 Found 1804 hits of ki for UniProtKB: P08173
Found 1804 hits of ki for UniProtKB: P08173 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kcal/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Muscarinic acetylcholine receptor M4
(Homo sapiens (Human)) | BDBM50296345

((1R,2R,4S,5R,7S)-7-(2-hydroxy-2,2-di-thiophen-2-yl...)Show SMILES C[N+]1(C)[C@@H]2C[C@H](C[C@@H]1[C@@H]1O[C@H]21)OC(=O)C(O)(c1cccs1)c1cccs1 |TLB:11:5:1:8.10| Show InChI InChI=1S/C19H22NO4S2/c1-20(2)12-9-11(10-13(20)17-16(12)24-17)23-18(21)19(22,14-5-3-7-25-14)15-6-4-8-26-15/h3-8,11-13,16-17,22H,9-10H2,1-2H3/q+1/t11-,12-,13-,16-,17+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.00780 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]-NMS from human recombinant M4 receptor expressed in CHO cells after 24 hrs by filter binding assay |
J Med Chem 54: 6888-904 (2011)
Article DOI: 10.1021/jm200884j
BindingDB Entry DOI: 10.7270/Q2CZ37JW |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M4
(Homo sapiens (Human)) | BDBM81768

(BENZYLIC ACID | CAS_6581-06-2 | NSC_5311391 | Quin...)Show InChI InChI=1S/C14H12O3/c15-13(16)14(17,11-7-3-1-4-8-11)12-9-5-2-6-10-12/h1-10,17H,(H,15,16) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
PDB
UniChem
Similars
| PubMed
| 0.0340 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Mayo Clinic Jacksonville
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 260: 576-80 (1992)
BindingDB Entry DOI: 10.7270/Q28P5Z0G |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M4
(Homo sapiens (Human)) | BDBM81768

(BENZYLIC ACID | CAS_6581-06-2 | NSC_5311391 | Quin...)Show InChI InChI=1S/C14H12O3/c15-13(16)14(17,11-7-3-1-4-8-11)12-9-5-2-6-10-12/h1-10,17H,(H,15,16) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 0.0370 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Mayo Foundation
Curated by PDSP Ki Database
| |
Biochem Pharmacol 45: 2352-4 (1993)
Article DOI: 10.1016/0006-2952(93)90211-e
BindingDB Entry DOI: 10.7270/Q2F76B2C |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1/M2/M3/M4/M5
(Homo sapiens (Human)) | BDBM50010096
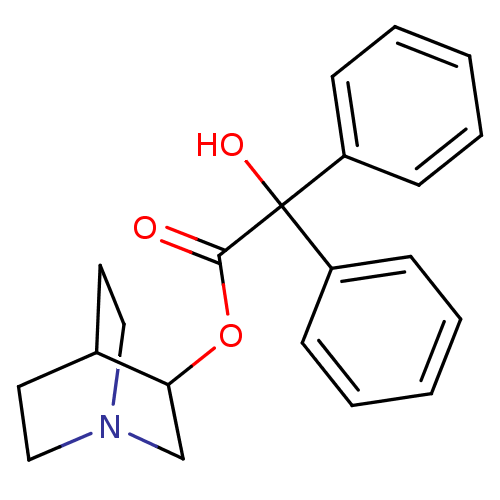
(CHEMBL12980 | Hydroxy-diphenyl-acetic acid 1-aza-b...)Show SMILES OC(C(=O)OC1CN2CCC1CC2)(c1ccccc1)c1ccccc1 |(5.41,-4.43,;4.65,-5.78,;6.2,-5.78,;6.97,-7.12,;6.97,-4.45,;8.52,-4.1,;8.52,-2.56,;9.86,-1.78,;11.19,-2.56,;11.19,-4.1,;9.86,-4.88,;9.09,-3.78,;9.09,-2.9,;3.1,-5.81,;2.36,-7.19,;.82,-7.2,;.01,-5.88,;.78,-4.52,;2.32,-4.49,;4.65,-7.23,;3.39,-7.96,;3.38,-9.41,;4.64,-10.15,;5.91,-9.42,;5.91,-7.97,)| Show InChI InChI=1S/C21H23NO3/c23-20(25-19-15-22-13-11-16(19)12-14-22)21(24,17-7-3-1-4-8-17)18-9-5-2-6-10-18/h1-10,16,19,24H,11-15H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 0.0450 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University
Curated by ChEMBL
| Assay Description
Ability of compound to displace (-)-[3H]3-Quinuclidinyl benzilate (-)-[3H]-QNB from Muscarinic acetylcholine receptors in the heart from Guinea Pig. |
J Med Chem 40: 3804-19 (1997)
Article DOI: 10.1021/jm970346t
BindingDB Entry DOI: 10.7270/Q2XG9TVC |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1/M2/M3/M4/M5
(Homo sapiens (Human)) | BDBM50010096

(CHEMBL12980 | Hydroxy-diphenyl-acetic acid 1-aza-b...)Show SMILES OC(C(=O)OC1CN2CCC1CC2)(c1ccccc1)c1ccccc1 |(5.41,-4.43,;4.65,-5.78,;6.2,-5.78,;6.97,-7.12,;6.97,-4.45,;8.52,-4.1,;8.52,-2.56,;9.86,-1.78,;11.19,-2.56,;11.19,-4.1,;9.86,-4.88,;9.09,-3.78,;9.09,-2.9,;3.1,-5.81,;2.36,-7.19,;.82,-7.2,;.01,-5.88,;.78,-4.52,;2.32,-4.49,;4.65,-7.23,;3.39,-7.96,;3.38,-9.41,;4.64,-10.15,;5.91,-9.42,;5.91,-7.97,)| Show InChI InChI=1S/C21H23NO3/c23-20(25-19-15-22-13-11-16(19)12-14-22)21(24,17-7-3-1-4-8-17)18-9-5-2-6-10-18/h1-10,16,19,24H,11-15H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 0.0510 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University
Curated by ChEMBL
| Assay Description
Ability of compound to displace (-)-[3H]3-Quinuclidinyl benzilate (-)-[3H]-QNB from Muscarinic acetylcholine receptors in cerebral cortex from Guinea... |
J Med Chem 40: 3804-19 (1997)
Article DOI: 10.1021/jm970346t
BindingDB Entry DOI: 10.7270/Q2XG9TVC |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1/M2/M3/M4/M5
(Homo sapiens (Human)) | BDBM50011851
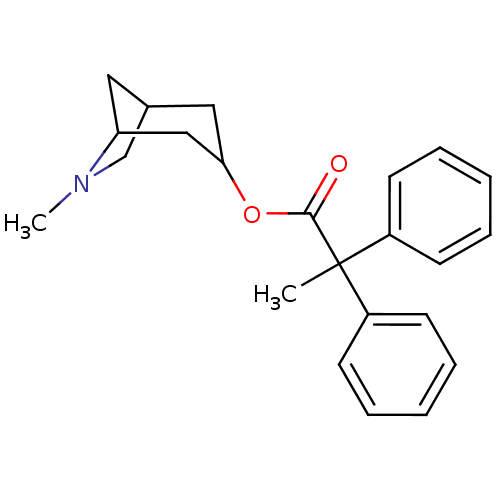
(2,2-Diphenyl-propionic acid 6-methyl-6-aza-bicyclo...)Show SMILES CN1CC2CC1CC(C2)OC(=O)C(C)(c1ccccc1)c1ccccc1 |TLB:9:7:1.2:4,THB:0:1:4:6.7.8| Show InChI InChI=1S/C23H27NO2/c1-23(18-9-5-3-6-10-18,19-11-7-4-8-12-19)22(25)26-21-14-17-13-20(15-21)24(2)16-17/h3-12,17,20-21H,13-16H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.0540 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute
Curated by ChEMBL
| Assay Description
Inhibition of [3H]QNB binding against muscarinic acetylcholine receptor in guinea pig ileum |
J Med Chem 34: 3164-71 (1991)
BindingDB Entry DOI: 10.7270/Q2V988Q7 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M4
(Homo sapiens (Human)) | BDBM50092313
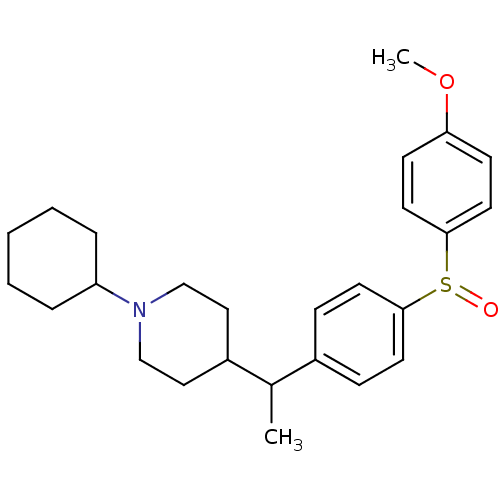
(1-Cyclohexyl-4-{1-[4-(4-methoxy-benzenesulfinyl)-p...)Show SMILES COc1ccc(cc1)S(=O)c1ccc(cc1)C(C)C1CCN(CC1)C1CCCCC1 Show InChI InChI=1S/C26H35NO2S/c1-20(22-16-18-27(19-17-22)23-6-4-3-5-7-23)21-8-12-25(13-9-21)30(28)26-14-10-24(29-2)11-15-26/h8-15,20,22-23H,3-7,16-19H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.0700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute
Curated by ChEMBL
| Assay Description
Binding affinity against Muscarinic acetylcholine receptor M4 |
Bioorg Med Chem Lett 10: 2209-12 (2001)
BindingDB Entry DOI: 10.7270/Q24T6HMP |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M4
(Homo sapiens (Human)) | BDBM50451113
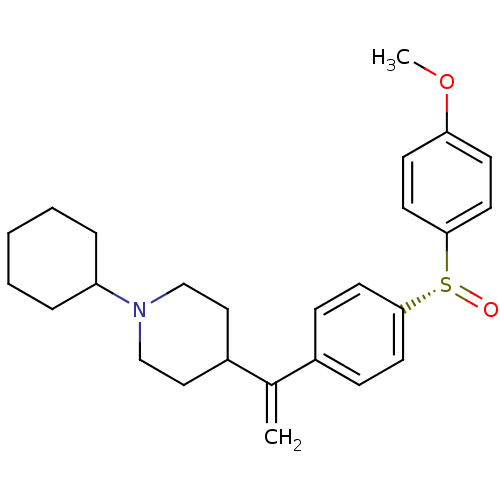
(CHEMBL2114068)Show SMILES COc1ccc(cc1)[S@+]([O-])c1ccc(cc1)C(=C)C1CCN(CC1)C1CCCCC1 |r| Show InChI InChI=1S/C26H33NO2S/c1-20(22-16-18-27(19-17-22)23-6-4-3-5-7-23)21-8-12-25(13-9-21)30(28)26-14-10-24(29-2)11-15-26/h8-15,22-23H,1,3-7,16-19H2,2H3/t30-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.0900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute
Curated by ChEMBL
| Assay Description
Binding affinity against Muscarinic acetylcholine receptor M4 |
Bioorg Med Chem Lett 10: 2209-12 (2001)
BindingDB Entry DOI: 10.7270/Q24T6HMP |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M4
(Homo sapiens (Human)) | BDBM86231

(ATR | ATROPINE | Atropine,(-) | CAS_51-55-8 | CHEM...)Show SMILES CN1C2CCC1CC(C2)OC(=O)C(CO)c1ccccc1 |THB:9:7:1:3.4| Show InChI InChI=1S/C17H23NO3/c1-18-13-7-8-14(18)10-15(9-13)21-17(20)16(11-19)12-5-3-2-4-6-12/h2-6,13-16,19H,7-11H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by PDSP Ki Database
| |
Prog Neuropsychopharmacol Biol Psychiatry 27: 1125-43 (2003)
Article DOI: 10.1016/j.pnpbp.2003.09.008
BindingDB Entry DOI: 10.7270/Q2M61HTM |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M4
(Homo sapiens (Human)) | BDBM50355622

(CHEMBL1910856)Show SMILES CC(C)(CCC(C(N)=O)(c1ccccc1)c1ccccc1)N1CC(C1)Oc1cccc(O)c1 Show InChI InChI=1S/C28H32N2O3/c1-27(2,30-19-25(20-30)33-24-15-9-14-23(31)18-24)16-17-28(26(29)32,21-10-5-3-6-11-21)22-12-7-4-8-13-22/h3-15,18,25,31H,16-17,19-20H2,1-2H3,(H2,29,32) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.115 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]-NMS from human recombinant M4 receptor expressed in CHO cells after 24 hrs by filter binding assay |
J Med Chem 54: 6888-904 (2011)
Article DOI: 10.1021/jm200884j
BindingDB Entry DOI: 10.7270/Q2CZ37JW |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M4
(Homo sapiens (Human)) | BDBM50241132

(3-Hydroxy-2-phenyl-propionic acid 9-methyl-3-oxa-9...)Show SMILES C[N+]1(C)[C@H]2C[C@@H](C[C@@H]1[C@H]1O[C@@H]21)OC(=O)[C@H](CO)c1ccccc1 |r,TLB:9:8:4.5.6:1,9:10:4.5.6:1,THB:11:5:1:8.10| Show InChI InChI=1S/C18H24NO4/c1-19(2)14-8-12(9-15(19)17-16(14)23-17)22-18(21)13(10-20)11-6-4-3-5-7-11/h3-7,12-17,20H,8-10H2,1-2H3/q+1/t12-,13-,14-,15+,16-,17+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 0.141 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universita di Firenze
Curated by ChEMBL
| Assay Description
Displacement of [3H]NMS from human cloned muscarinic M4 receptor expressed in CHO cells by scintillation counting |
J Med Chem 53: 201-7 (2010)
Article DOI: 10.1021/jm901048j
BindingDB Entry DOI: 10.7270/Q2D79CPP |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M4
(Homo sapiens (Human)) | BDBM50241132

(3-Hydroxy-2-phenyl-propionic acid 9-methyl-3-oxa-9...)Show SMILES C[N+]1(C)[C@H]2C[C@@H](C[C@@H]1[C@H]1O[C@@H]21)OC(=O)[C@H](CO)c1ccccc1 |r,TLB:9:8:4.5.6:1,9:10:4.5.6:1,THB:11:5:1:8.10| Show InChI InChI=1S/C18H24NO4/c1-19(2)14-8-12(9-15(19)17-16(14)23-17)22-18(21)13(10-20)11-6-4-3-5-7-11/h3-7,12-17,20H,8-10H2,1-2H3/q+1/t12-,13-,14-,15+,16-,17+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 0.141 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Camerino
Curated by ChEMBL
| Assay Description
Displacement of [3H]N-methylscopolamine from human muscarinic M4 receptor expressed in CHO cells after 120 mins by scintillation counting |
J Med Chem 55: 1783-7 (2012)
Article DOI: 10.1021/jm2013216
BindingDB Entry DOI: 10.7270/Q2GT5P63 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1/M2/M3/M4/M5
(Homo sapiens (Human)) | BDBM50010096

(CHEMBL12980 | Hydroxy-diphenyl-acetic acid 1-aza-b...)Show SMILES OC(C(=O)OC1CN2CCC1CC2)(c1ccccc1)c1ccccc1 |(5.41,-4.43,;4.65,-5.78,;6.2,-5.78,;6.97,-7.12,;6.97,-4.45,;8.52,-4.1,;8.52,-2.56,;9.86,-1.78,;11.19,-2.56,;11.19,-4.1,;9.86,-4.88,;9.09,-3.78,;9.09,-2.9,;3.1,-5.81,;2.36,-7.19,;.82,-7.2,;.01,-5.88,;.78,-4.52,;2.32,-4.49,;4.65,-7.23,;3.39,-7.96,;3.38,-9.41,;4.64,-10.15,;5.91,-9.42,;5.91,-7.97,)| Show InChI InChI=1S/C21H23NO3/c23-20(25-19-15-22-13-11-16(19)12-14-22)21(24,17-7-3-1-4-8-17)18-9-5-2-6-10-18/h1-10,16,19,24H,11-15H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University
Curated by ChEMBL
| Assay Description
Affinity for Muscarinic acetylcholine receptors in urinary bladder from Guinea Pig by (-)-[3H]3-Quinuclidinyl benzilate (-)-[3H]-QNB displacement. |
J Med Chem 40: 3804-19 (1997)
Article DOI: 10.1021/jm970346t
BindingDB Entry DOI: 10.7270/Q2XG9TVC |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1/M2/M3/M4/M5
(Homo sapiens (Human)) | BDBM50010096

(CHEMBL12980 | Hydroxy-diphenyl-acetic acid 1-aza-b...)Show SMILES OC(C(=O)OC1CN2CCC1CC2)(c1ccccc1)c1ccccc1 |(5.41,-4.43,;4.65,-5.78,;6.2,-5.78,;6.97,-7.12,;6.97,-4.45,;8.52,-4.1,;8.52,-2.56,;9.86,-1.78,;11.19,-2.56,;11.19,-4.1,;9.86,-4.88,;9.09,-3.78,;9.09,-2.9,;3.1,-5.81,;2.36,-7.19,;.82,-7.2,;.01,-5.88,;.78,-4.52,;2.32,-4.49,;4.65,-7.23,;3.39,-7.96,;3.38,-9.41,;4.64,-10.15,;5.91,-9.42,;5.91,-7.97,)| Show InChI InChI=1S/C21H23NO3/c23-20(25-19-15-22-13-11-16(19)12-14-22)21(24,17-7-3-1-4-8-17)18-9-5-2-6-10-18/h1-10,16,19,24H,11-15H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 0.240 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University
Curated by ChEMBL
| Assay Description
Ability of compound to displace (-)-[3H]3-Quinuclidinyl benzilate (-)-[3H]-QNB from Muscarinic acetylcholine receptors in the parotid gland from Guin... |
J Med Chem 40: 3804-19 (1997)
Article DOI: 10.1021/jm970346t
BindingDB Entry DOI: 10.7270/Q2XG9TVC |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1/M2/M3/M4/M5
(Homo sapiens (Human)) | BDBM50011851

(2,2-Diphenyl-propionic acid 6-methyl-6-aza-bicyclo...)Show SMILES CN1CC2CC1CC(C2)OC(=O)C(C)(c1ccccc1)c1ccccc1 |TLB:9:7:1.2:4,THB:0:1:4:6.7.8| Show InChI InChI=1S/C23H27NO2/c1-23(18-9-5-3-6-10-18,19-11-7-4-8-12-19)22(25)26-21-14-17-13-20(15-21)24(2)16-17/h3-12,17,20-21H,13-16H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.270 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibit the binding of [N-mnethyl-3H]-scopolamine [3H]-NMS) to Muscarinic acetylcholine receptor of human IRM-30 neuroblastoma cells |
J Med Chem 30: 805-9 (1987)
BindingDB Entry DOI: 10.7270/Q2B27XHZ |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1/M2/M3/M4/M5
(Homo sapiens (Human)) | BDBM50471463

(CHEMBL331497)Show SMILES Oc1cccc(c1)-c1ccoc1C1=CN2CCC1CC2 |t:14,(16.5,.02,;16.18,-1.49,;14.73,-1.97,;14.42,-3.5,;15.56,-4.5,;17.01,-4.02,;17.34,-2.51,;18.17,-5.05,;19.65,-4.72,;20.44,-6.06,;19.42,-7.2,;18.01,-6.58,;16.66,-7.35,;16.66,-8.89,;15.34,-9.66,;16.08,-8.32,;14.6,-7.9,;15.34,-6.58,;14,-7.35,;14,-8.89,)| Show InChI InChI=1S/C17H17NO2/c19-14-3-1-2-13(10-14)15-6-9-20-17(15)16-11-18-7-4-12(16)5-8-18/h1-3,6,9-12,19H,4-5,7-8H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.270 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University
Curated by ChEMBL
| Assay Description
Ability of compound to displace (-)-[3H]3-Quinuclidinyl benzilate (-)-[3H]-QNB from Muscarinic acetylcholine receptors in cerebral cortex from Guinea... |
J Med Chem 40: 3804-19 (1997)
Article DOI: 10.1021/jm970346t
BindingDB Entry DOI: 10.7270/Q2XG9TVC |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M4
(Homo sapiens (Human)) | BDBM86231

(ATR | ATROPINE | Atropine,(-) | CAS_51-55-8 | CHEM...)Show SMILES CN1C2CCC1CC(C2)OC(=O)C(CO)c1ccccc1 |THB:9:7:1:3.4| Show InChI InChI=1S/C17H23NO3/c1-18-13-7-8-14(18)10-15(9-13)21-17(20)16(11-19)12-5-3-2-4-6-12/h2-6,13-16,19H,7-11H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by PDSP Ki Database
| |
Prog Neuropsychopharmacol Biol Psychiatry 27: 1125-43 (2003)
Article DOI: 10.1016/j.pnpbp.2003.09.008
BindingDB Entry DOI: 10.7270/Q2M61HTM |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M4
(Homo sapiens (Human)) | BDBM86231

(ATR | ATROPINE | Atropine,(-) | CAS_51-55-8 | CHEM...)Show SMILES CN1C2CCC1CC(C2)OC(=O)C(CO)c1ccccc1 |THB:9:7:1:3.4| Show InChI InChI=1S/C17H23NO3/c1-18-13-7-8-14(18)10-15(9-13)21-17(20)16(11-19)12-5-3-2-4-6-12/h2-6,13-16,19H,7-11H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by PDSP Ki Database
| |
Eur J Pharmacol 390: 245-8 (2000)
Article DOI: 10.1016/s0014-2999(00)00037-6
BindingDB Entry DOI: 10.7270/Q2WM1BZZ |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1/M2/M3/M4/M5
(Homo sapiens (Human)) | BDBM50403547

(ATROPEN | ATROPINE)Show SMILES CN1[C@H]2CC[C@@H]1C[C@@H](C2)OC(=O)C(CO)c1ccccc1 |r,THB:9:7:1:3.4| Show InChI InChI=1S/C17H23NO3/c1-18-13-7-8-14(18)10-15(9-13)21-17(20)16(11-19)12-5-3-2-4-6-12/h2-6,13-16,19H,7-11H2,1H3/t13-,14+,15+,16? | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Similars
| DrugBank
Article
PubMed
| 0.320 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University
Curated by ChEMBL
| Assay Description
Ability of compound to displace (-)-[3H]3-Quinuclidinyl benzilate (-)-[3H]-QNB from Muscarinic acetylcholine receptors in cerebral cortex from Guinea... |
J Med Chem 40: 3804-19 (1997)
Article DOI: 10.1021/jm970346t
BindingDB Entry DOI: 10.7270/Q2XG9TVC |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M4
(Homo sapiens (Human)) | BDBM50451114
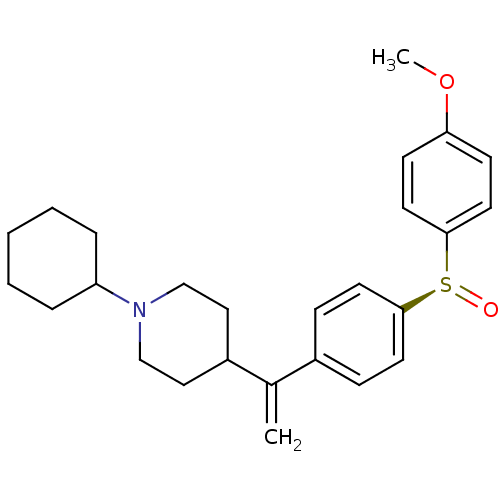
(CHEMBL2115128)Show SMILES COc1ccc(cc1)[S@@+]([O-])c1ccc(cc1)C(=C)C1CCN(CC1)C1CCCCC1 |r| Show InChI InChI=1S/C26H33NO2S/c1-20(22-16-18-27(19-17-22)23-6-4-3-5-7-23)21-8-12-25(13-9-21)30(28)26-14-10-24(29-2)11-15-26/h8-15,22-23H,1,3-7,16-19H2,2H3/t30-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.320 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute
Curated by ChEMBL
| Assay Description
Binding affinity against Muscarinic acetylcholine receptor M4 |
Bioorg Med Chem Lett 10: 2209-12 (2001)
BindingDB Entry DOI: 10.7270/Q24T6HMP |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M4
(Homo sapiens (Human)) | BDBM50092321

(1-Cyclohexyl-4-{1-[4-(4-methoxy-benzenesulfonyl)-p...)Show SMILES COc1ccc(cc1)S(=O)(=O)c1ccc(cc1)C(=C)C1CCN(CC1)C1CCCCC1 Show InChI InChI=1S/C26H33NO3S/c1-20(22-16-18-27(19-17-22)23-6-4-3-5-7-23)21-8-12-25(13-9-21)31(28,29)26-14-10-24(30-2)11-15-26/h8-15,22-23H,1,3-7,16-19H2,2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 0.330 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute
Curated by ChEMBL
| Assay Description
Binding affinity against Muscarinic acetylcholine receptor M4 |
Bioorg Med Chem Lett 10: 2209-12 (2001)
BindingDB Entry DOI: 10.7270/Q24T6HMP |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M4
(Homo sapiens (Human)) | BDBM86231

(ATR | ATROPINE | Atropine,(-) | CAS_51-55-8 | CHEM...)Show SMILES CN1C2CCC1CC(C2)OC(=O)C(CO)c1ccccc1 |THB:9:7:1:3.4| Show InChI InChI=1S/C17H23NO3/c1-18-13-7-8-14(18)10-15(9-13)21-17(20)16(11-19)12-5-3-2-4-6-12/h2-6,13-16,19H,7-11H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| 0.340 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Banyu Pharmaceutical Co., Ltd.
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 297: 790-7 (2001)
BindingDB Entry DOI: 10.7270/Q24T6GX9 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M4
(Homo sapiens (Human)) | BDBM50128835
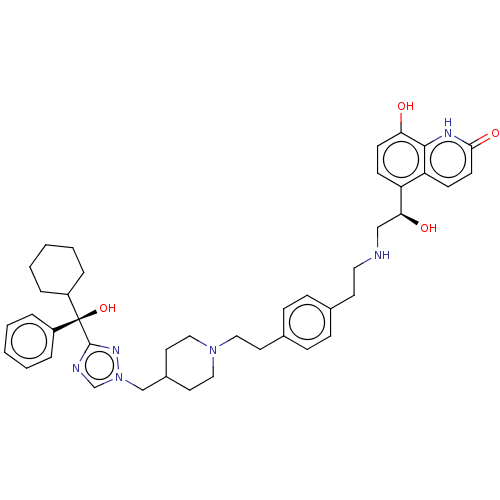
(CHEMBL3629360)Show SMILES O[C@@H](CNCCc1ccc(CCN2CCC(Cn3cnc(n3)[C@@](O)(C3CCCCC3)c3ccccc3)CC2)cc1)c1ccc(O)c2[nH]c(=O)ccc12 |r| Show InChI InChI=1S/C42H52N6O4/c49-37-17-15-35(36-16-18-39(51)45-40(36)37)38(50)27-43-23-19-30-11-13-31(14-12-30)20-24-47-25-21-32(22-26-47)28-48-29-44-41(46-48)42(52,33-7-3-1-4-8-33)34-9-5-2-6-10-34/h1,3-4,7-8,11-18,29,32,34,38,43,49-50,52H,2,5-6,9-10,19-28H2,(H,45,51)/t38-,42-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.350 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer
Curated by ChEMBL
| Assay Description
Antagonist activity at muscarinic M4 receptor (unknown origin) |
Bioorg Med Chem Lett 25: 5121-6 (2015)
Article DOI: 10.1016/j.bmcl.2015.10.008
BindingDB Entry DOI: 10.7270/Q2JQ12V7 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M4
(Homo sapiens (Human)) | BDBM50355610
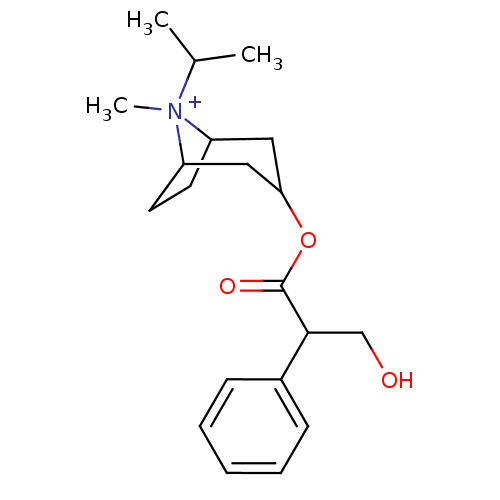
(CHEMBL1237108)Show SMILES CC(C)[N+]1(C)C2CCC1CC(C2)OC(=O)C(CO)c1ccccc1 |TLB:4:3:6.7:11.9.10,1:3:6.7:11.9.10,(10.09,.76,;9.35,2.05,;7.85,2.05,;10.09,3.35,;11.39,2.6,;10.09,4.89,;10.86,3.55,;10.3,2.58,;8.76,2.58,;7.43,3.35,;7.43,4.89,;8.76,5.66,;6.09,5.66,;6.09,7.2,;7.43,7.97,;4.76,7.97,;4.76,9.51,;3.43,10.28,;3.43,7.2,;3.43,5.66,;2.09,4.89,;.76,5.66,;.76,7.2,;2.09,7.97,)| Show InChI InChI=1S/C20H30NO3/c1-14(2)21(3)16-9-10-17(21)12-18(11-16)24-20(23)19(13-22)15-7-5-4-6-8-15/h4-8,14,16-19,22H,9-13H2,1-3H3/q+1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 0.382 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]-NMS from human recombinant M4 receptor expressed in CHO cells after 24 hrs by filter binding assay |
J Med Chem 54: 6888-904 (2011)
Article DOI: 10.1021/jm200884j
BindingDB Entry DOI: 10.7270/Q2CZ37JW |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M4
(Homo sapiens (Human)) | BDBM50557939

(CHEMBL4742721)Show SMILES OC(=O)C(F)(F)F.OC(=O)C(F)(F)F.F[B-]1(F)n2c(ccc2-c2cccs2)C=C2C=CC(\C=C\c3ccc(OCC(=O)NCCCCCC(=O)NCCNC(=O)CCc4cn(CCCCC5CCN(CC(=O)N6c7ccccc7NC(=O)c7ccccc67)CC5)cn4)cc3)=[N+]12 |c:29,t:27,99| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.417 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]NMS from human recombinant muscarinic receptor M4 expressed in CHO-K9 cell membranes measured after 3 hrs by radioligand competit... |
Citation and Details
Article DOI: 10.1039/d0md00137f
BindingDB Entry DOI: 10.7270/Q2ZS316G |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M4
(Homo sapiens (Human)) | BDBM50538317

(CHEMBL4636083)Show SMILES OC(=O)C(F)(F)F.OC(=O)C(F)(F)F.OC(=O)C(F)(F)F.OC(=O)C(F)(F)F.NCCN1CCN(CCCCC2CCN(CC(=O)N3c4ccccc4NC(=O)c4ccccc34)CC2)CC1 Show InChI InChI=1S/C30H42N6O2/c31-14-18-34-21-19-33(20-22-34)15-6-5-7-24-12-16-35(17-13-24)23-29(37)36-27-10-3-1-8-25(27)30(38)32-26-9-2-4-11-28(26)36/h1-4,8-11,24H,5-7,12-23,31H2,(H,32,38) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.447 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Regensburg
Curated by ChEMBL
| Assay Description
Displacement of [3H]-NMS from human muscarinic M4 receptor stably expressed in CHO-K9 cells by radioligand competitive binding assay |
J Med Chem 63: 4133-4154 (2020)
Article DOI: 10.1021/acs.jmedchem.9b02172
BindingDB Entry DOI: 10.7270/Q2R214WT |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1/M2/M3/M4/M5
(Homo sapiens (Human)) | BDBM50368152
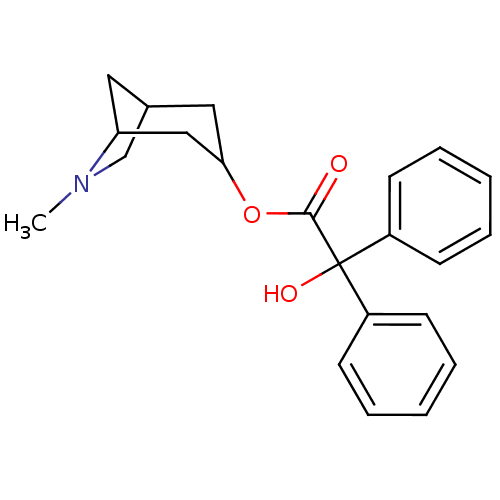
(CHEMBL318812)Show SMILES CN1CC2CC1CC(C2)OC(=O)C(O)(c1ccccc1)c1ccccc1 |THB:9:7:1.2:4,0:1:4:6.7.8| Show InChI InChI=1S/C22H25NO3/c1-23-15-16-12-19(23)14-20(13-16)26-21(24)22(25,17-8-4-2-5-9-17)18-10-6-3-7-11-18/h2-11,16,19-20,25H,12-15H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.449 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute
Curated by ChEMBL
| Assay Description
Inhibition of [3H]QNB binding against muscarinic acetylcholine receptor in guinea pig ileum |
J Med Chem 34: 3164-71 (1991)
BindingDB Entry DOI: 10.7270/Q2V988Q7 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M4
(Homo sapiens (Human)) | BDBM50265995

(CHEMBL4097258)Show SMILES OC(=O)C(F)(F)F.OC(=O)C(F)(F)F.OC(=O)C(F)(F)F.OC(=O)C(F)(F)F.CCC(=O)NCc1cc(cc(c1)C(=O)NCCNC(=O)CCc1cn(CCCCC2CCN(CC(=O)N3c4ccccc4NC(=O)c4ccccc34)CC2)cn1)C(=O)NCCNC(=O)CCc1cn(CCCCC2CCN(CC(=O)N3c4ccccc4NC(=O)c4ccccc34)CC2)cn1 Show InChI InChI=1S/C76H91N15O9/c1-2-68(92)81-46-55-43-56(73(97)79-35-33-77-69(93)27-25-58-47-88(51-82-58)37-13-11-15-53-29-39-86(40-30-53)49-71(95)90-64-21-7-3-17-60(64)75(99)84-62-19-5-9-23-66(62)90)45-57(44-55)74(98)80-36-34-78-70(94)28-26-59-48-89(52-83-59)38-14-12-16-54-31-41-87(42-32-54)50-72(96)91-65-22-8-4-18-61(65)76(100)85-63-20-6-10-24-67(63)91/h3-10,17-24,43-45,47-48,51-54H,2,11-16,25-42,46,49-50H2,1H3,(H,77,93)(H,78,94)(H,79,97)(H,80,98)(H,81,92)(H,84,99)(H,85,100) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.468 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Regensburg
Curated by ChEMBL
| Assay Description
Displacement of [3H]NMS from human muscarinic acetylcholine receptor M4 expressed in CHOK9 cells after 3 hrs by liquid scintillation counting assay |
J Med Chem 60: 3314-3334 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01892
BindingDB Entry DOI: 10.7270/Q29S1THC |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1/M2/M3/M4/M5
(Homo sapiens (Human)) | BDBM50368152

(CHEMBL318812)Show SMILES CN1CC2CC1CC(C2)OC(=O)C(O)(c1ccccc1)c1ccccc1 |THB:9:7:1.2:4,0:1:4:6.7.8| Show InChI InChI=1S/C22H25NO3/c1-23-15-16-12-19(23)14-20(13-16)26-21(24)22(25,17-8-4-2-5-9-17)18-10-6-3-7-11-18/h2-11,16,19-20,25H,12-15H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.475 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute
Curated by ChEMBL
| Assay Description
Inhibition of [3H]QNB binding against muscarinic acetylcholine receptor in guinea pig ileum |
J Med Chem 34: 3164-71 (1991)
BindingDB Entry DOI: 10.7270/Q2V988Q7 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M4
(Homo sapiens (Human)) | BDBM50176065
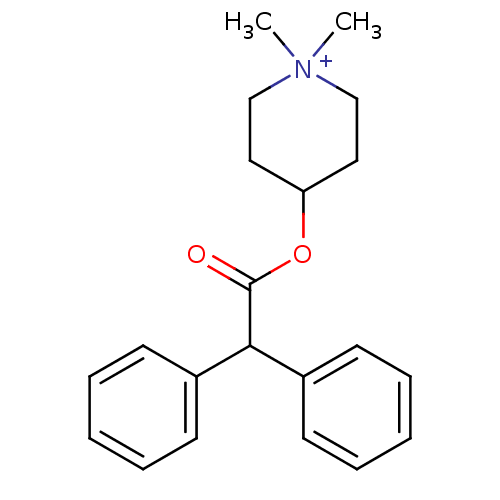
(4-DAMP | 4-Diphenylacetoxy-1,1-dimethyl-piperidini...)Show InChI InChI=1S/C21H26NO2/c1-22(2)15-13-19(14-16-22)24-21(23)20(17-9-5-3-6-10-17)18-11-7-4-8-12-18/h3-12,19-20H,13-16H2,1-2H3/q+1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
| 0.513 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Evaluation of antagonistic affinity against muscarinic receptor (M2) in guinea pig left atria |
Bioorg Med Chem Lett 5: 2325-2330 (1995)
Article DOI: 10.1016/0960-894X(95)00403-G
BindingDB Entry DOI: 10.7270/Q28G8MW2 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M4
(Homo sapiens (Human)) | BDBM50609878
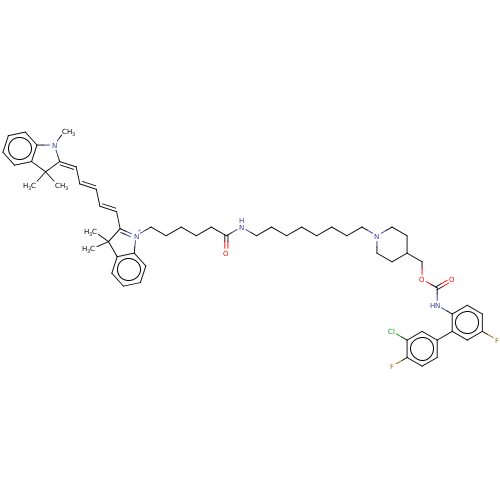
(CHEMBL5289601) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| | 0.560 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M4
(Homo sapiens (Human)) | BDBM50176065

(4-DAMP | 4-Diphenylacetoxy-1,1-dimethyl-piperidini...)Show InChI InChI=1S/C21H26NO2/c1-22(2)15-13-19(14-16-22)24-21(23)20(17-9-5-3-6-10-17)18-11-7-4-8-12-18/h3-12,19-20H,13-16H2,1-2H3/q+1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.580 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Case Western Reserve University
Curated by PDSP Ki Database
| |
Pharmacol Toxicol 83: 200-7 (1998)
Article DOI: 10.1111/j.1600-0773.1998.tb01469.x
BindingDB Entry DOI: 10.7270/Q21N7ZPP |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M4
(Homo sapiens (Human)) | BDBM86231

(ATR | ATROPINE | Atropine,(-) | CAS_51-55-8 | CHEM...)Show SMILES CN1C2CCC1CC(C2)OC(=O)C(CO)c1ccccc1 |THB:9:7:1:3.4| Show InChI InChI=1S/C17H23NO3/c1-18-13-7-8-14(18)10-15(9-13)21-17(20)16(11-19)12-5-3-2-4-6-12/h2-6,13-16,19H,7-11H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Mayo Clinic Jacksonville
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 260: 576-80 (1992)
BindingDB Entry DOI: 10.7270/Q28P5Z0G |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1/M2/M3/M4/M5
(Homo sapiens (Human)) | BDBM50471463

(CHEMBL331497)Show SMILES Oc1cccc(c1)-c1ccoc1C1=CN2CCC1CC2 |t:14,(16.5,.02,;16.18,-1.49,;14.73,-1.97,;14.42,-3.5,;15.56,-4.5,;17.01,-4.02,;17.34,-2.51,;18.17,-5.05,;19.65,-4.72,;20.44,-6.06,;19.42,-7.2,;18.01,-6.58,;16.66,-7.35,;16.66,-8.89,;15.34,-9.66,;16.08,-8.32,;14.6,-7.9,;15.34,-6.58,;14,-7.35,;14,-8.89,)| Show InChI InChI=1S/C17H17NO2/c19-14-3-1-2-13(10-14)15-6-9-20-17(15)16-11-18-7-4-12(16)5-8-18/h1-3,6,9-12,19H,4-5,7-8H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.610 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University
Curated by ChEMBL
| Assay Description
Ability of compound to displace (-)-[3H]3-Quinuclidinyl benzilate (-)-[3H]-QNB from Muscarinic acetylcholine receptors in the parotid gland from Guin... |
J Med Chem 40: 3804-19 (1997)
Article DOI: 10.1021/jm970346t
BindingDB Entry DOI: 10.7270/Q2XG9TVC |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M4
(Homo sapiens (Human)) | BDBM50240552
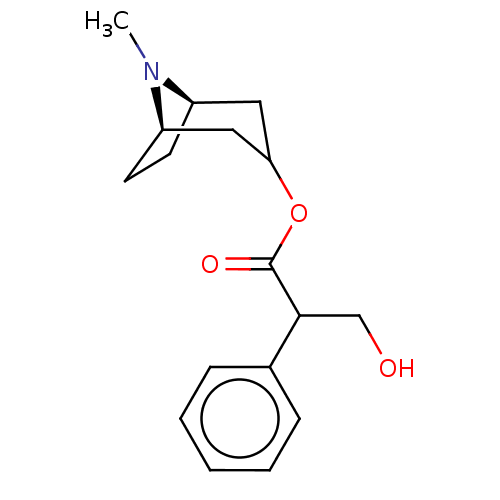
(CHEMBL195)Show SMILES [H][C@@]12CC[C@]([H])(CC(C1)OC(=O)C(CO)c1ccccc1)N2C |r,@@:7,TLB:9:7:21:2.3| Show InChI InChI=1S/C17H23NO3/c1-18-13-7-8-14(18)10-15(9-13)21-17(20)16(11-19)12-5-3-2-4-6-12/h2-6,13-16,19H,7-11H2,1H3/t13-,14-,16?/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.640 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Technology
Curated by ChEMBL
| Assay Description
Displacement of [3H]NMS from human recombinant muscarinic M4 receptor expressed in CHO cell membranes |
J Med Chem 34: 1879-84 (1991)
Article DOI: 10.1016/j.bmcl.2017.05.042
BindingDB Entry DOI: 10.7270/Q29G5KR1 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M4
(Homo sapiens (Human)) | BDBM82372
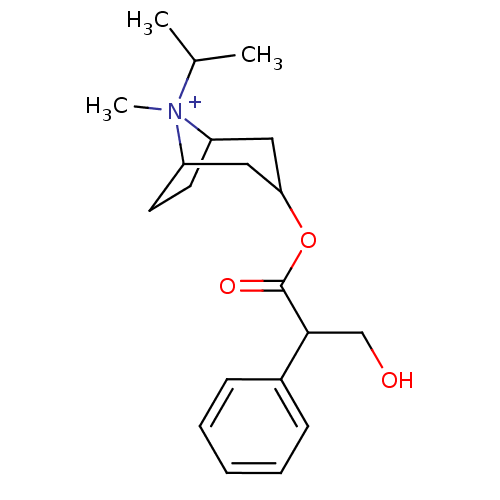
(CAS_22254-24-6 | Ipratropium | NSC_3746)Show SMILES CC(C)[N+]1(C)C2CCC1CC(C2)OC(=O)C(CO)c1ccccc1 |TLB:1:3:6.7:9.10.11,THB:4:3:6.7:9.10.11,12:10:3:6.7,(-1.51,-1.3,;-.6,-.05,;-1.22,1.36,;.93,-.22,;1.51,1.21,;1.98,-1.04,;1.38,-2.36,;.22,-3.07,;1.19,-1.74,;3.01,-1.74,;3.97,-2.54,;3.71,-1.01,;4.74,-3.87,;3.97,-5.2,;2.43,-5.2,;4.74,-6.54,;6.28,-6.54,;7.05,-7.87,;3.97,-7.87,;4.73,-9.21,;3.96,-10.54,;2.42,-10.54,;1.65,-9.2,;2.43,-7.87,)| Show InChI InChI=1S/C20H30NO3/c1-14(2)21(3)16-9-10-17(21)12-18(11-16)24-20(23)19(13-22)15-7-5-4-6-8-15/h4-8,14,16-19,22H,9-13H2,1-3H3/q+1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| 0.660 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Banyu Pharmaceutical Co., Ltd.
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 297: 790-7 (2001)
BindingDB Entry DOI: 10.7270/Q24T6GX9 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M4
(Homo sapiens (Human)) | BDBM50568852

(CHEMBL4851514)Show SMILES OC(=O)C(F)(F)F.OC(=O)C(F)(F)F.OC(=O)C(F)(F)F.OC(=O)C(F)(F)F.OC(=O)C(F)(F)F.C[C@H](NC(=O)[C@H](CCCNC(N)=N)NCCN1CCN(CCCCC2CCN(CC(=O)N3c4ccccc4NC(=O)c4ccccc34)CC2)CC1)C(=O)N[C@@H](CCCNC(N)=N)C(=O)NC(C)=O |r| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.661 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]-NMS from human muscarinic M4 receptor expressed in CHO cell membranes assessed as inhibition constant by radioligand competition... |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113159
BindingDB Entry DOI: 10.7270/Q2F47SWV |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M4
(Homo sapiens (Human)) | BDBM50540490

(CHEMBL4645580)Show SMILES [I-].C[N+](C)(C)C[C@H]1COC[C@](O1)(C1CCCCC1)c1ccccc1 |r| Show InChI InChI=1S/C20H32NO2.HI/c1-21(2,3)14-19-15-22-16-20(23-19,17-10-6-4-7-11-17)18-12-8-5-9-13-18;/h4,6-7,10-11,18-19H,5,8-9,12-16H2,1-3H3;1H/q+1;/p-1/t19-,20+;/m0./s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.676 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Camerino
Curated by ChEMBL
| Assay Description
Displacement of [3H]NMS from human recombinant muscarinic receptor M4 expressed in CHO-K1 cell membranes incubated for 2 hrs by scintillation countin... |
J Med Chem 63: 5763-5782 (2020)
Article DOI: 10.1021/acs.jmedchem.9b02100
BindingDB Entry DOI: 10.7270/Q20R9SX7 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1/M2/M3/M4/M5
(Homo sapiens (Human)) | BDBM50471463

(CHEMBL331497)Show SMILES Oc1cccc(c1)-c1ccoc1C1=CN2CCC1CC2 |t:14,(16.5,.02,;16.18,-1.49,;14.73,-1.97,;14.42,-3.5,;15.56,-4.5,;17.01,-4.02,;17.34,-2.51,;18.17,-5.05,;19.65,-4.72,;20.44,-6.06,;19.42,-7.2,;18.01,-6.58,;16.66,-7.35,;16.66,-8.89,;15.34,-9.66,;16.08,-8.32,;14.6,-7.9,;15.34,-6.58,;14,-7.35,;14,-8.89,)| Show InChI InChI=1S/C17H17NO2/c19-14-3-1-2-13(10-14)15-6-9-20-17(15)16-11-18-7-4-12(16)5-8-18/h1-3,6,9-12,19H,4-5,7-8H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.720 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University
Curated by ChEMBL
| Assay Description
Ability of compound to displace (-)-[3H]3-Quinuclidinyl benzilate (-)-[3H]-QNB from Muscarinic acetylcholine receptors in the heart from Guinea Pig. |
J Med Chem 40: 3804-19 (1997)
Article DOI: 10.1021/jm970346t
BindingDB Entry DOI: 10.7270/Q2XG9TVC |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M4
(Homo sapiens (Human)) | BDBM50568853
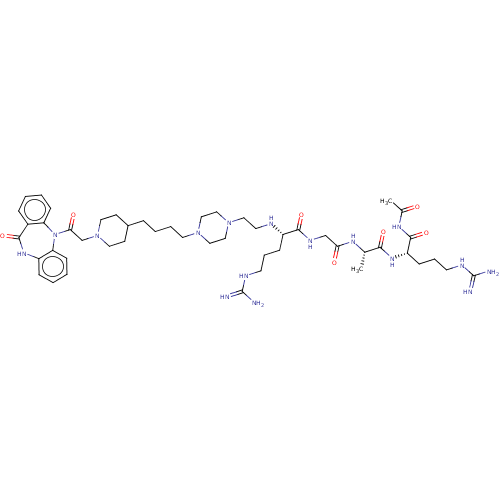
(CHEMBL4862735)Show SMILES OC(=O)C(F)(F)F.OC(=O)C(F)(F)F.OC(=O)C(F)(F)F.OC(=O)C(F)(F)F.OC(=O)C(F)(F)F.C[C@H](NC(=O)CNC(=O)[C@H](CCCNC(N)=N)NCCN1CCN(CCCCC2CCN(CC(=O)N3c4ccccc4NC(=O)c4ccccc34)CC2)CC1)C(=O)N[C@@H](CCCNC(N)=N)C(=O)NC(C)=O |r| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.724 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]-NMS from human muscarinic M4 receptor expressed in CHO cell membranes assessed as inhibition constant by radioligand competition... |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113159
BindingDB Entry DOI: 10.7270/Q2F47SWV |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M4
(Homo sapiens (Human)) | BDBM50568855
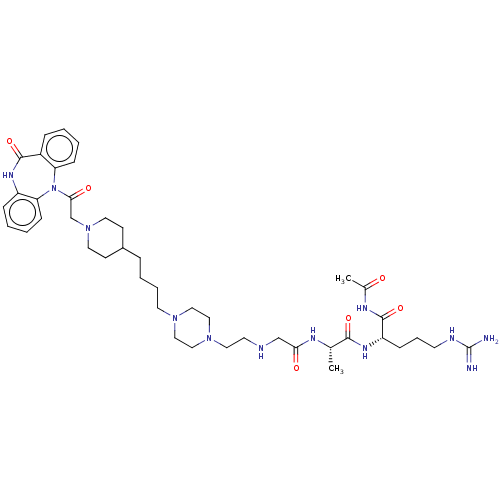
(CHEMBL4847898)Show SMILES OC(=O)C(F)(F)F.OC(=O)C(F)(F)F.OC(=O)C(F)(F)F.OC(=O)C(F)(F)F.C[C@H](NC(=O)CNCCN1CCN(CCCCC2CCN(CC(=O)N3c4ccccc4NC(=O)c4ccccc34)CC2)CC1)C(=O)N[C@@H](CCCNC(N)=N)C(=O)NC(C)=O |r| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.741 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]-NMS from human muscarinic M4 receptor expressed in CHO cell membranes assessed as inhibition constant by radioligand competition... |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113159
BindingDB Entry DOI: 10.7270/Q2F47SWV |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M4
(Homo sapiens (Human)) | BDBM50107696
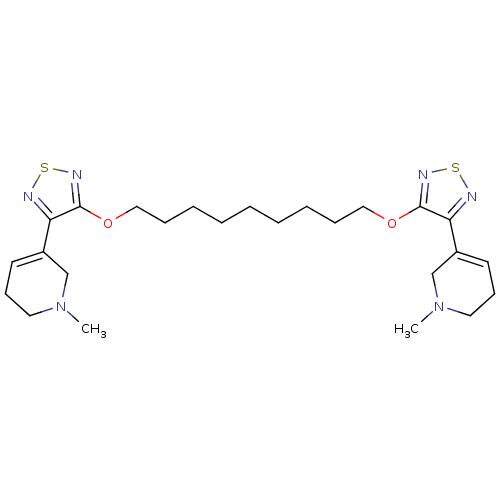
(1,9-di[4-(1-methyl-1,2,3,6-tetrahydro-5-pyridinyl)...)Show SMILES CN1CCC=C(C1)c1nsnc1OCCCCCCCCCOc1nsnc1C1=CCCN(C)C1 |c:4,t:31| Show InChI InChI=1S/C25H38N6O2S2/c1-30-14-10-12-20(18-30)22-24(28-34-26-22)32-16-8-6-4-3-5-7-9-17-33-25-23(27-35-29-25)21-13-11-15-31(2)19-21/h12-13H,3-11,14-19H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.776 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Camerino
Curated by ChEMBL
| Assay Description
Displacement of [3H]NMS from human M4 receptor expressed in CHO cells by microplate scintillation counting based radioligand binding assay |
J Med Chem 57: 9065-77 (2014)
Article DOI: 10.1021/jm501173q
BindingDB Entry DOI: 10.7270/Q2FT8NNB |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1/M2/M3/M4/M5
(Homo sapiens (Human)) | BDBM50471448

(CHEMBL123510)Show SMILES Oc1ccc(cc1)-c1ccoc1C1=CN2CCC1CC2 |t:14,(13.57,-.96,;14.73,-1.97,;14.42,-3.5,;15.56,-4.5,;17.01,-4.02,;17.34,-2.51,;16.18,-1.49,;18.17,-5.05,;19.65,-4.72,;20.44,-6.06,;19.42,-7.2,;18.01,-6.58,;16.66,-7.35,;16.66,-8.89,;15.34,-9.66,;14,-8.89,;14,-7.35,;15.34,-6.58,;14.6,-7.9,;16.08,-8.32,)| Show InChI InChI=1S/C17H17NO2/c19-14-3-1-12(2-4-14)15-7-10-20-17(15)16-11-18-8-5-13(16)6-9-18/h1-4,7,10-11,13,19H,5-6,8-9H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.790 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University
Curated by ChEMBL
| Assay Description
Ability of compound to displace (-)-[3H]3-Quinuclidinyl benzilate (-)-[3H]-QNB from Muscarinic acetylcholine receptors in cerebral cortex from Guinea... |
J Med Chem 40: 3804-19 (1997)
Article DOI: 10.1021/jm970346t
BindingDB Entry DOI: 10.7270/Q2XG9TVC |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M4
(Homo sapiens (Human)) | BDBM50568851
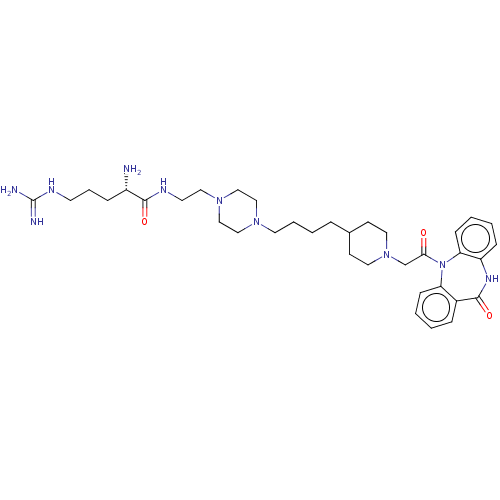
(CHEMBL4864727)Show SMILES OC(=O)C(F)(F)F.OC(=O)C(F)(F)F.OC(=O)C(F)(F)F.OC(=O)C(F)(F)F.OC(=O)C(F)(F)F.N[C@@H](CCCNC(N)=N)C(=O)NCCN1CCN(CCCCC2CCN(CC(=O)N3c4ccccc4NC(=O)c4ccccc34)CC2)CC1 |r| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.794 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]-NMS from human muscarinic M4 receptor expressed in CHO cell membranes assessed as inhibition constant by radioligand competition... |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113159
BindingDB Entry DOI: 10.7270/Q2F47SWV |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1/M2/M3/M4/M5
(Homo sapiens (Human)) | BDBM50471478

(CHEMBL339719)Show SMILES COC(=O)c1cccc(c1)-c1ccoc1C1=CN2CCC1CC2 |t:17,(15.66,2.56,;15.35,1.05,;16.5,.02,;17.95,.5,;16.18,-1.49,;14.73,-1.97,;14.42,-3.5,;15.56,-4.5,;17.01,-4.02,;17.34,-2.51,;18.17,-5.05,;19.65,-4.72,;20.44,-6.06,;19.42,-7.2,;18.01,-6.58,;16.66,-7.35,;16.66,-8.89,;15.34,-9.66,;14,-8.89,;14,-7.35,;15.34,-6.58,;14.6,-7.9,;16.08,-8.32,)| Show InChI InChI=1S/C19H19NO3/c1-22-19(21)15-4-2-3-14(11-15)16-7-10-23-18(16)17-12-20-8-5-13(17)6-9-20/h2-4,7,10-13H,5-6,8-9H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University
Curated by ChEMBL
| Assay Description
Ability of compound to displace (-)-[3H]3-Quinuclidinyl benzilate (-)-[3H]-QNB from Muscarinic acetylcholine receptors in the parotid gland from Guin... |
J Med Chem 40: 3804-19 (1997)
Article DOI: 10.1021/jm970346t
BindingDB Entry DOI: 10.7270/Q2XG9TVC |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M4
(Homo sapiens (Human)) | BDBM50015720
((hyoscine)3-Hydroxy-2-phenyl-propionic acid 9-meth...)Show SMILES CN1C2CC(CC1C1OC21)OC(=O)C(CO)c1ccccc1 |TLB:8:9:1:3.5.4,8:7:1:3.5.4,0:1:9.7:3.5.4,THB:10:4:9.7:1| Show InChI InChI=1S/C17H21NO4/c1-18-13-7-11(8-14(18)16-15(13)22-16)21-17(20)12(9-19)10-5-3-2-4-6-10/h2-6,11-16,19H,7-9H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Mayo Clinic Jacksonville
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 260: 576-80 (1992)
BindingDB Entry DOI: 10.7270/Q28P5Z0G |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M4
(Homo sapiens (Human)) | BDBM50540493

(CHEMBL4635500)Show SMILES CN(C)C[C@H]1COC[C@](O1)(C1CCCCC1)c1ccccc1 |r| Show InChI InChI=1S/C19H29NO2/c1-20(2)13-18-14-21-15-19(22-18,16-9-5-3-6-10-16)17-11-7-4-8-12-17/h3,5-6,9-10,17-18H,4,7-8,11-15H2,1-2H3/t18-,19+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.813 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Camerino
Curated by ChEMBL
| Assay Description
Displacement of [3H]NMS from human recombinant muscarinic receptor M4 expressed in CHO-K1 cell membranes incubated for 2 hrs by scintillation countin... |
J Med Chem 63: 5763-5782 (2020)
Article DOI: 10.1021/acs.jmedchem.9b02100
BindingDB Entry DOI: 10.7270/Q20R9SX7 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M4
(Homo sapiens (Human)) | BDBM81462
((R)-Trihexyphenidyl | (S)-Trihexyphenidyl | Benzhe...)Show InChI InChI=1S/C20H31NO/c22-20(18-10-4-1-5-11-18,19-12-6-2-7-13-19)14-17-21-15-8-3-9-16-21/h1,4-5,10-11,19,22H,2-3,6-9,12-17H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| DrugBank
PubMed
| 0.830 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Neurological Disorders and Stroke
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 256: 727-33 (1991)
BindingDB Entry DOI: 10.7270/Q2VD6WZ3 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1/M2/M3/M4/M5
(Homo sapiens (Human)) | BDBM50403547

(ATROPEN | ATROPINE)Show SMILES CN1[C@H]2CC[C@@H]1C[C@@H](C2)OC(=O)C(CO)c1ccccc1 |r,THB:9:7:1:3.4| Show InChI InChI=1S/C17H23NO3/c1-18-13-7-8-14(18)10-15(9-13)21-17(20)16(11-19)12-5-3-2-4-6-12/h2-6,13-16,19H,7-11H2,1H3/t13-,14+,15+,16? | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Similars
| DrugBank
Article
PubMed
| 0.850 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University
Curated by ChEMBL
| Assay Description
Ability of compound to displace (-)-[3H]3-Quinuclidinyl benzilate (-)-[3H]-QNB from Muscarinic acetylcholine receptors in the parotid gland from Guin... |
J Med Chem 40: 3804-19 (1997)
Article DOI: 10.1021/jm970346t
BindingDB Entry DOI: 10.7270/Q2XG9TVC |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M4
(Homo sapiens (Human)) | BDBM50609876

(CHEMBL5289667) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| | 0.860 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data