 Found 301 hits of ic50 for UniProtKB: P47820
Found 301 hits of ic50 for UniProtKB: P47820 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kcal/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Angiotensin-converting enzyme
(Rattus norvegicus) | BDBM50050127

((S)-3-(4-Hydroxy-phenyl)-2-[(2S,3R)-2-((S)-2-merca...)Show SMILES CC[C@@H](C)[C@H](NC(=O)[C@@H](S)C(C)C)C(=O)N[C@@H](Cc1ccc(O)cc1)C(O)=O Show InChI InChI=1S/C20H30N2O5S/c1-5-12(4)16(22-19(25)17(28)11(2)3)18(24)21-15(20(26)27)10-13-6-8-14(23)9-7-13/h6-9,11-12,15-17,23,28H,5,10H2,1-4H3,(H,21,24)(H,22,25)(H,26,27)/t12-,15+,16+,17+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.260 | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM
Curated by ChEMBL
| Assay Description
Inhibitory activity against angiotensin I converting enzyme |
J Med Chem 39: 1210-9 (1996)
Article DOI: 10.1021/jm950590p
BindingDB Entry DOI: 10.7270/Q2DF6RWV |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme
(Rattus norvegicus) | BDBM50050151

(1-[(S)-2-((S)-2-Mercapto-3-phenyl-propionylamino)-...)Show SMILES C[C@H](NC(=O)[C@@H](S)Cc1ccccc1)C(=O)N1CCCC1C(O)=O Show InChI InChI=1S/C17H22N2O4S/c1-11(16(21)19-9-5-8-13(19)17(22)23)18-15(20)14(24)10-12-6-3-2-4-7-12/h2-4,6-7,11,13-14,24H,5,8-10H2,1H3,(H,18,20)(H,22,23)/t11-,13?,14-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM
Curated by ChEMBL
| Assay Description
Inhibitory activity against angiotensin I converting enzyme |
J Med Chem 39: 1210-9 (1996)
Article DOI: 10.1021/jm950590p
BindingDB Entry DOI: 10.7270/Q2DF6RWV |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme
(Rattus norvegicus) | BDBM50050149

(1-[(S)-2-((S)-2-Mercapto-4-phenyl-butyrylamino)-pr...)Show SMILES C[C@H](NC(=O)[C@@H](S)CCc1ccccc1)C(=O)N1CCCC1C(O)=O Show InChI InChI=1S/C18H24N2O4S/c1-12(17(22)20-11-5-8-14(20)18(23)24)19-16(21)15(25)10-9-13-6-3-2-4-7-13/h2-4,6-7,12,14-15,25H,5,8-11H2,1H3,(H,19,21)(H,23,24)/t12-,14?,15-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.350 | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM
Curated by ChEMBL
| Assay Description
Inhibitory activity against angiotensin I converting enzyme |
J Med Chem 39: 1210-9 (1996)
Article DOI: 10.1021/jm950590p
BindingDB Entry DOI: 10.7270/Q2DF6RWV |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme
(Rattus norvegicus) | BDBM50050150
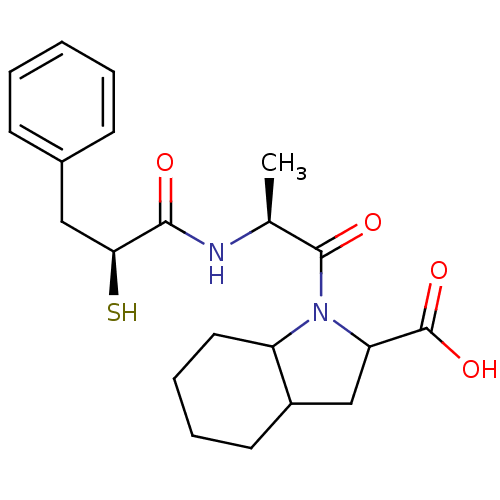
(1-[(S)-2-((S)-2-Mercapto-3-phenyl-propionylamino)-...)Show SMILES C[C@H](NC(=O)[C@@H](S)Cc1ccccc1)C(=O)N1C(CC2CCCCC12)C(O)=O Show InChI InChI=1S/C21H28N2O4S/c1-13(22-19(24)18(28)11-14-7-3-2-4-8-14)20(25)23-16-10-6-5-9-15(16)12-17(23)21(26)27/h2-4,7-8,13,15-18,28H,5-6,9-12H2,1H3,(H,22,24)(H,26,27)/t13-,15?,16?,17?,18-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM
Curated by ChEMBL
| Assay Description
Inhibitory activity against angiotensin I converting enzyme |
J Med Chem 39: 1210-9 (1996)
Article DOI: 10.1021/jm950590p
BindingDB Entry DOI: 10.7270/Q2DF6RWV |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme
(Rattus norvegicus) | BDBM50051796

((2S,5R)-5-(2-Hydroxy-phenyl)-1-{2-[(S)-2-mercapto-...)Show SMILES COc1ccc(C[C@H](S)C(=O)NCC(=O)N2[C@@H](CC[C@@H]2c2ccccc2O)C(O)=O)cc1 Show InChI InChI=1S/C23H26N2O6S/c1-31-15-8-6-14(7-9-15)12-20(32)22(28)24-13-21(27)25-17(10-11-18(25)23(29)30)16-4-2-3-5-19(16)26/h2-9,17-18,20,26,32H,10-13H2,1H3,(H,24,28)(H,29,30)/t17-,18+,20+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.420 | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM
Curated by ChEMBL
| Assay Description
In vitro inhibition of rat angiotensin I converting enzyme |
J Med Chem 39: 2594-608 (1996)
Article DOI: 10.1021/jm950783c
BindingDB Entry DOI: 10.7270/Q25B0353 |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme
(Rattus norvegicus) | BDBM50051798
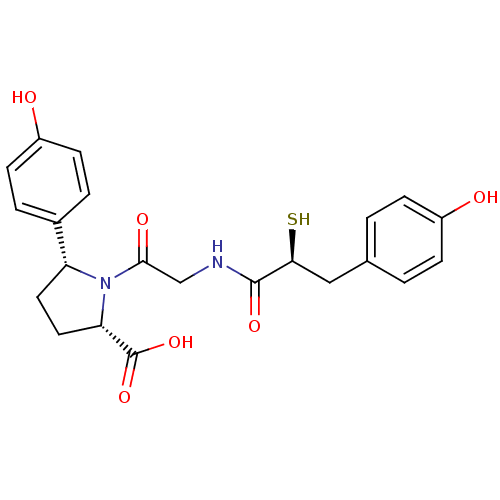
((2S,5R)-5-(4-Hydroxy-phenyl)-1-{2-[(S)-3-(4-hydrox...)Show SMILES OC(=O)[C@@H]1CC[C@@H](N1C(=O)CNC(=O)[C@@H](S)Cc1ccc(O)cc1)c1ccc(O)cc1 Show InChI InChI=1S/C22H24N2O6S/c25-15-5-1-13(2-6-15)11-19(31)21(28)23-12-20(27)24-17(9-10-18(24)22(29)30)14-3-7-16(26)8-4-14/h1-8,17-19,25-26,31H,9-12H2,(H,23,28)(H,29,30)/t17-,18+,19+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.460 | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM
Curated by ChEMBL
| Assay Description
In vitro inhibition of rat angiotensin I converting enzyme |
J Med Chem 39: 2594-608 (1996)
Article DOI: 10.1021/jm950783c
BindingDB Entry DOI: 10.7270/Q25B0353 |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme
(Rattus norvegicus) | BDBM50051784
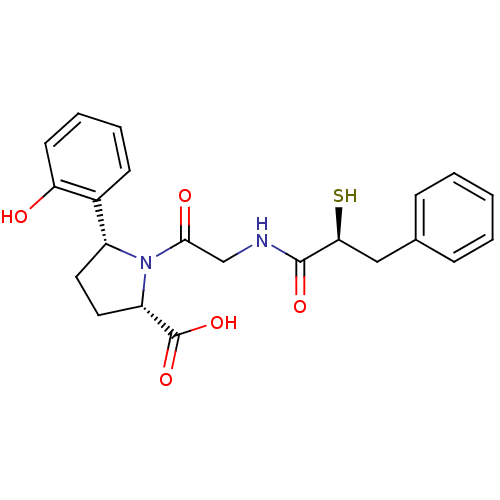
((2S,5R)-5-(2-Hydroxy-phenyl)-1-[2-((S)-2-mercapto-...)Show SMILES OC(=O)[C@@H]1CC[C@@H](N1C(=O)CNC(=O)[C@@H](S)Cc1ccccc1)c1ccccc1O Show InChI InChI=1S/C22H24N2O5S/c25-18-9-5-4-8-15(18)16-10-11-17(22(28)29)24(16)20(26)13-23-21(27)19(30)12-14-6-2-1-3-7-14/h1-9,16-17,19,25,30H,10-13H2,(H,23,27)(H,28,29)/t16-,17+,19+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM
Curated by ChEMBL
| Assay Description
In vitro inhibition of rat angiotensin I converting enzyme |
J Med Chem 39: 2594-608 (1996)
Article DOI: 10.1021/jm950783c
BindingDB Entry DOI: 10.7270/Q25B0353 |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme
(Rattus norvegicus) | BDBM50011193

(5-tert-Butyl-3-[2-(1-carboxy-3-phenyl-propylamino)...)Show SMILES C[C@H](N[C@@H](CCc1ccccc1)C(O)=O)C(=O)N1N=C(S[C@H]1C(O)=O)C(C)(C)C |c:19| Show InChI InChI=1S/C20H27N3O5S/c1-12(21-14(17(25)26)11-10-13-8-6-5-7-9-13)15(24)23-16(18(27)28)29-19(22-23)20(2,3)4/h5-9,12,14,16,21H,10-11H2,1-4H3,(H,25,26)(H,27,28)/t12-,14-,16-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.510 | n/a | n/a | n/a | n/a | n/a | n/a |
Research and Development Laboratories
Curated by ChEMBL
| Assay Description
Compound was tested for inhibitory activity against angiotensin I converting enzyme |
J Med Chem 34: 439-47 (1991)
BindingDB Entry DOI: 10.7270/Q28P5ZGR |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme
(Rattus norvegicus) | BDBM50051785
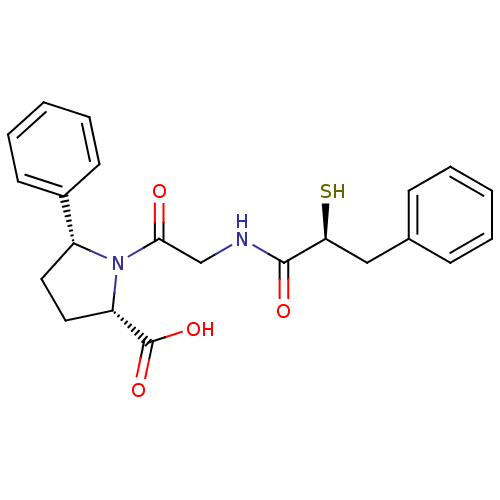
((2S,5R)-1-[2-((S)-2-Mercapto-3-phenyl-propionylami...)Show SMILES OC(=O)[C@@H]1CC[C@@H](N1C(=O)CNC(=O)[C@@H](S)Cc1ccccc1)c1ccccc1 Show InChI InChI=1S/C22H24N2O4S/c25-20(14-23-21(26)19(29)13-15-7-3-1-4-8-15)24-17(11-12-18(24)22(27)28)16-9-5-2-6-10-16/h1-10,17-19,29H,11-14H2,(H,23,26)(H,27,28)/t17-,18+,19+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.550 | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM
Curated by ChEMBL
| Assay Description
In vitro inhibition of rat angiotensin I converting enzyme |
J Med Chem 39: 2594-608 (1996)
Article DOI: 10.1021/jm950783c
BindingDB Entry DOI: 10.7270/Q25B0353 |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme
(Rattus norvegicus) | BDBM50050156

((S)-1-[(S)-2-((2S,3S)-2-Mercapto-3-methyl-pentanoy...)Show SMILES CC[C@H](C)[C@H](S)C(=O)N[C@@H](C(C)C)C(=O)N1CCC[C@H]1C(O)=O Show InChI InChI=1S/C16H28N2O4S/c1-5-10(4)13(23)14(19)17-12(9(2)3)15(20)18-8-6-7-11(18)16(21)22/h9-13,23H,5-8H2,1-4H3,(H,17,19)(H,21,22)/t10-,11-,12-,13-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.580 | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM
Curated by ChEMBL
| Assay Description
Inhibitory activity against angiotensin I converting enzyme |
J Med Chem 39: 1210-9 (1996)
Article DOI: 10.1021/jm950590p
BindingDB Entry DOI: 10.7270/Q2DF6RWV |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme
(Rattus norvegicus) | BDBM50051780
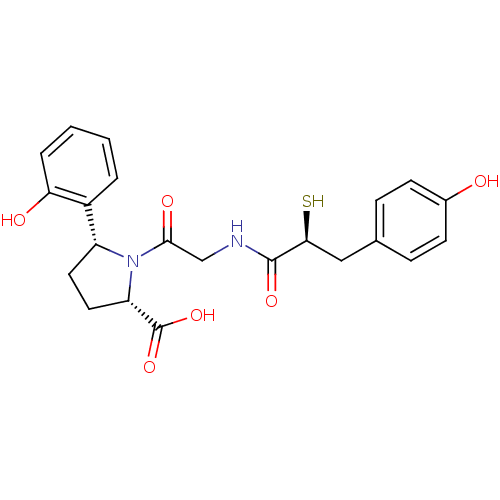
((2S,5R)-5-(2-Hydroxy-phenyl)-1-{2-[(S)-3-(4-hydrox...)Show SMILES OC(=O)[C@@H]1CC[C@@H](N1C(=O)CNC(=O)[C@@H](S)Cc1ccc(O)cc1)c1ccccc1O Show InChI InChI=1S/C22H24N2O6S/c25-14-7-5-13(6-8-14)11-19(31)21(28)23-12-20(27)24-16(9-10-17(24)22(29)30)15-3-1-2-4-18(15)26/h1-8,16-17,19,25-26,31H,9-12H2,(H,23,28)(H,29,30)/t16-,17+,19+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM
Curated by ChEMBL
| Assay Description
In vitro inhibition of rat angiotensin I converting enzyme |
J Med Chem 39: 2594-608 (1996)
Article DOI: 10.1021/jm950783c
BindingDB Entry DOI: 10.7270/Q25B0353 |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme
(Rattus norvegicus) | BDBM50011190
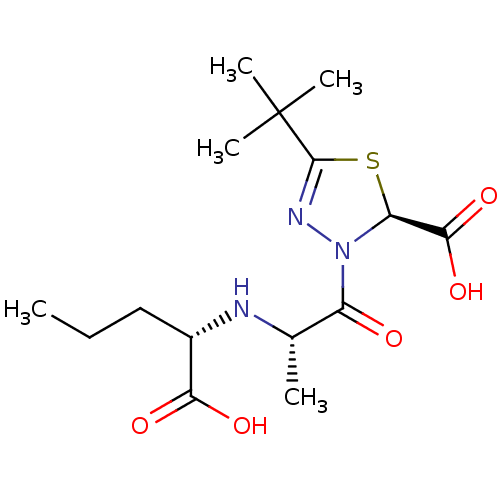
(5-tert-Butyl-3-[2-(1-carboxy-butylamino)-propionyl...)Show SMILES CCC[C@H](N[C@@H](C)C(=O)N1N=C(S[C@H]1C(O)=O)C(C)(C)C)C(O)=O |c:10| Show InChI InChI=1S/C15H25N3O5S/c1-6-7-9(12(20)21)16-8(2)10(19)18-11(13(22)23)24-14(17-18)15(3,4)5/h8-9,11,16H,6-7H2,1-5H3,(H,20,21)(H,22,23)/t8-,9-,11-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Research and Development Laboratories
Curated by ChEMBL
| Assay Description
Compound was tested for inhibitory activity against angiotensin I converting enzyme |
J Med Chem 34: 439-47 (1991)
BindingDB Entry DOI: 10.7270/Q28P5ZGR |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme
(Rattus norvegicus) | BDBM50051791
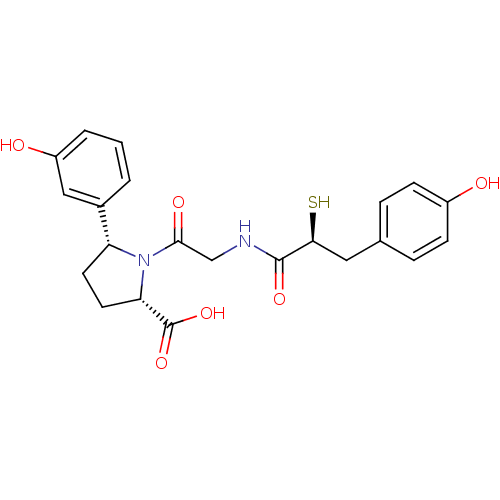
((2S,5R)-5-(3-Hydroxy-phenyl)-1-{2-[(S)-3-(4-hydrox...)Show SMILES OC(=O)[C@@H]1CC[C@@H](N1C(=O)CNC(=O)[C@@H](S)Cc1ccc(O)cc1)c1cccc(O)c1 Show InChI InChI=1S/C22H24N2O6S/c25-15-6-4-13(5-7-15)10-19(31)21(28)23-12-20(27)24-17(8-9-18(24)22(29)30)14-2-1-3-16(26)11-14/h1-7,11,17-19,25-26,31H,8-10,12H2,(H,23,28)(H,29,30)/t17-,18+,19+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM
Curated by ChEMBL
| Assay Description
In vitro inhibition of rat angiotensin I converting enzyme |
J Med Chem 39: 2594-608 (1996)
Article DOI: 10.1021/jm950783c
BindingDB Entry DOI: 10.7270/Q25B0353 |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme
(Rattus norvegicus) | BDBM50011191
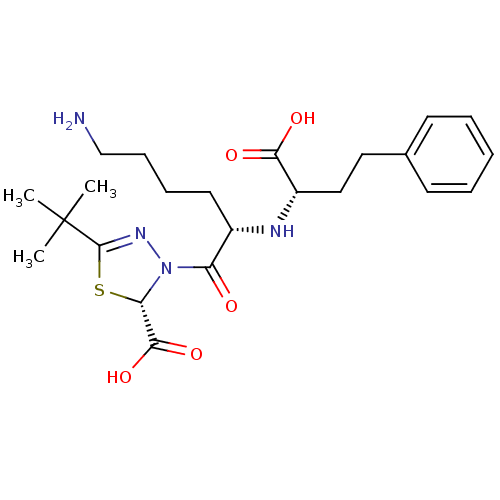
(3-[6-Amino-2-(1-carboxy-3-phenyl-propylamino)-hexa...)Show SMILES CC(C)(C)C1=NN([C@@H](S1)C(O)=O)C(=O)[C@H](CCCCN)N[C@@H](CCc1ccccc1)C(O)=O |t:4| Show InChI InChI=1S/C23H34N4O5S/c1-23(2,3)22-26-27(19(33-22)21(31)32)18(28)16(11-7-8-14-24)25-17(20(29)30)13-12-15-9-5-4-6-10-15/h4-6,9-10,16-17,19,25H,7-8,11-14,24H2,1-3H3,(H,29,30)(H,31,32)/t16-,17-,19-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
Research and Development Laboratories
Curated by ChEMBL
| Assay Description
Compound was tested for inhibitory activity against angiotensin I converting enzyme |
J Med Chem 34: 439-47 (1991)
BindingDB Entry DOI: 10.7270/Q28P5ZGR |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme
(Rattus norvegicus) | BDBM50050144
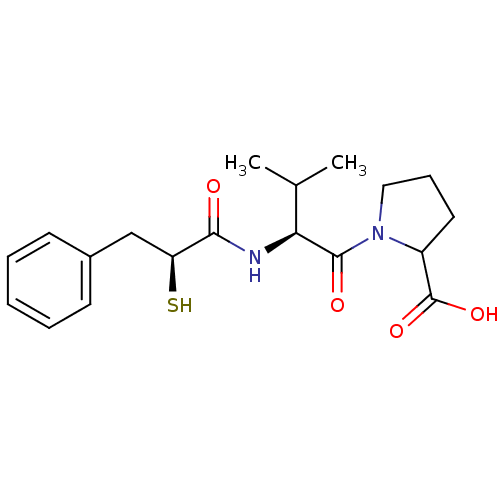
(1-[(S)-2-((S)-2-Mercapto-3-phenyl-propionylamino)-...)Show SMILES CC(C)[C@H](NC(=O)[C@@H](S)Cc1ccccc1)C(=O)N1CCCC1C(O)=O Show InChI InChI=1S/C19H26N2O4S/c1-12(2)16(18(23)21-10-6-9-14(21)19(24)25)20-17(22)15(26)11-13-7-4-3-5-8-13/h3-5,7-8,12,14-16,26H,6,9-11H2,1-2H3,(H,20,22)(H,24,25)/t14?,15-,16-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.850 | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM
Curated by ChEMBL
| Assay Description
Inhibitory activity against angiotensin I converting enzyme |
J Med Chem 39: 1210-9 (1996)
Article DOI: 10.1021/jm950590p
BindingDB Entry DOI: 10.7270/Q2DF6RWV |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme
(Rattus norvegicus) | BDBM50017122
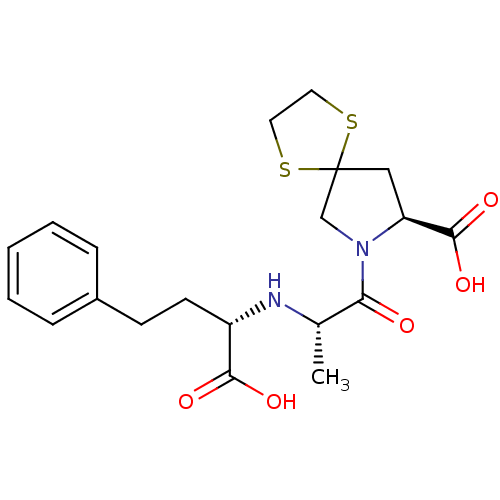
(7-[2-(1-Carboxy-3-phenyl-propylamino)-propionyl]-1...)Show SMILES C[C@H](N[C@@H](CCc1ccccc1)C(O)=O)C(=O)N1CC2(C[C@H]1C(O)=O)SCCS2 Show InChI InChI=1S/C20H26N2O5S2/c1-13(21-15(18(24)25)8-7-14-5-3-2-4-6-14)17(23)22-12-20(28-9-10-29-20)11-16(22)19(26)27/h2-6,13,15-16,21H,7-12H2,1H3,(H,24,25)(H,26,27)/t13-,15-,16-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Corporation
Curated by ChEMBL
| Assay Description
Compound tested in vivo for inhibition of Angiotensin I converting enzyme in rat |
J Med Chem 32: 1600-6 (1989)
BindingDB Entry DOI: 10.7270/Q2M61KVW |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme
(Rattus norvegicus) | BDBM50051803
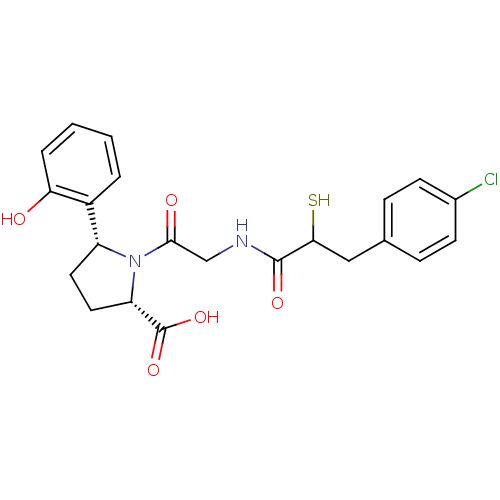
((2S,5R)-1-{2-[3-(4-Chloro-phenyl)-2-mercapto-propi...)Show SMILES OC(=O)[C@@H]1CC[C@@H](N1C(=O)CNC(=O)C(S)Cc1ccc(Cl)cc1)c1ccccc1O Show InChI InChI=1S/C22H23ClN2O5S/c23-14-7-5-13(6-8-14)11-19(31)21(28)24-12-20(27)25-16(9-10-17(25)22(29)30)15-3-1-2-4-18(15)26/h1-8,16-17,19,26,31H,9-12H2,(H,24,28)(H,29,30)/t16-,17+,19?/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM
Curated by ChEMBL
| Assay Description
In vitro inhibition of rat angiotensin I converting enzyme |
J Med Chem 39: 2594-608 (1996)
Article DOI: 10.1021/jm950783c
BindingDB Entry DOI: 10.7270/Q25B0353 |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme
(Rattus norvegicus) | BDBM50011192

((R)-1-[(S)-2-((S)-1-Carboxy-3-phenyl-propylamino)-...)Show SMILES CC(NC(CCc1ccccc1)C(O)=O)C(=O)N1CCC[C@H]1C(O)=O Show InChI InChI=1S/C18H24N2O5/c1-12(16(21)20-11-5-8-15(20)18(24)25)19-14(17(22)23)10-9-13-6-3-2-4-7-13/h2-4,6-7,12,14-15,19H,5,8-11H2,1H3,(H,22,23)(H,24,25)/t12?,14?,15-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MMDB
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.950 | n/a | n/a | n/a | n/a | n/a | n/a |
Research and Development Laboratories
Curated by ChEMBL
| Assay Description
Compound was tested for inhibitory activity against angiotensin I converting enzyme |
J Med Chem 34: 439-47 (1991)
BindingDB Entry DOI: 10.7270/Q28P5ZGR |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme
(Rattus norvegicus) | BDBM50050125

(1-[(S)-2-((S)-2-Mercapto-3-phenyl-propionylamino)-...)Show SMILES OC(=O)C1CCCN1C(=O)[C@H](Cc1ccccc1)NC(=O)[C@@H](S)Cc1ccccc1 Show InChI InChI=1S/C23H26N2O4S/c26-21(20(30)15-17-10-5-2-6-11-17)24-18(14-16-8-3-1-4-9-16)22(27)25-13-7-12-19(25)23(28)29/h1-6,8-11,18-20,30H,7,12-15H2,(H,24,26)(H,28,29)/t18-,19?,20-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM
Curated by ChEMBL
| Assay Description
Inhibitory activity against angiotensin I converting enzyme |
J Med Chem 39: 1210-9 (1996)
Article DOI: 10.1021/jm950590p
BindingDB Entry DOI: 10.7270/Q2DF6RWV |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme
(Rattus norvegicus) | BDBM50287432
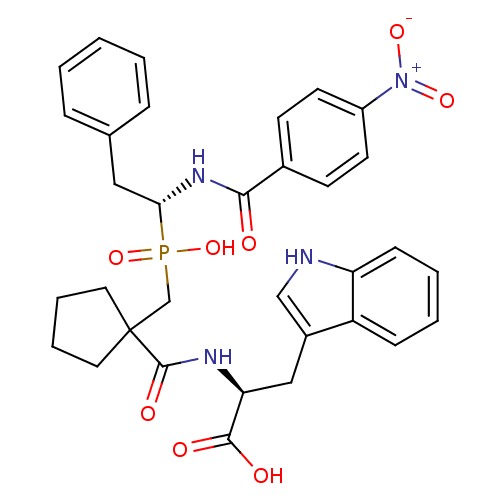
((S)-2-[(1-{Hydroxy-[(R)-1-(4-nitro-benzoylamino)-2...)Show SMILES OC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)C1(CP(O)(=O)[C@H](Cc2ccccc2)NC(=O)c2ccc(cc2)[N+]([O-])=O)CCCC1 Show InChI InChI=1S/C33H35N4O8P/c38-30(23-12-14-25(15-13-23)37(42)43)36-29(18-22-8-2-1-3-9-22)46(44,45)21-33(16-6-7-17-33)32(41)35-28(31(39)40)19-24-20-34-27-11-5-4-10-26(24)27/h1-5,8-15,20,28-29,34H,6-7,16-19,21H2,(H,35,41)(H,36,38)(H,39,40)(H,44,45)/t28-,29+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was measured for the inhibition of Angiotensin I converting enzyme |
Bioorg Med Chem Lett 6: 1629-1634 (1996)
Article DOI: 10.1016/0960-894X(96)00297-1
BindingDB Entry DOI: 10.7270/Q2697437 |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme
(Rattus norvegicus) | BDBM50051790
((2S,5R)-5-(3-Hydroxy-phenyl)-1-[2-((S)-2-mercapto-...)Show SMILES OC(=O)[C@@H]1CC[C@@H](N1C(=O)CNC(=O)[C@@H](S)Cc1ccccc1)c1cccc(O)c1 Show InChI InChI=1S/C22H24N2O5S/c25-16-8-4-7-15(12-16)17-9-10-18(22(28)29)24(17)20(26)13-23-21(27)19(30)11-14-5-2-1-3-6-14/h1-8,12,17-19,25,30H,9-11,13H2,(H,23,27)(H,28,29)/t17-,18+,19+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM
Curated by ChEMBL
| Assay Description
In vitro inhibition of rat angiotensin I converting enzyme |
J Med Chem 39: 2594-608 (1996)
Article DOI: 10.1021/jm950783c
BindingDB Entry DOI: 10.7270/Q25B0353 |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme
(Rattus norvegicus) | BDBM50011194
(3-[2-(1-Carboxy-3-phenyl-propylamino)-propionyl]-5...)Show SMILES C[C@H](N[C@@H](CCc1ccccc1)C(O)=O)C(=O)N1N=C(S[C@H]1C(O)=O)c1ccccc1 |c:19| Show InChI InChI=1S/C22H23N3O5S/c1-14(23-17(21(27)28)13-12-15-8-4-2-5-9-15)19(26)25-20(22(29)30)31-18(24-25)16-10-6-3-7-11-16/h2-11,14,17,20,23H,12-13H2,1H3,(H,27,28)(H,29,30)/t14-,17-,20-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Research and Development Laboratories
Curated by ChEMBL
| Assay Description
Compound was tested for inhibitory activity against angiotensin I converting enzyme |
J Med Chem 34: 439-47 (1991)
BindingDB Entry DOI: 10.7270/Q28P5ZGR |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme
(Rattus norvegicus) | BDBM50050162
((S)-3-(4-Hydroxy-phenyl)-2-[(S)-2-((S)-2-mercapto-...)Show SMILES OC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@@H](S)CCc1ccccc1 Show InChI InChI=1S/C28H30N2O5S/c31-22-14-11-21(12-15-22)18-24(28(34)35)30-26(32)23(17-20-9-5-2-6-10-20)29-27(33)25(36)16-13-19-7-3-1-4-8-19/h1-12,14-15,23-25,31,36H,13,16-18H2,(H,29,33)(H,30,32)(H,34,35)/t23-,24-,25-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM
Curated by ChEMBL
| Assay Description
Inhibitory activity against angiotensin I converting enzyme |
J Med Chem 39: 1210-9 (1996)
Article DOI: 10.1021/jm950590p
BindingDB Entry DOI: 10.7270/Q2DF6RWV |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme
(Rattus norvegicus) | BDBM50050148

((S)-3-(4-Hydroxy-phenyl)-2-[(S)-2-((S)-2-mercapto-...)Show SMILES CC(C)[C@H](NC(=O)[C@@H](S)CCc1ccccc1)C(=O)N[C@@H](Cc1ccc(O)cc1)C(O)=O Show InChI InChI=1S/C24H30N2O5S/c1-15(2)21(26-22(28)20(32)13-10-16-6-4-3-5-7-16)23(29)25-19(24(30)31)14-17-8-11-18(27)12-9-17/h3-9,11-12,15,19-21,27,32H,10,13-14H2,1-2H3,(H,25,29)(H,26,28)(H,30,31)/t19-,20-,21-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM
Curated by ChEMBL
| Assay Description
Inhibitory activity against angiotensin I converting enzyme |
J Med Chem 39: 1210-9 (1996)
Article DOI: 10.1021/jm950590p
BindingDB Entry DOI: 10.7270/Q2DF6RWV |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme
(Rattus norvegicus) | BDBM50050134
(1-[2-((S)-2-Mercapto-4-phenyl-butyrylamino)-2-meth...)Show SMILES CC(C)(NC(=O)[C@@H](S)CCc1ccccc1)C(=O)N1CCCC1C(O)=O Show InChI InChI=1S/C19H26N2O4S/c1-19(2,18(25)21-12-6-9-14(21)17(23)24)20-16(22)15(26)11-10-13-7-4-3-5-8-13/h3-5,7-8,14-15,26H,6,9-12H2,1-2H3,(H,20,22)(H,23,24)/t14?,15-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM
Curated by ChEMBL
| Assay Description
Inhibitory activity against angiotensin I converting enzyme |
J Med Chem 39: 1210-9 (1996)
Article DOI: 10.1021/jm950590p
BindingDB Entry DOI: 10.7270/Q2DF6RWV |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme
(Rattus norvegicus) | BDBM50050136
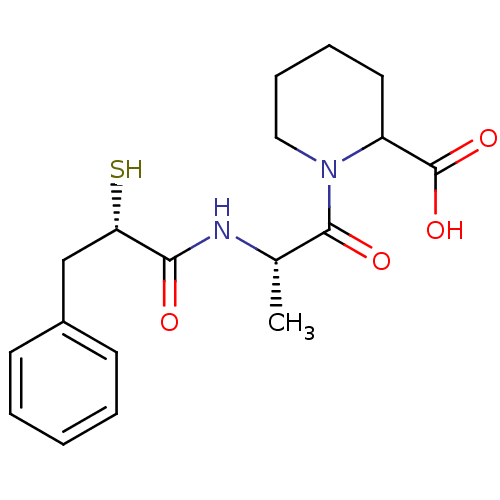
(1-[(S)-2-((S)-2-Mercapto-3-phenyl-propionylamino)-...)Show SMILES C[C@H](NC(=O)[C@@H](S)Cc1ccccc1)C(=O)N1CCCCC1C(O)=O Show InChI InChI=1S/C18H24N2O4S/c1-12(17(22)20-10-6-5-9-14(20)18(23)24)19-16(21)15(25)11-13-7-3-2-4-8-13/h2-4,7-8,12,14-15,25H,5-6,9-11H2,1H3,(H,19,21)(H,23,24)/t12-,14?,15-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM
Curated by ChEMBL
| Assay Description
Inhibitory activity against angiotensin I converting enzyme |
J Med Chem 39: 1210-9 (1996)
Article DOI: 10.1021/jm950590p
BindingDB Entry DOI: 10.7270/Q2DF6RWV |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme
(Rattus norvegicus) | BDBM50050146

(1-[(2S,3R)-2-((S)-2-Mercapto-3-phenyl-propionylami...)Show SMILES CC[C@@H](C)[C@H](NC(=O)[C@@H](S)Cc1ccccc1)C(=O)N1CCCC1C(O)=O Show InChI InChI=1S/C20H28N2O4S/c1-3-13(2)17(19(24)22-11-7-10-15(22)20(25)26)21-18(23)16(27)12-14-8-5-4-6-9-14/h4-6,8-9,13,15-17,27H,3,7,10-12H2,1-2H3,(H,21,23)(H,25,26)/t13-,15?,16+,17+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM
Curated by ChEMBL
| Assay Description
Inhibitory activity against angiotensin I converting enzyme |
J Med Chem 39: 1210-9 (1996)
Article DOI: 10.1021/jm950590p
BindingDB Entry DOI: 10.7270/Q2DF6RWV |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme
(Rattus norvegicus) | BDBM50021135
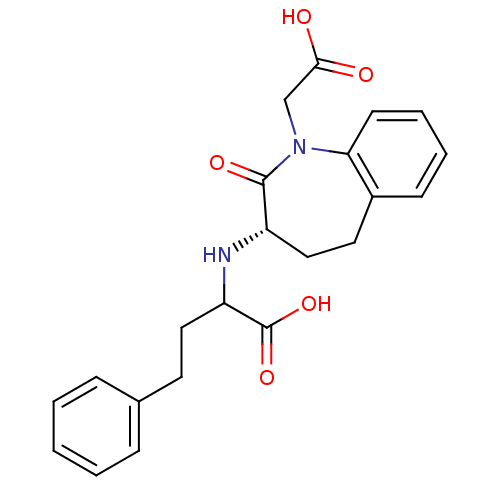
(2-(1-Carboxymethyl-2-oxo-2,3,4,5-tetrahydro-1H-ben...)Show SMILES OC(=O)CN1c2ccccc2CC[C@H](NC(CCc2ccccc2)C(O)=O)C1=O Show InChI InChI=1S/C22H24N2O5/c25-20(26)14-24-19-9-5-4-8-16(19)11-13-17(21(24)27)23-18(22(28)29)12-10-15-6-2-1-3-7-15/h1-9,17-18,23H,10-14H2,(H,25,26)(H,28,29)/t17-,18?/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of Angiotensin I converting enzyme in rat |
J Med Chem 28: 1517-21 (1985)
BindingDB Entry DOI: 10.7270/Q2736RGG |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme
(Rattus norvegicus) | BDBM50021153
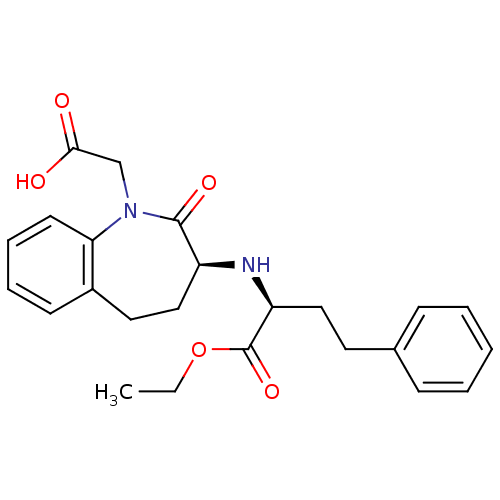
(1H-1-Benzazepine-1-acetic acid, 3-((1-(ethoxycarbo...)Show SMILES CCOC(=O)[C@H](CCc1ccccc1)N[C@H]1CCc2ccccc2N(CC(O)=O)C1=O Show InChI InChI=1S/C24H28N2O5/c1-2-31-24(30)20(14-12-17-8-4-3-5-9-17)25-19-15-13-18-10-6-7-11-21(18)26(23(19)29)16-22(27)28/h3-11,19-20,25H,2,12-16H2,1H3,(H,27,28)/t19-,20-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against Angiotensin I converting enzyme |
J Med Chem 28: 1603-6 (1985)
BindingDB Entry DOI: 10.7270/Q2ZK5H7D |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme
(Rattus norvegicus) | BDBM50369209
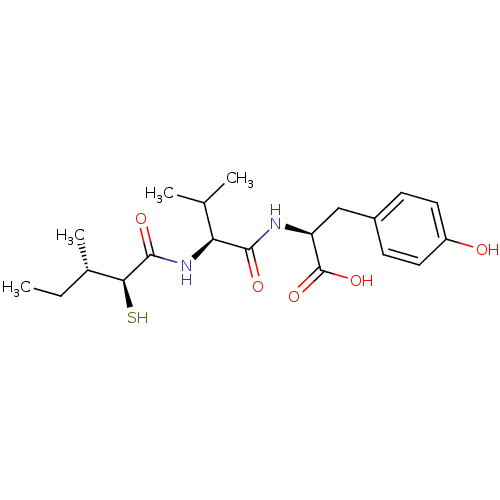
(CHEMBL1794012)Show SMILES CC[C@H](C)[C@H](S)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](Cc1ccc(O)cc1)C(O)=O Show InChI InChI=1S/C20H30N2O5S/c1-5-12(4)17(28)19(25)22-16(11(2)3)18(24)21-15(20(26)27)10-13-6-8-14(23)9-7-13/h6-9,11-12,15-17,23,28H,5,10H2,1-4H3,(H,21,24)(H,22,25)(H,26,27)/t12-,15-,16-,17-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM
Curated by ChEMBL
| Assay Description
Inhibitory activity against angiotensin I converting enzyme |
J Med Chem 39: 1210-9 (1996)
Article DOI: 10.1021/jm950590p
BindingDB Entry DOI: 10.7270/Q2DF6RWV |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme
(Rattus norvegicus) | BDBM50051799
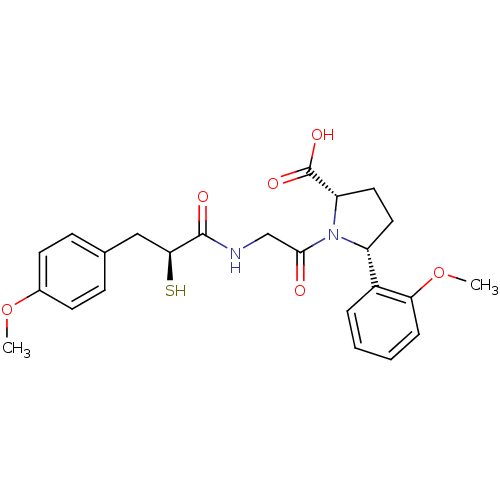
((2S,5R)-1-{2-[(S)-2-Mercapto-3-(4-methoxy-phenyl)-...)Show SMILES COc1ccc(C[C@H](S)C(=O)NCC(=O)N2[C@@H](CC[C@@H]2c2ccccc2OC)C(O)=O)cc1 Show InChI InChI=1S/C24H28N2O6S/c1-31-16-9-7-15(8-10-16)13-21(33)23(28)25-14-22(27)26-18(11-12-19(26)24(29)30)17-5-3-4-6-20(17)32-2/h3-10,18-19,21,33H,11-14H2,1-2H3,(H,25,28)(H,29,30)/t18-,19+,21+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM
Curated by ChEMBL
| Assay Description
In vitro inhibition of rat angiotensin I converting enzyme |
J Med Chem 39: 2594-608 (1996)
Article DOI: 10.1021/jm950783c
BindingDB Entry DOI: 10.7270/Q25B0353 |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme
(Rattus norvegicus) | BDBM50367254

(ENALAPRILAT)Show SMILES C[C@H](N[C@@H](CCc1ccccc1)C(O)=O)C(=O)N1CCC[C@H]1C(O)=O |r| Show InChI InChI=1S/C18H24N2O5/c1-12(16(21)20-11-5-8-15(20)18(24)25)19-14(17(22)23)10-9-13-6-3-2-4-7-13/h2-4,6-7,12,14-15,19H,5,8-11H2,1H3,(H,22,23)(H,24,25)/t12-,14-,15-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PubMed
| n/a | n/a | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of rat Angiotensin I converting enzyme (ACE), using Hip-Gly-Gly as synthetic substrate. |
J Med Chem 30: 992-8 (1987)
BindingDB Entry DOI: 10.7270/Q29C6Z02 |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme
(Rattus norvegicus) | BDBM50226811

(CHEMBL3349972)Show SMILES [H][C@]12C[C@]([H])(N(C(=O)[C@H](C)N[C@@H](CCc3ccccc3)C(O)=O)[C@@]1([H])CCCC2)C(O)=O Show InChI InChI=1S/C22H30N2O5/c1-14(23-17(21(26)27)12-11-15-7-3-2-4-8-15)20(25)24-18-10-6-5-9-16(18)13-19(24)22(28)29/h2-4,7-8,14,16-19,23H,5-6,9-13H2,1H3,(H,26,27)(H,28,29)/t14-,16-,17-,18-,19-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of rat Angiotensin I converting enzyme (ACE), using Hip-Gly-Gly as synthetic substrate. |
J Med Chem 30: 992-8 (1987)
BindingDB Entry DOI: 10.7270/Q29C6Z02 |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme
(Rattus norvegicus) | BDBM50051786
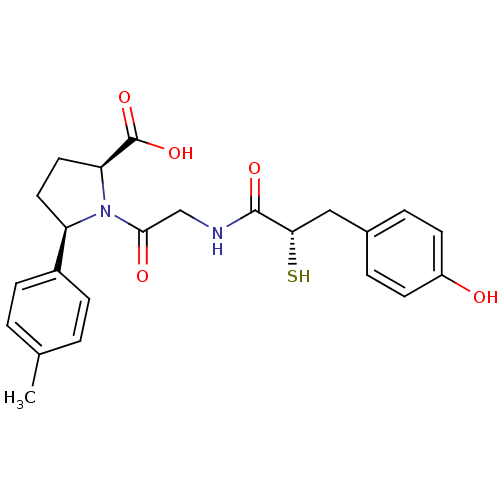
((2S,5R)-1-{2-[(S)-3-(4-Hydroxy-phenyl)-2-mercapto-...)Show SMILES Cc1ccc(cc1)[C@H]1CC[C@H](N1C(=O)CNC(=O)[C@@H](S)Cc1ccc(O)cc1)C(O)=O Show InChI InChI=1S/C23H26N2O5S/c1-14-2-6-16(7-3-14)18-10-11-19(23(29)30)25(18)21(27)13-24-22(28)20(31)12-15-4-8-17(26)9-5-15/h2-9,18-20,26,31H,10-13H2,1H3,(H,24,28)(H,29,30)/t18-,19+,20+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM
Curated by ChEMBL
| Assay Description
In vitro inhibition of rat angiotensin I converting enzyme |
J Med Chem 39: 2594-608 (1996)
Article DOI: 10.1021/jm950783c
BindingDB Entry DOI: 10.7270/Q25B0353 |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme
(Rattus norvegicus) | BDBM50050143

((S)-3-(4-Hydroxy-phenyl)-2-[(2S,3S)-2-((S)-2-merca...)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@@H](S)Cc1ccccc1)C(=O)N[C@@H](Cc1ccc(O)cc1)C(O)=O Show InChI InChI=1S/C24H30N2O5S/c1-3-15(2)21(26-22(28)20(32)14-16-7-5-4-6-8-16)23(29)25-19(24(30)31)13-17-9-11-18(27)12-10-17/h4-12,15,19-21,27,32H,3,13-14H2,1-2H3,(H,25,29)(H,26,28)(H,30,31)/t15-,19-,20-,21-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM
Curated by ChEMBL
| Assay Description
Inhibitory activity against angiotensin I converting enzyme |
J Med Chem 39: 1210-9 (1996)
Article DOI: 10.1021/jm950590p
BindingDB Entry DOI: 10.7270/Q2DF6RWV |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme
(Rattus norvegicus) | BDBM50287430
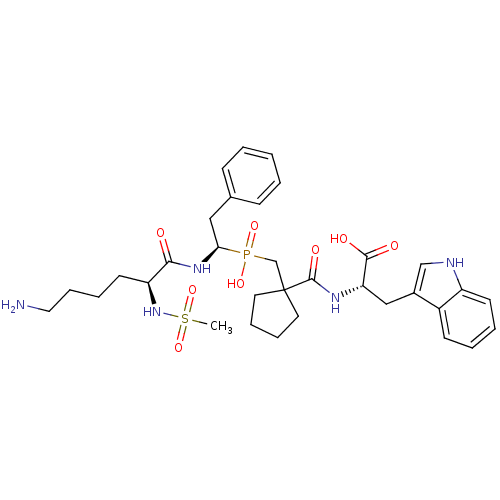
((S)-2-[(1-{[(R)-1-((S)-6-Amino-2-methanesulfonylam...)Show SMILES CS(=O)(=O)N[C@@H](CCCCN)C(=O)N[C@@H](Cc1ccccc1)P(O)(=O)CC1(CCCC1)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(O)=O |r| Show InChI InChI=1S/C33H46N5O8PS/c1-48(45,46)38-27(15-7-10-18-34)30(39)37-29(19-23-11-3-2-4-12-23)47(43,44)22-33(16-8-9-17-33)32(42)36-28(31(40)41)20-24-21-35-26-14-6-5-13-25(24)26/h2-6,11-14,21,27-29,35,38H,7-10,15-20,22,34H2,1H3,(H,36,42)(H,37,39)(H,40,41)(H,43,44)/t27-,28-,29+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was measured for the inhibition of Angiotensin I converting enzyme |
Bioorg Med Chem Lett 6: 1629-1634 (1996)
Article DOI: 10.1016/0960-894X(96)00297-1
BindingDB Entry DOI: 10.7270/Q2697437 |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme
(Rattus norvegicus) | BDBM50405541
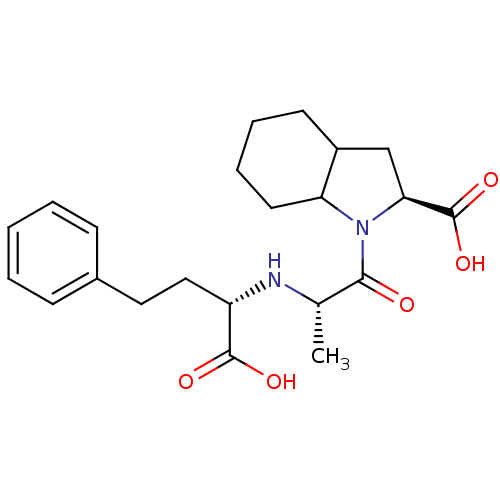
(CHEMBL2079670)Show SMILES C[C@H](N[C@@H](CCc1ccccc1)C(O)=O)C(=O)N1C2CCCCC2C[C@H]1C(O)=O |r| Show InChI InChI=1S/C22H30N2O5/c1-14(23-17(21(26)27)12-11-15-7-3-2-4-8-15)20(25)24-18-10-6-5-9-16(18)13-19(24)22(28)29/h2-4,7-8,14,16-19,23H,5-6,9-13H2,1H3,(H,26,27)(H,28,29)/t14-,16?,17-,18?,19-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of rat Angiotensin I converting enzyme (ACE), using Hip-Gly-Gly as synthetic substrate. |
J Med Chem 30: 992-8 (1987)
BindingDB Entry DOI: 10.7270/Q29C6Z02 |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme
(Rattus norvegicus) | BDBM50405179
(CHEMBL2114418)Show SMILES OC(=O)CN1c2ccccc2SC[C@H](N[C@@H](CCc2ccccc2)C(O)=O)C1=O Show InChI InChI=1S/C21H22N2O5S/c24-19(25)12-23-17-8-4-5-9-18(17)29-13-16(20(23)26)22-15(21(27)28)11-10-14-6-2-1-3-7-14/h1-9,15-16,22H,10-13H2,(H,24,25)(H,27,28)/t15-,16-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 2.90 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of Angiotensin I converting enzyme in rat |
J Med Chem 28: 1517-21 (1985)
BindingDB Entry DOI: 10.7270/Q2736RGG |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme
(Rattus norvegicus) | BDBM50050138
((S)-3-(4-Hydroxy-phenyl)-2-[(S)-2-((S)-2-mercapto-...)Show SMILES CC(C)C[C@H](NC(=O)[C@@H](S)Cc1ccccc1)C(=O)N[C@@H](Cc1ccc(O)cc1)C(O)=O Show InChI InChI=1S/C24H30N2O5S/c1-15(2)12-19(25-23(29)21(32)14-16-6-4-3-5-7-16)22(28)26-20(24(30)31)13-17-8-10-18(27)11-9-17/h3-11,15,19-21,27,32H,12-14H2,1-2H3,(H,25,29)(H,26,28)(H,30,31)/t19-,20-,21-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM
Curated by ChEMBL
| Assay Description
Inhibitory activity against angiotensin I converting enzyme |
J Med Chem 39: 1210-9 (1996)
Article DOI: 10.1021/jm950590p
BindingDB Entry DOI: 10.7270/Q2DF6RWV |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme
(Rattus norvegicus) | BDBM21642

((2S)-1-[(2S)-2-methyl-3-sulfanylpropanoyl]pyrrolid...)Show InChI InChI=1S/C9H15NO3S/c1-6(5-14)8(11)10-4-2-3-7(10)9(12)13/h6-7,14H,2-5H2,1H3,(H,12,13)/t6-,7+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Laboratori Guidotti S.p.A.
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against rat serum angiotensin I converting enzyme using hippuryl-glycyl-glycine as substrate |
J Med Chem 36: 699-707 (1993)
BindingDB Entry DOI: 10.7270/Q21N81RS |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme
(Rattus norvegicus) | BDBM50051800
(1-{2-[(S)-3-(4-Hydroxy-phenyl)-2-mercapto-propiony...)Show SMILES Cc1cccc(c1)C1CCC(N1C(=O)CNC(=O)[C@@H](S)Cc1ccc(O)cc1)C(O)=O Show InChI InChI=1S/C23H26N2O5S/c1-14-3-2-4-16(11-14)18-9-10-19(23(29)30)25(18)21(27)13-24-22(28)20(31)12-15-5-7-17(26)8-6-15/h2-8,11,18-20,26,31H,9-10,12-13H2,1H3,(H,24,28)(H,29,30)/t18?,19?,20-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.10 | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM
Curated by ChEMBL
| Assay Description
In vitro inhibition of rat angiotensin I converting enzyme |
J Med Chem 39: 2594-608 (1996)
Article DOI: 10.1021/jm950783c
BindingDB Entry DOI: 10.7270/Q25B0353 |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme
(Rattus norvegicus) | BDBM50051778
((2S,5R)-1-[2-((S)-2-Mercapto-3-phenyl-propionylami...)Show SMILES Cc1ccccc1[C@H]1CC[C@H](N1C(=O)CNC(=O)[C@@H](S)Cc1ccccc1)C(O)=O Show InChI InChI=1S/C23H26N2O4S/c1-15-7-5-6-10-17(15)18-11-12-19(23(28)29)25(18)21(26)14-24-22(27)20(30)13-16-8-3-2-4-9-16/h2-10,18-20,30H,11-14H2,1H3,(H,24,27)(H,28,29)/t18-,19+,20+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.10 | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM
Curated by ChEMBL
| Assay Description
In vitro inhibition of rat angiotensin I converting enzyme |
J Med Chem 39: 2594-608 (1996)
Article DOI: 10.1021/jm950783c
BindingDB Entry DOI: 10.7270/Q25B0353 |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme
(Rattus norvegicus) | BDBM21642

((2S)-1-[(2S)-2-methyl-3-sulfanylpropanoyl]pyrrolid...)Show InChI InChI=1S/C9H15NO3S/c1-6(5-14)8(11)10-4-2-3-7(10)9(12)13/h6-7,14H,2-5H2,1H3,(H,12,13)/t6-,7+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade Federal do Piau£
Curated by ChEMBL
| Assay Description
Inhibition of Wistar rat plasma angiotensin 1-converting enzyme using H-hippuryl-His-Leu-OH as substrate after 20 mins by fluorescence assay |
Eur J Med Chem 139: 401-411 (2017)
Article DOI: 10.1016/j.ejmech.2017.08.019
BindingDB Entry DOI: 10.7270/Q2T43WMD |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme
(Rattus norvegicus) | BDBM50027141
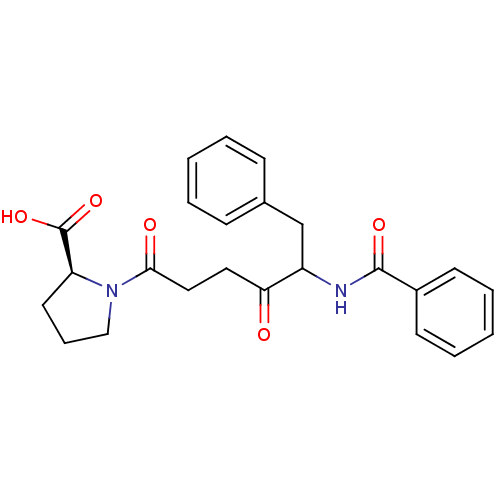
(1-(5-Benzoylamino-4-oxo-6-phenyl-hexanoyl)-pyrroli...)Show SMILES OC(=O)[C@@H]1CCCN1C(=O)CCC(=O)C(Cc1ccccc1)NC(=O)c1ccccc1 Show InChI InChI=1S/C24H26N2O5/c27-21(13-14-22(28)26-15-7-12-20(26)24(30)31)19(16-17-8-3-1-4-9-17)25-23(29)18-10-5-2-6-11-18/h1-6,8-11,19-20H,7,12-16H2,(H,25,29)(H,30,31)/t19?,20-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of rat Angiotensin I converting enzyme (ACE), using Hip-Gly-Gly as synthetic substrate. |
J Med Chem 30: 992-8 (1987)
BindingDB Entry DOI: 10.7270/Q29C6Z02 |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme
(Rattus norvegicus) | BDBM50051795
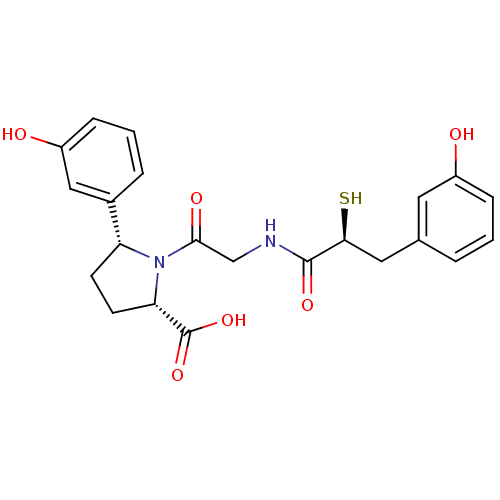
((2S,5R)-5-(3-Hydroxy-phenyl)-1-{2-[(S)-3-(3-hydrox...)Show SMILES OC(=O)[C@@H]1CC[C@@H](N1C(=O)CNC(=O)[C@@H](S)Cc1cccc(O)c1)c1cccc(O)c1 Show InChI InChI=1S/C22H24N2O6S/c25-15-5-1-3-13(9-15)10-19(31)21(28)23-12-20(27)24-17(7-8-18(24)22(29)30)14-4-2-6-16(26)11-14/h1-6,9,11,17-19,25-26,31H,7-8,10,12H2,(H,23,28)(H,29,30)/t17-,18+,19+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM
Curated by ChEMBL
| Assay Description
In vitro inhibition of rat angiotensin I converting enzyme |
J Med Chem 39: 2594-608 (1996)
Article DOI: 10.1021/jm950783c
BindingDB Entry DOI: 10.7270/Q25B0353 |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme
(Rattus norvegicus) | BDBM50050133

(1-[(S)-2-((2S,3S)-2-Mercapto-3-methyl-pentanoylami...)Show SMILES CC[C@H](C)[C@H](S)C(=O)N[C@@H](C)C(=O)N1C(Cc2ccccc12)C(O)=O Show InChI InChI=1S/C18H24N2O4S/c1-4-10(2)15(25)16(21)19-11(3)17(22)20-13-8-6-5-7-12(13)9-14(20)18(23)24/h5-8,10-11,14-15,25H,4,9H2,1-3H3,(H,19,21)(H,23,24)/t10-,11-,14?,15-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.30 | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM
Curated by ChEMBL
| Assay Description
Inhibitory activity against angiotensin I converting enzyme |
J Med Chem 39: 1210-9 (1996)
Article DOI: 10.1021/jm950590p
BindingDB Entry DOI: 10.7270/Q2DF6RWV |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme
(Rattus norvegicus) | BDBM50050145

((S)-3-(4-Hydroxy-phenyl)-2-[(2S,3S)-2-((S)-2-merca...)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@@H](S)c1ccccc1)C(=O)N[C@@H](Cc1ccc(O)cc1)C(O)=O Show InChI InChI=1S/C23H28N2O5S/c1-3-14(2)19(25-22(28)20(31)16-7-5-4-6-8-16)21(27)24-18(23(29)30)13-15-9-11-17(26)12-10-15/h4-12,14,18-20,26,31H,3,13H2,1-2H3,(H,24,27)(H,25,28)(H,29,30)/t14-,18-,19-,20-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.5 | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM
Curated by ChEMBL
| Assay Description
Inhibitory activity against angiotensin I converting enzyme |
J Med Chem 39: 1210-9 (1996)
Article DOI: 10.1021/jm950590p
BindingDB Entry DOI: 10.7270/Q2DF6RWV |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme
(Rattus norvegicus) | BDBM50051794
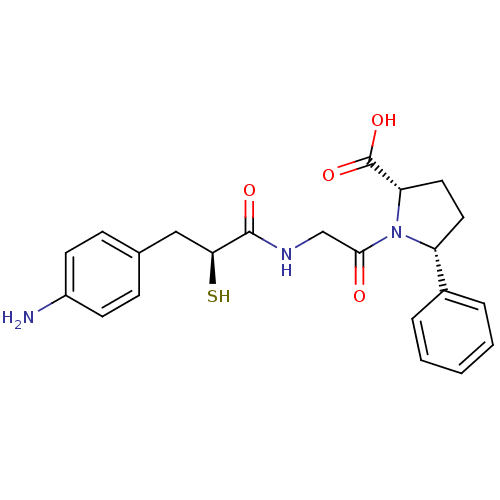
((2S,5R)-1-{2-[(S)-3-(4-Amino-phenyl)-2-mercapto-pr...)Show SMILES Nc1ccc(C[C@H](S)C(=O)NCC(=O)N2[C@@H](CC[C@@H]2c2ccccc2)C(O)=O)cc1 Show InChI InChI=1S/C22H25N3O4S/c23-16-8-6-14(7-9-16)12-19(30)21(27)24-13-20(26)25-17(10-11-18(25)22(28)29)15-4-2-1-3-5-15/h1-9,17-19,30H,10-13,23H2,(H,24,27)(H,28,29)/t17-,18+,19+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.5 | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM
Curated by ChEMBL
| Assay Description
In vitro inhibition of rat angiotensin I converting enzyme |
J Med Chem 39: 2594-608 (1996)
Article DOI: 10.1021/jm950783c
BindingDB Entry DOI: 10.7270/Q25B0353 |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme
(Rattus norvegicus) | BDBM50421824
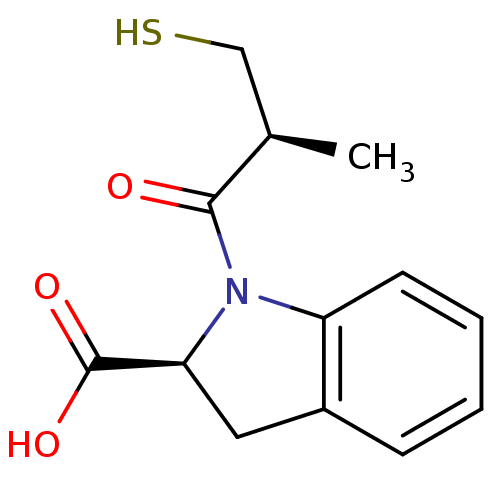
(CHEMBL75752)Show InChI InChI=1S/C13H15NO3S/c1-8(7-18)12(15)14-10-5-3-2-4-9(10)6-11(14)13(16)17/h2-5,8,11,18H,6-7H2,1H3,(H,16,17)/t8-,11+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 3.70 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of rat Angiotensin I converting enzyme (ACE), using Hip-Gly-Gly as synthetic substrate. |
J Med Chem 30: 992-8 (1987)
BindingDB Entry DOI: 10.7270/Q29C6Z02 |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme
(Rattus norvegicus) | BDBM50027641

(1-(2-Mercapto-propionyl)-2,3-dihydro-1H-indole-2-c...)Show InChI InChI=1S/C12H13NO3S/c1-7(17)11(14)13-9-5-3-2-4-8(9)6-10(13)12(15)16/h2-5,7,10,17H,6H2,1H3,(H,15,16)/t7-,10-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 3.70 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against Angiotensin I converting enzyme |
J Med Chem 26: 394-403 (1983)
BindingDB Entry DOI: 10.7270/Q2T15473 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data