 Found 36 hits of ic50 for UniProtKB: P60953
Found 36 hits of ic50 for UniProtKB: P60953 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kcal/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Cell division control protein 42 homolog
(Homo sapiens (Human)) | BDBM50319716
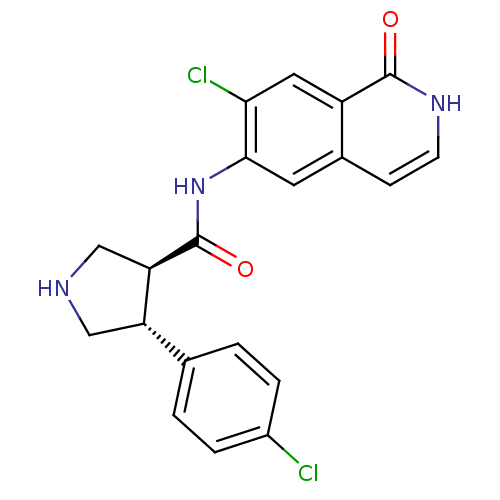
((3S,4R)-N-(7-chloro-1-oxo-1,2-dihydroisoquinolin-6...)Show SMILES Clc1ccc(cc1)[C@@H]1CNC[C@H]1C(=O)Nc1cc2cc[nH]c(=O)c2cc1Cl |r| Show InChI InChI=1S/C20H17Cl2N3O2/c21-13-3-1-11(2-4-13)15-9-23-10-16(15)20(27)25-18-7-12-5-6-24-19(26)14(12)8-17(18)22/h1-8,15-16,23H,9-10H2,(H,24,26)(H,25,27)/t15-,16+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 54 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer-Ingelheim Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of CDC42 |
Bioorg Med Chem Lett 20: 3746-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.04.069
BindingDB Entry DOI: 10.7270/Q2XD12MD |
More data for this
Ligand-Target Pair | |
Cell division control protein 42 homolog
(Homo sapiens (Human)) | BDBM50391237

(CHEMBL5290216)Show InChI InChI=1S/C11H13N5/c12-10-9(7-15-11(13)16-10)14-6-8-4-2-1-3-5-8/h1-5,7,14H,6H2,(H4,12,13,15,16) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 80 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
In vitro inhibition of angiotensin I converting enzyme in rabbit lung with hippuryl-histidyl-leucine as substrate |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c02221 |
More data for this
Ligand-Target Pair | |
Cell division control protein 42 homolog
(Homo sapiens (Human)) | BDBM50319634
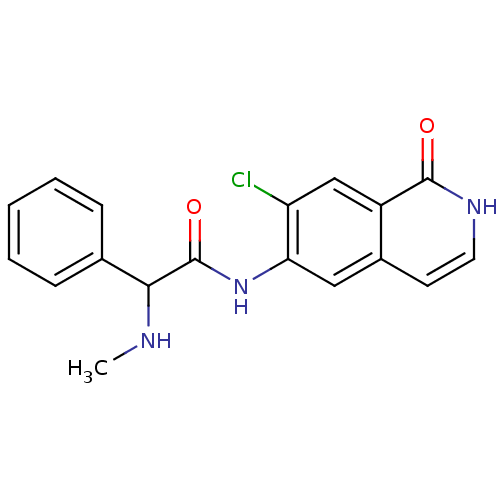
(CHEMBL1084892 | N-(7-chloro-1-oxo-1,2-dihydroisoqu...)Show InChI InChI=1S/C18H16ClN3O2/c1-20-16(11-5-3-2-4-6-11)18(24)22-15-9-12-7-8-21-17(23)13(12)10-14(15)19/h2-10,16,20H,1H3,(H,21,23)(H,22,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of CDc42 |
Bioorg Med Chem Lett 20: 3235-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.04.070
BindingDB Entry DOI: 10.7270/Q21N819Z |
More data for this
Ligand-Target Pair | |
Cell division control protein 42 homolog
(Homo sapiens (Human)) | BDBM50300554

(((2S,3R,4S,5S)-5-(2-amino-6-oxo-1H-purin-9(6H)-yl)...)Show SMILES Nc1nc2n(cnc2c(=O)[nH]1)[C@H]1O[C@@H](COP(O)(=O)OP(O)(O)=O)[C@H](O)[C@@H]1O |r| Show InChI InChI=1S/C10H15N5O11P2/c11-10-13-7-4(8(18)14-10)12-2-15(7)9-6(17)5(16)3(25-9)1-24-28(22,23)26-27(19,20)21/h2-3,5-6,9,16-17H,1H2,(H,22,23)(H2,19,20,21)(H3,11,13,14,18)/t3-,5-,6-,9-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Exonhit Therapeutics
Curated by ChEMBL
| Assay Description
Inhibition of Cdc42 GTPase activity assessed as incorporation of BODIPY-GTP after 40 mins by nucleotide binding competition assay |
Bioorg Med Chem Lett 19: 5594-8 (2009)
Article DOI: 10.1016/j.bmcl.2009.08.037
BindingDB Entry DOI: 10.7270/Q27P8ZF4 |
More data for this
Ligand-Target Pair | |
Cell division control protein 42 homolog
(Homo sapiens (Human)) | BDBM50319633
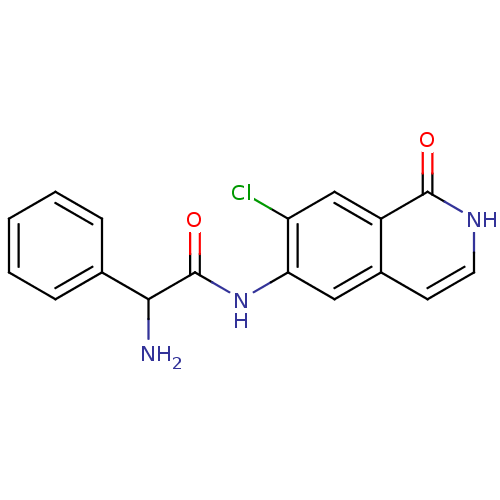
(2-amino-N-(7-chloro-1-oxo-1,2-dihydroisoquinolin-6...)Show InChI InChI=1S/C17H14ClN3O2/c18-13-9-12-11(6-7-20-16(12)22)8-14(13)21-17(23)15(19)10-4-2-1-3-5-10/h1-9,15H,19H2,(H,20,22)(H,21,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of CDc42 |
Bioorg Med Chem Lett 20: 3235-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.04.070
BindingDB Entry DOI: 10.7270/Q21N819Z |
More data for this
Ligand-Target Pair | |
Cell division control protein 42 homolog
(Homo sapiens (Human)) | BDBM50308894
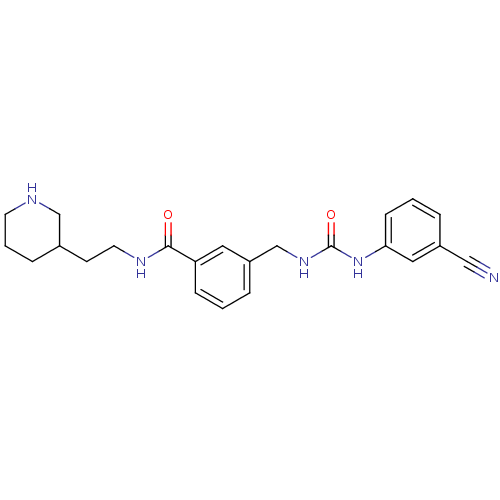
(3-[3-(3-Cyano-phenyl)-ureidomethyl]-N-(2-piperidin...)Show SMILES O=C(NCc1cccc(c1)C(=O)NCCC1CCCNC1)Nc1cccc(c1)C#N Show InChI InChI=1S/C23H27N5O2/c24-14-18-4-2-8-21(13-18)28-23(30)27-16-19-5-1-7-20(12-19)22(29)26-11-9-17-6-3-10-25-15-17/h1-2,4-5,7-8,12-13,17,25H,3,6,9-11,15-16H2,(H,26,29)(H2,27,28,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of Cdc42 |
J Med Chem 53: 759-77 (2010)
Article DOI: 10.1021/jm9014263
BindingDB Entry DOI: 10.7270/Q2V125RD |
More data for this
Ligand-Target Pair | |
Cell division control protein 42 homolog
(Homo sapiens (Human)) | BDBM50308897

(3-[3-(4-Cyano-phenyl)-ureidomethyl]-N-(2-piperidin...)Show SMILES O=C(NCc1cccc(c1)C(=O)NCCC1CCCNC1)Nc1ccc(cc1)C#N Show InChI InChI=1S/C23H27N5O2/c24-14-17-6-8-21(9-7-17)28-23(30)27-16-19-3-1-5-20(13-19)22(29)26-12-10-18-4-2-11-25-15-18/h1,3,5-9,13,18,25H,2,4,10-12,15-16H2,(H,26,29)(H2,27,28,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of Cdc42 |
J Med Chem 53: 759-77 (2010)
Article DOI: 10.1021/jm9014263
BindingDB Entry DOI: 10.7270/Q2V125RD |
More data for this
Ligand-Target Pair | |
Cell division control protein 42 homolog
(Homo sapiens (Human)) | BDBM50308862

(3-[3-(4-Cyano-phenyl)-ureidomethyl]-N-(1,2,3,4-tet...)Show SMILES O=C(NCc1cccc(c1)C(=O)Nc1ccc2CCNCc2c1)Nc1ccc(cc1)C#N Show InChI InChI=1S/C25H23N5O2/c26-14-17-4-7-22(8-5-17)30-25(32)28-15-18-2-1-3-20(12-18)24(31)29-23-9-6-19-10-11-27-16-21(19)13-23/h1-9,12-13,27H,10-11,15-16H2,(H,29,31)(H2,28,30,32) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of Cdc42 |
J Med Chem 53: 759-77 (2010)
Article DOI: 10.1021/jm9014263
BindingDB Entry DOI: 10.7270/Q2V125RD |
More data for this
Ligand-Target Pair | |
Cell division control protein 42 homolog
(Homo sapiens (Human)) | BDBM50308861
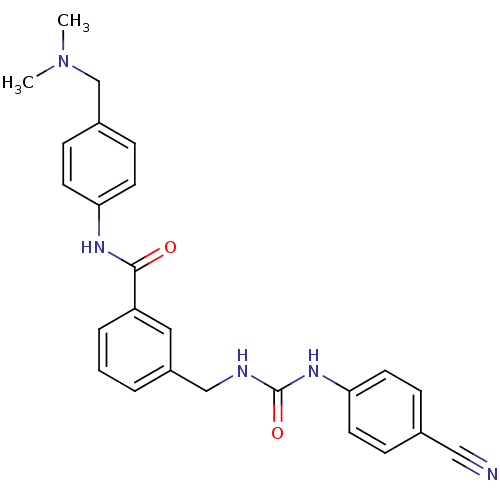
(3-[3-(4-Cyano-phenyl)-ureidomethyl]-N-(4-dimethyla...)Show SMILES CN(C)Cc1ccc(NC(=O)c2cccc(CNC(=O)Nc3ccc(cc3)C#N)c2)cc1 Show InChI InChI=1S/C25H25N5O2/c1-30(2)17-19-8-12-22(13-9-19)28-24(31)21-5-3-4-20(14-21)16-27-25(32)29-23-10-6-18(15-26)7-11-23/h3-14H,16-17H2,1-2H3,(H,28,31)(H2,27,29,32) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of Cdc42 |
J Med Chem 53: 759-77 (2010)
Article DOI: 10.1021/jm9014263
BindingDB Entry DOI: 10.7270/Q2V125RD |
More data for this
Ligand-Target Pair | |
Cell division control protein 42 homolog
(Homo sapiens (Human)) | BDBM50308898

(3-[3-(4-Amido-phenyl)-ureidomethyl]-N-(1,2,3,4-tet...)Show SMILES NC(=O)c1ccc(NC(=O)NCc2cccc(c2)C(=O)Nc2ccc3CCNCc3c2)cc1 Show InChI InChI=1S/C25H25N5O3/c26-23(31)18-5-7-21(8-6-18)30-25(33)28-14-16-2-1-3-19(12-16)24(32)29-22-9-4-17-10-11-27-15-20(17)13-22/h1-9,12-13,27H,10-11,14-15H2,(H2,26,31)(H,29,32)(H2,28,30,33) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of Cdc42 |
J Med Chem 53: 759-77 (2010)
Article DOI: 10.1021/jm9014263
BindingDB Entry DOI: 10.7270/Q2V125RD |
More data for this
Ligand-Target Pair | |
Cell division control protein 42 homolog
(Homo sapiens (Human)) | BDBM14027
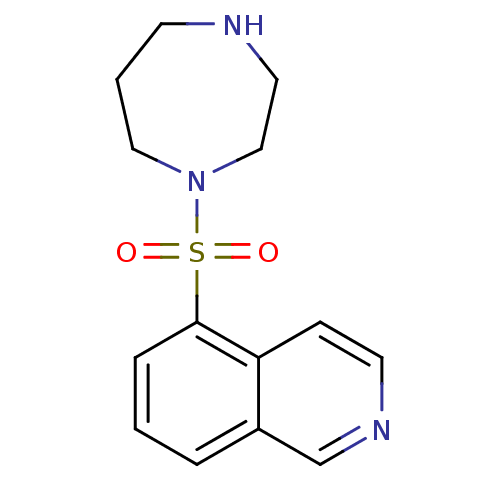
(5-(1,4-diazepan-1-ylsulfonyl)isoquinoline | 5-(1,4...)Show InChI InChI=1S/C14H17N3O2S/c18-20(19,17-9-2-6-15-8-10-17)14-4-1-3-12-11-16-7-5-13(12)14/h1,3-5,7,11,15H,2,6,8-10H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of Cdc42 |
J Med Chem 53: 759-77 (2010)
Article DOI: 10.1021/jm9014263
BindingDB Entry DOI: 10.7270/Q2V125RD |
More data for this
Ligand-Target Pair | |
Cell division control protein 42 homolog
(Homo sapiens (Human)) | BDBM50308866
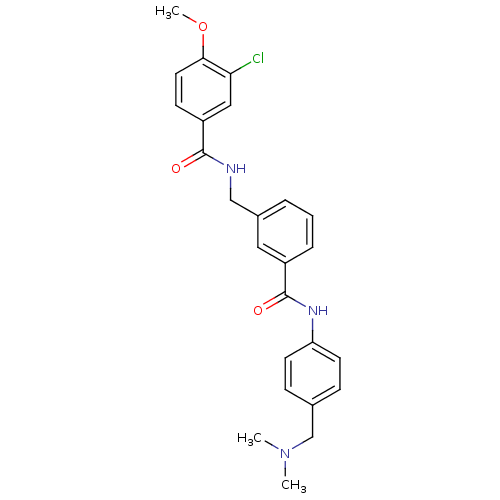
(3-Chloro-N-[3-(4-dimethylaminomethyl-phenylcarbamo...)Show SMILES COc1ccc(cc1Cl)C(=O)NCc1cccc(c1)C(=O)Nc1ccc(CN(C)C)cc1 Show InChI InChI=1S/C25H26ClN3O3/c1-29(2)16-17-7-10-21(11-8-17)28-25(31)19-6-4-5-18(13-19)15-27-24(30)20-9-12-23(32-3)22(26)14-20/h4-14H,15-16H2,1-3H3,(H,27,30)(H,28,31) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of Cdc42 |
J Med Chem 53: 759-77 (2010)
Article DOI: 10.1021/jm9014263
BindingDB Entry DOI: 10.7270/Q2V125RD |
More data for this
Ligand-Target Pair | |
Cell division control protein 42 homolog
(Homo sapiens (Human)) | BDBM50308875
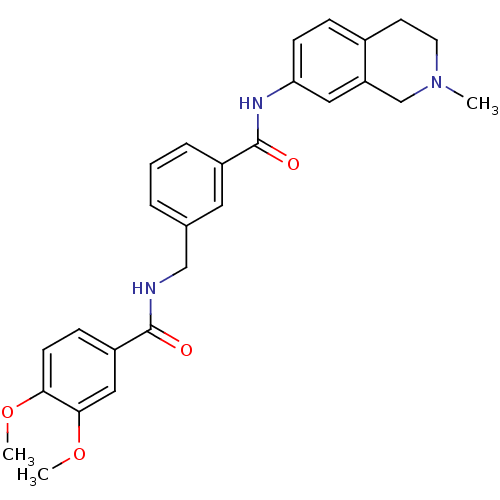
(3,4-Dimethoxy-N-[3-(2-methyl-1,2,3,4-tetrahydro-is...)Show SMILES COc1ccc(cc1OC)C(=O)NCc1cccc(c1)C(=O)Nc1ccc2CCN(C)Cc2c1 Show InChI InChI=1S/C27H29N3O4/c1-30-12-11-19-7-9-23(14-22(19)17-30)29-27(32)20-6-4-5-18(13-20)16-28-26(31)21-8-10-24(33-2)25(15-21)34-3/h4-10,13-15H,11-12,16-17H2,1-3H3,(H,28,31)(H,29,32) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of Cdc42 |
J Med Chem 53: 759-77 (2010)
Article DOI: 10.1021/jm9014263
BindingDB Entry DOI: 10.7270/Q2V125RD |
More data for this
Ligand-Target Pair | |
Cell division control protein 42 homolog
(Homo sapiens (Human)) | BDBM50308876
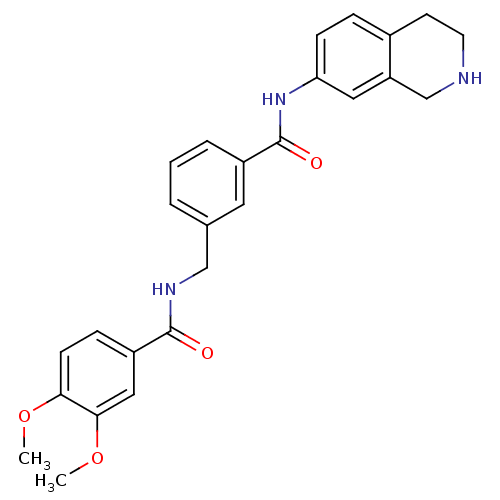
(3,4-Dimethoxy-N-[3-(1,2,3,4-tetrahydro-isoquinolin...)Show SMILES COc1ccc(cc1OC)C(=O)NCc1cccc(c1)C(=O)Nc1ccc2CCNCc2c1 Show InChI InChI=1S/C26H27N3O4/c1-32-23-9-7-20(14-24(23)33-2)25(30)28-15-17-4-3-5-19(12-17)26(31)29-22-8-6-18-10-11-27-16-21(18)13-22/h3-9,12-14,27H,10-11,15-16H2,1-2H3,(H,28,30)(H,29,31) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of Cdc42 |
J Med Chem 53: 759-77 (2010)
Article DOI: 10.1021/jm9014263
BindingDB Entry DOI: 10.7270/Q2V125RD |
More data for this
Ligand-Target Pair | |
Cell division control protein 42 homolog
(Homo sapiens (Human)) | BDBM50308900
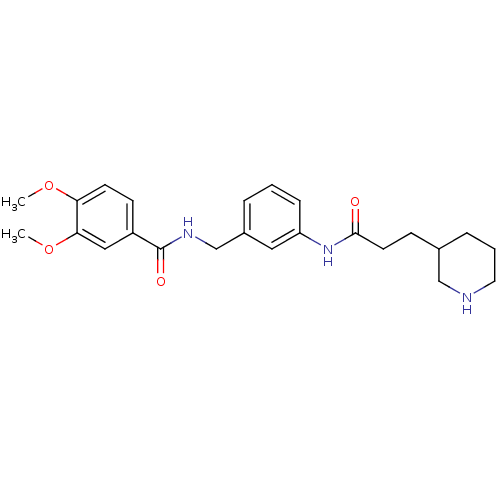
(3,4-Dimethoxy-N-[3-(3-piperidin-3-yl-propionylamin...)Show SMILES COc1ccc(cc1OC)C(=O)NCc1cccc(NC(=O)CCC2CCCNC2)c1 Show InChI InChI=1S/C24H31N3O4/c1-30-21-10-9-19(14-22(21)31-2)24(29)26-16-18-5-3-7-20(13-18)27-23(28)11-8-17-6-4-12-25-15-17/h3,5,7,9-10,13-14,17,25H,4,6,8,11-12,15-16H2,1-2H3,(H,26,29)(H,27,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | >5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of Cdc42 |
J Med Chem 53: 759-77 (2010)
Article DOI: 10.1021/jm9014263
BindingDB Entry DOI: 10.7270/Q2V125RD |
More data for this
Ligand-Target Pair | |
Cell division control protein 42 homolog
(Homo sapiens (Human)) | BDBM50308877
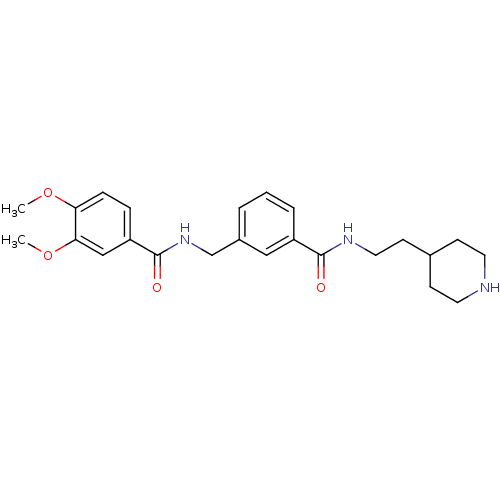
(3,4-Dimethoxy-N-[3-(2-piperidin-4-yl-ethylcarbamoy...)Show SMILES COc1ccc(cc1OC)C(=O)NCc1cccc(c1)C(=O)NCCC1CCNCC1 Show InChI InChI=1S/C24H31N3O4/c1-30-21-7-6-20(15-22(21)31-2)24(29)27-16-18-4-3-5-19(14-18)23(28)26-13-10-17-8-11-25-12-9-17/h3-7,14-15,17,25H,8-13,16H2,1-2H3,(H,26,28)(H,27,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of Cdc42 |
J Med Chem 53: 759-77 (2010)
Article DOI: 10.1021/jm9014263
BindingDB Entry DOI: 10.7270/Q2V125RD |
More data for this
Ligand-Target Pair | |
Cell division control protein 42 homolog
(Homo sapiens (Human)) | BDBM50308872
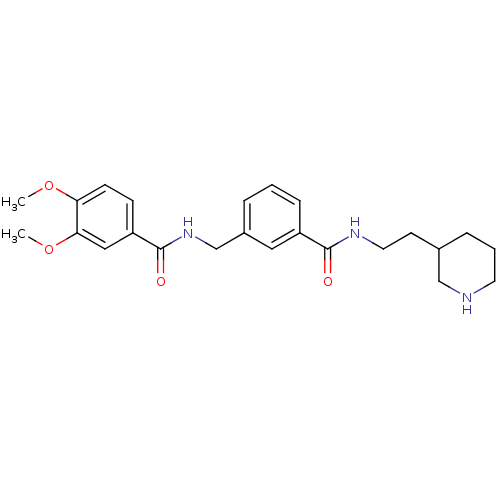
(3,4-Dimethoxy-N-[3-(2-piperidin-3-yl-ethylcarbamoy...)Show SMILES COc1ccc(cc1OC)C(=O)NCc1cccc(c1)C(=O)NCCC1CCCNC1 Show InChI InChI=1S/C24H31N3O4/c1-30-21-9-8-20(14-22(21)31-2)24(29)27-16-18-5-3-7-19(13-18)23(28)26-12-10-17-6-4-11-25-15-17/h3,5,7-9,13-14,17,25H,4,6,10-12,15-16H2,1-2H3,(H,26,28)(H,27,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of Cdc42 |
J Med Chem 53: 759-77 (2010)
Article DOI: 10.1021/jm9014263
BindingDB Entry DOI: 10.7270/Q2V125RD |
More data for this
Ligand-Target Pair | |
Cell division control protein 42 homolog
(Homo sapiens (Human)) | BDBM50308871
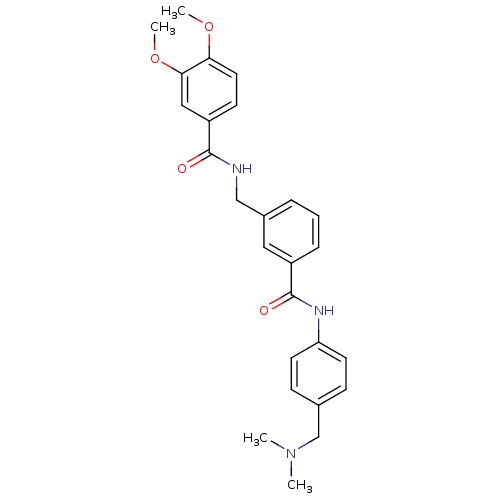
(CHEMBL599792 | N-[3-(4-Dimethylaminomethyl-phenylc...)Show SMILES COc1ccc(cc1OC)C(=O)NCc1cccc(c1)C(=O)Nc1ccc(CN(C)C)cc1 Show InChI InChI=1S/C26H29N3O4/c1-29(2)17-18-8-11-22(12-9-18)28-26(31)20-7-5-6-19(14-20)16-27-25(30)21-10-13-23(32-3)24(15-21)33-4/h5-15H,16-17H2,1-4H3,(H,27,30)(H,28,31) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of Cdc42 |
J Med Chem 53: 759-77 (2010)
Article DOI: 10.1021/jm9014263
BindingDB Entry DOI: 10.7270/Q2V125RD |
More data for this
Ligand-Target Pair | |
Cell division control protein 42 homolog
(Homo sapiens (Human)) | BDBM50319632
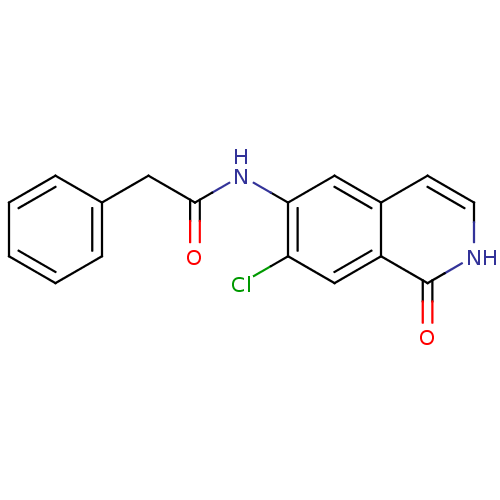
(CHEMBL1084890 | N-(7-chloro-1-oxo-1,2-dihydroisoqu...)Show InChI InChI=1S/C17H13ClN2O2/c18-14-10-13-12(6-7-19-17(13)22)9-15(14)20-16(21)8-11-4-2-1-3-5-11/h1-7,9-10H,8H2,(H,19,22)(H,20,21) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 5.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of CDc42 |
Bioorg Med Chem Lett 20: 3235-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.04.070
BindingDB Entry DOI: 10.7270/Q21N819Z |
More data for this
Ligand-Target Pair | |
Cell division control protein 42 homolog
(Homo sapiens (Human)) | BDBM50300547
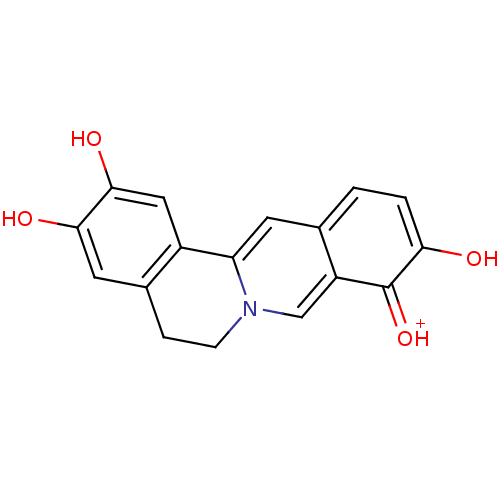
(2,3,9,10-Tetrahydroxy-5,6-dihydro-isoquino[3,2-a]i...)Show InChI InChI=1S/C17H13NO4/c19-14-2-1-9-5-13-11-7-16(21)15(20)6-10(11)3-4-18(13)8-12(9)17(14)22/h1-2,5-8,19-21H,3-4H2/p+1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Exonhit Therapeutics
Curated by ChEMBL
| Assay Description
Inhibition of Cdc42 GTPase activity assessed as incorporation of BODIPY-GTP after 40 mins by nucleotide binding competition assay |
Bioorg Med Chem Lett 19: 5594-8 (2009)
Article DOI: 10.1016/j.bmcl.2009.08.037
BindingDB Entry DOI: 10.7270/Q27P8ZF4 |
More data for this
Ligand-Target Pair | |
Cell division control protein 42 homolog
(Homo sapiens (Human)) | BDBM50324673

(CHEMBL1222039 | Secramine B)Show SMILES CO\N=C1/C[C@H]2Oc3c4c(CN[C@H](CO)C[C@]24[C@@H](C1)SCc1ccc(OC)cc1)cc(OCC1CC1)c3Br |r| Show InChI InChI=1S/C29H35BrN2O5S/c1-34-22-7-5-18(6-8-22)16-38-25-11-20(32-35-2)10-24-29(25)12-21(14-33)31-13-19-9-23(36-15-17-3-4-17)27(30)28(37-24)26(19)29/h5-9,17,21,24-25,31,33H,3-4,10-16H2,1-2H3/b32-20+/t21-,24+,25+,29+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School
Curated by ChEMBL
| Assay Description
Inhibition of GEF-independent nucleotide exchange activity of prenylated Cdc42 after 3 mins in presence of 4 mM Mg2+ chelator EDTA |
Nat Chem Biol 2: 39-46 (2006)
Article DOI: 10.1038/nchembio751
BindingDB Entry DOI: 10.7270/Q23B60BR |
More data for this
Ligand-Target Pair | |
Cell division control protein 42 homolog
(Homo sapiens (Human)) | BDBM14027

(5-(1,4-diazepan-1-ylsulfonyl)isoquinoline | 5-(1,4...)Show InChI InChI=1S/C14H17N3O2S/c18-20(19,17-9-2-6-15-8-10-17)14-4-1-3-12-11-16-7-5-13(12)14/h1,3-5,7,11,15H,2,6,8-10H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >8.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of CDc42 |
Bioorg Med Chem Lett 20: 3235-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.04.070
BindingDB Entry DOI: 10.7270/Q21N819Z |
More data for this
Ligand-Target Pair | |
Cell division control protein 42 homolog
(Homo sapiens (Human)) | BDBM50319637
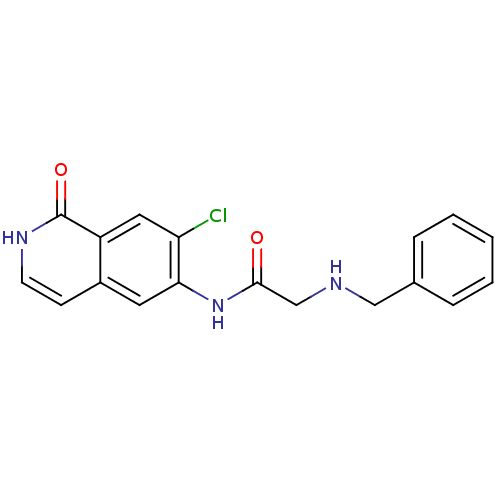
(2-(benzylamino)-N-(7-chloro-1-oxo-1,2-dihydroisoqu...)Show InChI InChI=1S/C18H16ClN3O2/c19-15-9-14-13(6-7-21-18(14)24)8-16(15)22-17(23)11-20-10-12-4-2-1-3-5-12/h1-9,20H,10-11H2,(H,21,24)(H,22,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >8.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of CDc42 |
Bioorg Med Chem Lett 20: 3235-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.04.070
BindingDB Entry DOI: 10.7270/Q21N819Z |
More data for this
Ligand-Target Pair | |
Cell division control protein 42 homolog
(Homo sapiens (Human)) | BDBM50319636
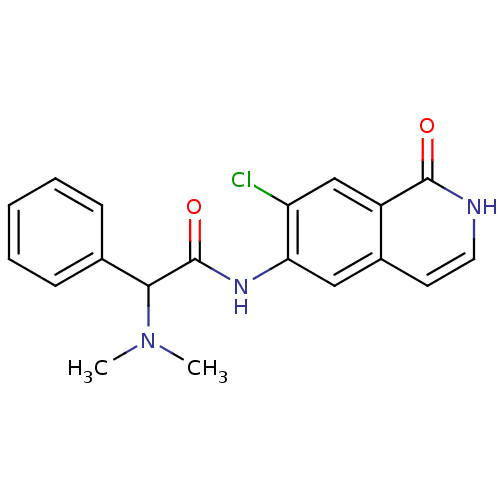
(CHEMBL1084107 | N-(7-chloro-1-oxo-1,2-dihydroisoqu...)Show SMILES CN(C)C(C(=O)Nc1cc2cc[nH]c(=O)c2cc1Cl)c1ccccc1 Show InChI InChI=1S/C19H18ClN3O2/c1-23(2)17(12-6-4-3-5-7-12)19(25)22-16-10-13-8-9-21-18(24)14(13)11-15(16)20/h3-11,17H,1-2H3,(H,21,24)(H,22,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >8.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of CDc42 |
Bioorg Med Chem Lett 20: 3235-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.04.070
BindingDB Entry DOI: 10.7270/Q21N819Z |
More data for this
Ligand-Target Pair | |
Cell division control protein 42 homolog
(Homo sapiens (Human)) | BDBM14029
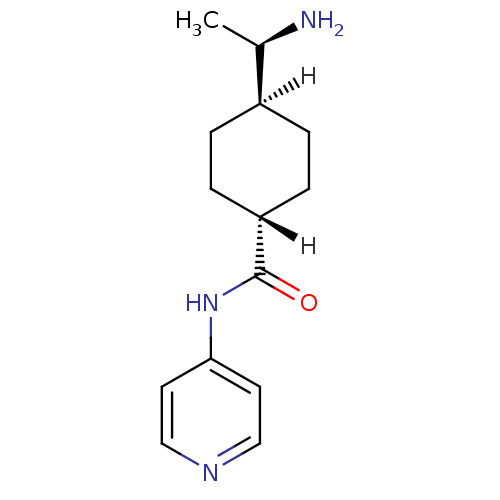
((R)-TRANS-4-(1-AMINOETHYL)-N-(4-PYRIDYL) CYCLOHEXA...)Show SMILES [H][C@@]1(CC[C@@]([H])(CC1)C(=O)Nc1ccncc1)[C@@H](C)N |r,wU:4.4,1.18,17.20,wD:4.8,1.0,(1.92,.41,;1.06,-.86,;-.27,-1.63,;-1.61,-.86,;-1.61,.68,;-1.61,2.22,;-.27,1.45,;1.06,.68,;-2.94,1.45,;-2.94,2.99,;-4.27,.68,;-5.61,1.45,;-5.61,2.99,;-6.94,3.76,;-8.28,2.99,;-8.28,1.45,;-6.94,.68,;2.6,-.86,;3.37,.47,;3.37,-2.2,)| Show InChI InChI=1S/C14H21N3O/c1-10(15)11-2-4-12(5-3-11)14(18)17-13-6-8-16-9-7-13/h6-12H,2-5,15H2,1H3,(H,16,17,18)/t10-,11-,12-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >8.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of CDc42 |
Bioorg Med Chem Lett 20: 3235-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.04.070
BindingDB Entry DOI: 10.7270/Q21N819Z |
More data for this
Ligand-Target Pair | |
Cell division control protein 42 homolog
(Homo sapiens (Human)) | BDBM50319635
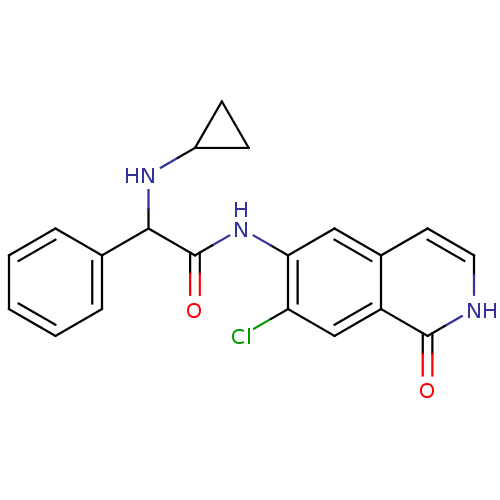
(CHEMBL1084106 | N-(7-chloro-1-oxo-1,2-dihydroisoqu...)Show SMILES Clc1cc2c(cc[nH]c2=O)cc1NC(=O)C(NC1CC1)c1ccccc1 Show InChI InChI=1S/C20H18ClN3O2/c21-16-11-15-13(8-9-22-19(15)25)10-17(16)24-20(26)18(23-14-6-7-14)12-4-2-1-3-5-12/h1-5,8-11,14,18,23H,6-7H2,(H,22,25)(H,24,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >8.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of CDc42 |
Bioorg Med Chem Lett 20: 3235-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.04.070
BindingDB Entry DOI: 10.7270/Q21N819Z |
More data for this
Ligand-Target Pair | |
Cell division control protein 42 homolog
(Homo sapiens (Human)) | BDBM50324672

(CHEMBL1222038 | Secramine A)Show SMILES COc1ccc(CS[C@@H]2C\C(C[C@H]3Oc4c5c(CN[C@H](CO)C[C@]235)cc(OCC2CC2)c4Br)=N\OCc2ccccc2)cc1 |r| Show InChI InChI=1S/C35H39BrN2O5S/c1-40-28-11-9-24(10-12-28)21-44-31-15-26(38-42-20-22-5-3-2-4-6-22)14-30-35(31)16-27(18-39)37-17-25-13-29(41-19-23-7-8-23)33(36)34(43-30)32(25)35/h2-6,9-13,23,27,30-31,37,39H,7-8,14-21H2,1H3/b38-26+/t27-,30+,31+,35+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 9.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School
Curated by ChEMBL
| Assay Description
Inhibition of GEF-independent nucleotide exchange activity of prenylated Cdc42 after 3 mins in presence of 4 mM Mg2+ chelator EDTA |
Nat Chem Biol 2: 39-46 (2006)
Article DOI: 10.1038/nchembio751
BindingDB Entry DOI: 10.7270/Q23B60BR |
More data for this
Ligand-Target Pair | |
Cell division control protein 42 homolog
(Homo sapiens (Human)) | BDBM50300551
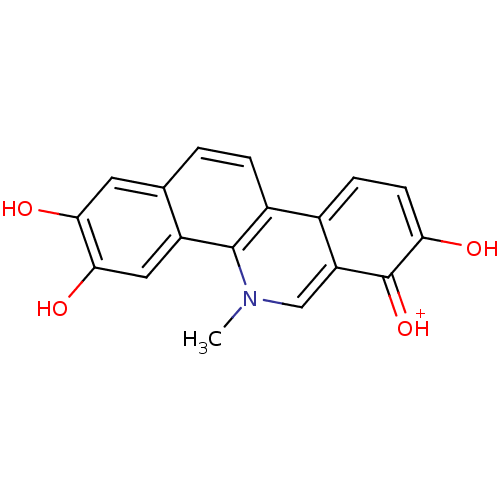
(2,3,7,8-Tetrahydroxy-5-methyl-benzo[c]phenanthridi...)Show SMILES Cn1cc2c(ccc(O)c2=[OH+])c2ccc3cc(O)c(O)cc3c12 Show InChI InChI=1S/C18H13NO4/c1-19-8-13-10(4-5-14(20)18(13)23)11-3-2-9-6-15(21)16(22)7-12(9)17(11)19/h2-8,20-22H,1H3/p+1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Exonhit Therapeutics
Curated by ChEMBL
| Assay Description
Inhibition of Cdc42 GTPase activity assessed as incorporation of BODIPY-GTP after 40 mins by nucleotide binding competition assay |
Bioorg Med Chem Lett 19: 5594-8 (2009)
Article DOI: 10.1016/j.bmcl.2009.08.037
BindingDB Entry DOI: 10.7270/Q27P8ZF4 |
More data for this
Ligand-Target Pair | |
Cell division control protein 42 homolog
(Homo sapiens (Human)) | BDBM50300548

(3,4,10,11-Tetrahydroxy-8-methyl-isoquino[3,2-a]iso...)Show InChI InChI=1S/C18H13NO4/c1-9-13-8-17(22)16(21)7-10(13)6-14-11-2-3-15(20)18(23)12(11)4-5-19(9)14/h2-8H,1H3,(H3,20,21,22,23)/p+1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Exonhit Therapeutics
Curated by ChEMBL
| Assay Description
Inhibition of Cdc42 GTPase activity assessed as incorporation of BODIPY-GTP after 40 mins by nucleotide binding competition assay |
Bioorg Med Chem Lett 19: 5594-8 (2009)
Article DOI: 10.1016/j.bmcl.2009.08.037
BindingDB Entry DOI: 10.7270/Q27P8ZF4 |
More data for this
Ligand-Target Pair | |
Cell division control protein 42 homolog
(Homo sapiens (Human)) | BDBM25525
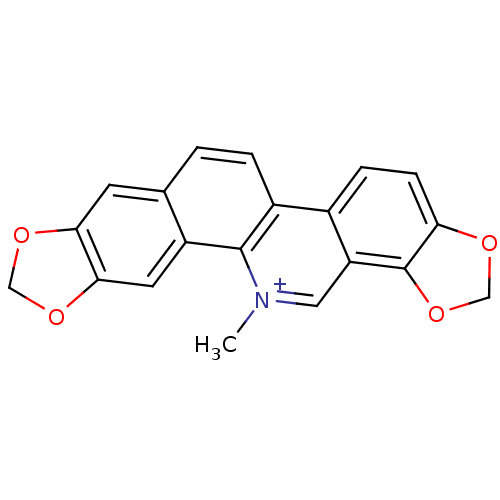
(24-methyl-5,7,18,20-tetraoxa-24-azahexacyclo[11.11...)Show InChI InChI=1S/C20H14NO4/c1-21-8-15-12(4-5-16-20(15)25-10-22-16)13-3-2-11-6-17-18(24-9-23-17)7-14(11)19(13)21/h2-8H,9-10H2,1H3/q+1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Exonhit Therapeutics
Curated by ChEMBL
| Assay Description
Inhibition of Cdc42 GTPase activity assessed as incorporation of BODIPY-GTP after 40 mins by nucleotide binding competition assay |
Bioorg Med Chem Lett 19: 5594-8 (2009)
Article DOI: 10.1016/j.bmcl.2009.08.037
BindingDB Entry DOI: 10.7270/Q27P8ZF4 |
More data for this
Ligand-Target Pair | |
Cell division control protein 42 homolog
(Homo sapiens (Human)) | BDBM50300552

(2,3-Dihydroxy-7,8-dimethoxy-5-methyl-benzo[c]phena...)Show SMILES COc1ccc2c(cn(C)c3c4cc(O)c(=[OH+])cc4ccc23)c1OC Show InChI InChI=1S/C20H17NO4/c1-21-10-15-12(6-7-18(24-2)20(15)25-3)13-5-4-11-8-16(22)17(23)9-14(11)19(13)21/h4-10,23H,1-3H3/p+1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Exonhit Therapeutics
Curated by ChEMBL
| Assay Description
Inhibition of Cdc42 GTPase activity assessed as incorporation of BODIPY-GTP after 40 mins by nucleotide binding competition assay |
Bioorg Med Chem Lett 19: 5594-8 (2009)
Article DOI: 10.1016/j.bmcl.2009.08.037
BindingDB Entry DOI: 10.7270/Q27P8ZF4 |
More data for this
Ligand-Target Pair | |
Cell division control protein 42 homolog
(Homo sapiens (Human)) | BDBM50300550
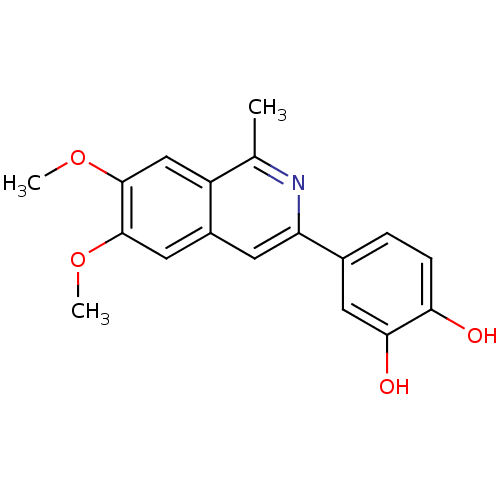
(3-(3,4-Dihydroxy-phenyl)-6,7-dimethoxy-1-methyl-is...)Show InChI InChI=1S/C18H17NO4/c1-10-13-9-18(23-3)17(22-2)8-12(13)6-14(19-10)11-4-5-15(20)16(21)7-11/h4-9,20-21H,1-3H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Exonhit Therapeutics
Curated by ChEMBL
| Assay Description
Inhibition of Cdc42 GTPase activity assessed as incorporation of BODIPY-GTP after 40 mins by nucleotide binding competition assay |
Bioorg Med Chem Lett 19: 5594-8 (2009)
Article DOI: 10.1016/j.bmcl.2009.08.037
BindingDB Entry DOI: 10.7270/Q27P8ZF4 |
More data for this
Ligand-Target Pair | |
Cell division control protein 42 homolog
(Homo sapiens (Human)) | BDBM50300549
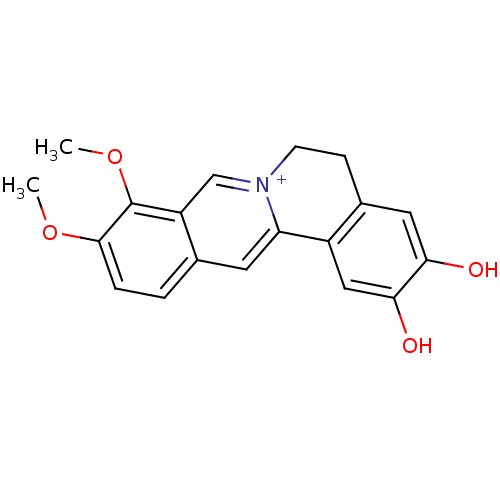
(2,3-Dihydroxy-9,10-dimethoxy-5,6-dihydro-isoquino[...)Show InChI InChI=1S/C19H17NO4/c1-23-18-4-3-11-7-15-13-9-17(22)16(21)8-12(13)5-6-20(15)10-14(11)19(18)24-2/h3-4,7-10,22H,5-6H2,1-2H3/p+1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Exonhit Therapeutics
Curated by ChEMBL
| Assay Description
Inhibition of Cdc42 GTPase activity assessed as incorporation of BODIPY-GTP after 40 mins by nucleotide binding competition assay |
Bioorg Med Chem Lett 19: 5594-8 (2009)
Article DOI: 10.1016/j.bmcl.2009.08.037
BindingDB Entry DOI: 10.7270/Q27P8ZF4 |
More data for this
Ligand-Target Pair | |
Cell division control protein 42 homolog
(Homo sapiens (Human)) | BDBM50300545

(CHEMBL573174 | tetrahydroxycanadine hydrochloride)Show InChI InChI=1S/C17H17NO4/c19-14-2-1-9-5-13-11-7-16(21)15(20)6-10(11)3-4-18(13)8-12(9)17(14)22/h1-2,6-7,13,19-22H,3-5,8H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Exonhit Therapeutics
Curated by ChEMBL
| Assay Description
Inhibition of Cdc42 GTPase activity assessed as incorporation of BODIPY-GTP after 40 mins by nucleotide binding competition assay |
Bioorg Med Chem Lett 19: 5594-8 (2009)
Article DOI: 10.1016/j.bmcl.2009.08.037
BindingDB Entry DOI: 10.7270/Q27P8ZF4 |
More data for this
Ligand-Target Pair | |
Cell division control protein 42 homolog
(Homo sapiens (Human)) | BDBM50300546
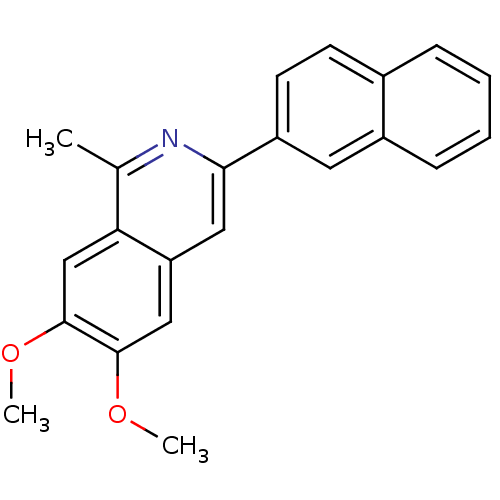
(6,7-Dimethoxy-1-methyl-3-naphthalen-2-yl-isoquinol...)Show InChI InChI=1S/C22H19NO2/c1-14-19-13-22(25-3)21(24-2)12-18(19)11-20(23-14)17-9-8-15-6-4-5-7-16(15)10-17/h4-13H,1-3H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Exonhit Therapeutics
Curated by ChEMBL
| Assay Description
Inhibition of Cdc42 GTPase activity assessed as incorporation of BODIPY-GTP after 40 mins by nucleotide binding competition assay |
Bioorg Med Chem Lett 19: 5594-8 (2009)
Article DOI: 10.1016/j.bmcl.2009.08.037
BindingDB Entry DOI: 10.7270/Q27P8ZF4 |
More data for this
Ligand-Target Pair | |
Cell division control protein 42 homolog
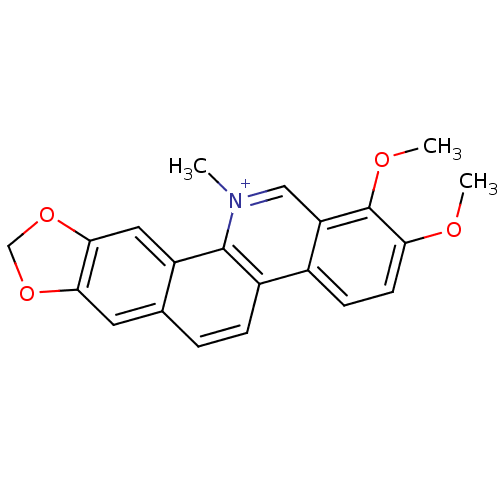
(Homo sapiens (Human)) | BDBM25524
(17,18-dimethoxy-21-methyl-5,7-dioxa-21-azapentacyc...)Show SMILES COc1ccc2c(c[n+](C)c3c4cc5OCOc5cc4ccc23)c1OC Show InChI InChI=1S/C21H18NO4/c1-22-10-16-13(6-7-17(23-2)21(16)24-3)14-5-4-12-8-18-19(26-11-25-18)9-15(12)20(14)22/h4-10H,11H2,1-3H3/q+1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Exonhit Therapeutics
Curated by ChEMBL
| Assay Description
Inhibition of Cdc42 GTPase activity assessed as incorporation of BODIPY-GTP after 40 mins by nucleotide binding competition assay |
Bioorg Med Chem Lett 19: 5594-8 (2009)
Article DOI: 10.1016/j.bmcl.2009.08.037
BindingDB Entry DOI: 10.7270/Q27P8ZF4 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data