

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cathepsin K (Rattus norvegicus) | BDBM50138858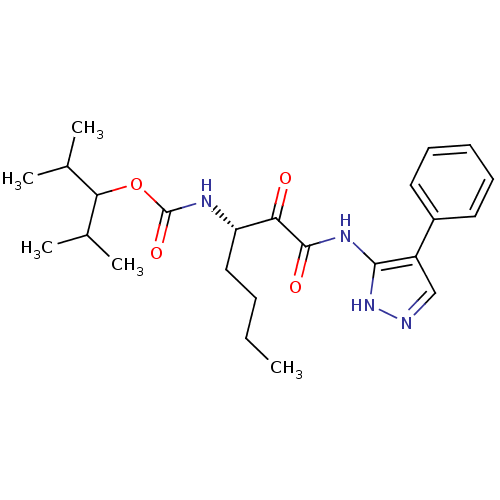 (CHEMBL154579 | [(S)-1-(4-Phenyl-1H-pyrazol-3-ylami...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of cystiene protease cathepsin K of rat | J Med Chem 47: 588-99 (2004) Article DOI: 10.1021/jm030373l BindingDB Entry DOI: 10.7270/Q29S1QF7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin K (Rattus norvegicus) | BDBM50138869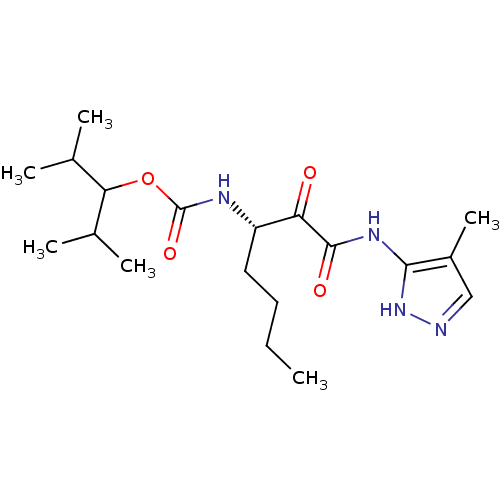 (CHEMBL154959 | [(S)-1-(4-Methyl-1H-pyrazol-3-ylami...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.5 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of cystiene protease cathepsin K of rat | J Med Chem 47: 588-99 (2004) Article DOI: 10.1021/jm030373l BindingDB Entry DOI: 10.7270/Q29S1QF7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin K (Rattus norvegicus) | BDBM50138866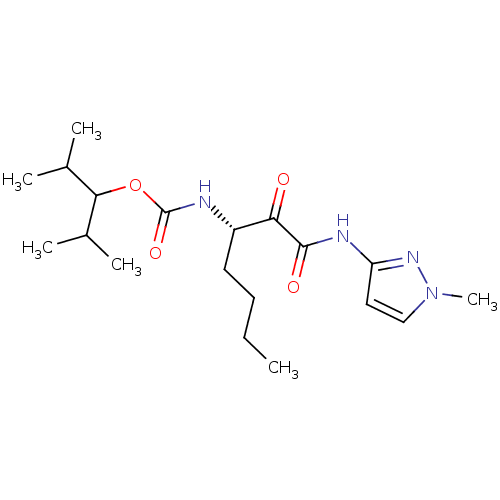 (CHEMBL156764 | [(S)-1-(1-Methyl-1H-pyrazol-3-ylami...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of cystiene protease cathepsin K of rat | J Med Chem 47: 588-99 (2004) Article DOI: 10.1021/jm030373l BindingDB Entry DOI: 10.7270/Q29S1QF7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin K (Rattus norvegicus) | BDBM50223929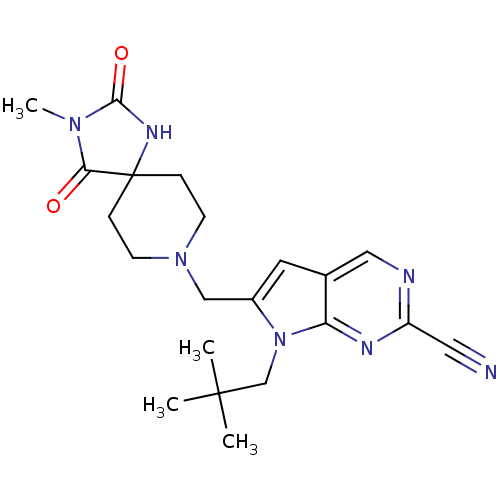 (7-(2,2-Dimethyl-propyl)-6-(3-methyl-2,4-dioxo-1,3,...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of rat recombinant cathepsin K expressed in Sf21 cells by fluorescence assay | J Med Chem 51: 5459-62 (2008) Article DOI: 10.1021/jm800626a BindingDB Entry DOI: 10.7270/Q2SF2W0R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin K (Rattus norvegicus) | BDBM50138879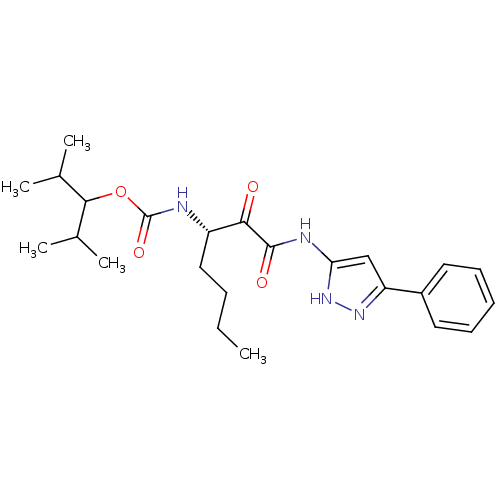 (CHEMBL345982 | [(S)-1-(5-Phenyl-1H-pyrazol-3-ylami...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of cystiene protease cathepsin K of rat | J Med Chem 47: 588-99 (2004) Article DOI: 10.1021/jm030373l BindingDB Entry DOI: 10.7270/Q29S1QF7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin K (Rattus norvegicus) | BDBM50138868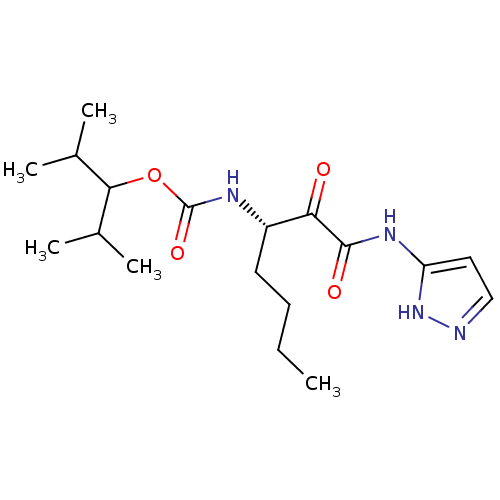 (CHEMBL154862 | [(S)-1-(1H-Pyrazol-3-ylaminooxalyl)...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of cystiene protease cathepsin K of rat | J Med Chem 47: 588-99 (2004) Article DOI: 10.1021/jm030373l BindingDB Entry DOI: 10.7270/Q29S1QF7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin K (Rattus norvegicus) | BDBM50138842 (2,4-dimethylpentan-3-yl(S)-1,2-dioxo-1-((R)-1-phen...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibitory activity of the compound against rat cathepsin K | Bioorg Med Chem Lett 14: 719-22 (2004) BindingDB Entry DOI: 10.7270/Q2QV3KX1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin K (Rattus norvegicus) | BDBM50223934 (7-(2,2-Dimethyl-propyl)-6-(2,4-dioxo-8-propyl-1,3,...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 27 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of rat recombinant cathepsin K expressed in Sf21 cells by fluorescence assay | J Med Chem 51: 5459-62 (2008) Article DOI: 10.1021/jm800626a BindingDB Entry DOI: 10.7270/Q2SF2W0R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin K (Rattus norvegicus) | BDBM50223913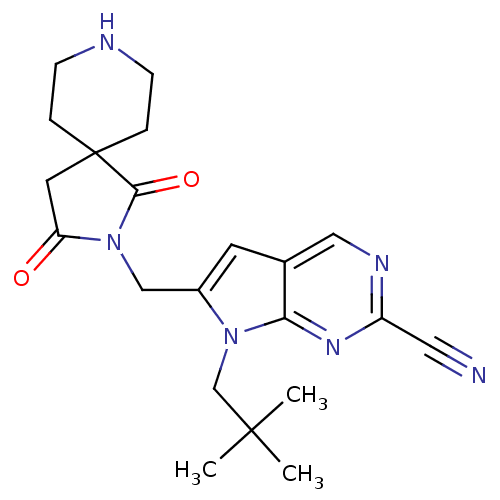 (6-((1,3-dioxo-2,8-diazaspiro[4.5]decan-2-yl)methyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of rat recombinant cathepsin K expressed in Sf21 cells by fluorescence assay | J Med Chem 51: 5459-62 (2008) Article DOI: 10.1021/jm800626a BindingDB Entry DOI: 10.7270/Q2SF2W0R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin K (Rattus norvegicus) | BDBM50244510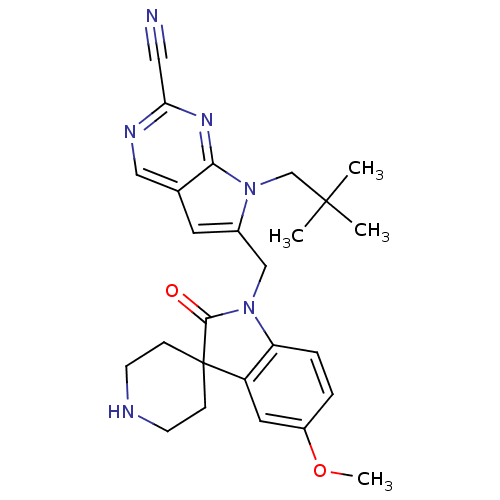 (7-(2,2-dimethylpropyl)-6-[(5-methoxy-2-oxospiro[in...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 44 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of rat recombinant cathepsin K expressed in Sf21 cells by fluorescence assay | J Med Chem 51: 5459-62 (2008) Article DOI: 10.1021/jm800626a BindingDB Entry DOI: 10.7270/Q2SF2W0R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin K (Rattus norvegicus) | BDBM50318880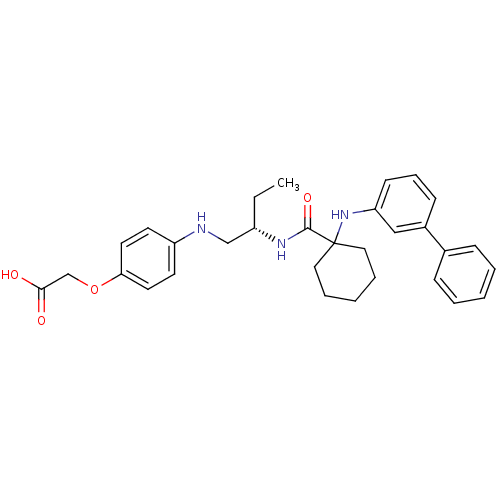 ((S)-2-(4-(2-(1-(biphenyl-3-ylamino)cyclohexanecarb...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 58 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen, Inc. Curated by ChEMBL | Assay Description Inhibition of rat cathepsin K | J Med Chem 53: 4332-53 (2010) Article DOI: 10.1021/jm9018756 BindingDB Entry DOI: 10.7270/Q21R6QPF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin K (Rattus norvegicus) | BDBM50244460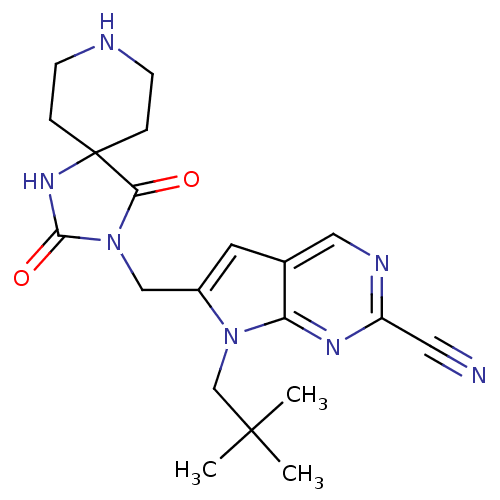 (7-(2,2-dimethylpropyl)-6-[(2,4-dioxo-1,3,8-triazas...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 76 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of rat recombinant cathepsin K expressed in Sf21 cells by fluorescence assay | J Med Chem 51: 5459-62 (2008) Article DOI: 10.1021/jm800626a BindingDB Entry DOI: 10.7270/Q2SF2W0R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin K (Rattus norvegicus) | BDBM50223919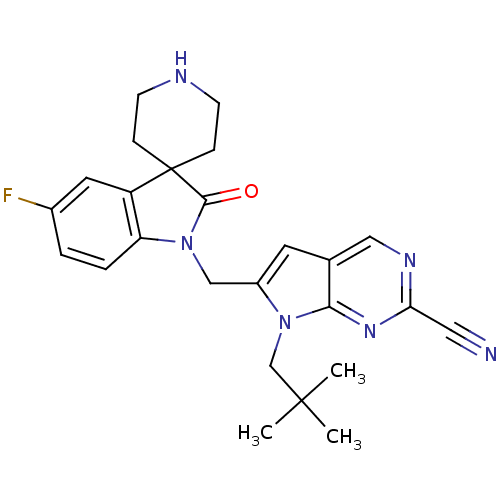 (7-(2,2-dimethylpropyl)-6-[(5-fluoro-2-oxospiro[ind...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 78 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of rat recombinant cathepsin K expressed in Sf21 cells by fluorescence assay | J Med Chem 51: 5459-62 (2008) Article DOI: 10.1021/jm800626a BindingDB Entry DOI: 10.7270/Q2SF2W0R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin K (Rattus norvegicus) | BDBM50177494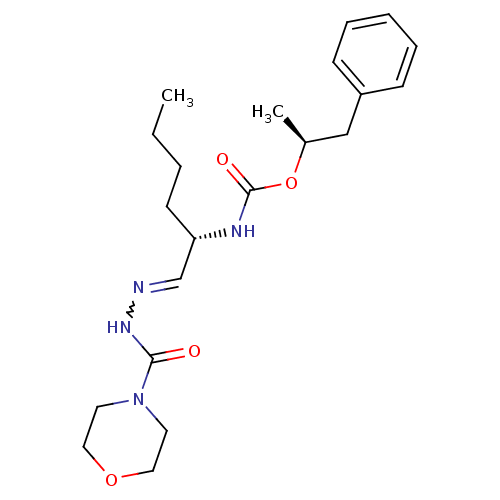 (CHEMBL204605 | {(S)-1-[(morpholine-4-carbonyl)-hyd...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 130 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of rat cathepsin K | Bioorg Med Chem Lett 16: 978-83 (2006) Article DOI: 10.1016/j.bmcl.2005.10.108 BindingDB Entry DOI: 10.7270/Q2X929VS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin K (Rattus norvegicus) | BDBM50138867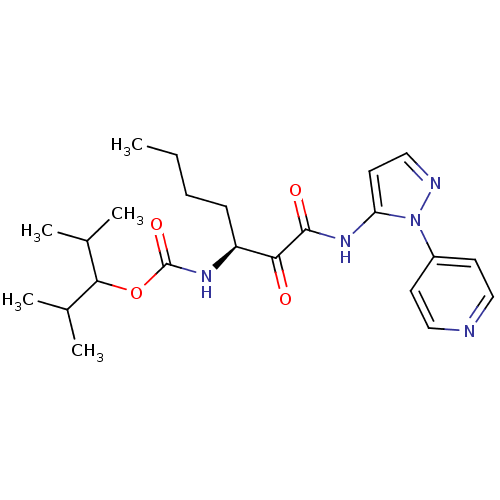 (CHEMBL157911 | [(S)-1-(2-Pyridin-4-yl-2H-pyrazol-3...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 470 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of cystiene protease cathepsin K of rat | J Med Chem 47: 588-99 (2004) Article DOI: 10.1021/jm030373l BindingDB Entry DOI: 10.7270/Q29S1QF7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin K (Rattus norvegicus) | BDBM50138854 (CHEMBL345356 | [(S)-1-(2-Phenyl-2H-pyrazol-3-ylami...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 790 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of cystiene protease cathepsin K of rat | J Med Chem 47: 588-99 (2004) Article DOI: 10.1021/jm030373l BindingDB Entry DOI: 10.7270/Q29S1QF7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin K (Rattus norvegicus) | BDBM50138849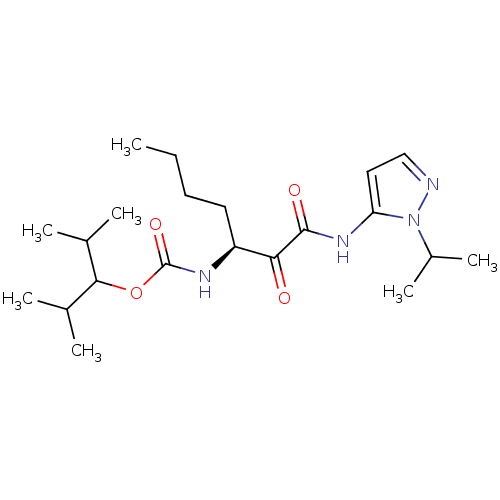 (CHEMBL157224 | [(S)-1-(2-Isopropyl-2H-pyrazol-3-yl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of cystiene protease cathepsin K of rat | J Med Chem 47: 588-99 (2004) Article DOI: 10.1021/jm030373l BindingDB Entry DOI: 10.7270/Q29S1QF7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin K (Rattus norvegicus) | BDBM50138865 (CHEMBL155148 | [(S)-1-(2-Cyclobutyl-2H-pyrazol-3-y...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of cystiene protease cathepsin K of rat | J Med Chem 47: 588-99 (2004) Article DOI: 10.1021/jm030373l BindingDB Entry DOI: 10.7270/Q29S1QF7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin K (Rattus norvegicus) | BDBM50138847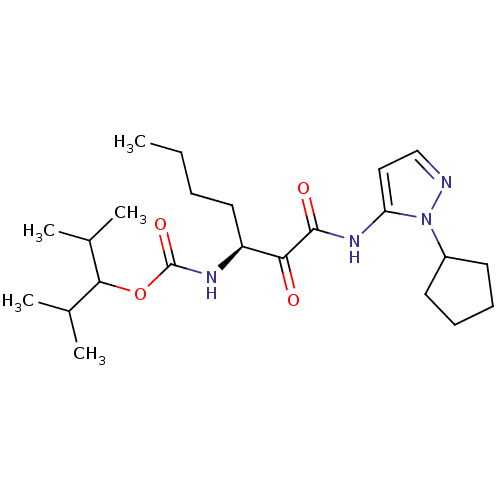 (CHEMBL155123 | [(S)-1-(2-Cyclopentyl-2H-pyrazol-3-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of cystiene protease cathepsin K of rat | J Med Chem 47: 588-99 (2004) Article DOI: 10.1021/jm030373l BindingDB Entry DOI: 10.7270/Q29S1QF7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin K (Rattus norvegicus) | BDBM50138850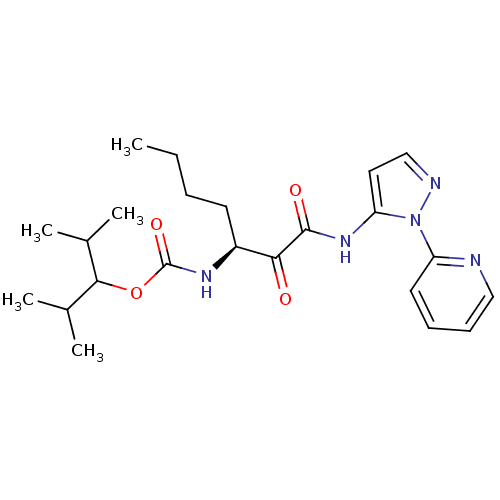 (CHEMBL155297 | [(S)-1-(2-Pyridin-2-yl-2H-pyrazol-3...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of cystiene protease cathepsin K of rat | J Med Chem 47: 588-99 (2004) Article DOI: 10.1021/jm030373l BindingDB Entry DOI: 10.7270/Q29S1QF7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin K (Rattus norvegicus) | BDBM50138877 (CHEMBL346102 | [(S)-1-(2-Cyclohexyl-2H-pyrazol-3-y...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of cystiene protease cathepsin K of rat | J Med Chem 47: 588-99 (2004) Article DOI: 10.1021/jm030373l BindingDB Entry DOI: 10.7270/Q29S1QF7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin K (Rattus norvegicus) | BDBM50138855 (CHEMBL345136 | [(S)-1-(2-Ethyl-2H-pyrazol-3-ylamin...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of cystiene protease cathepsin K of rat | J Med Chem 47: 588-99 (2004) Article DOI: 10.1021/jm030373l BindingDB Entry DOI: 10.7270/Q29S1QF7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin K (Rattus norvegicus) | BDBM50138844 (CHEMBL155183 | {(S)-1-[2-(3,3-Dimethyl-butyl)-2H-p...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of cystiene protease cathepsin K of rat | J Med Chem 47: 588-99 (2004) Article DOI: 10.1021/jm030373l BindingDB Entry DOI: 10.7270/Q29S1QF7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin K (Rattus norvegicus) | BDBM50138851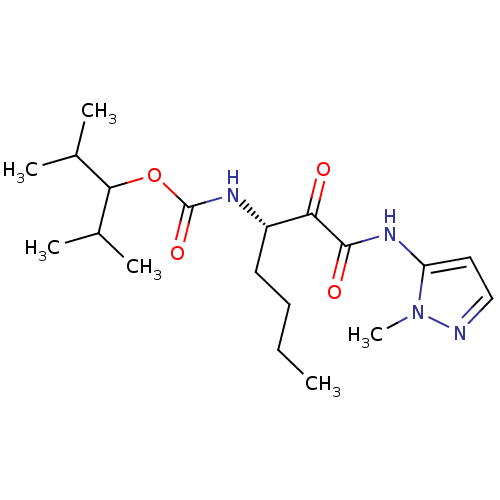 (CHEMBL152633 | [(S)-1-(2-Methyl-2H-pyrazol-3-ylami...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of cystiene protease cathepsin K of rat | J Med Chem 47: 588-99 (2004) Article DOI: 10.1021/jm030373l BindingDB Entry DOI: 10.7270/Q29S1QF7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin K (Rattus norvegicus) | BDBM50138848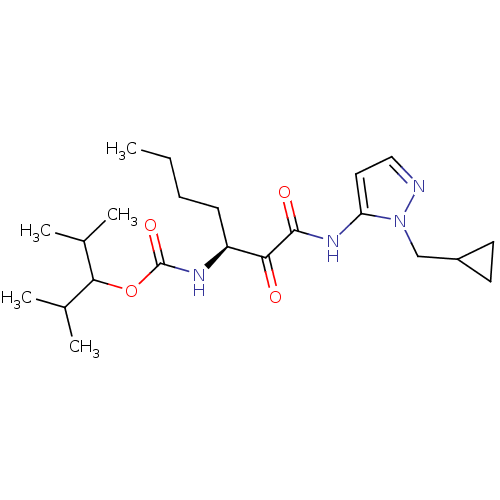 (CHEMBL155592 | [(S)-1-(2-Cyclopropylmethyl-2H-pyra...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of cystiene protease cathepsin K of rat | J Med Chem 47: 588-99 (2004) Article DOI: 10.1021/jm030373l BindingDB Entry DOI: 10.7270/Q29S1QF7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin K (Rattus norvegicus) | BDBM50138872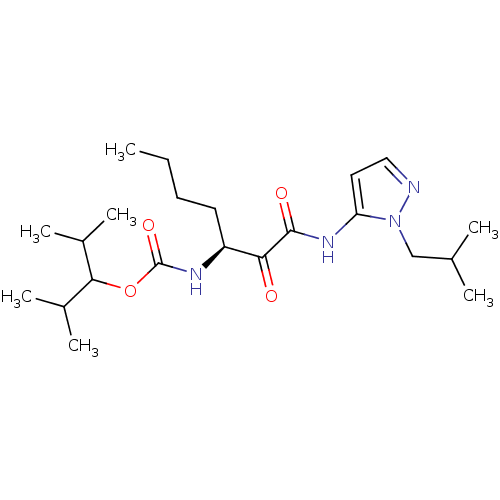 (CHEMBL346739 | [(S)-1-(2-Isobutyl-2H-pyrazol-3-yla...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of cystiene protease cathepsin K of rat | J Med Chem 47: 588-99 (2004) Article DOI: 10.1021/jm030373l BindingDB Entry DOI: 10.7270/Q29S1QF7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin K (Rattus norvegicus) | BDBM25135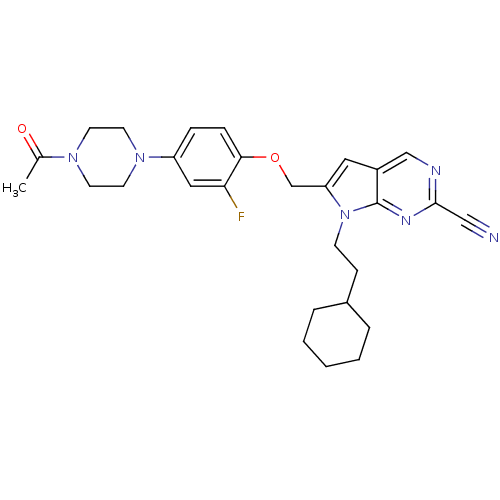 (2-cyano-pyrropyrimidine, 7a | 7-(2-cyclohexylethyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | 7.0 | 22 |
Novartis | Assay Description The substrate hydrolysis with or without inhibitor was monitored at an excitation wavelength of 360nm and an emission wavelength of 460 nm on a fluor... | J Med Chem 51: 5502-5 (2008) Article DOI: 10.1021/jm800839j BindingDB Entry DOI: 10.7270/Q2125QZR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin K (Rattus norvegicus) | BDBM25134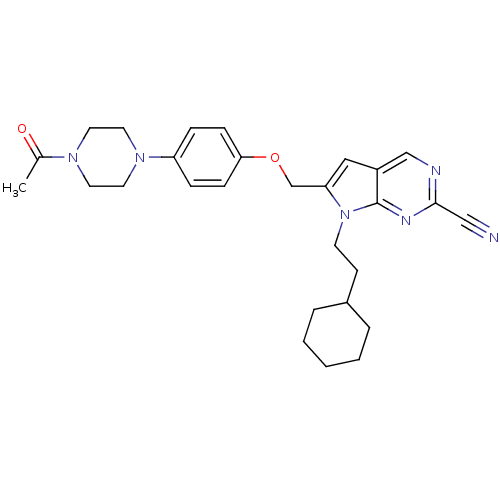 (2-cyano-pyrropyrimidine, 2 | 7-(2-cyclohexylethyl)...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | 7.0 | 22 |
Novartis | Assay Description The substrate hydrolysis with or without inhibitor was monitored at an excitation wavelength of 360nm and an emission wavelength of 460 nm on a fluor... | J Med Chem 51: 5502-5 (2008) Article DOI: 10.1021/jm800839j BindingDB Entry DOI: 10.7270/Q2125QZR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin K (Rattus norvegicus) | BDBM25141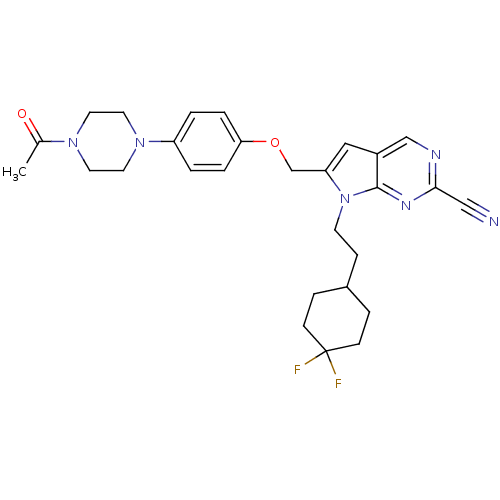 (2-cyano-pyrropyrimidine, 7g | 7-[2-(4,4-difluorocy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | 7.0 | 22 |
Novartis | Assay Description The substrate hydrolysis with or without inhibitor was monitored at an excitation wavelength of 360nm and an emission wavelength of 460 nm on a fluor... | J Med Chem 51: 5502-5 (2008) Article DOI: 10.1021/jm800839j BindingDB Entry DOI: 10.7270/Q2125QZR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||