 Found 366 hits of ic50 for UniProtKB: P04775
Found 366 hits of ic50 for UniProtKB: P04775 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kcal/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Sodium channel protein type 2 subunit alpha
(Rattus norvegicus) | BDBM50051090
(CHEMBL3318149)Show SMILES [H][C@]12CSSC[C@]3([H])NC(=O)[C@@]([H])(NC(=O)[C@H](Cc4c[nH]c5ccccc45)NC(=O)[C@H](CCSC)NC(=O)[C@H](Cc4c[nH]c5ccccc45)NC(=O)[C@H](CCCCN)NC(=O)[C@H](CCC(N)=O)NC(=O)[C@H](CSSC[C@]([H])(NC(=O)[C@]([H])(CSSC[C@H](NC(=O)[C@H](Cc4c[nH]c5ccccc45)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](CCCNC(N)=N)NC1=O)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](Cc1c[nH]c4ccccc14)C(=O)OC)NC(=O)[C@H](CCCCN)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](CO)NC(=O)[C@H](CC(O)=O)NC3=O)C(=O)N[C@@H](CCC(O)=O)C(=O)NCC(=O)N[C@@H](CCSC)C(=O)N[C@@H](C(C)C)C(=O)N2)NC(=O)[C@@H](N)Cc1ccc(O)cc1)[C@@H](C)O |r| | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue Pharma L.P.
Curated by ChEMBL
| Assay Description
Inhibition of rat Nav1.7 expressed in HEK-293 cells at -130 to -110 mV holding potential by whole-cell voltage clamp analysis |
J Med Chem 57: 6623-31 (2014)
Article DOI: 10.1021/jm500687u
BindingDB Entry DOI: 10.7270/Q22B90P2 |
More data for this
Ligand-Target Pair | |
Sodium channel protein type 2 subunit alpha
(Rattus norvegicus) | BDBM50051091
(CHEMBL3318150)Show SMILES [H][C@]12CSSC[C@]3([H])NC(=O)[C@@]([H])(NC(=O)[C@H](Cc4c[nH]c5ccccc45)NC(=O)[C@H](CCSC)NC(=O)[C@H](Cc4c[nH]c5ccccc45)NC(=O)[C@H](CCCCN)NC(=O)[C@H](CCC(N)=O)NC(=O)[C@H](CSSC[C@]([H])(NC(=O)[C@]([H])(CSSC[C@H](NC(=O)[C@H](Cc4c[nH]c5ccccc45)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](CCCNC(N)=N)NC1=O)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](Cc1c[nH]c4ccccc14)C(=O)Nc1ccccc1)NC(=O)[C@H](CCCCN)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](CO)NC(=O)[C@H](CC(O)=O)NC3=O)C(=O)N[C@@H](CCC(O)=O)C(=O)NCC(=O)N[C@@H](CCSC)C(=O)N[C@@H](C(C)C)C(=O)N2)NC(=O)[C@@H](N)Cc1ccc(O)cc1)[C@@H](C)O |r| | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue Pharma L.P.
Curated by ChEMBL
| Assay Description
Inhibition of rat Nav1.7 expressed in HEK-293 cells at -130 to -110 mV holding potential by whole-cell voltage clamp analysis |
J Med Chem 57: 6623-31 (2014)
Article DOI: 10.1021/jm500687u
BindingDB Entry DOI: 10.7270/Q22B90P2 |
More data for this
Ligand-Target Pair | |
Sodium channel protein type 2 subunit alpha
(Rattus norvegicus) | BDBM50051089
(CHEMBL3318148)Show SMILES [H][C@]12CSSC[C@]3([H])NC(=O)[C@@]([H])(NC(=O)[C@H](Cc4c[nH]c5ccccc45)NC(=O)[C@H](CCSC)NC(=O)[C@H](Cc4c[nH]c5ccccc45)NC(=O)[C@H](CCCCN)NC(=O)[C@H](CCC(N)=O)NC(=O)[C@H](CSSC[C@]([H])(NC(=O)[C@]([H])(CSSC[C@H](NC(=O)[C@H](Cc4c[nH]c5ccccc45)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](CCCNC(N)=N)NC1=O)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](Cc1c[nH]c4ccccc14)C(=O)NC)NC(=O)[C@H](CCCCN)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](CO)NC(=O)[C@H](CC(O)=O)NC3=O)C(=O)N[C@@H](CCC(O)=O)C(=O)NCC(=O)N[C@@H](CCSC)C(=O)N[C@@H](C(C)C)C(=O)N2)NC(=O)[C@@H](N)Cc1ccc(O)cc1)[C@@H](C)O |r| | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue Pharma L.P.
Curated by ChEMBL
| Assay Description
Inhibition of rat Nav1.7 expressed in HEK-293 cells at -130 to -110 mV holding potential by whole-cell voltage clamp analysis |
J Med Chem 57: 6623-31 (2014)
Article DOI: 10.1021/jm500687u
BindingDB Entry DOI: 10.7270/Q22B90P2 |
More data for this
Ligand-Target Pair | |
Sodium channel protein type 2 subunit alpha
(Rattus norvegicus) | BDBM50344821
(10-hydroxymethyl-5-imino-(2S)-12,13-dioxa-4,6-diaz...)Show SMILES NC1=N[C@H](O)[C@H]2[C@H]3O[C@]4(O)O[C@@H]([C@@H](O)[C@@]2(N1)[C@@H]4O)[C@]3(O)CO |r,t:1,TLB:13:12:16:6.7.5,18:11:16:6.7.5,18:6:16:11.10.12,19:18:7:16.14.5,20:18:7:16.14.5,THB:12:11:7:16.14.5,3:5:16:11.10.12| Show InChI InChI=1S/C11H17N3O8/c12-8-13-6(17)2-4-9(19,1-15)5-3(16)10(2,14-8)7(18)11(20,21-4)22-5/h2-7,15-20H,1H2,(H3,12,13,14)/t2-,3-,4-,5+,6-,7+,9+,10-,11+/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Cambridge NeuroScience Inc.
Curated by ChEMBL
| Assay Description
In vitro inhibition of [14C]- guanidinium influx in Chinese hamster ovary (CHO) cells expressing rat brain sodium channel type IIA (CNaIIA-1) |
J Med Chem 41: 3048-61 (1998)
Article DOI: 10.1021/jm980124a
BindingDB Entry DOI: 10.7270/Q2R49RF5 |
More data for this
Ligand-Target Pair | |
Sodium channel protein type 2 subunit alpha
(Rattus norvegicus) | BDBM50051088
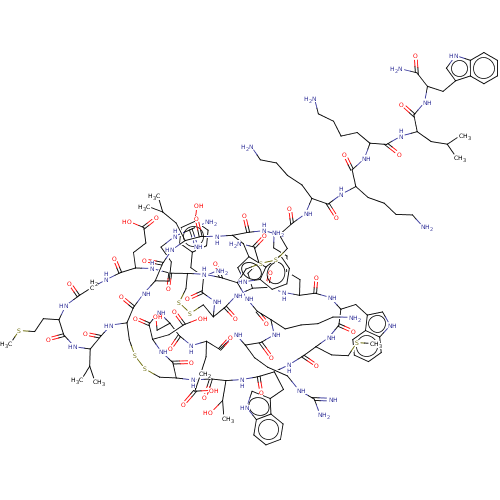
(CHEMBL3318147)Show SMILES [H][C@]12CSSC[C@]3([H])NC(=O)[C@@]([H])(NC(=O)[C@H](Cc4c[nH]c5ccccc45)NC(=O)[C@H](CCSC)NC(=O)[C@H](Cc4c[nH]c5ccccc45)NC(=O)[C@H](CCCCN)NC(=O)[C@H](CCC(N)=O)NC(=O)[C@H](CSSC[C@]([H])(NC(=O)[C@]([H])(CSSC[C@H](NC(=O)[C@H](Cc4c[nH]c5ccccc45)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](CCCNC(N)=N)NC1=O)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](Cc1c[nH]c4ccccc14)C(N)=O)NC(=O)[C@H](CCCCN)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](CO)NC(=O)[C@H](CC(O)=O)NC3=O)C(=O)N[C@@H](CCC(O)=O)C(=O)NCC(=O)N[C@@H](CCSC)C(=O)N[C@@H](C(C)C)C(=O)N2)NC(=O)[C@@H](N)Cc1ccc(O)cc1)[C@@H](C)O |r| | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue Pharma L.P.
Curated by ChEMBL
| Assay Description
Inhibition of rat Nav1.7 expressed in HEK-293 cells at -130 to -110 mV holding potential by whole-cell voltage clamp analysis |
J Med Chem 57: 6623-31 (2014)
Article DOI: 10.1021/jm500687u
BindingDB Entry DOI: 10.7270/Q22B90P2 |
More data for this
Ligand-Target Pair | |
Sodium channel protein type 2 subunit alpha
(Rattus norvegicus) | BDBM50051075
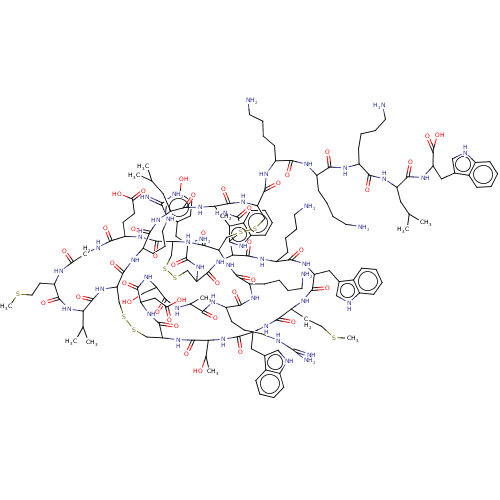
(CHEMBL3318134)Show SMILES [H][C@]12CSSC[C@]3([H])NC(=O)[C@@]([H])(NC(=O)[C@H](Cc4c[nH]c5ccccc45)NC(=O)[C@H](CCSC)NC(=O)[C@H](Cc4c[nH]c5ccccc45)NC(=O)[C@H](CCCCN)NC(=O)[C@H](CCC(N)=O)NC(=O)[C@H](CSSC[C@]([H])(NC(=O)[C@]([H])(CSSC[C@H](NC(=O)[C@H](Cc4c[nH]c5ccccc45)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](CCCNC(N)=N)NC1=O)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](Cc1c[nH]c4ccccc14)C(O)=O)NC(=O)[C@H](CCCCN)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](C)NC(=O)[C@H](CO)NC(=O)[C@H](CC(O)=O)NC3=O)C(=O)N[C@@H](CCC(O)=O)C(=O)NCC(=O)N[C@@H](CCSC)C(=O)N[C@@H](C(C)C)C(=O)N2)NC(=O)[C@@H](N)Cc1ccc(O)cc1)[C@@H](C)O |r| | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue Pharma L.P.
Curated by ChEMBL
| Assay Description
Inhibition of rat Nav1.7 expressed in HEK-293 cells at -130 to -110 mV holding potential by whole-cell voltage clamp analysis |
J Med Chem 57: 6623-31 (2014)
Article DOI: 10.1021/jm500687u
BindingDB Entry DOI: 10.7270/Q22B90P2 |
More data for this
Ligand-Target Pair | |
Sodium channel protein type 1/2/3 subunit alpha
(Rattus norvegicus) | BDBM50223720
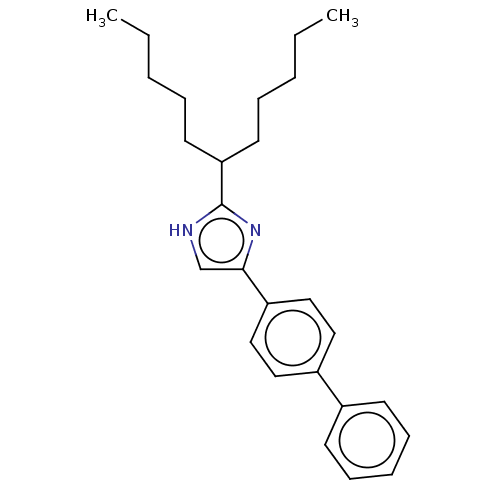
(CHEMBL332257)Show SMILES CCCCCC(CCCCC)c1nc(c[nH]1)-c1ccc(cc1)-c1ccccc1 Show InChI InChI=1S/C26H34N2/c1-3-5-8-14-24(15-9-6-4-2)26-27-20-25(28-26)23-18-16-22(17-19-23)21-12-10-7-11-13-21/h7,10-13,16-20,24H,3-6,8-9,14-15H2,1-2H3,(H,27,28) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Institut Henri Beaufour
Curated by ChEMBL
| Assay Description
Concentration required to inhibit [3H]BTX binding to Sodium channel of rat brain |
Bioorg Med Chem Lett 14: 3521-3 (2004)
BindingDB Entry DOI: 10.7270/Q2474D2N |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 13/Cyclin-K
(Homo sapiens (Human)) | BDBM50367676
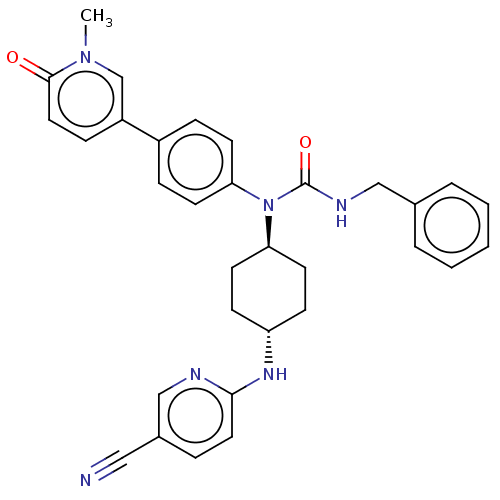
(CHEMBL4160662)Show SMILES Cn1cc(ccc1=O)-c1ccc(cc1)N([C@H]1CC[C@@H](CC1)Nc1ccc(cn1)C#N)C(=O)NCc1ccccc1 |r,wU:15.16,wD:18.23,(17.35,-24.38,;18.11,-25.71,;19.66,-25.71,;20.43,-27.05,;19.66,-28.38,;18.11,-28.38,;17.35,-27.05,;15.8,-27.05,;21.97,-27.05,;22.74,-28.38,;24.28,-28.38,;25.05,-27.05,;24.28,-25.71,;22.74,-25.71,;26.6,-27.05,;27.37,-25.71,;26.6,-24.38,;27.37,-23.04,;28.91,-23.04,;29.68,-24.38,;28.91,-25.71,;29.68,-21.71,;31.22,-21.71,;31.99,-20.37,;33.54,-20.37,;34.3,-21.71,;33.54,-23.04,;31.99,-23.04,;35.85,-21.71,;37.39,-21.71,;27.37,-28.38,;26.6,-29.71,;28.91,-28.38,;29.68,-29.71,;31.22,-29.71,;31.99,-28.38,;33.54,-28.38,;34.3,-29.71,;33.54,-31.05,;31.99,-31.05,)| Show InChI InChI=1S/C32H32N6O2/c1-37-22-26(10-18-31(37)39)25-8-13-28(14-9-25)38(32(40)35-20-23-5-3-2-4-6-23)29-15-11-27(12-16-29)36-30-17-7-24(19-33)21-34-30/h2-10,13-14,17-18,21-22,27,29H,11-12,15-16,20H2,1H3,(H,34,36)(H,35,40)/t27-,29- | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal FLAG-tagged human full-length CDK13 (1 to 1512 residues)/N-terminal His-tagged CycK (1 to 580 residues) expressed in Sf9 cel... |
J Med Chem 61: 7710-7728 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00683
BindingDB Entry DOI: 10.7270/Q2HX1G6F |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 13/Cyclin-K
(Homo sapiens (Human)) | BDBM50597426
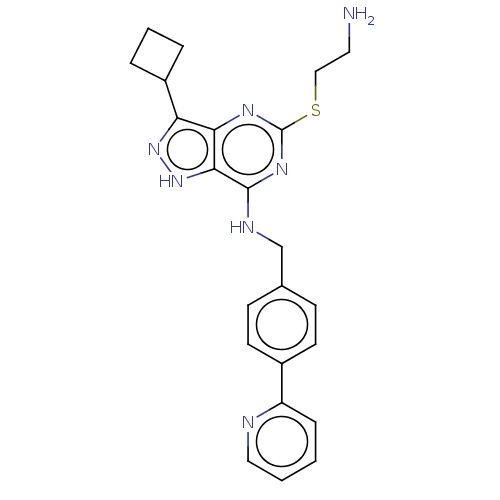
(CHEMBL5169449)Show SMILES NCCSc1nc(NCc2ccc(cc2)-c2ccccn2)c2[nH]nc(C3CCC3)c2n1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c02184
BindingDB Entry DOI: 10.7270/Q26114CS |
More data for this
Ligand-Target Pair | |
Sodium channel protein type 2 subunit alpha
(Rattus norvegicus) | BDBM50344821

(10-hydroxymethyl-5-imino-(2S)-12,13-dioxa-4,6-diaz...)Show SMILES NC1=N[C@H](O)[C@H]2[C@H]3O[C@]4(O)O[C@@H]([C@@H](O)[C@@]2(N1)[C@@H]4O)[C@]3(O)CO |r,t:1,TLB:13:12:16:6.7.5,18:11:16:6.7.5,18:6:16:11.10.12,19:18:7:16.14.5,20:18:7:16.14.5,THB:12:11:7:16.14.5,3:5:16:11.10.12| Show InChI InChI=1S/C11H17N3O8/c12-8-13-6(17)2-4-9(19,1-15)5-3(16)10(2,14-8)7(18)11(20,21-4)22-5/h2-7,15-20H,1H2,(H3,12,13,14)/t2-,3-,4-,5+,6-,7+,9+,10-,11+/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PubMed
| n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company
Curated by ChEMBL
| Assay Description
Inhibition of Na+ influx in chinese hamster ovary cells expressing rat brain sodium channel type IIA |
J Med Chem 37: 268-74 (1994)
BindingDB Entry DOI: 10.7270/Q2CJ8F39 |
More data for this
Ligand-Target Pair | |
Sodium channel protein type 1/2/3 subunit alpha
(Rattus norvegicus) | BDBM50223731
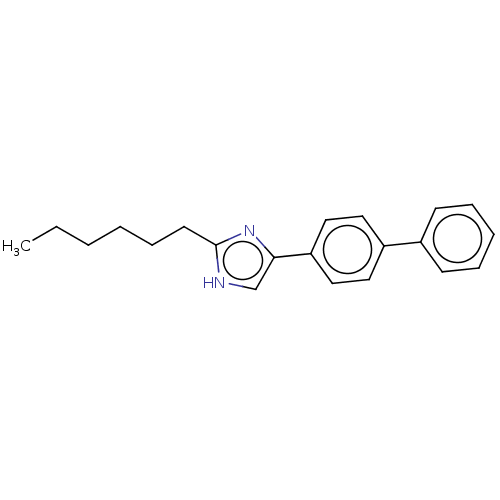
(CHEMBL118603)Show InChI InChI=1S/C21H24N2/c1-2-3-4-8-11-21-22-16-20(23-21)19-14-12-18(13-15-19)17-9-6-5-7-10-17/h5-7,9-10,12-16H,2-4,8,11H2,1H3,(H,22,23) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
Institut Henri Beaufour
Curated by ChEMBL
| Assay Description
Concentration required to inhibit [3H]BTX binding to Sodium channel of rat brain |
Bioorg Med Chem Lett 14: 3521-3 (2004)
BindingDB Entry DOI: 10.7270/Q2474D2N |
More data for this
Ligand-Target Pair | |
Sodium channel protein type 1/2/3 subunit alpha
(Rattus norvegicus) | BDBM50223721
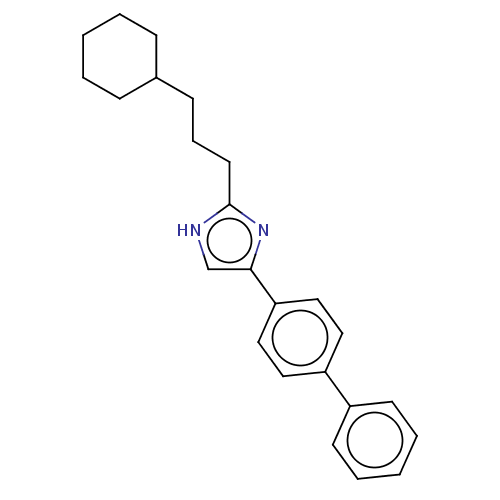
(CHEMBL331513)Show SMILES C(CC1CCCCC1)Cc1nc(c[nH]1)-c1ccc(cc1)-c1ccccc1 Show InChI InChI=1S/C24H28N2/c1-3-8-19(9-4-1)10-7-13-24-25-18-23(26-24)22-16-14-21(15-17-22)20-11-5-2-6-12-20/h2,5-6,11-12,14-19H,1,3-4,7-10,13H2,(H,25,26) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
Institut Henri Beaufour
Curated by ChEMBL
| Assay Description
Concentration required to inhibit [3H]BTX binding to Sodium channel of rat brain |
Bioorg Med Chem Lett 14: 3521-3 (2004)
BindingDB Entry DOI: 10.7270/Q2474D2N |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 13/Cyclin-K
(Homo sapiens (Human)) | BDBM50139171
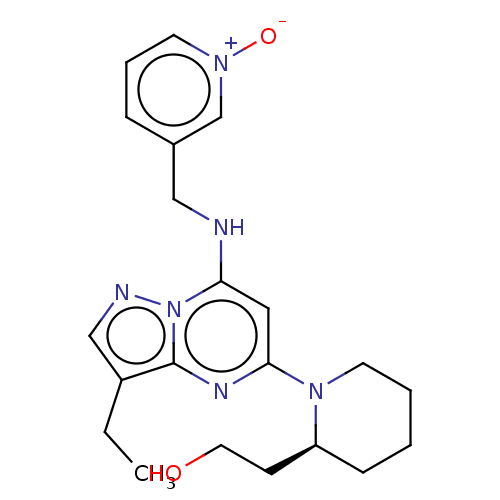
(Dinaciclib | MK-7965 | SCH-727965 | US11643396, Ex...)Show SMILES CCc1cnn2c(NCc3ccc[n+]([O-])c3)cc(nc12)N1CCCC[C@H]1CCO |r| Show InChI InChI=1S/C21H28N6O2/c1-2-17-14-23-27-19(22-13-16-6-5-9-25(29)15-16)12-20(24-21(17)27)26-10-4-3-7-18(26)8-11-28/h5-6,9,12,14-15,18,22,28H,2-4,7-8,10-11,13H2,1H3/t18-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c02184
BindingDB Entry DOI: 10.7270/Q26114CS |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 13/Cyclin-K
(Homo sapiens (Human)) | BDBM50597425
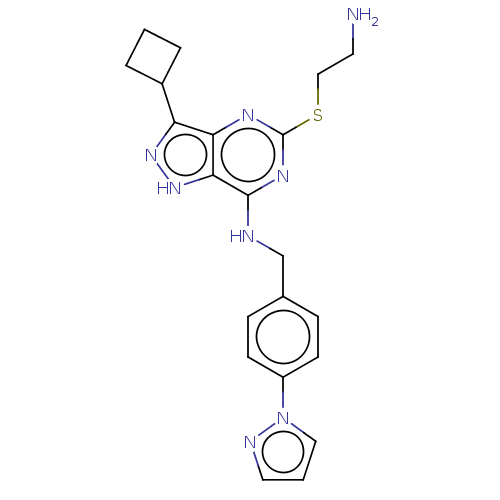
(CHEMBL5194202)Show SMILES NCCSc1nc(NCc2ccc(cc2)-n2cccn2)c2[nH]nc(C3CCC3)c2n1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c02184
BindingDB Entry DOI: 10.7270/Q26114CS |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 13/Cyclin-K
(Homo sapiens (Human)) | BDBM50597424
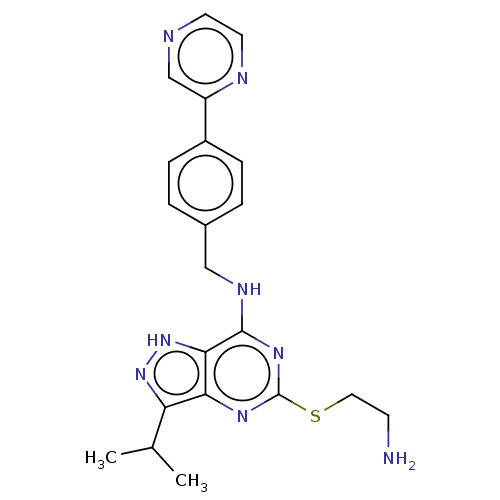
(CHEMBL5187378)Show SMILES CC(C)c1n[nH]c2c(NCc3ccc(cc3)-c3cnccn3)nc(SCCN)nc12 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c02184
BindingDB Entry DOI: 10.7270/Q26114CS |
More data for this
Ligand-Target Pair | |
Sodium channel protein type 2 subunit alpha
(Rattus norvegicus) | BDBM50051077
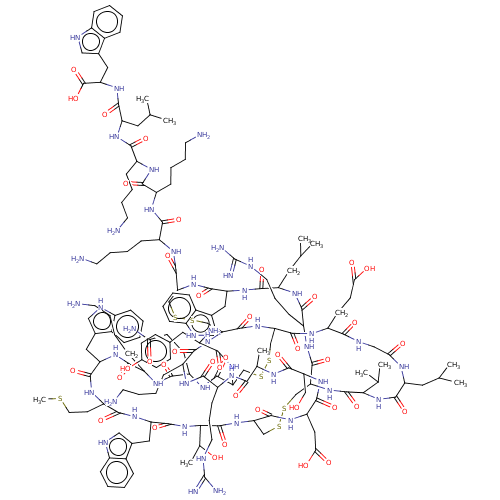
(CHEMBL3318136)Show SMILES [H][C@]12CSSC[C@]3([H])NC(=O)[C@@]([H])(NC(=O)[C@H](Cc4c[nH]c5ccccc45)NC(=O)[C@H](CCSC)NC(=O)[C@H](Cc4c[nH]c5ccccc45)NC(=O)[C@H](CCCCN)NC(=O)[C@H](CCC(N)=O)NC(=O)[C@H](CSSC[C@]([H])(NC(=O)[C@]([H])(CSSC[C@H](NC(=O)[C@H](Cc4c[nH]c5ccccc45)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](CCCNC(N)=N)NC1=O)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](Cc1c[nH]c4ccccc14)C(O)=O)NC(=O)[C@H](CCCCN)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](C)NC(=O)[C@H](CO)NC(=O)[C@H](CC(O)=O)NC3=O)C(=O)N[C@@H](CCC(O)=O)C(=O)NCC(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](C(C)C)C(=O)N2)NC(=O)[C@@H](N)Cc1ccc(O)cc1)[C@@H](C)O |r| | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 24 | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue Pharma L.P.
Curated by ChEMBL
| Assay Description
Inhibition of rat Nav1.7 expressed in HEK-293 cells at -130 to -110 mV holding potential by whole-cell voltage clamp analysis |
J Med Chem 57: 6623-31 (2014)
Article DOI: 10.1021/jm500687u
BindingDB Entry DOI: 10.7270/Q22B90P2 |
More data for this
Ligand-Target Pair | |
Sodium channel protein type 1/2/3 subunit alpha
(Rattus norvegicus) | BDBM50223726
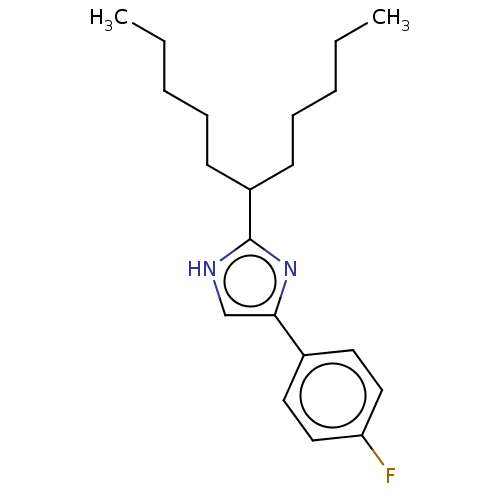
(CHEMBL117331)Show InChI InChI=1S/C20H29FN2/c1-3-5-7-9-17(10-8-6-4-2)20-22-15-19(23-20)16-11-13-18(21)14-12-16/h11-15,17H,3-10H2,1-2H3,(H,22,23) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
Institut Henri Beaufour
Curated by ChEMBL
| Assay Description
Concentration required to inhibit [3H]BTX binding to Sodium channel of rat brain |
Bioorg Med Chem Lett 14: 3521-3 (2004)
BindingDB Entry DOI: 10.7270/Q2474D2N |
More data for this
Ligand-Target Pair | |
Sodium channel protein type 1/2/3 subunit alpha
(Rattus norvegicus) | BDBM50223735
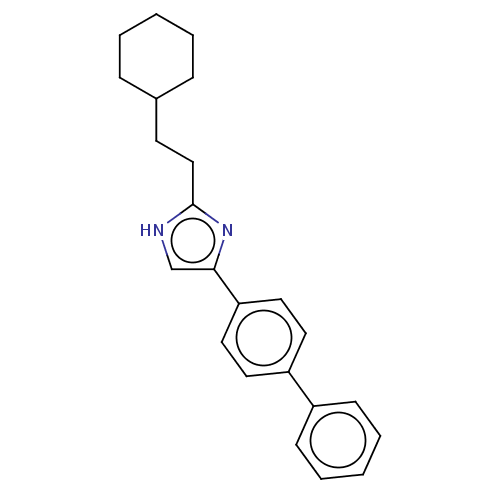
(CHEMBL119139)Show SMILES C(Cc1nc(c[nH]1)-c1ccc(cc1)-c1ccccc1)C1CCCCC1 Show InChI InChI=1S/C23H26N2/c1-3-7-18(8-4-1)11-16-23-24-17-22(25-23)21-14-12-20(13-15-21)19-9-5-2-6-10-19/h2,5-6,9-10,12-15,17-18H,1,3-4,7-8,11,16H2,(H,24,25) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 26 | n/a | n/a | n/a | n/a | n/a | n/a |
Institut Henri Beaufour
Curated by ChEMBL
| Assay Description
Concentration required to inhibit [3H]BTX binding to Sodium channel of rat brain |
Bioorg Med Chem Lett 14: 3521-3 (2004)
BindingDB Entry DOI: 10.7270/Q2474D2N |
More data for this
Ligand-Target Pair | |
Sodium channel protein type 2 subunit alpha
(Rattus norvegicus) | BDBM50051084
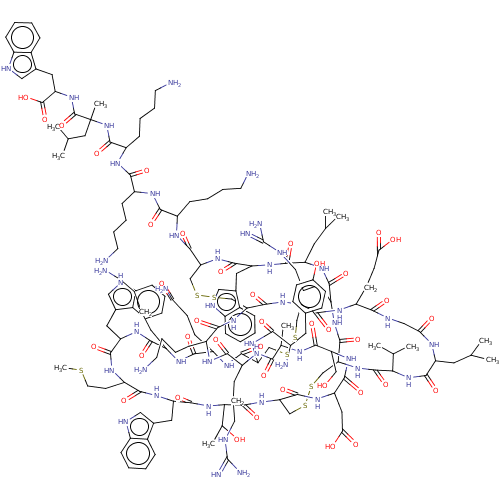
(CHEMBL3318143)Show SMILES [H][C@]12CSSC[C@]3([H])NC(=O)[C@@]([H])(NC(=O)[C@H](Cc4c[nH]c5ccccc45)NC(=O)[C@H](CCSC)NC(=O)[C@H](Cc4c[nH]c5ccccc45)NC(=O)[C@H](CCCCN)NC(=O)[C@H](CCC(N)=O)NC(=O)[C@H](CSSC[C@]([H])(NC(=O)[C@]([H])(CSSC[C@H](NC(=O)[C@H](Cc4c[nH]c5ccccc45)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](CCCNC(N)=N)NC1=O)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CCCCN)C(=O)N[C@@](C)(CC(C)C)C(=O)N[C@@H](Cc1c[nH]c4ccccc14)C(O)=O)NC(=O)[C@H](CCCCN)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](C)NC(=O)[C@H](CO)NC(=O)[C@H](CC(O)=O)NC3=O)C(=O)N[C@@H](CCC(O)=O)C(=O)NCC(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](C(C)C)C(=O)N2)NC(=O)[C@@H](N)Cc1ccc(O)cc1)[C@@H](C)O |r| | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 28 | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue Pharma L.P.
Curated by ChEMBL
| Assay Description
Inhibition of rat Nav1.7 expressed in HEK-293 cells at -130 to -110 mV holding potential by whole-cell voltage clamp analysis |
J Med Chem 57: 6623-31 (2014)
Article DOI: 10.1021/jm500687u
BindingDB Entry DOI: 10.7270/Q22B90P2 |
More data for this
Ligand-Target Pair | |
Sodium channel protein type 1/2/3 subunit alpha
(Rattus norvegicus) | BDBM50223734
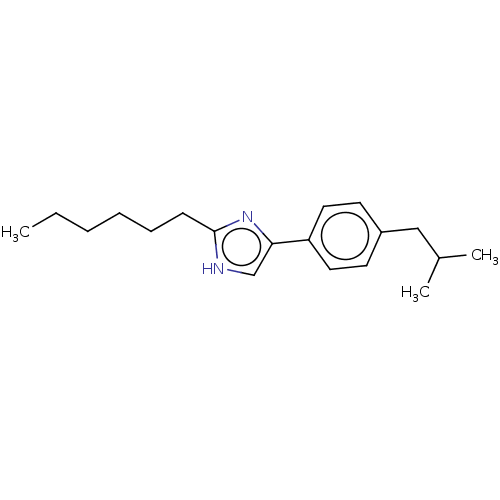
(CHEMBL331942)Show InChI InChI=1S/C19H28N2/c1-4-5-6-7-8-19-20-14-18(21-19)17-11-9-16(10-12-17)13-15(2)3/h9-12,14-15H,4-8,13H2,1-3H3,(H,20,21) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 28 | n/a | n/a | n/a | n/a | n/a | n/a |
Institut Henri Beaufour
Curated by ChEMBL
| Assay Description
Concentration required to inhibit [3H]BTX binding to Sodium channel of rat brain |
Bioorg Med Chem Lett 14: 3521-3 (2004)
BindingDB Entry DOI: 10.7270/Q2474D2N |
More data for this
Ligand-Target Pair | |
Sodium channel protein type 1/2/3 subunit alpha
(Rattus norvegicus) | BDBM50223733
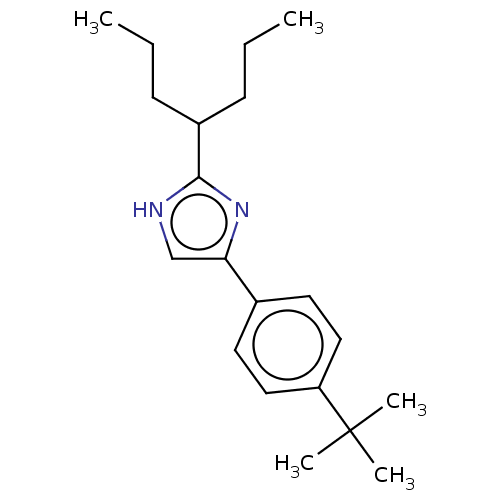
(CHEMBL120306)Show InChI InChI=1S/C20H30N2/c1-6-8-16(9-7-2)19-21-14-18(22-19)15-10-12-17(13-11-15)20(3,4)5/h10-14,16H,6-9H2,1-5H3,(H,21,22) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Institut Henri Beaufour
Curated by ChEMBL
| Assay Description
Concentration required to inhibit [3H]BTX binding to Sodium channel of rat brain |
Bioorg Med Chem Lett 14: 3521-3 (2004)
BindingDB Entry DOI: 10.7270/Q2474D2N |
More data for this
Ligand-Target Pair | |
Sodium channel protein type 2 subunit alpha
(Rattus norvegicus) | BDBM50051079
(CHEMBL3318138)Show SMILES [H][C@]12CSSC[C@]3([H])NC(=O)[C@@]([H])(NC(=O)[C@H](Cc4c[nH]c5ccccc45)NC(=O)[C@H](CCSC)NC(=O)[C@H](Cc4c[nH]c5ccccc45)NC(=O)[C@H](CCCCN)NC(=O)[C@H](CCC(N)=O)NC(=O)[C@H](CSSC[C@]([H])(NC(=O)[C@]([H])(CSSC[C@H](NC(=O)[C@H](Cc4c[nH]c5ccccc45)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](CCCNC(N)=N)NC1=O)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H]([C@@H](C)CC)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](Cc1c[nH]c4ccccc14)C(O)=O)NC(=O)[C@H](CCCCN)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](C)NC(=O)[C@H](CO)NC(=O)[C@H](CC(O)=O)NC3=O)C(=O)N[C@@H](CCC(O)=O)C(=O)NCC(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](C(C)C)C(=O)N2)NC(=O)[C@@H](N)Cc1ccc(O)cc1)[C@@H](C)O |r| | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 33 | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue Pharma L.P.
Curated by ChEMBL
| Assay Description
Inhibition of rat Nav1.7 expressed in HEK-293 cells at -130 to -110 mV holding potential by whole-cell voltage clamp analysis |
J Med Chem 57: 6623-31 (2014)
Article DOI: 10.1021/jm500687u
BindingDB Entry DOI: 10.7270/Q22B90P2 |
More data for this
Ligand-Target Pair | |
Sodium channel protein type 2 subunit alpha
(Rattus norvegicus) | BDBM50118517
(CHEMBL3617067)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@H](CCCCN)NC(=O)[C@H](CS)NC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](Cc1cnc[nH]1)NC(=O)[C@H](CCCCN)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](CO)NC(=O)[C@H](CS)NC(=O)[C@@H](NC(=O)[C@H](CC(C)C)NC(=O)[C@H](CC(N)=O)NC(=O)[C@H](CO)NC(=O)[C@H](CO)NC(=O)[C@H](CCCCN)NC(=O)[C@H](CS)NC(=O)[C@H](CS)NC(=O)[C@H](CCC(N)=O)NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](CC(N)=O)NC(=O)[C@H](CO)NC(=O)[C@@H]1CCCN1C(=O)[C@H](CC(N)=O)NC(=O)[C@H](CS)NC(=O)CNC(=O)[C@H](CCCCN)NC(=O)CNC(=O)[C@H](Cc1ccccc1)NC(=O)CNC(=O)[C@H](CC(C)C)NC(=O)[C@H](CS)NC(=O)[C@@H](N)CCC(O)=O)C(C)C)C(N)=O |r| Show InChI InChI=1S/C166H263N53O50S6/c1-9-83(8)132(133(176)238)218-144(249)99(43-46-129(234)235)198-145(250)103(57-85-37-39-88(224)40-38-85)201-139(244)94(32-17-21-49-169)195-157(262)116(75-272)214-146(251)104(58-86-64-184-91-29-14-13-28-89(86)91)202-141(246)97(35-24-52-183-166(179)180)193-147(252)105(59-87-65-181-79-188-87)203-140(245)93(31-16-20-48-168)192-138(243)96(34-23-51-182-165(177)178)194-152(257)110(69-220)210-160(265)119(78-275)216-163(268)131(82(6)7)217-151(256)101(55-81(4)5)200-148(253)106(60-122(173)226)204-153(258)111(70-221)209-155(260)112(71-222)208-142(247)95(33-18-22-50-170)196-158(263)117(76-273)215-161(266)118(77-274)213-143(248)98(42-44-121(172)225)197-150(255)108(63-130(236)237)206-149(254)107(61-123(174)227)205-154(259)113(72-223)211-162(267)120-36-25-53-219(120)164(269)109(62-124(175)228)207-156(261)114(73-270)191-127(231)68-185-135(240)92(30-15-19-47-167)189-125(229)66-187-137(242)102(56-84-26-11-10-12-27-84)190-126(230)67-186-136(241)100(54-80(2)3)199-159(264)115(74-271)212-134(239)90(171)41-45-128(232)233/h10-14,26-29,37-40,64-65,79-83,90,92-120,131-132,184,220-224,270-275H,9,15-25,30-36,41-63,66-78,167-171H2,1-8H3,(H2,172,225)(H2,173,226)(H2,174,227)(H2,175,228)(H2,176,238)(H,181,188)(H,185,240)(H,186,241)(H,187,242)(H,189,229)(H,190,230)(H,191,231)(H,192,243)(H,193,252)(H,194,257)(H,195,262)(H,196,263)(H,197,255)(H,198,250)(H,199,264)(H,200,253)(H,201,244)(H,202,246)(H,203,245)(H,204,258)(H,205,259)(H,206,254)(H,207,261)(H,208,247)(H,209,260)(H,210,265)(H,211,267)(H,212,239)(H,213,248)(H,214,251)(H,215,266)(H,216,268)(H,217,256)(H,218,249)(H,232,233)(H,234,235)(H,236,237)(H4,177,178,182)(H4,179,180,183)/t83-,90-,92-,93-,94-,95-,96-,97-,98-,99-,100-,101-,102-,103-,104-,105-,106-,107-,108-,109-,110-,111-,112-,113-,114-,115-,116-,117-,118-,119-,120-,131-,132-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 36 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of rat NaV1.2 channel |
J Med Chem 58: 7093-118 (2015)
Article DOI: 10.1021/jm501981g
BindingDB Entry DOI: 10.7270/Q2XS5X6P |
More data for this
Ligand-Target Pair | |
Sodium channel protein type 2 subunit alpha
(Rattus norvegicus) | BDBM50051082
(CHEMBL3318141)Show SMILES [H][C@]12CSSC[C@]3([H])NC(=O)[C@@]([H])(NC(=O)[C@H](Cc4c[nH]c5ccccc45)NC(=O)[C@H](CCSC)NC(=O)[C@H](Cc4c[nH]c5ccccc45)NC(=O)[C@H](CCCCN)NC(=O)[C@H](CCC(N)=O)NC(=O)[C@H](CSSC[C@]([H])(NC(=O)[C@]([H])(CSSC[C@H](NC(=O)[C@H](Cc4c[nH]c5ccccc45)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](CCCNC(N)=N)NC1=O)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H]([C@@H](C)CC)C(=O)N[C@@H]([C@@H](C)CC)C(=O)N[C@@H](Cc1c[nH]c4ccccc14)C(O)=O)NC(=O)[C@H](CCCCN)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](C)NC(=O)[C@H](CO)NC(=O)[C@H](CC(O)=O)NC3=O)C(=O)N[C@@H](CCC(O)=O)C(=O)NCC(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](C(C)C)C(=O)N2)NC(=O)[C@@H](N)Cc1ccc(O)cc1)[C@@H](C)O |r| | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 45 | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue Pharma L.P.
Curated by ChEMBL
| Assay Description
Inhibition of rat Nav1.7 expressed in HEK-293 cells at -130 to -110 mV holding potential by whole-cell voltage clamp analysis |
J Med Chem 57: 6623-31 (2014)
Article DOI: 10.1021/jm500687u
BindingDB Entry DOI: 10.7270/Q22B90P2 |
More data for this
Ligand-Target Pair | |
Sodium channel protein type 2 subunit alpha
(Rattus norvegicus) | BDBM50051076
(CHEMBL3318135)Show SMILES [H][C@]12CSSC[C@]3([H])NC(=O)[C@@]([H])(NC(=O)[C@H](Cc4c[nH]c5ccccc45)NC(=O)[C@H](CCSC)NC(=O)[C@H](Cc4c[nH]c5ccccc45)NC(=O)[C@H](CCCCN)NC(=O)[C@H](CCC(N)=O)NC(=O)[C@H](CSSC[C@]([H])(NC(=O)[C@]([H])(CSSC[C@H](NC(=O)[C@H](Cc4c[nH]c5ccccc45)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](CCCNC(N)=N)NC1=O)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](Cc1c[nH]c4ccccc14)C(O)=O)NC(=O)[C@H](CCCCN)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](CO)NC(=O)[C@H](CC(O)=O)NC3=O)C(=O)N[C@@H](CCC(O)=O)C(=O)NCC(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](C(C)C)C(=O)N2)NC(=O)[C@@H](N)Cc1ccc(O)cc1)[C@@H](C)O |r| | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 45 | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue Pharma L.P.
Curated by ChEMBL
| Assay Description
Inhibition of rat Nav1.7 expressed in HEK-293 cells at -130 to -110 mV holding potential by whole-cell voltage clamp analysis |
J Med Chem 57: 6623-31 (2014)
Article DOI: 10.1021/jm500687u
BindingDB Entry DOI: 10.7270/Q22B90P2 |
More data for this
Ligand-Target Pair | |
Sodium channel protein type 2 subunit alpha
(Rattus norvegicus) | BDBM50262566
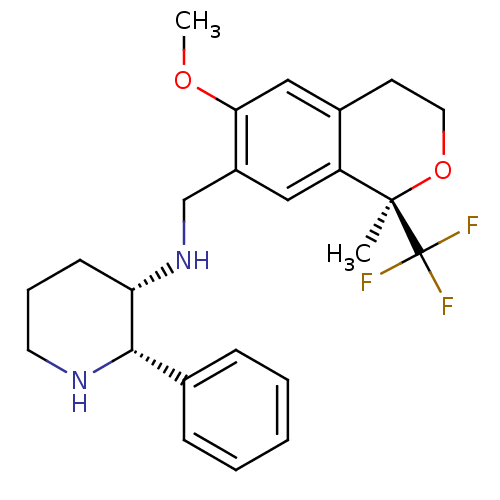
((2S,3S)-3-[(1R)-6-Methoxy-1-methyl-1-trifluorometh...)Show SMILES COc1cc2CCO[C@](C)(c2cc1CN[C@H]1CCCN[C@H]1c1ccccc1)C(F)(F)F |r| Show InChI InChI=1S/C24H29F3N2O2/c1-23(24(25,26)27)19-13-18(21(30-2)14-17(19)10-12-31-23)15-29-20-9-6-11-28-22(20)16-7-4-3-5-8-16/h3-5,7-8,13-14,20,22,28-29H,6,9-12,15H2,1-2H3/t20-,22-,23+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 48 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research & Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]batrachotoxinin from rat sodium channel type 2A subunit expressed in CHO-CNa2A cells by whole cell patch clamp technique |
Bioorg Med Chem 16: 7193-205 (2008)
Article DOI: 10.1016/j.bmc.2008.06.047
BindingDB Entry DOI: 10.7270/Q2RV0NHN |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 13/Cyclin-K
(Homo sapiens (Human)) | BDBM50539904
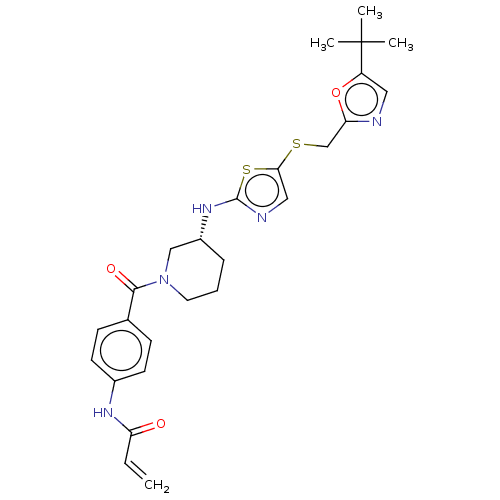
(CHEMBL4647764)Show SMILES CC(C)(C)c1cnc(CSc2cnc(N[C@@H]3CCCN(C3)C(=O)c3ccc(NC(=O)C=C)cc3)s2)o1 |r| Show InChI InChI=1S/C26H31N5O3S2/c1-5-21(32)29-18-10-8-17(9-11-18)24(33)31-12-6-7-19(15-31)30-25-28-14-23(36-25)35-16-22-27-13-20(34-22)26(2,3)4/h5,8-11,13-14,19H,1,6-7,12,15-16H2,2-4H3,(H,28,30)(H,29,32)/t19-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 49 | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School
Curated by ChEMBL
| Assay Description
Inhibition of human CDK13/cyclin K using Pol2-CTD as substrate by [gamma-33P]ATP-based radioisotope filter binding assay |
J Med Chem 63: 6708-6726 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01929
BindingDB Entry DOI: 10.7270/Q25M698Z |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 13/Cyclin-K
(Homo sapiens (Human)) | BDBM50573506
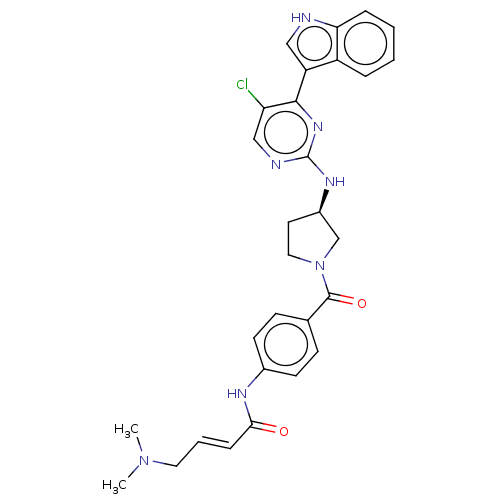
(CHEMBL4861833)Show SMILES CN(C)C\C=C\C(=O)Nc1ccc(cc1)C(=O)N1CC[C@H](C1)Nc1ncc(Cl)c(n1)-c1c[nH]c2ccccc12 |r| | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human recombinant CDK13/CyclinK assessed as reduction in substrate phosphorylation using His-c-Myc as substrate preincubated with enzym... |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113481
BindingDB Entry DOI: 10.7270/Q22B92TP |
More data for this
Ligand-Target Pair | |
Sodium channel protein type 2 subunit alpha
(Rattus norvegicus) | BDBM50051081
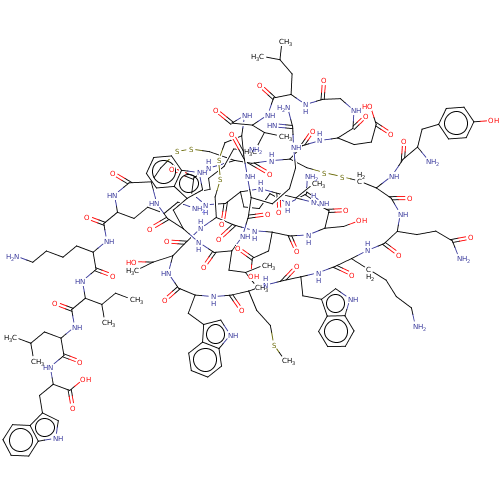
(CHEMBL3318140)Show SMILES [H][C@]12CSSC[C@]3([H])NC(=O)[C@@]([H])(NC(=O)[C@H](Cc4c[nH]c5ccccc45)NC(=O)[C@H](CCSC)NC(=O)[C@H](Cc4c[nH]c5ccccc45)NC(=O)[C@H](CCCCN)NC(=O)[C@H](CCC(N)=O)NC(=O)[C@H](CSSC[C@]([H])(NC(=O)[C@]([H])(CSSC[C@H](NC(=O)[C@H](Cc4c[nH]c5ccccc45)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](CCCNC(N)=N)NC1=O)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H]([C@@H](C)CC)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](Cc1c[nH]c4ccccc14)C(O)=O)NC(=O)[C@H](CCCCN)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](C)NC(=O)[C@H](CO)NC(=O)[C@H](CC(O)=O)NC3=O)C(=O)N[C@@H](CCC(O)=O)C(=O)NCC(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](C(C)C)C(=O)N2)NC(=O)[C@@H](N)Cc1ccc(O)cc1)[C@@H](C)O |r| | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue Pharma L.P.
Curated by ChEMBL
| Assay Description
Inhibition of rat Nav1.7 expressed in HEK-293 cells at -130 to -110 mV holding potential by whole-cell voltage clamp analysis |
J Med Chem 57: 6623-31 (2014)
Article DOI: 10.1021/jm500687u
BindingDB Entry DOI: 10.7270/Q22B90P2 |
More data for this
Ligand-Target Pair | |
Sodium channel protein type 2 subunit alpha
(Rattus norvegicus) | BDBM50051086
(CHEMBL3318145)Show SMILES [H][C@]12CSSC[C@]3([H])NC(=O)[C@@]([H])(NC(=O)[C@H](Cc4c[nH]c5ccccc45)NC(=O)[C@H](CCSC)NC(=O)[C@H](Cc4c[nH]c5ccccc45)NC(=O)[C@H](CCCCN)NC(=O)[C@H](CCC(N)=O)NC(=O)[C@H](CSSC[C@]([H])(NC(=O)[C@]([H])(CSSC[C@H](NC(=O)[C@H](Cc4c[nH]c5ccccc45)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](CCCNC(N)=N)NC1=O)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CCCCN)C(=O)N(C)[C@@H](CC(C)C)C(=O)N[C@@H](Cc1c[nH]c4ccccc14)C(O)=O)NC(=O)[C@H](CCCCN)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](C)NC(=O)[C@H](CO)NC(=O)[C@H](CC(O)=O)NC3=O)C(=O)N[C@@H](CCC(O)=O)C(=O)NCC(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](C(C)C)C(=O)N2)NC(=O)[C@@H](N)Cc1ccc(O)cc1)[C@@H](C)O |r| | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 51 | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue Pharma L.P.
Curated by ChEMBL
| Assay Description
Inhibition of rat Nav1.7 expressed in HEK-293 cells at -130 to -110 mV holding potential by whole-cell voltage clamp analysis |
J Med Chem 57: 6623-31 (2014)
Article DOI: 10.1021/jm500687u
BindingDB Entry DOI: 10.7270/Q22B90P2 |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 13/Cyclin-K
(Homo sapiens (Human)) | BDBM2579

((2S,3R,4R,6R)-3-methoxy-2-methyl-4-(methylamino)-2...)Show SMILES CN[C@@H]1C[C@H]2O[C@@](C)([C@@H]1OC)n1c3ccccc3c3c4CNC(=O)c4c4c5ccccc5n2c4c13 |r| Show InChI InChI=1S/C28H26N4O3/c1-28-26(34-3)17(29-2)12-20(35-28)31-18-10-6-4-8-14(18)22-23-16(13-30-27(23)33)21-15-9-5-7-11-19(15)32(28)25(21)24(22)31/h4-11,17,20,26,29H,12-13H2,1-3H3,(H,30,33)/t17-,20-,26-,28+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 57 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal FLAG-tagged human full-length CDK13 (1 to 1512 residues)/N-terminal His-tagged CycK (1 to 580 residues) expressed in Sf9 cel... |
J Med Chem 61: 7710-7728 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00683
BindingDB Entry DOI: 10.7270/Q2HX1G6F |
More data for this
Ligand-Target Pair | |
Sodium channel protein type 2 subunit alpha
(Rattus norvegicus) | BDBM50066062
(1,3-Bis-(4-sec-butyl-phenyl)-imidazolidin-2-yliden...)Show SMILES CCC(C)c1ccc(cc1)N1CCN(C1=N)c1ccc(cc1)C(C)CC Show InChI InChI=1S/C23H31N3/c1-5-17(3)19-7-11-21(12-8-19)25-15-16-26(23(25)24)22-13-9-20(10-14-22)18(4)6-2/h7-14,17-18,24H,5-6,15-16H2,1-4H3 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 60 | n/a | n/a | n/a | n/a | n/a | n/a |
Cambridge NeuroScience Inc.
Curated by ChEMBL
| Assay Description
In vitro inhibition of [14C]- guanidinium influx in Chinese hamster ovary (CHO) cells expressing rat brain sodium channel type IIA (CNaIIA-1) |
J Med Chem 41: 3048-61 (1998)
Article DOI: 10.1021/jm980124a
BindingDB Entry DOI: 10.7270/Q2R49RF5 |
More data for this
Ligand-Target Pair | |
Sodium channel protein type 2 subunit alpha
(Rattus norvegicus) | BDBM50066059
(1,3-Bis-(4-sec-butyl-phenyl)-tetrahydro-pyrimidin-...)Show SMILES CCC(C)c1ccc(cc1)N1CCCN(C1=N)c1ccc(cc1)C(C)CC Show InChI InChI=1S/C24H33N3/c1-5-18(3)20-8-12-22(13-9-20)26-16-7-17-27(24(26)25)23-14-10-21(11-15-23)19(4)6-2/h8-15,18-19,25H,5-7,16-17H2,1-4H3 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 60 | n/a | n/a | n/a | n/a | n/a | n/a |
Cambridge NeuroScience Inc.
Curated by ChEMBL
| Assay Description
In vitro inhibition of [14C]- guanidinium influx in Chinese hamster ovary (CHO) cells expressing rat brain sodium channel type IIA (CNaIIA-1) |
J Med Chem 41: 3048-61 (1998)
Article DOI: 10.1021/jm980124a
BindingDB Entry DOI: 10.7270/Q2R49RF5 |
More data for this
Ligand-Target Pair | |
Sodium channel protein type 2 subunit alpha
(Rattus norvegicus) | BDBM50051083
(CHEMBL3318142)Show SMILES [H][C@]12CSSC[C@]3([H])NC(=O)[C@@]([H])(NC(=O)[C@H](Cc4c[nH]c5ccccc45)NC(=O)[C@H](CCSC)NC(=O)[C@H](Cc4c[nH]c5ccccc45)NC(=O)[C@H](CCCCN)NC(=O)[C@H](CCC(N)=O)NC(=O)[C@H](CSSC[C@]([H])(NC(=O)[C@]([H])(CSSC[C@H](NC(=O)[C@H](Cc4c[nH]c5ccccc45)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](CCCNC(N)=N)NC1=O)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](Cc1c[nH]c4ccccc14)C(O)=O)NC(=O)[C@H](CCCCN)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](C)NC(=O)[C@H](CO)NC(=O)[C@H](CC(O)=O)NC3=O)C(=O)N[C@@H](CCC(O)=O)C(=O)NCC(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](C(C)C)C(=O)N2)NC(=O)[C@@H](N)Cc1ccc(O)cc1)[C@@H](C)O |r| | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 63 | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue Pharma L.P.
Curated by ChEMBL
| Assay Description
Inhibition of rat Nav1.7 expressed in HEK-293 cells at -130 to -110 mV holding potential by whole-cell voltage clamp analysis |
J Med Chem 57: 6623-31 (2014)
Article DOI: 10.1021/jm500687u
BindingDB Entry DOI: 10.7270/Q22B90P2 |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 13/Cyclin-K
(Homo sapiens (Human)) | BDBM50597419
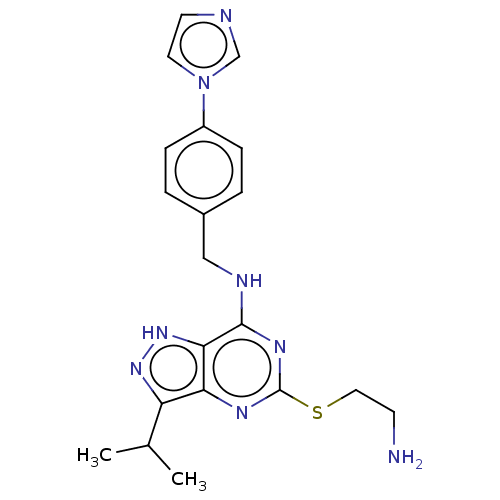
(CHEMBL5182767)Show SMILES CC(C)c1n[nH]c2c(NCc3ccc(cc3)-n3ccnc3)nc(SCCN)nc12 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 67 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c02184
BindingDB Entry DOI: 10.7270/Q26114CS |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 13/Cyclin-K
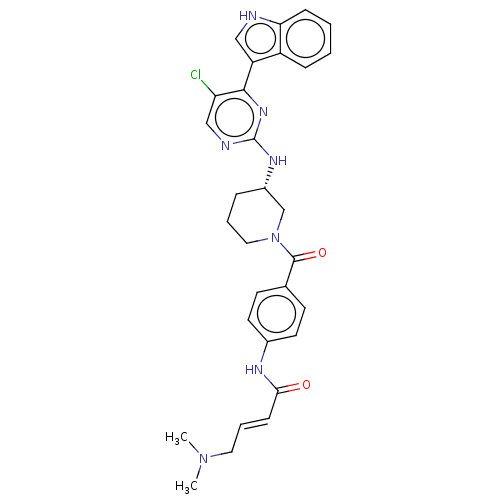
(Homo sapiens (Human)) | BDBM50540080
(CHEMBL4648857)Show SMILES CN(C)C\C=C\C(=O)Nc1ccc(cc1)C(=O)N1CCC[C@@H](C1)Nc1ncc(Cl)c(n1)-c1c[nH]c2ccccc12 |r| Show InChI InChI=1S/C30H32ClN7O2/c1-37(2)15-6-10-27(39)34-21-13-11-20(12-14-21)29(40)38-16-5-7-22(19-38)35-30-33-18-25(31)28(36-30)24-17-32-26-9-4-3-8-23(24)26/h3-4,6,8-14,17-18,22,32H,5,7,15-16,19H2,1-2H3,(H,34,39)(H,33,35,36)/b10-6+/t22-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 69 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01719
BindingDB Entry DOI: 10.7270/Q2HQ43WX |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 13/Cyclin-K
(Homo sapiens (Human)) | BDBM50528813
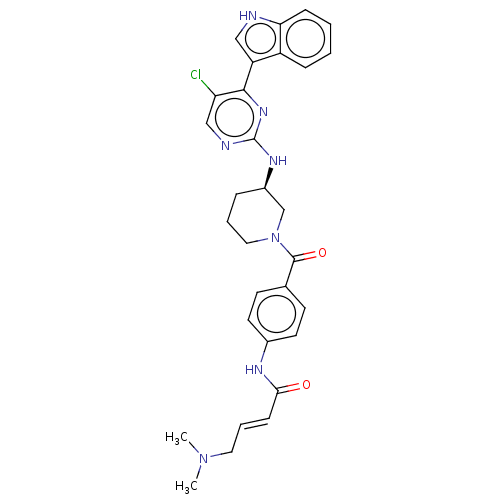
(CHEMBL4163879)Show SMILES CN(C)C\C=C\C(=O)Nc1ccc(cc1)C(=O)N1CCC[C@H](C1)Nc1ncc(Cl)c(n1)-c1c[nH]c2ccccc12 |r| Show InChI InChI=1S/C30H32ClN7O2/c1-37(2)15-6-10-27(39)34-21-13-11-20(12-14-21)29(40)38-16-5-7-22(19-38)35-30-33-18-25(31)28(36-30)24-17-32-26-9-4-3-8-23(24)26/h3-4,6,8-14,17-18,22,32H,5,7,15-16,19H2,1-2H3,(H,34,39)(H,33,35,36)/b10-6+/t22-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 69 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of CDK13/human cyclin K expressed in baculovirus-infected insect cells using pol2 CTD-peptide substrate and [gammaP]ATP by scintillation c... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c00766
BindingDB Entry DOI: 10.7270/Q2GX4G9M |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 13/Cyclin-K
(Homo sapiens (Human)) | BDBM50528813

(CHEMBL4163879)Show SMILES CN(C)C\C=C\C(=O)Nc1ccc(cc1)C(=O)N1CCC[C@H](C1)Nc1ncc(Cl)c(n1)-c1c[nH]c2ccccc12 |r| Show InChI InChI=1S/C30H32ClN7O2/c1-37(2)15-6-10-27(39)34-21-13-11-20(12-14-21)29(40)38-16-5-7-22(19-38)35-30-33-18-25(31)28(36-30)24-17-32-26-9-4-3-8-23(24)26/h3-4,6,8-14,17-18,22,32H,5,7,15-16,19H2,1-2H3,(H,34,39)(H,33,35,36)/b10-6+/t22-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 69 | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company
Curated by ChEMBL
| Assay Description
Competitive irreversible inhibition of CDK13/cyclinK (unknown origin) in presence of Km ATP |
Bioorg Med Chem Lett 29: (2019)
Article DOI: 10.1016/j.bmcl.2019.126637
BindingDB Entry DOI: 10.7270/Q23F4T3T |
More data for this
Ligand-Target Pair | |
Sodium channel protein type 1/2/3 subunit alpha
(Rattus norvegicus) | BDBM50223722
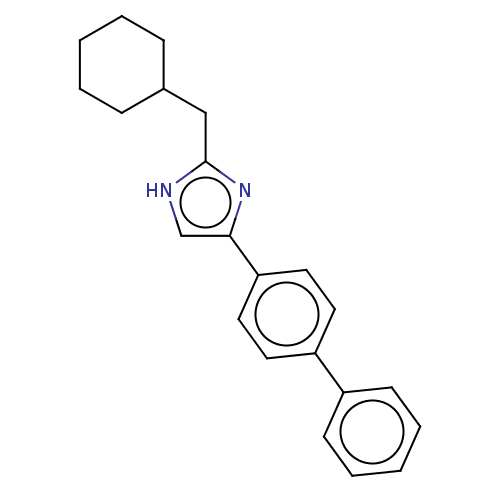
(CHEMBL334104)Show SMILES C(C1CCCCC1)c1nc(c[nH]1)-c1ccc(cc1)-c1ccccc1 Show InChI InChI=1S/C22H24N2/c1-3-7-17(8-4-1)15-22-23-16-21(24-22)20-13-11-19(12-14-20)18-9-5-2-6-10-18/h2,5-6,9-14,16-17H,1,3-4,7-8,15H2,(H,23,24) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 70 | n/a | n/a | n/a | n/a | n/a | n/a |
Institut Henri Beaufour
Curated by ChEMBL
| Assay Description
Concentration required to inhibit [3H]BTX binding to Sodium channel of rat brain |
Bioorg Med Chem Lett 14: 3521-3 (2004)
BindingDB Entry DOI: 10.7270/Q2474D2N |
More data for this
Ligand-Target Pair | |
Sodium channel protein type 1/2/3 subunit alpha
(Rattus norvegicus) | BDBM50143966
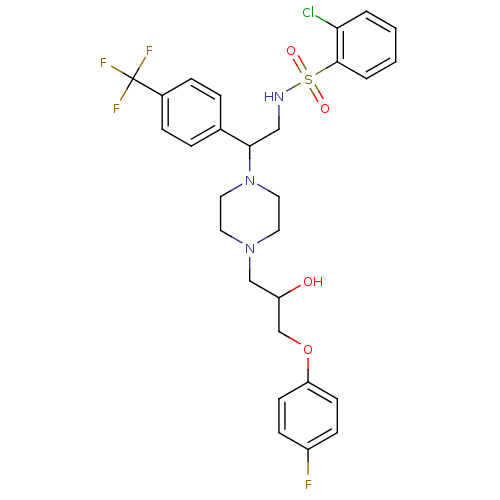
(2-Chloro-N-[2-{4-[3-(4-fluoro-phenoxy)-2-hydroxy-p...)Show SMILES OC(COc1ccc(F)cc1)CN1CCN(CC1)C(CNS(=O)(=O)c1ccccc1Cl)c1ccc(cc1)C(F)(F)F Show InChI InChI=1S/C28H30ClF4N3O4S/c29-25-3-1-2-4-27(25)41(38,39)34-17-26(20-5-7-21(8-6-20)28(31,32)33)36-15-13-35(14-16-36)18-23(37)19-40-24-11-9-22(30)10-12-24/h1-12,23,26,34,37H,13-19H2 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 76 | n/a | n/a | n/a | n/a | n/a | n/a |
ArQule Inc
Curated by ChEMBL
| Assay Description
In vitro inhibition of [3H]-batrachotoxin binding to neurotoxin site 2 of sodium (Na+) channel in rat cerebral cortex membrane |
Bioorg Med Chem Lett 14: 2025-30 (2004)
Article DOI: 10.1016/j.bmcl.2004.02.078
BindingDB Entry DOI: 10.7270/Q22J6B8R |
More data for this
Ligand-Target Pair | |
Sodium channel protein type 1/2/3 subunit alpha
(Rattus norvegicus) | BDBM50223723
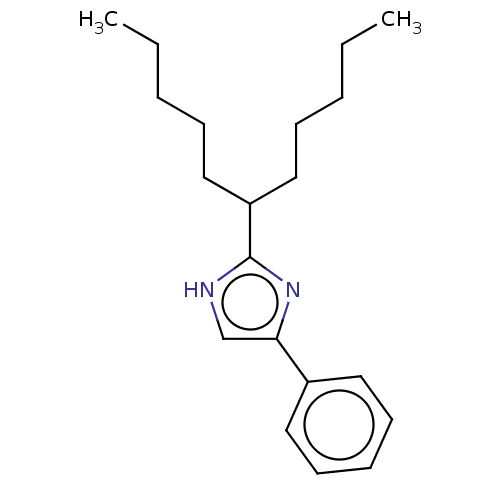
(CHEMBL119494)Show InChI InChI=1S/C20H30N2/c1-3-5-8-14-18(15-9-6-4-2)20-21-16-19(22-20)17-12-10-7-11-13-17/h7,10-13,16,18H,3-6,8-9,14-15H2,1-2H3,(H,21,22) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 86 | n/a | n/a | n/a | n/a | n/a | n/a |
Institut Henri Beaufour
Curated by ChEMBL
| Assay Description
Concentration required to inhibit [3H]BTX binding to Sodium channel of rat brain |
Bioorg Med Chem Lett 14: 3521-3 (2004)
BindingDB Entry DOI: 10.7270/Q2474D2N |
More data for this
Ligand-Target Pair | |
Sodium channel protein type 1/2/3 subunit alpha
(Rattus norvegicus) | BDBM50143963
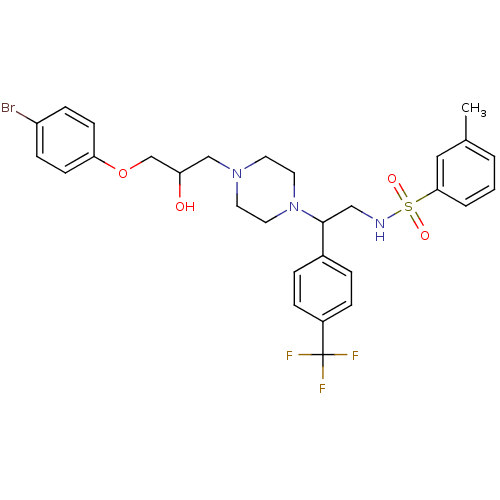
(CHEMBL64869 | N-[2-{4-[3-(4-Bromo-phenoxy)-2-hydro...)Show SMILES Cc1cccc(c1)S(=O)(=O)NCC(N1CCN(CC(O)COc2ccc(Br)cc2)CC1)c1ccc(cc1)C(F)(F)F Show InChI InChI=1S/C29H33BrF3N3O4S/c1-21-3-2-4-27(17-21)41(38,39)34-18-28(22-5-7-23(8-6-22)29(31,32)33)36-15-13-35(14-16-36)19-25(37)20-40-26-11-9-24(30)10-12-26/h2-12,17,25,28,34,37H,13-16,18-20H2,1H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 95 | n/a | n/a | n/a | n/a | n/a | n/a |
ArQule Inc
Curated by ChEMBL
| Assay Description
Inhibitory concentration against [3H]-batrachotoxin binding to neurotoxin site 2 of sodium (Na+) channel in vitro in rat cerebral cortex membrane at ... |
Bioorg Med Chem Lett 14: 2025-30 (2004)
Article DOI: 10.1016/j.bmcl.2004.02.078
BindingDB Entry DOI: 10.7270/Q22J6B8R |
More data for this
Ligand-Target Pair | |
Sodium channel protein type 2 subunit alpha
(Rattus norvegicus) | BDBM50051080
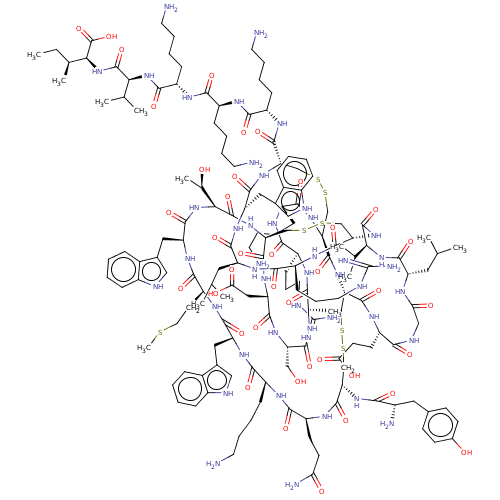
(CHEMBL3318139)Show SMILES [H][C@]12CSSC[C@]3([H])NC(=O)[C@@]([H])(NC(=O)[C@H](Cc4c[nH]c5ccccc45)NC(=O)[C@H](CCSC)NC(=O)[C@H](Cc4c[nH]c5ccccc45)NC(=O)[C@H](CCCCN)NC(=O)[C@H](CCC(N)=O)NC(=O)[C@H](CSSC[C@]([H])(NC(=O)[C@]([H])(CSSC[C@H](NC(=O)[C@H](Cc4c[nH]c5ccccc45)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](CCCNC(N)=N)NC1=O)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H]([C@@H](C)CC)C(O)=O)NC(=O)[C@H](CCCCN)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](C)NC(=O)[C@H](CO)NC(=O)[C@H](CC(O)=O)NC3=O)C(=O)N[C@@H](CCC(O)=O)C(=O)NCC(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](C(C)C)C(=O)N2)NC(=O)[C@@H](N)Cc1ccc(O)cc1)[C@@H](C)O |r| | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 98 | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue Pharma L.P.
Curated by ChEMBL
| Assay Description
Inhibition of rat Nav1.7 expressed in HEK-293 cells at -130 to -110 mV holding potential by whole-cell voltage clamp analysis |
J Med Chem 57: 6623-31 (2014)
Article DOI: 10.1021/jm500687u
BindingDB Entry DOI: 10.7270/Q22B90P2 |
More data for this
Ligand-Target Pair | |
Sodium channel protein type 1/2/3 subunit alpha
(Rattus norvegicus) | BDBM50223725
(CHEMBL118682)Show InChI InChI=1S/C22H26N2/c1-3-8-20(9-4-2)22-23-16-21(24-22)19-14-12-18(13-15-19)17-10-6-5-7-11-17/h5-7,10-16,20H,3-4,8-9H2,1-2H3,(H,23,24) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
Institut Henri Beaufour
Curated by ChEMBL
| Assay Description
Concentration required to inhibit [3H]BTX binding to Sodium channel of rat brain |
Bioorg Med Chem Lett 14: 3521-3 (2004)
BindingDB Entry DOI: 10.7270/Q2474D2N |
More data for this
Ligand-Target Pair | |
Sodium channel protein type 2 subunit alpha
(Rattus norvegicus) | BDBM50051073
(CHEMBL3318132)Show SMILES [H][C@]12CSSC[C@]3([H])NC(=O)[C@@]([H])(NC(=O)[C@H](Cc4c[nH]c5ccccc45)NC(=O)[C@H](CCSC)NC(=O)[C@H](Cc4c[nH]c5ccccc45)NC(=O)[C@H](CCCCN)NC(=O)[C@H](CCC(N)=O)NC(=O)[C@H](CSSC[C@]([H])(NC(=O)[C@]([H])(CSSC[C@H](NC(=O)[C@H](Cc4c[nH]c5ccccc45)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](CCCNC(N)=N)NC1=O)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](Cc1c[nH]c4ccccc14)C(O)=O)NC(=O)[C@H](CCCCN)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](CO)NC(=O)[C@H](CC(O)=O)NC3=O)C(=O)N[C@@H](CCC(O)=O)C(=O)NCC(=O)N[C@@H](CCSC)C(=O)N[C@@H](C(C)C)C(=O)N2)NC(=O)[C@@H](N)Cc1ccc(O)cc1)[C@@H](C)O |r| | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 105 | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue Pharma L.P.
Curated by ChEMBL
| Assay Description
Inhibition of rat Nav1.7 expressed in HEK-293 cells at -130 to -110 mV holding potential by whole-cell voltage clamp analysis |
J Med Chem 57: 6623-31 (2014)
Article DOI: 10.1021/jm500687u
BindingDB Entry DOI: 10.7270/Q22B90P2 |
More data for this
Ligand-Target Pair | |
Sodium channel protein type 2 subunit alpha
(Rattus norvegicus) | BDBM50066417

(CHEMBL112806 | N-(4-Benzyloxy-phenyl)-N'-(4-tert-b...)Show SMILES CC(C)(C)c1ccc(NC(N)=Nc2ccc(OCc3ccccc3)cc2)cc1 |w:11.11| Show InChI InChI=1S/C24H27N3O/c1-24(2,3)19-9-11-20(12-10-19)26-23(25)27-21-13-15-22(16-14-21)28-17-18-7-5-4-6-8-18/h4-16H,17H2,1-3H3,(H3,25,26,27) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
Cambridge NeuroScience, Inc.
Curated by ChEMBL
| Assay Description
Sodium channel block, determined by % block of [14C]guanidinium flux in CHO line expressing rat type IIA sodium channels derived from rat brain |
J Med Chem 41: 3298-302 (1998)
Article DOI: 10.1021/jm980134b
BindingDB Entry DOI: 10.7270/Q2Q52Q8W |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 13/Cyclin-K
(Homo sapiens (Human)) | BDBM50573513
(CHEMBL4866757)Show SMILES C\C=C\C(=O)Nc1ccc(nc1)N1CCC[C@H](C1)Nc1ncc(Cl)c(n1)-c1c[nH]c2ccccc12 |r| | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 120 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human recombinant CDK13/CyclinK assessed as reduction in substrate phosphorylation using His-c-Myc as substrate preincubated with enzym... |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113481
BindingDB Entry DOI: 10.7270/Q22B92TP |
More data for this
Ligand-Target Pair | |
Sodium channel protein type 1/2/3 subunit alpha
(Rattus norvegicus) | BDBM50082319
((R)-1-Phenoxy-3-[4-(4-phenoxy-phenyl)-piperidin-1-...)Show SMILES O[C@@H](COc1ccccc1)CN1CCC(CC1)c1ccc(Oc2ccccc2)cc1 Show InChI InChI=1S/C26H29NO3/c28-23(20-29-24-7-3-1-4-8-24)19-27-17-15-22(16-18-27)21-11-13-26(14-12-21)30-25-9-5-2-6-10-25/h1-14,22-23,28H,15-20H2/t23-/m1/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 120 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of veratridine-induced depolarization in rat cerebrocortical synaptosomes using the voltage sensitive fluorescent dye Rhodamine 6G |
Bioorg Med Chem Lett 9: 2999-3002 (1999)
BindingDB Entry DOI: 10.7270/Q2D21Z3B |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 13/Cyclin-K
(Homo sapiens (Human)) | BDBM50597407
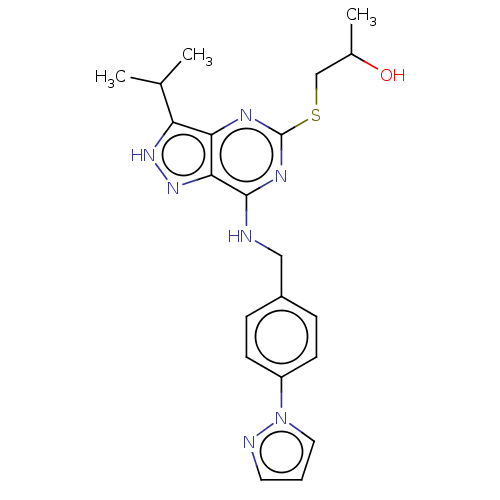
(CHEMBL5191136)Show SMILES CC(O)CSc1nc(NCc2ccc(cc2)-n2cccn2)c2n[nH]c(C(C)C)c2n1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 122 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c02184
BindingDB Entry DOI: 10.7270/Q26114CS |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 13/Cyclin-K
(Homo sapiens (Human)) | BDBM50573511

(CHEMBL4864697)Show SMILES C\C=C\C(=O)Nc1ccc(cc1)N1CCC[C@H](C1)Nc1ncc(Cl)c(n1)-c1c[nH]c2ccccc12 |r| | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 123 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human recombinant CDK13/CyclinK assessed as reduction in substrate phosphorylation using His-c-Myc as substrate preincubated with enzym... |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113481
BindingDB Entry DOI: 10.7270/Q22B92TP |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data