

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM433523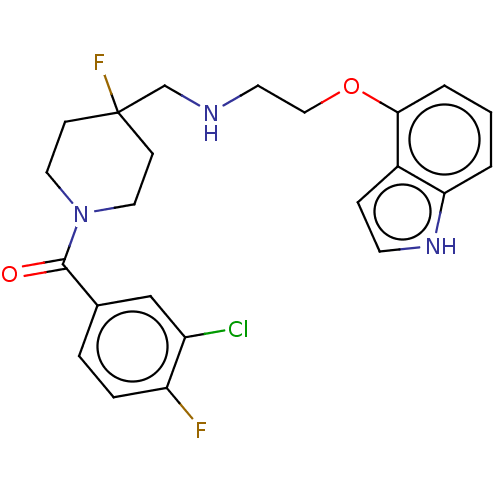 (US10562853, Compound 44) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 0.0000210 | n/a | n/a | n/a | n/a |
TBA | Assay Description Biased agonist activity at Gal4-VP16 transcription factor linked human 5-HT1A receptor expressed in human U2OS cells assessed as induction of beta-ar... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c00814 BindingDB Entry DOI: 10.7270/Q24T6NZT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM433476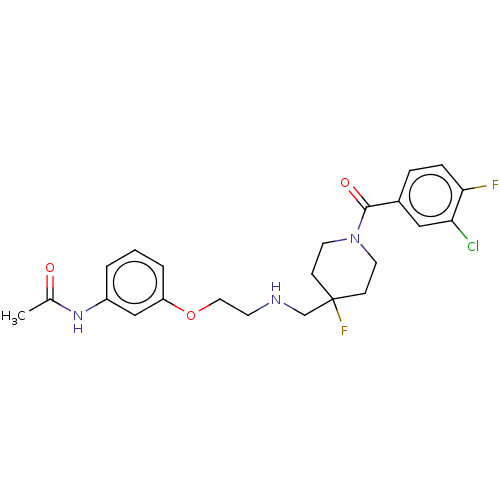 (US10562853, Compound 2) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 0.000209 | n/a | n/a | n/a | n/a |
TBA | Assay Description Biased agonist activity at Gal4-VP16 transcription factor linked human 5-HT1A receptor expressed in human U2OS cells assessed as induction of beta-ar... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c00814 BindingDB Entry DOI: 10.7270/Q24T6NZT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM433499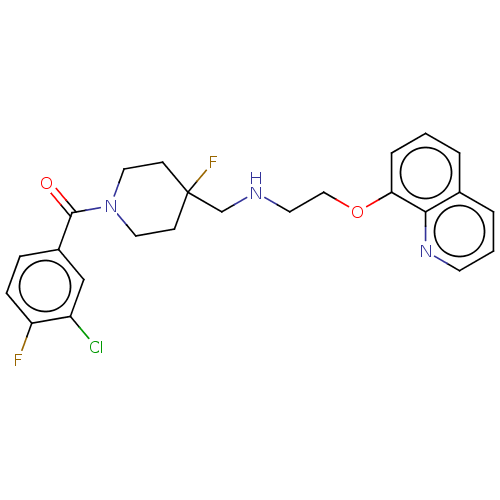 (US10562853, Compound 16) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 0.00148 | n/a | n/a | n/a | n/a |
TBA | Assay Description Biased agonist activity at Gal4-VP16 transcription factor linked human 5-HT1A receptor expressed in human U2OS cells assessed as induction of beta-ar... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c00814 BindingDB Entry DOI: 10.7270/Q24T6NZT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50546857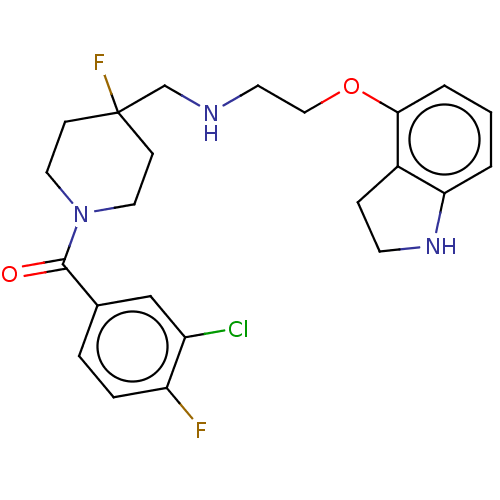 (CHEMBL4757755) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 0.00363 | n/a | n/a | n/a | n/a |
TBA | Assay Description Biased agonist activity at human 5-HT1A receptor stably expressed in CHO-K1 cells assessed as increase in ERK1/2 phosphorylation after 15 mins by Alp... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c00814 BindingDB Entry DOI: 10.7270/Q24T6NZT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50546857 (CHEMBL4757755) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 0.00513 | n/a | n/a | n/a | n/a |
TBA | Assay Description Biased agonist activity at Gal4-VP16 transcription factor linked human 5-HT1A receptor expressed in human U2OS cells assessed as induction of beta-ar... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c00814 BindingDB Entry DOI: 10.7270/Q24T6NZT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50180054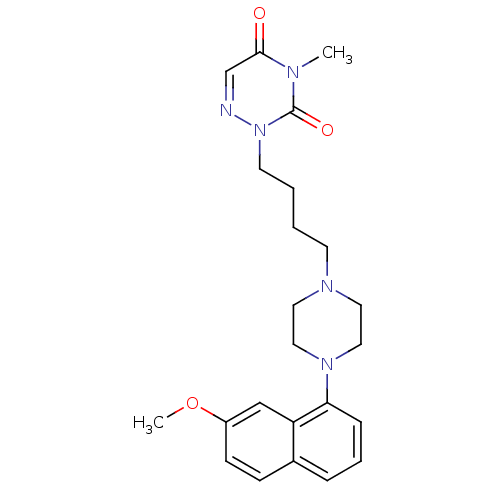 (CHEMBL199824 | [O-methyl-11C]2-{4-[4-(7-methoxynap...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 0.0100 | n/a | n/a | n/a | n/a |
Columbia University College of Physicians and Surgeons Curated by ChEMBL | Assay Description Agonistic activity at human 5HT1A in CHO cells by the inhibition of forskolin-stimulated cAMP accumulation | J Med Chem 49: 125-34 (2006) Article DOI: 10.1021/jm050725j BindingDB Entry DOI: 10.7270/Q2R49QBD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM433537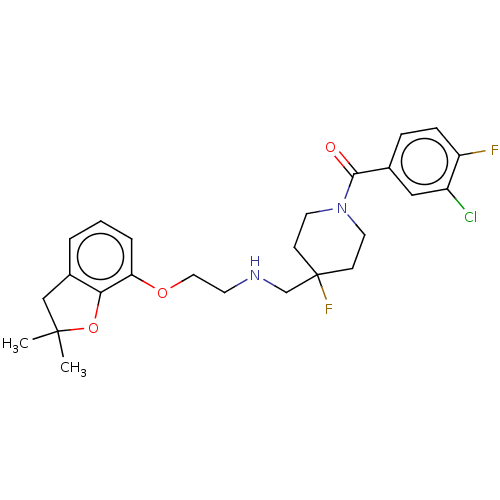 (US10562853, Compound 60) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 0.0102 | n/a | n/a | n/a | n/a |
TBA | Assay Description Biased agonist activity at human 5-HT1A receptor stably expressed in CHO-K1 cells assessed as increase in ERK1/2 phosphorylation after 15 mins by Alp... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c00814 BindingDB Entry DOI: 10.7270/Q24T6NZT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50546857 (CHEMBL4757755) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 0.0132 | n/a | n/a | n/a | n/a |
TBA | Assay Description Biased agonist activity at human 5-HT1A receptor stably expressed in CHO-K1 cells assessed as inhibition of forskolin-induced cAMP accumulation after... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c00814 BindingDB Entry DOI: 10.7270/Q24T6NZT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM433476 (US10562853, Compound 2) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 0.0138 | n/a | n/a | n/a | n/a |
TBA | Assay Description Biased agonist activity at human 5-HT1A receptor stably expressed in CHO-K1 cells assessed as inhibition of forskolin-induced cAMP accumulation after... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c00814 BindingDB Entry DOI: 10.7270/Q24T6NZT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM433477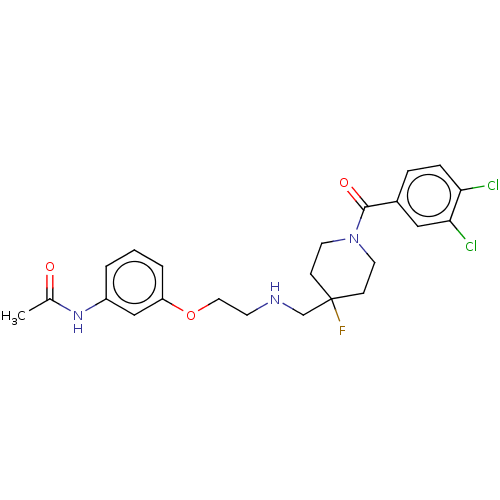 (US10562853, Compound 3) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 0.0251 | n/a | n/a | n/a | n/a |
TBA | Assay Description Biased agonist activity at Gal4-VP16 transcription factor linked human 5-HT1A receptor expressed in human U2OS cells assessed as induction of beta-ar... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c00814 BindingDB Entry DOI: 10.7270/Q24T6NZT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM433523 (US10562853, Compound 44) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 0.0295 | n/a | n/a | n/a | n/a |
TBA | Assay Description Biased agonist activity at human 5-HT1A receptor stably expressed in CHO-K1 cells assessed as increase in ERK1/2 phosphorylation after 15 mins by Alp... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c00814 BindingDB Entry DOI: 10.7270/Q24T6NZT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50417427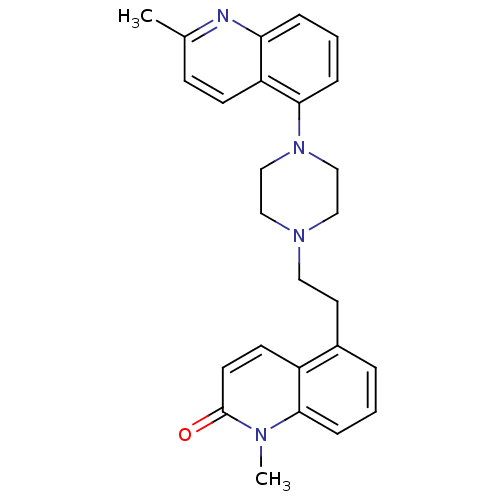 (CHEMBL1290716) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 0.0316 | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Agonist activity at human 5-HT1A receptor expressed in HEK293 cells assessed as stimulation of GTPgammaS binding by scintillation proximity assay | Bioorg Med Chem Lett 20: 7092-6 (2010) Article DOI: 10.1016/j.bmcl.2010.09.085 BindingDB Entry DOI: 10.7270/Q2MC919V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50180054 (CHEMBL199824 | [O-methyl-11C]2-{4-[4-(7-methoxynap...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 0.0500 | n/a | n/a | n/a | n/a |
Columbia University College of Physicians and Surgeons Curated by ChEMBL | Assay Description Agonistic activity at human 5HT1A receptor by [35S]GTP-gamma-S binding | J Med Chem 49: 125-34 (2006) Article DOI: 10.1021/jm050725j BindingDB Entry DOI: 10.7270/Q2R49QBD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM433477 (US10562853, Compound 3) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 0.0661 | n/a | n/a | n/a | n/a |
TBA | Assay Description Biased agonist activity at human 5-HT1A receptor stably expressed in CHO-K1 cells assessed as inhibition of forskolin-induced cAMP accumulation after... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c00814 BindingDB Entry DOI: 10.7270/Q24T6NZT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM433531 (US10562853, Compound 52) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 0.0741 | n/a | n/a | n/a | n/a |
TBA | Assay Description Biased agonist activity at human 5-HT1A receptor stably expressed in CHO-K1 cells assessed as increase in ERK1/2 phosphorylation after 15 mins by Alp... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c00814 BindingDB Entry DOI: 10.7270/Q24T6NZT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50024580 (CHEMBL3330612) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 0.0800 | n/a | n/a | n/a | n/a |
Stony Brook University Curated by ChEMBL | Assay Description Agonist activity at human 5HT1AR expressed in CHO cells assessed as increase in [35S]GTPgammaS binding by liquid scintillation spectrometry | Bioorg Med Chem Lett 24: 4759-62 (2014) Article DOI: 10.1016/j.bmcl.2014.07.048 BindingDB Entry DOI: 10.7270/Q2NZ896M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM433523 (US10562853, Compound 44) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 0.0871 | n/a | n/a | n/a | n/a |
TBA | Assay Description Biased agonist activity at human 5-HT1A receptor stably expressed in CHO-K1 cells assessed as inhibition of forskolin-induced cAMP accumulation after... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c00814 BindingDB Entry DOI: 10.7270/Q24T6NZT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM433531 (US10562853, Compound 52) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 0.0891 | n/a | n/a | n/a | n/a |
TBA | Assay Description Biased agonist activity at Gal4-VP16 transcription factor linked human 5-HT1A receptor expressed in human U2OS cells assessed as induction of beta-ar... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c00814 BindingDB Entry DOI: 10.7270/Q24T6NZT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50542266 (CHEMBL4633397) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a |
University of Chinese Academy of Sciences Curated by ChEMBL | Assay Description Agonist activity at 5HT1A receptor (unknown origin) by calcium-dye based FLIPR assay | Bioorg Med Chem Lett 30: (2020) Article DOI: 10.1016/j.bmcl.2020.127027 BindingDB Entry DOI: 10.7270/Q2VD731V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50542256 (CHEMBL4648027) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a |
University of Chinese Academy of Sciences Curated by ChEMBL | Assay Description Agonist activity at 5HT1A receptor (unknown origin) by calcium-dye based FLIPR assay | Bioorg Med Chem Lett 30: (2020) Article DOI: 10.1016/j.bmcl.2020.127027 BindingDB Entry DOI: 10.7270/Q2VD731V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50542251 (CHEMBL4647011) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a |
University of Chinese Academy of Sciences Curated by ChEMBL | Assay Description Agonist activity at 5HT1A receptor (unknown origin) by calcium-dye based FLIPR assay | Bioorg Med Chem Lett 30: (2020) Article DOI: 10.1016/j.bmcl.2020.127027 BindingDB Entry DOI: 10.7270/Q2VD731V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM86757 (CAS_0 | NSC_11603174 | [11C]MMP) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a |
Stony Brook University Curated by ChEMBL | Assay Description Agonist activity at human 5HT1AR expressed in CHO cells assessed as increase in [35S]GTPgammaS binding by liquid scintillation spectrometry | Bioorg Med Chem Lett 24: 4759-62 (2014) Article DOI: 10.1016/j.bmcl.2014.07.048 BindingDB Entry DOI: 10.7270/Q2NZ896M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM433531 (US10562853, Compound 52) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 0.112 | n/a | n/a | n/a | n/a |
TBA | Assay Description Biased agonist activity at human 5-HT1A receptor stably expressed in CHO-K1 cells assessed as inhibition of forskolin-induced cAMP accumulation after... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c00814 BindingDB Entry DOI: 10.7270/Q24T6NZT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50151982 (5-{4-[4-(5-Cyano-1H-indol-3-yl)-butyl]-piperazin-1...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 0.120 | n/a | n/a | n/a | n/a |
East China University of Science and Technology Curated by ChEMBL | Assay Description Agonist activity at human 5-HT1A receptor expressed in HEK293 cells after 60 mins by Eu-cAMP solution based ultra LANCE assay | Bioorg Med Chem 26: 3117-3125 (2018) Article DOI: 10.1016/j.bmc.2018.04.037 BindingDB Entry DOI: 10.7270/Q28P631Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50151982 (5-{4-[4-(5-Cyano-1H-indol-3-yl)-butyl]-piperazin-1...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 0.120 | n/a | n/a | n/a | n/a |
CSIR-Central Drug Research Institute Curated by ChEMBL | Assay Description Agonist activity at human 5H1A receptor expressed in HEK293 cells incubated for 60 mins by Eu-cAMP tracer based LANCE ultra cAMP assay | Bioorg Med Chem 27: 895-930 (2019) Article DOI: 10.1016/j.bmc.2019.01.025 BindingDB Entry DOI: 10.7270/Q2N87F3N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50094670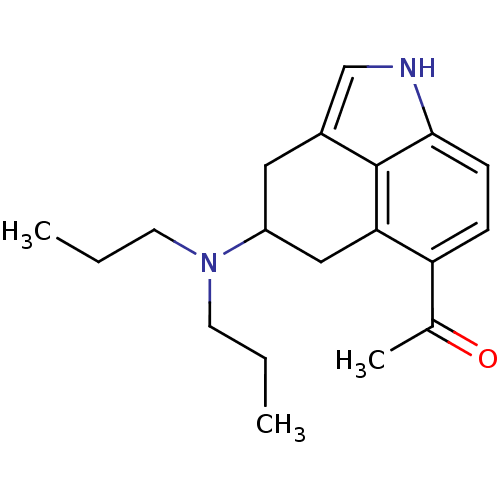 (1-(4-Dipropylamino-1,3,4,5-tetrahydro-benzo[cd]ind...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | n/a | n/a | 0.130 | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description EC50 for inhibition of 50 microM forskolin-stimulated cAMP accumulation against 5-hydroxytryptamine 1A receptor | J Med Chem 43: 4701-10 (2001) BindingDB Entry DOI: 10.7270/Q2HT2NM7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM433480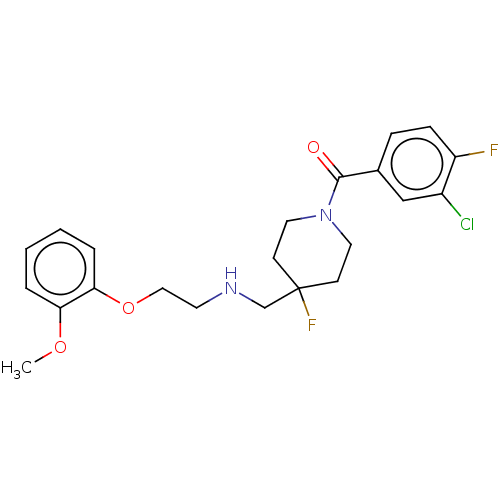 (US10562853, Compound 6) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 0.138 | n/a | n/a | n/a | n/a |
TBA | Assay Description Biased agonist activity at human 5-HT1A receptor stably expressed in CHO-K1 cells assessed as inhibition of forskolin-induced cAMP accumulation after... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c00814 BindingDB Entry DOI: 10.7270/Q24T6NZT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50272610 (CHEMBL4129019) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | <0.140 | n/a | n/a | n/a | n/a |
East China University of Science and Technology Curated by ChEMBL | Assay Description Agonist activity at human 5-HT1A receptor expressed in HEK293 cells after 60 mins by Eu-cAMP solution based ultra LANCE assay | Bioorg Med Chem 26: 3117-3125 (2018) Article DOI: 10.1016/j.bmc.2018.04.037 BindingDB Entry DOI: 10.7270/Q28P631Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50272735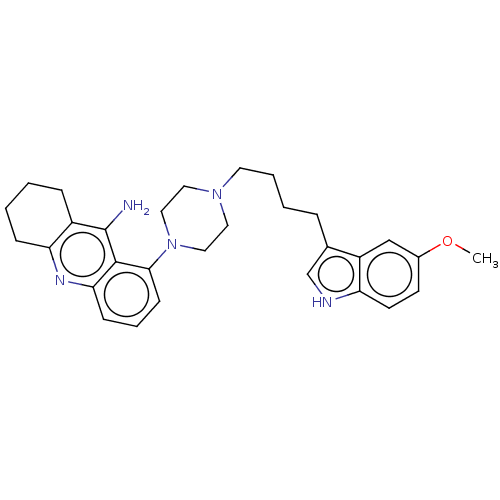 (CHEMBL4126958) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | <0.140 | n/a | n/a | n/a | n/a |
East China University of Science and Technology Curated by ChEMBL | Assay Description Agonist activity at human 5-HT1A receptor expressed in HEK293 cells after 60 mins by Eu-cAMP solution based ultra LANCE assay | Bioorg Med Chem 26: 3117-3125 (2018) Article DOI: 10.1016/j.bmc.2018.04.037 BindingDB Entry DOI: 10.7270/Q28P631Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM433476 (US10562853, Compound 2) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 0.178 | n/a | n/a | n/a | n/a |
TBA | Assay Description Biased agonist activity at human 5-HT1A receptor stably expressed in CHO-K1 cells assessed as increase in ERK1/2 phosphorylation after 15 mins by Alp... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c00814 BindingDB Entry DOI: 10.7270/Q24T6NZT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM433526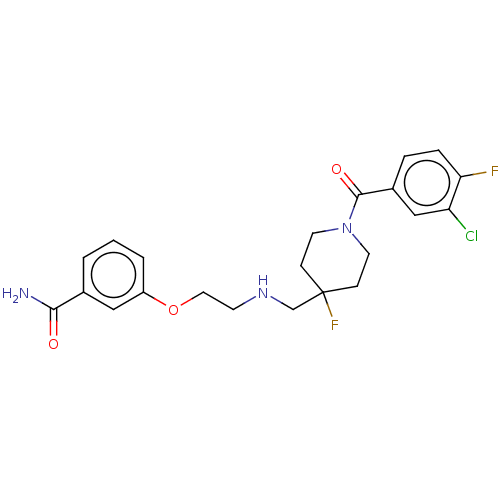 (US10562853, Compound 47) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 0.182 | n/a | n/a | n/a | n/a |
TBA | Assay Description Biased agonist activity at human 5-HT1A receptor stably expressed in CHO-K1 cells assessed as increase in ERK1/2 phosphorylation after 15 mins by Alp... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c00814 BindingDB Entry DOI: 10.7270/Q24T6NZT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50024574 (CHEMBL3330616) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a |
Stony Brook University Curated by ChEMBL | Assay Description Agonist activity at human 5HT1AR expressed in CHO cells assessed as increase in [35S]GTPgammaS binding by liquid scintillation spectrometry | Bioorg Med Chem Lett 24: 4759-62 (2014) Article DOI: 10.1016/j.bmcl.2014.07.048 BindingDB Entry DOI: 10.7270/Q2NZ896M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM319716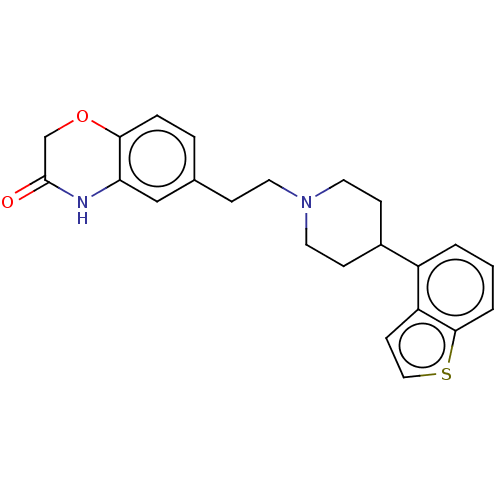 (6-(2-(4-(benzo[b]thiophen-4-yl)piperidin-1-yl)ethy...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | n/a | 0.223 | n/a | n/a | n/a | n/a |
SHANGHAI INSTITUTE OF MATERIA MEDICA, CHINESE ACADEMY OF SCIENCES; SUZHOU VIGONVITA LIFE SCIENCES CO., LTD.; TOPHARMAN SHANDONG CO., LTD. US Patent | Assay Description The 5-HT1A receptor agonism activity test (The agonism activity of test compounds on 5-HT1A receptor expressing human recombinant 5-HT1A receptor in ... | US Patent US10174011 (2019) BindingDB Entry DOI: 10.7270/Q2VH5QX7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM319709 (5-(2-(4-(6-fluorobenzo[b]thiophen-4-yl)piperazin-1...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | n/a | 0.235 | n/a | n/a | n/a | n/a |
SHANGHAI INSTITUTE OF MATERIA MEDICA, CHINESE ACADEMY OF SCIENCES; SUZHOU VIGONVITA LIFE SCIENCES CO., LTD.; TOPHARMAN SHANDONG CO., LTD. US Patent | Assay Description The 5-HT1A receptor agonism activity test (The agonism activity of test compounds on 5-HT1A receptor expressing human recombinant 5-HT1A receptor in ... | US Patent US10174011 (2019) BindingDB Entry DOI: 10.7270/Q2VH5QX7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50024605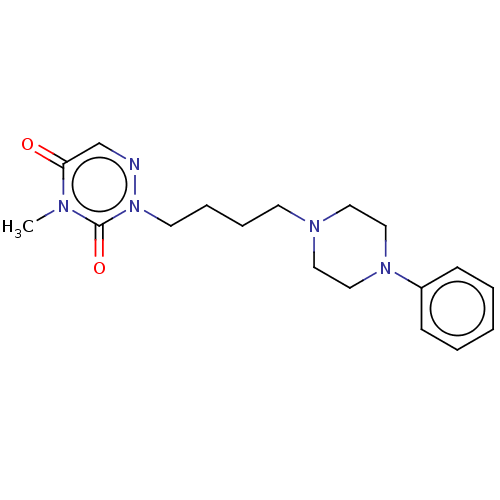 (CHEMBL3330603 | US9290463, E) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a |
Stony Brook University Curated by ChEMBL | Assay Description Agonist activity at human 5HT1AR expressed in CHO cells assessed as increase in [35S]GTPgammaS binding by liquid scintillation spectrometry | Bioorg Med Chem Lett 24: 4759-62 (2014) Article DOI: 10.1016/j.bmcl.2014.07.048 BindingDB Entry DOI: 10.7270/Q2NZ896M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50182020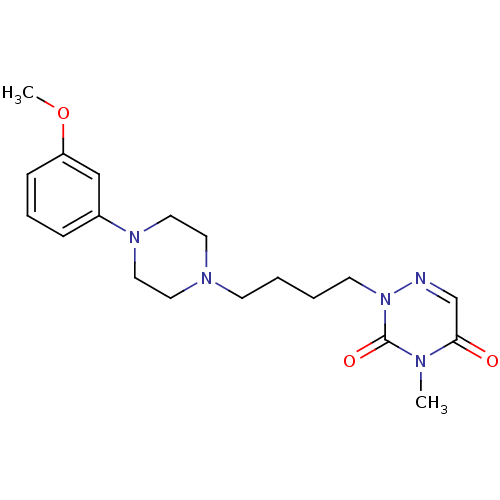 (2-(4-(4-(3-methoxyphenyl)piperazin-1-yl)butyl)-4-m...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a |
Columbia University College of Physicians and Surgeons Curated by ChEMBL | Assay Description Agonist activity assessed by stimulation of [35S]GTPgammaS binding to human 5HT1A receptor expressed in CHO cells | Bioorg Med Chem Lett 16: 2101-4 (2006) Article DOI: 10.1016/j.bmcl.2006.01.052 BindingDB Entry DOI: 10.7270/Q2ZC82FS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM433480 (US10562853, Compound 6) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 0.302 | n/a | n/a | n/a | n/a |
TBA | Assay Description Biased agonist activity at human 5-HT1A receptor stably expressed in CHO-K1 cells co-expressing Galpha16/GPCR assessed as increase in calcium mobiliz... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c00814 BindingDB Entry DOI: 10.7270/Q24T6NZT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM433537 (US10562853, Compound 60) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 0.324 | n/a | n/a | n/a | n/a |
TBA | Assay Description Biased agonist activity at Gal4-VP16 transcription factor linked human 5-HT1A receptor expressed in human U2OS cells assessed as induction of beta-ar... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c00814 BindingDB Entry DOI: 10.7270/Q24T6NZT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50274835 (CHEMBL511857 | trans-1-(4-(3-methoxyphenyl)cyclohe...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 0.324 | n/a | n/a | n/a | n/a |
Karolinska Institute and Karolinska University Hospital Curated by ChEMBL | Assay Description Agonist activity at human 5-HT1A receptor expressed in CHO cells after 90 mins by beta-arrestin-2 recruitment assay | Bioorg Med Chem 23: 4824-30 (2015) Article DOI: 10.1016/j.bmc.2015.05.042 BindingDB Entry DOI: 10.7270/Q21R6S8W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM433499 (US10562853, Compound 16) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 0.339 | n/a | n/a | n/a | n/a |
TBA | Assay Description Biased agonist activity at human 5-HT1A receptor stably expressed in CHO-K1 cells assessed as inhibition of forskolin-induced cAMP accumulation after... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c00814 BindingDB Entry DOI: 10.7270/Q24T6NZT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50150086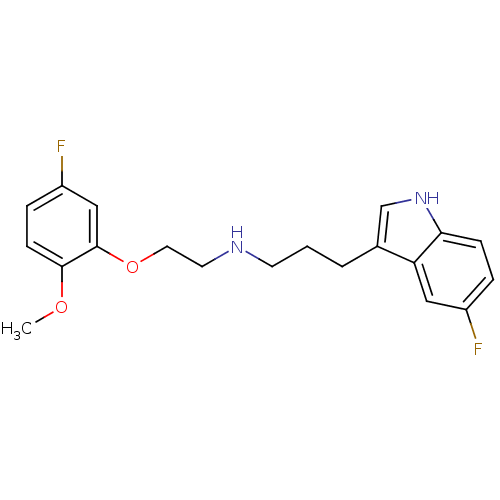 (CHEMBL127171 | [3-(5-Fluoro-1H-indol-3-yl)-propyl]...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 0.340 | n/a | n/a | n/a | n/a |
Wyeth Research Curated by ChEMBL | Assay Description Effective concentration against binding of radioligand [35]GTPgammaS in CHO cells expressing human 5-hydroxytryptamine 1A receptor | J Med Chem 47: 3823-42 (2004) Article DOI: 10.1021/jm0304010 BindingDB Entry DOI: 10.7270/Q2S46RDW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50272617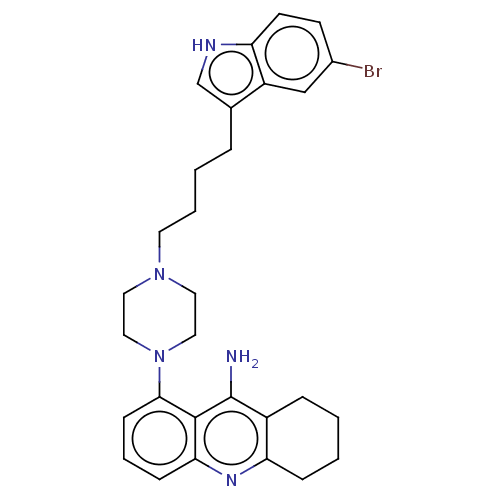 (CHEMBL4128200) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 0.350 | n/a | n/a | n/a | n/a |
East China University of Science and Technology Curated by ChEMBL | Assay Description Agonist activity at human 5-HT1A receptor expressed in HEK293 cells after 60 mins by Eu-cAMP solution based ultra LANCE assay | Bioorg Med Chem 26: 3117-3125 (2018) Article DOI: 10.1016/j.bmc.2018.04.037 BindingDB Entry DOI: 10.7270/Q28P631Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50272627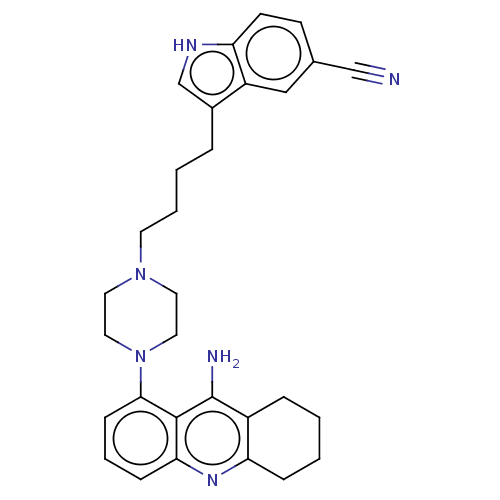 (CHEMBL4126644) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 0.360 | n/a | n/a | n/a | n/a |
East China University of Science and Technology Curated by ChEMBL | Assay Description Agonist activity at human 5-HT1A receptor expressed in HEK293 cells after 60 mins by Eu-cAMP solution based ultra LANCE assay | Bioorg Med Chem 26: 3117-3125 (2018) Article DOI: 10.1016/j.bmc.2018.04.037 BindingDB Entry DOI: 10.7270/Q28P631Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM433480 (US10562853, Compound 6) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 0.372 | n/a | n/a | n/a | n/a |
TBA | Assay Description Biased agonist activity at Gal4-VP16 transcription factor linked human 5-HT1A receptor expressed in human U2OS cells assessed as induction of beta-ar... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c00814 BindingDB Entry DOI: 10.7270/Q24T6NZT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM319733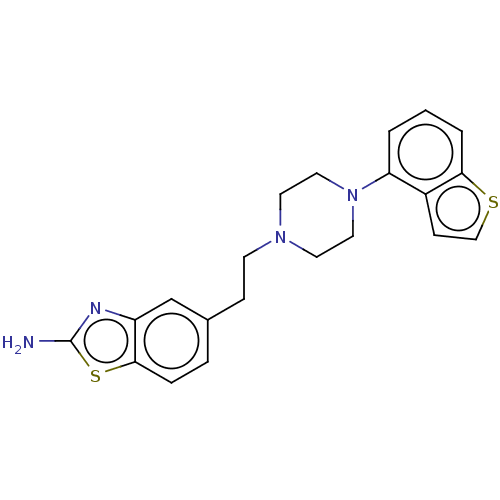 (5-(2-(4-(benzo[b]thiophen-4-yl)piperazin-1-yl)ethy...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | n/a | 0.379 | n/a | n/a | n/a | n/a |
SHANGHAI INSTITUTE OF MATERIA MEDICA, CHINESE ACADEMY OF SCIENCES; SUZHOU VIGONVITA LIFE SCIENCES CO., LTD.; TOPHARMAN SHANDONG CO., LTD. US Patent | Assay Description The 5-HT1A receptor agonism activity test (The agonism activity of test compounds on 5-HT1A receptor expressing human recombinant 5-HT1A receptor in ... | US Patent US10174011 (2019) BindingDB Entry DOI: 10.7270/Q2VH5QX7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM319734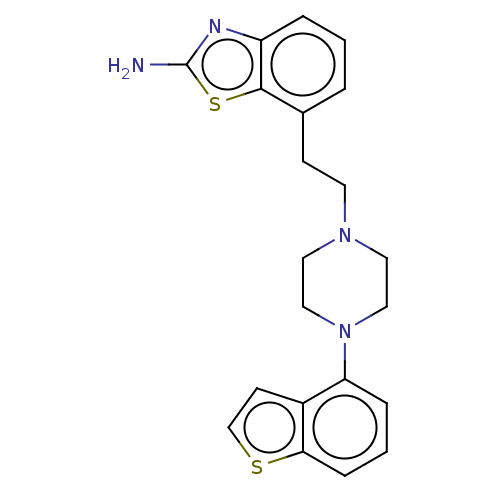 (7-(2-(4-(benzo[b]thiophen-4-yl)piperazin-1-yl)ethy...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | n/a | 0.397 | n/a | n/a | n/a | n/a |
SHANGHAI INSTITUTE OF MATERIA MEDICA, CHINESE ACADEMY OF SCIENCES; SUZHOU VIGONVITA LIFE SCIENCES CO., LTD.; TOPHARMAN SHANDONG CO., LTD. US Patent | Assay Description The 5-HT1A receptor agonism activity test (The agonism activity of test compounds on 5-HT1A receptor expressing human recombinant 5-HT1A receptor in ... | US Patent US10174011 (2019) BindingDB Entry DOI: 10.7270/Q2VH5QX7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM433483 (US10562853, Compound 8) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 0.398 | n/a | n/a | n/a | n/a |
TBA | Assay Description Biased agonist activity at human 5-HT1A receptor stably expressed in CHO-K1 cells assessed as increase in ERK1/2 phosphorylation after 15 mins by Alp... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c00814 BindingDB Entry DOI: 10.7270/Q24T6NZT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM433501 (US10562853, Compound 18) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 0.427 | n/a | n/a | n/a | n/a |
TBA | Assay Description Biased agonist activity at human recombinant 5-HT1A receptor stably expressed in CHO-K1 cells assessed as increase in ERK1/2 phosphorylation levels i... | Citation and Details Article DOI: 10.1021/acs.jmedchem.9b00062 BindingDB Entry DOI: 10.7270/Q23F4T9H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM433499 (US10562853, Compound 16) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 0.501 | n/a | n/a | n/a | n/a |
TBA | Assay Description Biased agonist activity at human 5-HT1A receptor stably expressed in CHO-K1 cells assessed as increase in ERK1/2 phosphorylation after 15 mins by Alp... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c00814 BindingDB Entry DOI: 10.7270/Q24T6NZT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50555884 (CHEMBL4741908) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 0.510 | n/a | n/a | n/a | n/a |
TBA | Assay Description Agonist activity at 5-HT1A receptor (unknown origin) preincubated for 60 mins followed by addition of Eu-cAMP and measured after 60 mins by plate rea... | Citation and Details Article DOI: 10.1016/j.ejmech.2020.112572 BindingDB Entry DOI: 10.7270/Q20K2D7B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 1428 total ) | Next | Last >> |