ccccc2)n1Cc1ccc(cc1F)-c1ccccc1S(=O)(=O)NC(=O)OCC(C)C Show InChI InChI=1S/C41H42FN3O7S/c1-5-14-37-43-35(6-2)38(40(47)51-26-31-17-10-11-19-33(31)39(46)28-15-8-7-9-16-28)45(37)24-30-22-21-29(23-34(30)42)32-18-12-13-20-36(32)53(49,50)44-41(48)52-25-27(3)4/h7-13,15-23,27H,5-6,14,24-26H2,1-4H3,(H,44,48) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity for angiotensin II AT-2 receptor |
Bioorg Med Chem Lett 4: 2235-2240 (1994)
Article DOI: 10.1016/S0960-894X(00)80077-3
BindingDB Entry DOI: 10.7270/Q2DZ088W |
More data for this
Ligand-Target Pair | |
Type-2 angiotensin II receptor
(Homo sapiens (Human)) | BDBM50283238
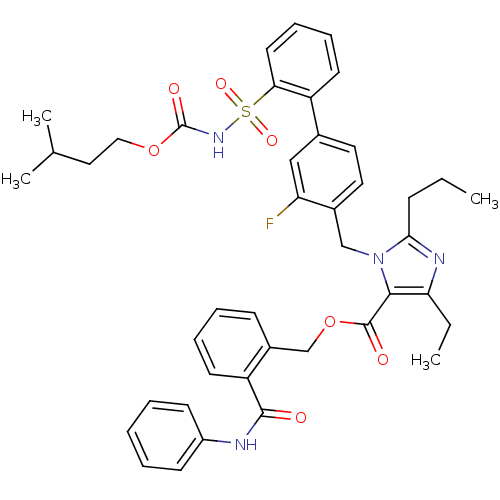
(Biphenylsulfonylcarbamate compound | CHEMBL70867)Show SMILES CCCc1nc(CC)c(C(=O)OCc2ccccc2C(=O)Nc2ccccc2)n1Cc1ccc(cc1F)-c1ccccc1S(=O)(=O)NC(=O)OCCC(C)C Show InChI InChI=1S/C42H45FN4O7S/c1-5-14-38-45-36(6-2)39(41(49)54-27-31-15-10-11-19-34(31)40(48)44-32-16-8-7-9-17-32)47(38)26-30-22-21-29(25-35(30)43)33-18-12-13-20-37(33)55(51,52)46-42(50)53-24-23-28(3)4/h7-13,15-22,25,28H,5-6,14,23-24,26-27H2,1-4H3,(H,44,48)(H,46,50) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity for angiotensin II AT-2 receptor |
Bioorg Med Chem Lett 4: 2235-2240 (1994)
Article DOI: 10.1016/S0960-894X(00)80077-3
BindingDB Entry DOI: 10.7270/Q2DZ088W |
More data for this
Ligand-Target Pair | |
Type-2 angiotensin II receptor
(Homo sapiens (Human)) | BDBM50032362
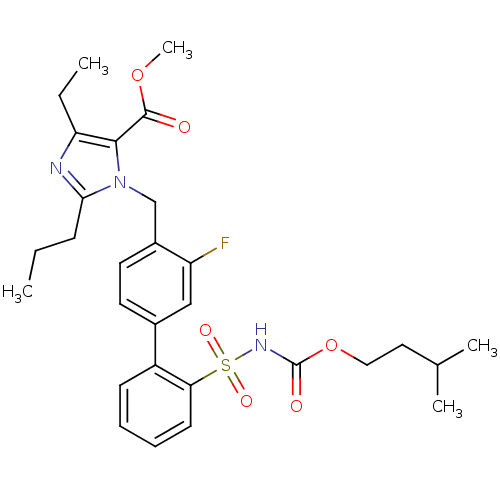
(3-(2'-Isopentyloxycarbonylsulfamoyl-3-fluoro-biphe...)Show SMILES CCCc1nc(CC)c(C(=O)OC)n1Cc1ccc(cc1F)-c1ccccc1S(=O)(=O)NC(=O)OCCC(C)C Show InChI InChI=1S/C29H36FN3O6S/c1-6-10-26-31-24(7-2)27(28(34)38-5)33(26)18-21-14-13-20(17-23(21)30)22-11-8-9-12-25(22)40(36,37)32-29(35)39-16-15-19(3)4/h8-9,11-14,17,19H,6-7,10,15-16,18H2,1-5H3,(H,32,35) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Merck Pharmaceutical Company
Curated by ChEMBL
| Assay Description
Inhibition of Angiotensin II receptor, type 2 |
J Med Chem 39: 625-56 (1996)
Article DOI: 10.1021/jm9504722
BindingDB Entry DOI: 10.7270/Q29P3299 |
More data for this
Ligand-Target Pair | |
Type-2 angiotensin II receptor
(Homo sapiens (Human)) | BDBM50283220

(Biphenylsulfonylcarbamate compound | CHEMBL302579)Show SMILES CCCc1nc(CC)c(C(=O)OCc2ccccc2NC(=O)c2ccccc2)n1Cc1ccc(cc1F)-c1ccccc1S(=O)(=O)NC(=O)OCCC(C)C Show InChI InChI=1S/C42H45FN4O7S/c1-5-14-38-44-35(6-2)39(41(49)54-27-32-17-10-12-19-36(32)45-40(48)29-15-8-7-9-16-29)47(38)26-31-22-21-30(25-34(31)43)33-18-11-13-20-37(33)55(51,52)46-42(50)53-24-23-28(3)4/h7-13,15-22,25,28H,5-6,14,23-24,26-27H2,1-4H3,(H,45,48)(H,46,50) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity for angiotensin II AT-2 receptor |
Bioorg Med Chem Lett 4: 2235-2240 (1994)
Article DOI: 10.1016/S0960-894X(00)80077-3
BindingDB Entry DOI: 10.7270/Q2DZ088W |
More data for this
Ligand-Target Pair | |
Type-2 angiotensin II receptor
(Homo sapiens (Human)) | BDBM50283204

(Biphenylsulfonylcarbamate compound | CHEMBL311625)Show SMILES CCCc1nc(CC)c(C(=O)OCCCS(=O)c2ccccc2)n1Cc1ccc(cc1F)-c1ccccc1S(=O)(=O)NC(=O)OCCC(C)C Show InChI InChI=1S/C37H44FN3O7S2/c1-5-13-34-39-32(6-2)35(36(42)47-21-12-23-49(44)29-14-8-7-9-15-29)41(34)25-28-19-18-27(24-31(28)38)30-16-10-11-17-33(30)50(45,46)40-37(43)48-22-20-26(3)4/h7-11,14-19,24,26H,5-6,12-13,20-23,25H2,1-4H3,(H,40,43) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity for angiotensin II AT-2 receptor |
Bioorg Med Chem Lett 4: 2235-2240 (1994)
Article DOI: 10.1016/S0960-894X(00)80077-3
BindingDB Entry DOI: 10.7270/Q2DZ088W |
More data for this
Ligand-Target Pair | |
Type-2 angiotensin II receptor
(Homo sapiens (Human)) | BDBM50283213

(Biphenylsulfonylcarbamate compound | CHEMBL307318)Show SMILES CCCCCOC(=O)NS(=O)(=O)c1ccccc1-c1ccc(Cn2c(CCC)nc(CC)c2C(=O)OCCC(=O)Nc2ccccc2)c(F)c1 Show InChI InChI=1S/C37H43FN4O7S/c1-4-7-13-22-49-37(45)41-50(46,47)32-18-12-11-17-29(32)26-19-20-27(30(38)24-26)25-42-33(14-5-2)40-31(6-3)35(42)36(44)48-23-21-34(43)39-28-15-9-8-10-16-28/h8-12,15-20,24H,4-7,13-14,21-23,25H2,1-3H3,(H,39,43)(H,41,45) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity for angiotensin II AT-2 receptor |
Bioorg Med Chem Lett 4: 2235-2240 (1994)
Article DOI: 10.1016/S0960-894X(00)80077-3
BindingDB Entry DOI: 10.7270/Q2DZ088W |
More data for this
Ligand-Target Pair | |
Type-2 angiotensin II receptor
(Homo sapiens (Human)) | BDBM50285752
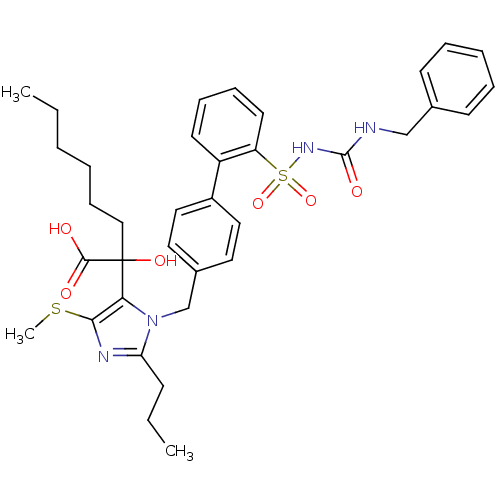
(5-alpha-hydroxyacid imidazolyl biphenyl sulfonyl u...)Show SMILES CCCCCCC(O)(C(O)=O)c1c(SC)nc(CCC)n1Cc1ccc(cc1)-c1ccccc1S(=O)(=O)NC(=O)NCc1ccccc1 Show InChI InChI=1S/C36H44N4O6S2/c1-4-6-7-13-23-36(44,34(41)42)32-33(47-3)38-31(14-5-2)40(32)25-27-19-21-28(22-20-27)29-17-11-12-18-30(29)48(45,46)39-35(43)37-24-26-15-9-8-10-16-26/h8-12,15-22,44H,4-7,13-14,23-25H2,1-3H3,(H,41,42)(H2,37,39,43) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro binding affinity at Angiotensin II Type 2 receptor in rabbit uterus membrane by [125I]-AII displacement. |
Bioorg Med Chem Lett 5: 2611-2616 (1995)
Article DOI: 10.1016/0960-894X(95)00479-D
BindingDB Entry DOI: 10.7270/Q2GT5N57 |
More data for this
Ligand-Target Pair | |
Type-2 angiotensin II receptor
(Homo sapiens (Human)) | BDBM50285763
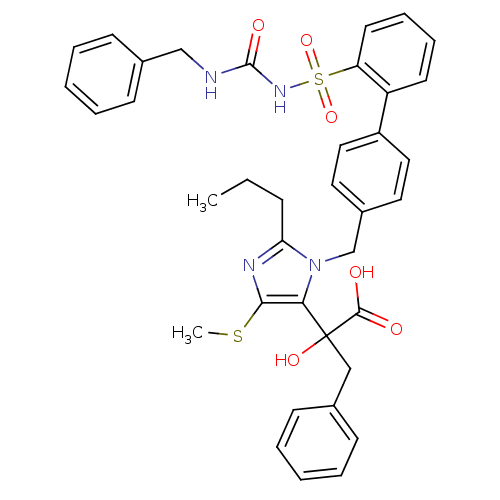
(5-alpha-hydroxyacid imidazolyl biphenyl sulfonyl u...)Show SMILES CCCc1nc(SC)c(n1Cc1ccc(cc1)-c1ccccc1S(=O)(=O)NC(=O)NCc1ccccc1)C(O)(Cc1ccccc1)C(O)=O Show InChI InChI=1S/C37H38N4O6S2/c1-3-12-32-39-34(48-2)33(37(45,35(42)43)23-26-13-6-4-7-14-26)41(32)25-28-19-21-29(22-20-28)30-17-10-11-18-31(30)49(46,47)40-36(44)38-24-27-15-8-5-9-16-27/h4-11,13-22,45H,3,12,23-25H2,1-2H3,(H,42,43)(H2,38,40,44) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro binding affinity at Angiotensin II Type 2 receptor in rabbit uterus membrane by [125I]-AII displacement. |
Bioorg Med Chem Lett 5: 2611-2616 (1995)
Article DOI: 10.1016/0960-894X(95)00479-D
BindingDB Entry DOI: 10.7270/Q2GT5N57 |
More data for this
Ligand-Target Pair | |
Type-2 angiotensin II receptor
(Homo sapiens (Human)) | BDBM50282404

(5-Benzyloxy-2-(2-cyclopentyl-2-phenyl-acetyl)-6-me...)Show SMILES COc1ccc2CN(C(Cc2c1OCc1ccccc1)C(O)=O)C(=O)C(C1CCCC1)c1ccccc1 Show InChI InChI=1S/C31H33NO5/c1-36-27-17-16-24-19-32(30(33)28(23-14-8-9-15-23)22-12-6-3-7-13-22)26(31(34)35)18-25(24)29(27)37-20-21-10-4-2-5-11-21/h2-7,10-13,16-17,23,26,28H,8-9,14-15,18-20H2,1H3,(H,34,35) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
| n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity against angiotensin II type 2 (AT2) receptor in rabbit uterine membranes. |
Bioorg Med Chem Lett 4: 57-62 (1994)
Article DOI: 10.1016/S0960-894X(01)81122-7
BindingDB Entry DOI: 10.7270/Q2SJ1KJV |
More data for this
Ligand-Target Pair | |
Type-2 angiotensin II receptor
(Homo sapiens (Human)) | BDBM50285757

(5-alpha-hydroxyacid imidazolyl biphenyl sulfonyl u...)Show SMILES CCCc1nc(SC)c(n1Cc1ccc(cc1)-c1ccccc1S(=O)(=O)NC(=O)NCc1ccccc1)C(O)(C(C)C)C(O)=O Show InChI InChI=1S/C33H38N4O6S2/c1-5-11-28-35-30(44-4)29(33(41,22(2)3)31(38)39)37(28)21-24-16-18-25(19-17-24)26-14-9-10-15-27(26)45(42,43)36-32(40)34-20-23-12-7-6-8-13-23/h6-10,12-19,22,41H,5,11,20-21H2,1-4H3,(H,38,39)(H2,34,36,40) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro binding affinity at Angiotensin II Type 2 receptor in rabbit uterus membrane by [125I]-AII displacement. |
Bioorg Med Chem Lett 5: 2611-2616 (1995)
Article DOI: 10.1016/0960-894X(95)00479-D
BindingDB Entry DOI: 10.7270/Q2GT5N57 |
More data for this
Ligand-Target Pair | |
Type-2 angiotensin II receptor
(Homo sapiens (Human)) | BDBM50030693

(CHEMBL338687 | Pentanoic acid {4-bromo-3-[3-ethyl-...)Show SMILES CCCCC(=O)Nc1ccc(Br)c(c1)-n1nc(CC)n(Cc2ccc(cc2F)-c2ccccc2S(=O)(=O)NC(=O)OC(C)C)c1=O Show InChI InChI=1S/C32H35BrFN5O6S/c1-5-7-12-30(40)35-23-15-16-25(33)27(18-23)39-32(42)38(29(6-2)36-39)19-22-14-13-21(17-26(22)34)24-10-8-9-11-28(24)46(43,44)37-31(41)45-20(3)4/h8-11,13-18,20H,5-7,12,19H2,1-4H3,(H,35,40)(H,37,41) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against Angiotensin II receptor type 2 in human adrenal membrane preparations. |
J Med Chem 38: 3741-58 (1995)
BindingDB Entry DOI: 10.7270/Q2Q81C35 |
More data for this
Ligand-Target Pair | |
Type-2 angiotensin II receptor
(Homo sapiens (Human)) | BDBM50608290

(CHEMBL5272027) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Type-2 angiotensin II receptor
(Homo sapiens (Human)) | BDBM50030684
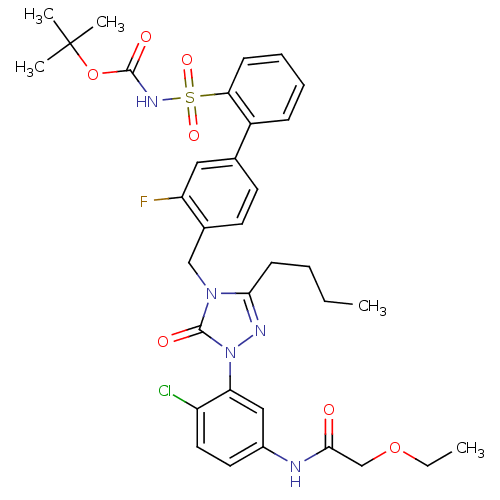
(CHEMBL339722 | N-{3-[3-Butyl-4-(3-fluoro-2-(N-t-bu...)Show SMILES CCCCc1nn(-c2cc(NC(=O)COCC)ccc2Cl)c(=O)n1Cc1ccc(cc1F)-c1ccccc1S(=O)(=O)NC(=O)OC(C)(C)C Show InChI InChI=1S/C34H39ClFN5O7S/c1-6-8-13-30-38-41(28-19-24(16-17-26(28)35)37-31(42)21-47-7-2)33(44)40(30)20-23-15-14-22(18-27(23)36)25-11-9-10-12-29(25)49(45,46)39-32(43)48-34(3,4)5/h9-12,14-19H,6-8,13,20-21H2,1-5H3,(H,37,42)(H,39,43) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against Angiotensin II receptor, type 2 in human adrenal membrane preparations. For this assay, only 0.02%BSA was presen... |
J Med Chem 38: 3741-58 (1995)
BindingDB Entry DOI: 10.7270/Q2Q81C35 |
More data for this
Ligand-Target Pair | |
Type-2 angiotensin II receptor
(Homo sapiens (Human)) | BDBM50009338

((S)-2-((S)-1-((S)-2-((S)-2-((S)-2-((S)-2-((S)-5-(d...)Show SMILES CNCC(=O)N[C@@H](CCCN=C(N)N)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](Cc1ccc(O)cc1)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](Cc1cnc[nH]1)C(=O)N1CCC[C@H]1C(=O)N[C@@H](C)C(O)=O |r,wU:60.63,wD:24.23,36.36,43.43,56.59,17.16,6.5,(6.84,-31.4,;5.3,-31.4,;4.53,-30.07,;2.99,-30.07,;2.22,-31.4,;2.22,-28.73,;2.99,-27.4,;4.53,-27.4,;5.3,-26.07,;4.53,-24.73,;5.3,-23.4,;6.84,-23.4,;7.61,-24.73,;7.61,-22.07,;2.22,-26.07,;2.99,-24.73,;.68,-26.07,;-.09,-24.73,;.68,-23.4,;2.22,-23.4,;-.09,-22.07,;-1.63,-24.73,;-2.4,-23.4,;-2.4,-26.07,;-3.94,-26.07,;-4.71,-24.73,;-6.25,-24.73,;-7.02,-23.4,;-8.56,-23.4,;-9.33,-24.73,;-10.87,-24.73,;-8.56,-26.07,;-7.02,-26.07,;-4.71,-27.4,;-6.25,-27.4,;-3.94,-28.73,;-4.71,-30.07,;-6.25,-30.07,;-7.02,-28.73,;-7.02,-31.4,;-3.94,-31.4,;-2.4,-31.4,;-4.71,-32.73,;-3.94,-34.07,;-4.71,-35.4,;-6.25,-35.4,;-7.15,-34.16,;-8.62,-34.63,;-8.62,-36.17,;-7.15,-36.65,;-2.4,-34.07,;-1.63,-32.73,;-1.63,-35.4,;-2.26,-36.81,;-1.12,-37.84,;.22,-37.07,;-.1,-35.56,;.93,-34.42,;.45,-32.95,;2.42,-34.82,;3.5,-33.73,;3.11,-32.24,;5,-34.13,;6.09,-33.04,;5.39,-35.61,)| Show InChI InChI=1S/C42H65N13O10/c1-22(2)33(53-35(58)28(50-32(57)20-45-6)9-7-15-47-42(43)44)38(61)51-29(17-25-11-13-27(56)14-12-25)36(59)54-34(23(3)4)39(62)52-30(18-26-19-46-21-48-26)40(63)55-16-8-10-31(55)37(60)49-24(5)41(64)65/h11-14,19,21-24,28-31,33-34,45,56H,7-10,15-18,20H2,1-6H3,(H,46,48)(H,49,60)(H,50,57)(H,51,61)(H,52,62)(H,53,58)(H,54,59)(H,64,65)(H4,43,44,47)/t24-,28-,29-,30-,31-,33-,34-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Washington University School of Medicine
Curated by ChEMBL
| Assay Description
In vitro binding affinity at angiotensin II (type 2) receptor in rabbit uterus. |
J Med Chem 36: 1902-13 (1993)
BindingDB Entry DOI: 10.7270/Q2N58KF4 |
More data for this
Ligand-Target Pair | |
Type-2 angiotensin II receptor
(Homo sapiens (Human)) | BDBM50030687
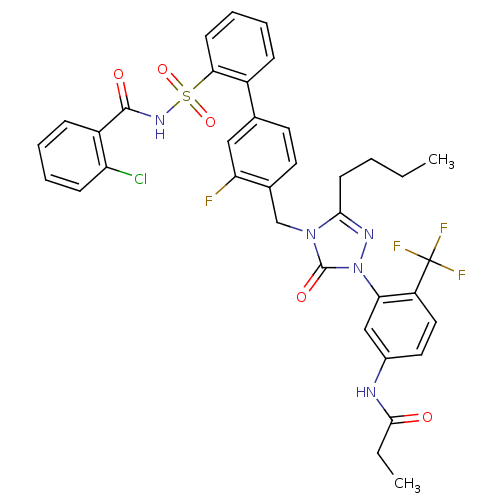
(CHEMBL339256 | N-(3-{3-Butyl-4-[2'-(2-chloro-benzo...)Show SMILES CCCCc1nn(-c2cc(NC(=O)CC)ccc2C(F)(F)F)c(=O)n1Cc1ccc(cc1F)-c1ccccc1S(=O)(=O)NC(=O)c1ccccc1Cl Show InChI InChI=1S/C36H32ClF4N5O5S/c1-3-5-14-32-43-46(30-20-24(42-33(47)4-2)17-18-27(30)36(39,40)41)35(49)45(32)21-23-16-15-22(19-29(23)38)25-10-7-9-13-31(25)52(50,51)44-34(48)26-11-6-8-12-28(26)37/h6-13,15-20H,3-5,14,21H2,1-2H3,(H,42,47)(H,44,48) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against Angiotensin II receptor, type 2 in human adrenal membrane preparations. For this assay, only 0.02%BSA was presen... |
J Med Chem 38: 3741-58 (1995)
BindingDB Entry DOI: 10.7270/Q2Q81C35 |
More data for this
Ligand-Target Pair | |
Type-2 angiotensin II receptor
(Homo sapiens (Human)) | BDBM50030700
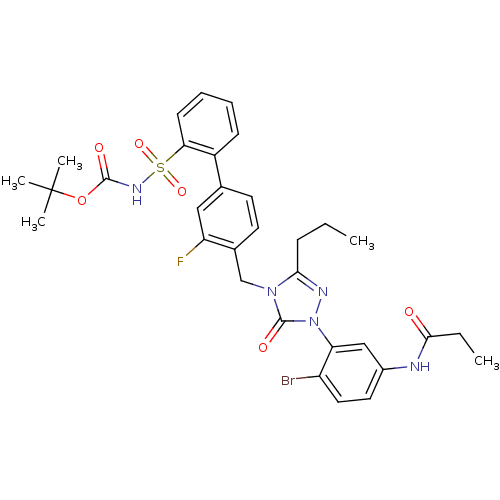
(CHEMBL436396 | N-{4-Bromo-3-[4-(3-fluoro-2'-(N-t-b...)Show SMILES CCCc1nn(-c2cc(NC(=O)CC)ccc2Br)c(=O)n1Cc1ccc(cc1F)-c1ccccc1S(=O)(=O)NC(=O)OC(C)(C)C Show InChI InChI=1S/C32H35BrFN5O6S/c1-6-10-28-36-39(26-18-22(15-16-24(26)33)35-29(40)7-2)31(42)38(28)19-21-14-13-20(17-25(21)34)23-11-8-9-12-27(23)46(43,44)37-30(41)45-32(3,4)5/h8-9,11-18H,6-7,10,19H2,1-5H3,(H,35,40)(H,37,41) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against Angiotensin II receptor type 2 in human adrenal membrane preparations. |
J Med Chem 38: 3741-58 (1995)
BindingDB Entry DOI: 10.7270/Q2Q81C35 |
More data for this
Ligand-Target Pair | |
Type-2 angiotensin II receptor
(Homo sapiens (Human)) | BDBM50280907

((S)-1,4-Bis-diphenylcarbamoyl-piperazine-2-carboxy...)Show SMILES OC(=O)[C@@H]1CN(CCN1C(=O)N(c1ccccc1)c1ccccc1)C(=O)N(c1ccccc1)c1ccccc1 Show InChI InChI=1S/C31H28N4O4/c36-29(37)28-23-32(30(38)34(24-13-5-1-6-14-24)25-15-7-2-8-16-25)21-22-33(28)31(39)35(26-17-9-3-10-18-26)27-19-11-4-12-20-27/h1-20,28H,21-23H2,(H,36,37)/t28-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro binding affinity of compound was measured against Angiotensin II receptor, type 2 |
Bioorg Med Chem Lett 4: 1479-1484 (1994)
Article DOI: 10.1016/S0960-894X(01)80517-5
BindingDB Entry DOI: 10.7270/Q2BC401G |
More data for this
Ligand-Target Pair | |
Type-2 angiotensin II receptor
(Homo sapiens (Human)) | BDBM50030691

(CHEMBL122380 | Pentanoic acid (4-bromo-3-{4-[2'-(2...)Show SMILES CCCCC(=O)Nc1ccc(Br)c(c1)-n1nc(CC)n(Cc2ccc(cc2F)-c2ccccc2S(=O)(=O)NC(=O)c2cc(F)ccc2F)c1=O Show InChI InChI=1S/C35H31BrF3N5O5S/c1-3-5-10-33(45)40-24-14-15-27(36)30(19-24)44-35(47)43(32(4-2)41-44)20-22-12-11-21(17-29(22)39)25-8-6-7-9-31(25)50(48,49)42-34(46)26-18-23(37)13-16-28(26)38/h6-9,11-19H,3-5,10,20H2,1-2H3,(H,40,45)(H,42,46) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against Angiotensin II receptor type 2 in human adrenal membrane preparations. |
J Med Chem 38: 3741-58 (1995)
BindingDB Entry DOI: 10.7270/Q2Q81C35 |
More data for this
Ligand-Target Pair | |
Type-2 angiotensin II receptor
(Homo sapiens (Human)) | BDBM50030708
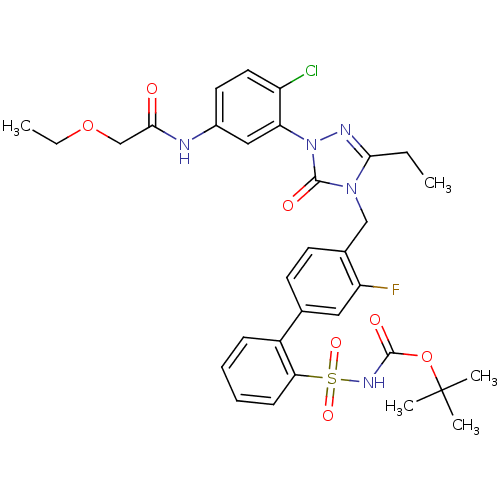
(CHEMBL125760 | N-{4-Chloro-3-[3-ethyl-4-(3-fluoro-...)Show SMILES CCOCC(=O)Nc1ccc(Cl)c(c1)-n1nc(CC)n(Cc2ccc(cc2F)-c2ccccc2S(=O)(=O)NC(=O)OC(C)(C)C)c1=O Show InChI InChI=1S/C32H35ClFN5O7S/c1-6-28-36-39(26-17-22(14-15-24(26)33)35-29(40)19-45-7-2)31(42)38(28)18-21-13-12-20(16-25(21)34)23-10-8-9-11-27(23)47(43,44)37-30(41)46-32(3,4)5/h8-17H,6-7,18-19H2,1-5H3,(H,35,40)(H,37,41) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against Angiotensin II receptor type 2 in human adrenal membrane preparations. |
J Med Chem 38: 3741-58 (1995)
BindingDB Entry DOI: 10.7270/Q2Q81C35 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data