 Found 622 hits of ic50 for UniProtKB: P07948
Found 622 hits of ic50 for UniProtKB: P07948 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kcal/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Tyrosine-protein kinase Lyn
(Homo sapiens (Human)) | BDBM50345579
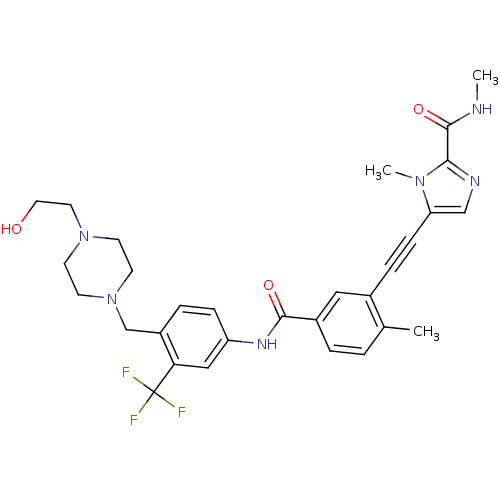
(5-((5-(4-((4-(2-hydroxyethyl)piperazin-1-yl)methyl...)Show SMILES CNC(=O)c1ncc(C#Cc2cc(ccc2C)C(=O)Nc2ccc(CN3CCN(CCO)CC3)c(c2)C(F)(F)F)n1C Show InChI InChI=1S/C30H33F3N6O3/c1-20-4-5-22(16-21(20)7-9-25-18-35-27(37(25)3)29(42)34-2)28(41)36-24-8-6-23(26(17-24)30(31,32)33)19-39-12-10-38(11-13-39)14-15-40/h4-6,8,16-18,40H,10-15,19H2,1-3H3,(H,34,42)(H,36,41) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | <0.150 | n/a | n/a | n/a | n/a | n/a | n/a |
ARIAD Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human LYN using poly[Glu:Tyr] by Hotspot assay |
Bioorg Med Chem Lett 21: 3743-8 (2011)
Article DOI: 10.1016/j.bmcl.2011.04.060
BindingDB Entry DOI: 10.7270/Q21G0MM8 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Lyn
(Homo sapiens (Human)) | BDBM50322535
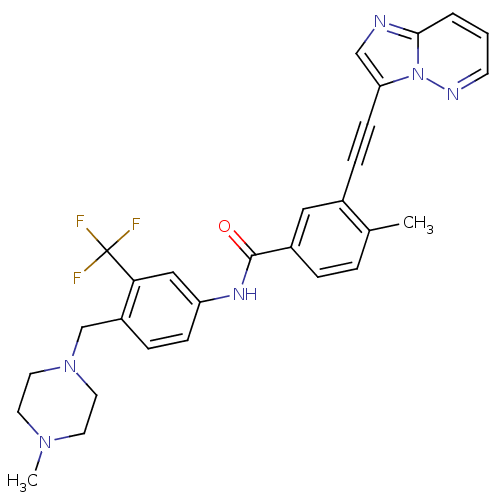
(3-(imidazo[1,2-b]pyridazin-3-ylethynyl)-4-methyl-N...)Show SMILES CN1CCN(Cc2ccc(NC(=O)c3ccc(C)c(c3)C#Cc3cnc4cccnn34)cc2C(F)(F)F)CC1 Show InChI InChI=1S/C29H27F3N6O/c1-20-5-6-22(16-21(20)8-10-25-18-33-27-4-3-11-34-38(25)27)28(39)35-24-9-7-23(26(17-24)29(30,31)32)19-37-14-12-36(2)13-15-37/h3-7,9,11,16-18H,12-15,19H2,1-2H3,(H,35,39) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| n/a | n/a | 0.160 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Binding affinity to LYN (unknown origin) |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.8b00081
BindingDB Entry DOI: 10.7270/Q2WM1J37 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Lyn
(Homo sapiens (Human)) | BDBM50322535

(3-(imidazo[1,2-b]pyridazin-3-ylethynyl)-4-methyl-N...)Show SMILES CN1CCN(Cc2ccc(NC(=O)c3ccc(C)c(c3)C#Cc3cnc4cccnn34)cc2C(F)(F)F)CC1 Show InChI InChI=1S/C29H27F3N6O/c1-20-5-6-22(16-21(20)8-10-25-18-33-27-4-3-11-34-38(25)27)28(39)35-24-9-7-23(26(17-24)29(30,31)32)19-37-14-12-36(2)13-15-37/h3-7,9,11,16-18H,12-15,19H2,1-2H3,(H,35,39) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| n/a | n/a | 0.240 | n/a | n/a | n/a | n/a | n/a | n/a |
The First Affiliated Hospital of Zhengzhou University
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human Lyn using peptide substrate poly[Glu:Tyr] (4:1) in presence of [33-P]ATP by kinase hotspot assay |
Eur J Med Chem 126: 476-490 (2017)
Article DOI: 10.1016/j.ejmech.2016.11.052
BindingDB Entry DOI: 10.7270/Q2445PQT |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Lyn
(Homo sapiens (Human)) | BDBM243041

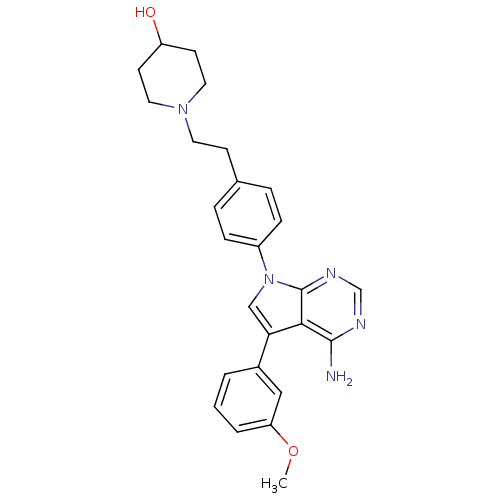
(A-419259)Show SMILES CN1CCN(CC1)[C@H]1CCC(CC1)n1cc(-c2ccc(Oc3ccccc3)cc2)c2c(N)ncnc12 |r,wD:7.7,(26.44,-17.33,;25.67,-18.66,;25.16,-19.93,;25.3,-21.48,;23.96,-22.25,;24.33,-20.97,;24.33,-19.43,;22.48,-22.65,;22.48,-21.11,;21.14,-20.34,;19.81,-21.11,;19.81,-22.65,;21.14,-23.42,;18.32,-20.71,;19.23,-19.47,;18.32,-18.22,;18.8,-16.76,;20.3,-16.44,;20.78,-14.97,;19.75,-13.83,;20.22,-12.36,;21.73,-12.04,;22.76,-13.19,;24.27,-12.87,;24.74,-11.4,;23.71,-10.26,;22.21,-10.58,;18.24,-14.15,;17.77,-15.61,;16.86,-18.7,;15.52,-17.93,;15.52,-16.39,;14.19,-18.7,;14.19,-20.24,;15.52,-21.01,;16.86,-20.24,)| Show InChI InChI=1S/C29H34N6O/c1-33-15-17-34(18-16-33)22-9-11-23(12-10-22)35-19-26(27-28(30)31-20-32-29(27)35)21-7-13-25(14-8-21)36-24-5-3-2-4-6-24/h2-8,13-14,19-20,22-23H,9-12,15-18H2,1H3,(H2,30,31,32)/t22-,23? | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | 22 |
Ankara University
| Assay Description
Kinase activity (Src-family kinases, Hck, Lyn, Fyn, and c-Src) and the effect of the molecules were determined by ProFluor Src-Family Kinase Assay Ki... |
J Enzyme Inhib Med Chem 28: 1080-7 (2013)
Article DOI: 10.3109/14756366.2012.715288
BindingDB Entry DOI: 10.7270/Q2KD1WTX |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Lyn
(Homo sapiens (Human)) | BDBM50413999
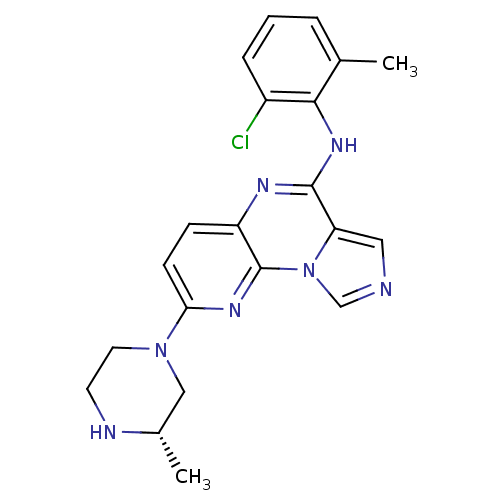
(CGP77675 | CHEMBL475584)Show SMILES COc1cccc(c1)-c1cn(-c2ccc(CCN3CCC(O)CC3)cc2)c2ncnc(N)c12 Show InChI InChI=1S/C26H29N5O2/c1-33-22-4-2-3-19(15-22)23-16-31(26-24(23)25(27)28-17-29-26)20-7-5-18(6-8-20)9-12-30-13-10-21(32)11-14-30/h2-8,15-17,21,32H,9-14H2,1H3,(H2,27,28,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Indo-Soviet Friendship College of Pharmacy (ISFCP)
Curated by ChEMBL
| Assay Description
Inhibition of Lyn (unknown origin) using Src-family kinase bisamide rhodamine 110 peptide substrate after 1 hr by fluorescence assay |
Eur J Med Chem 157: 503-526 (2018)
Article DOI: 10.1016/j.ejmech.2018.08.023
BindingDB Entry DOI: 10.7270/Q2DF6TX4 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Lyn
(Homo sapiens (Human)) | BDBM2579

((2S,3R,4R,6R)-3-methoxy-2-methyl-4-(methylamino)-2...)Show SMILES CN[C@@H]1C[C@H]2O[C@@](C)([C@@H]1OC)n1c3ccccc3c3c4CNC(=O)c4c4c5ccccc5n2c4c13 |r| Show InChI InChI=1S/C28H26N4O3/c1-28-26(34-3)17(29-2)12-20(35-28)31-18-10-6-4-8-14(18)22-23-16(13-30-27(23)33)21-15-9-5-7-11-19(15)32(28)25(21)24(22)31/h4-11,17,20,26,29H,12-13H2,1-3H3,(H,30,33)/t17-,20-,26-,28+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.473 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Institute of Science and Technology (KIST)
Curated by ChEMBL
| Assay Description
Inhibition of LYN (unknown origin) |
Eur J Med Chem 141: 657-675 (2017)
Article DOI: 10.1016/j.ejmech.2017.10.003
BindingDB Entry DOI: 10.7270/Q2CV4M86 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Lyn
(Homo sapiens (Human)) | BDBM2579

((2S,3R,4R,6R)-3-methoxy-2-methyl-4-(methylamino)-2...)Show SMILES CN[C@@H]1C[C@H]2O[C@@](C)([C@@H]1OC)n1c3ccccc3c3c4CNC(=O)c4c4c5ccccc5n2c4c13 |r| Show InChI InChI=1S/C28H26N4O3/c1-28-26(34-3)17(29-2)12-20(35-28)31-18-10-6-4-8-14(18)22-23-16(13-30-27(23)33)21-15-9-5-7-11-19(15)32(28)25(21)24(22)31/h4-11,17,20,26,29H,12-13H2,1-3H3,(H,30,33)/t17-,20-,26-,28+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.490 | n/a | n/a | n/a | n/a | n/a | n/a |
National Cancer Institute
Curated by ChEMBL
| Assay Description
Inhibition of LYN |
J Med Chem 52: 3191-204 (2009)
Article DOI: 10.1021/jm800861c
BindingDB Entry DOI: 10.7270/Q23J3DWT |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Lyn
(Homo sapiens (Human)) | BDBM50151366
((2-Chloro-6-methyl-phenyl)-[8-((S)-3-methyl-pipera...)Show SMILES C[C@H]1CN(CCN1)c1ccc2nc(Nc3c(C)cccc3Cl)c3cncn3c2n1 Show InChI InChI=1S/C21H22ClN7/c1-13-4-3-5-15(22)19(13)27-20-17-10-23-12-29(17)21-16(25-20)6-7-18(26-21)28-9-8-24-14(2)11-28/h3-7,10,12,14,24H,8-9,11H2,1-2H3,(H,25,27)/t14-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of Lyn kinase |
J Med Chem 47: 4517-29 (2004)
Article DOI: 10.1021/jm030217e
BindingDB Entry DOI: 10.7270/Q2R210VQ |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Lyn
(Homo sapiens (Human)) | BDBM2579

((2S,3R,4R,6R)-3-methoxy-2-methyl-4-(methylamino)-2...)Show SMILES CN[C@@H]1C[C@H]2O[C@@](C)([C@@H]1OC)n1c3ccccc3c3c4CNC(=O)c4c4c5ccccc5n2c4c13 |r| Show InChI InChI=1S/C28H26N4O3/c1-28-26(34-3)17(29-2)12-20(35-28)31-18-10-6-4-8-14(18)22-23-16(13-30-27(23)33)21-15-9-5-7-11-19(15)32(28)25(21)24(22)31/h4-11,17,20,26,29H,12-13H2,1-3H3,(H,30,33)/t17-,20-,26-,28+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.728 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human LYN using poly[Glu:Tyr] (4:1) as substrate by [gamma-33P]-ATP assay |
Citation and Details
Article DOI: 10.1016/j.bmc.2018.02.022
BindingDB Entry DOI: 10.7270/Q2DJ5KB8 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Lyn
(Homo sapiens (Human)) | BDBM2579

((2S,3R,4R,6R)-3-methoxy-2-methyl-4-(methylamino)-2...)Show SMILES CN[C@@H]1C[C@H]2O[C@@](C)([C@@H]1OC)n1c3ccccc3c3c4CNC(=O)c4c4c5ccccc5n2c4c13 |r| Show InChI InChI=1S/C28H26N4O3/c1-28-26(34-3)17(29-2)12-20(35-28)31-18-10-6-4-8-14(18)22-23-16(13-30-27(23)33)21-15-9-5-7-11-19(15)32(28)25(21)24(22)31/h4-11,17,20,26,29H,12-13H2,1-3H3,(H,30,33)/t17-,20-,26-,28+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.805 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Florida
Curated by ChEMBL
| Assay Description
Inhibition of human LYN using poly[Glu:Tyr] (4:1) as substrate by [gamma-33P]-ATP assay |
Eur J Med Chem 161: 456-467 (2019)
Article DOI: 10.1016/j.ejmech.2018.10.052
BindingDB Entry DOI: 10.7270/Q2W380MT |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Lyn
(Homo sapiens (Human)) | BDBM2579

((2S,3R,4R,6R)-3-methoxy-2-methyl-4-(methylamino)-2...)Show SMILES CN[C@@H]1C[C@H]2O[C@@](C)([C@@H]1OC)n1c3ccccc3c3c4CNC(=O)c4c4c5ccccc5n2c4c13 |r| Show InChI InChI=1S/C28H26N4O3/c1-28-26(34-3)17(29-2)12-20(35-28)31-18-10-6-4-8-14(18)22-23-16(13-30-27(23)33)21-15-9-5-7-11-19(15)32(28)25(21)24(22)31/h4-11,17,20,26,29H,12-13H2,1-3H3,(H,30,33)/t17-,20-,26-,28+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.964 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human LYN-B using poly[Glu:Tyr] (4:1) as substrate by [gamma-33P]-ATP assay |
Citation and Details
Article DOI: 10.1016/j.bmc.2018.02.022
BindingDB Entry DOI: 10.7270/Q2DJ5KB8 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Lyn
(Homo sapiens (Human)) | BDBM2579

((2S,3R,4R,6R)-3-methoxy-2-methyl-4-(methylamino)-2...)Show SMILES CN[C@@H]1C[C@H]2O[C@@](C)([C@@H]1OC)n1c3ccccc3c3c4CNC(=O)c4c4c5ccccc5n2c4c13 |r| Show InChI InChI=1S/C28H26N4O3/c1-28-26(34-3)17(29-2)12-20(35-28)31-18-10-6-4-8-14(18)22-23-16(13-30-27(23)33)21-15-9-5-7-11-19(15)32(28)25(21)24(22)31/h4-11,17,20,26,29H,12-13H2,1-3H3,(H,30,33)/t17-,20-,26-,28+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | <1 | n/a | n/a | n/a | n/a | n/a | n/a |
Hanyang University
Curated by ChEMBL
| Assay Description
Inhibition of Lyn (unknown origin) after 40 mins by scintillation counting analysis |
Eur J Med Chem 102: 600-10 (2015)
Article DOI: 10.1016/j.ejmech.2015.08.031
BindingDB Entry DOI: 10.7270/Q2XG9SXV |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Lyn
(Homo sapiens (Human)) | BDBM2579

((2S,3R,4R,6R)-3-methoxy-2-methyl-4-(methylamino)-2...)Show SMILES CN[C@@H]1C[C@H]2O[C@@](C)([C@@H]1OC)n1c3ccccc3c3c4CNC(=O)c4c4c5ccccc5n2c4c13 |r| Show InChI InChI=1S/C28H26N4O3/c1-28-26(34-3)17(29-2)12-20(35-28)31-18-10-6-4-8-14(18)22-23-16(13-30-27(23)33)21-15-9-5-7-11-19(15)32(28)25(21)24(22)31/h4-11,17,20,26,29H,12-13H2,1-3H3,(H,30,33)/t17-,20-,26-,28+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | <1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113511
BindingDB Entry DOI: 10.7270/Q2MS3XQV |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Lyn
(Homo sapiens (Human)) | BDBM97672
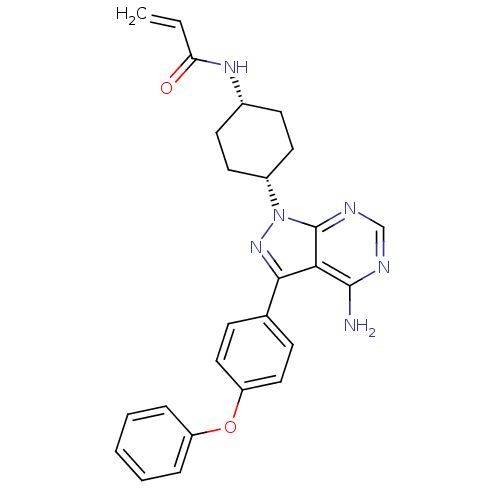
(US8476284, 40 | US9133201, 10 | US9181263, 9 | US9...)Show SMILES Nc1ncnc2n(nc(-c3ccc(Oc4ccccc4)cc3)c12)[C@@H]1CC[C@@H](CC1)NC(=O)C=C |r,wU:23.26,26.33,(-3.76,2.88,;-3.76,1.34,;-5.09,.57,;-5.09,-.97,;-3.76,-1.74,;-2.43,-.97,;-.96,-1.45,;-.06,-.2,;-.96,1.05,;-.56,2.53,;.92,2.93,;1.32,4.42,;.23,5.51,;.63,7,;2.12,7.4,;3.21,6.31,;4.7,6.7,;5.09,8.19,;4.01,9.28,;2.52,8.88,;-1.25,5.11,;-1.65,3.62,;-2.43,.57,;-.56,-2.93,;-1.65,-4.02,;-1.25,-5.51,;.23,-5.91,;1.32,-4.82,;.92,-3.33,;.63,-7.4,;2.12,-7.79,;2.52,-9.28,;3.21,-6.7,;4.7,-7.1,)| Show InChI InChI=1S/C26H26N6O2/c1-2-22(33)30-18-10-12-19(13-11-18)32-26-23(25(27)28-16-29-26)24(31-32)17-8-14-21(15-9-17)34-20-6-4-3-5-7-20/h2-9,14-16,18-19H,1,10-13H2,(H,30,33)(H2,27,28,29)/t18-,19+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacyclics LLC
US Patent
| Assay Description
IC50s were determined using the in vitro HotSpot kinase assay (purified enzymes, 33P-ATP, an appropriate substrate and 1 uM ATP.). Reaction condition... |
US Patent US9278100 (2016)
BindingDB Entry DOI: 10.7270/Q20C4TMX |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Lyn
(Homo sapiens (Human)) | BDBM97672

(US8476284, 40 | US9133201, 10 | US9181263, 9 | US9...)Show SMILES Nc1ncnc2n(nc(-c3ccc(Oc4ccccc4)cc3)c12)[C@@H]1CC[C@@H](CC1)NC(=O)C=C |r,wU:23.26,26.33,(-3.76,2.88,;-3.76,1.34,;-5.09,.57,;-5.09,-.97,;-3.76,-1.74,;-2.43,-.97,;-.96,-1.45,;-.06,-.2,;-.96,1.05,;-.56,2.53,;.92,2.93,;1.32,4.42,;.23,5.51,;.63,7,;2.12,7.4,;3.21,6.31,;4.7,6.7,;5.09,8.19,;4.01,9.28,;2.52,8.88,;-1.25,5.11,;-1.65,3.62,;-2.43,.57,;-.56,-2.93,;-1.65,-4.02,;-1.25,-5.51,;.23,-5.91,;1.32,-4.82,;.92,-3.33,;.63,-7.4,;2.12,-7.79,;2.52,-9.28,;3.21,-6.7,;4.7,-7.1,)| Show InChI InChI=1S/C26H26N6O2/c1-2-22(33)30-18-10-12-19(13-11-18)32-26-23(25(27)28-16-29-26)24(31-32)17-8-14-21(15-9-17)34-20-6-4-3-5-7-20/h2-9,14-16,18-19H,1,10-13H2,(H,30,33)(H2,27,28,29)/t18-,19+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
PHARMACYCLICS LLC
US Patent
| Assay Description
IC50s were determined using the in vitro HotSpot kinase assay (purified enzymes, 33P-ATP, an appropriate substrate and 1 μM ATP.). For enzyme in... |
US Patent US9181263 (2015)
BindingDB Entry DOI: 10.7270/Q2765D5Z |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Lyn
(Homo sapiens (Human)) | BDBM13216

(BMS-354825 | CHEMBL1421 | DASATINIB | N-(2-Chloro-...)Show SMILES Cc1nc(Nc2ncc(s2)C(=O)Nc2c(C)cccc2Cl)cc(n1)N1CCN(CCO)CC1 Show InChI InChI=1S/C22H26ClN7O2S/c1-14-4-3-5-16(23)20(14)28-21(32)17-13-24-22(33-17)27-18-12-19(26-15(2)25-18)30-8-6-29(7-9-30)10-11-31/h3-5,12-13,31H,6-11H2,1-2H3,(H,28,32)(H,24,25,26,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Silesia in Katowice
Curated by ChEMBL
| Assay Description
Inhibition of Lyn B (unknown origin) preincubated for 10 mins followed by substrate addition and measured after 1 hr by ADP-Glo luminescence assay |
Eur J Med Chem 163: 610-625 (2019)
Article DOI: 10.1016/j.ejmech.2018.12.012
BindingDB Entry DOI: 10.7270/Q29P353V |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Tyrosine-protein kinase Lyn
(Homo sapiens (Human)) | BDBM13216

(BMS-354825 | CHEMBL1421 | DASATINIB | N-(2-Chloro-...)Show SMILES Cc1nc(Nc2ncc(s2)C(=O)Nc2c(C)cccc2Cl)cc(n1)N1CCN(CCO)CC1 Show InChI InChI=1S/C22H26ClN7O2S/c1-14-4-3-5-16(23)20(14)28-21(32)17-13-24-22(33-17)27-18-12-19(26-15(2)25-18)30-8-6-29(7-9-30)10-11-31/h3-5,12-13,31H,6-11H2,1-2H3,(H,28,32)(H,24,25,26,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Silesia in Katowice
Curated by ChEMBL
| Assay Description
Inhibition of Lyn B (unknown origin) preincubated for 10 mins followed by substrate addition and measured after 1 hr by ADP-Glo luminescence assay |
Eur J Med Chem 163: 610-625 (2019)
Article DOI: 10.1016/j.ejmech.2018.12.012
BindingDB Entry DOI: 10.7270/Q29P353V |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Tyrosine-protein kinase Lyn
(Homo sapiens (Human)) | BDBM2579

((2S,3R,4R,6R)-3-methoxy-2-methyl-4-(methylamino)-2...)Show SMILES CN[C@@H]1C[C@H]2O[C@@](C)([C@@H]1OC)n1c3ccccc3c3c4CNC(=O)c4c4c5ccccc5n2c4c13 |r| Show InChI InChI=1S/C28H26N4O3/c1-28-26(34-3)17(29-2)12-20(35-28)31-18-10-6-4-8-14(18)22-23-16(13-30-27(23)33)21-15-9-5-7-11-19(15)32(28)25(21)24(22)31/h4-11,17,20,26,29H,12-13H2,1-3H3,(H,30,33)/t17-,20-,26-,28+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Hanyang University
Curated by ChEMBL
| Assay Description
Inhibition of LYN |
Bioorg Med Chem 19: 6760-7 (2011)
Article DOI: 10.1016/j.bmc.2011.09.042
BindingDB Entry DOI: 10.7270/Q2G44QPH |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Lyn
(Homo sapiens (Human)) | BDBM50059889
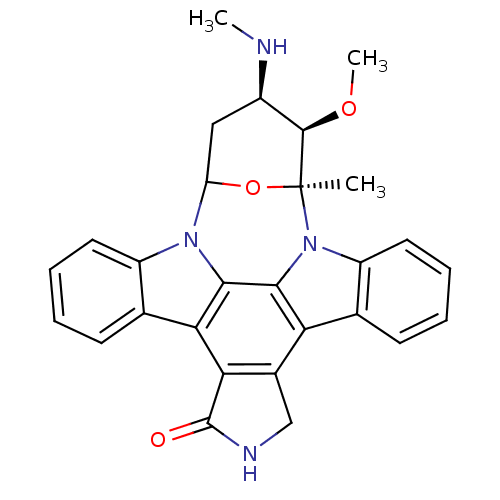
((staurosporine)3-methoxy-2-methyl-4-methylamino-(2...)Show SMILES CN[C@@H]1CC2O[C@@](C)([C@@H]1OC)n1c3ccccc3c3c4CNC(=O)c4c4c5ccccc5n2c4c13 Show InChI InChI=1S/C28H26N4O3/c1-28-26(34-3)17(29-2)12-20(35-28)31-18-10-6-4-8-14(18)22-23-16(13-30-27(23)33)21-15-9-5-7-11-19(15)32(28)25(21)24(22)31/h4-11,17,20,26,29H,12-13H2,1-3H3,(H,30,33)/t17-,20?,26-,28+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Osaka Prefecture University
Curated by ChEMBL
| Assay Description
Inhibition of human Lyn |
Bioorg Med Chem Lett 19: 6557-60 (2009)
Article DOI: 10.1016/j.bmcl.2009.10.038
BindingDB Entry DOI: 10.7270/Q2TT4R1D |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Lyn
(Homo sapiens (Human)) | BDBM2579

((2S,3R,4R,6R)-3-methoxy-2-methyl-4-(methylamino)-2...)Show SMILES CN[C@@H]1C[C@H]2O[C@@](C)([C@@H]1OC)n1c3ccccc3c3c4CNC(=O)c4c4c5ccccc5n2c4c13 |r| Show InChI InChI=1S/C28H26N4O3/c1-28-26(34-3)17(29-2)12-20(35-28)31-18-10-6-4-8-14(18)22-23-16(13-30-27(23)33)21-15-9-5-7-11-19(15)32(28)25(21)24(22)31/h4-11,17,20,26,29H,12-13H2,1-3H3,(H,30,33)/t17-,20-,26-,28+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.39 | n/a | n/a | n/a | n/a | n/a | n/a |
The Ohio State University
Curated by ChEMBL
| Assay Description
Inhibition of human LYN using poly[Glu:Tyr] (4:1) as substrate in presence of [gamma-32P]ATP |
Bioorg Med Chem 25: 2156-2166 (2017)
Article DOI: 10.1016/j.bmc.2017.02.030
BindingDB Entry DOI: 10.7270/Q2571FGM |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Lyn
(Homo sapiens (Human)) | BDBM50357321

(CHEMBL1916879)Show SMILES CN(C)C\C=C\C(=O)N(C)c1ccc2nc(Nc3cc(NC(=O)c4cccc(c4)C(F)(F)F)ccc3C)c3cncn3c2c1 Show InChI InChI=1S/C32H30F3N7O2/c1-20-10-11-23(37-31(44)21-7-5-8-22(15-21)32(33,34)35)16-26(20)39-30-28-18-36-19-42(28)27-17-24(12-13-25(27)38-30)41(4)29(43)9-6-14-40(2)3/h5-13,15-19H,14H2,1-4H3,(H,37,44)(H,38,39)/b9-6+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.55 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of LYN-A expressed in Sf9 cells after 60 mins by TR-FRET Assay |
Bioorg Med Chem Lett 21: 6258-63 (2011)
Article DOI: 10.1016/j.bmcl.2011.09.008
BindingDB Entry DOI: 10.7270/Q21836XD |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Lyn
(Homo sapiens (Human)) | BDBM50322535

(3-(imidazo[1,2-b]pyridazin-3-ylethynyl)-4-methyl-N...)Show SMILES CN1CCN(Cc2ccc(NC(=O)c3ccc(C)c(c3)C#Cc3cnc4cccnn34)cc2C(F)(F)F)CC1 Show InChI InChI=1S/C29H27F3N6O/c1-20-5-6-22(16-21(20)8-10-25-18-33-27-4-3-11-34-38(25)27)28(39)35-24-9-7-23(26(17-24)29(30,31)32)19-37-14-12-36(2)13-15-37/h3-7,9,11,16-18H,12-15,19H2,1-2H3,(H,35,39) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Tsinghua University
Curated by ChEMBL
| Assay Description
Inhibition of human LYN using poly(Glu,Tyr)4:1 substrate and [gamma-33P]-ATP incubated for 40 mins by scintillation counting method |
J Med Chem 63: 8567-8583 (2020)
Article DOI: 10.1021/acs.jmedchem.0c00967
BindingDB Entry DOI: 10.7270/Q2H41W0S |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Lyn
(Homo sapiens (Human)) | BDBM50126732
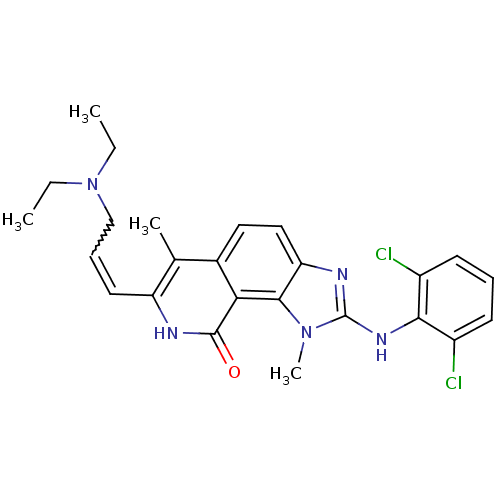
(2-(2,6-Dichloro-phenylamino)-7-(3-diethylamino-pro...)Show SMILES CCN(CC)CC=Cc1[nH]c(=O)c2c3n(C)c(Nc4c(Cl)cccc4Cl)nc3ccc2c1C |w:6.5| Show InChI InChI=1S/C25H27Cl2N5O/c1-5-32(6-2)14-8-11-19-15(3)16-12-13-20-23(21(16)24(33)28-19)31(4)25(29-20)30-22-17(26)9-7-10-18(22)27/h7-13H,5-6,14H2,1-4H3,(H,28,33)(H,29,30) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of Protein tyrosine kinase Lyn |
J Med Chem 46: 1337-49 (2003)
Article DOI: 10.1021/jm020446l
BindingDB Entry DOI: 10.7270/Q2DN44FW |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Lyn
(Homo sapiens (Human)) | BDBM50529757

(CHEMBL4436086)Show SMILES Oc1cccc(Nc2nc(\C=C\c3cc4OCOc4cc3Br)nc3ccccc23)c1 Show InChI InChI=1S/C23H16BrN3O3/c24-18-12-21-20(29-13-30-21)10-14(18)8-9-22-26-19-7-2-1-6-17(19)23(27-22)25-15-4-3-5-16(28)11-15/h1-12,28H,13H2,(H,25,26,27)/b9-8+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Silesia in Katowice
Curated by ChEMBL
| Assay Description
Inhibition of Lyn B (unknown origin) preincubated for 10 mins followed by substrate addition and measured after 1 hr by ADP-Glo luminescence assay |
Eur J Med Chem 163: 610-625 (2019)
Article DOI: 10.1016/j.ejmech.2018.12.012
BindingDB Entry DOI: 10.7270/Q29P353V |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Lyn
(Homo sapiens (Human)) | BDBM50529757

(CHEMBL4436086)Show SMILES Oc1cccc(Nc2nc(\C=C\c3cc4OCOc4cc3Br)nc3ccccc23)c1 Show InChI InChI=1S/C23H16BrN3O3/c24-18-12-21-20(29-13-30-21)10-14(18)8-9-22-26-19-7-2-1-6-17(19)23(27-22)25-15-4-3-5-16(28)11-15/h1-12,28H,13H2,(H,25,26,27)/b9-8+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Silesia in Katowice
Curated by ChEMBL
| Assay Description
Inhibition of Lyn B (unknown origin) preincubated for 10 mins followed by substrate addition and measured after 1 hr by ADP-Glo luminescence assay |
Eur J Med Chem 163: 610-625 (2019)
Article DOI: 10.1016/j.ejmech.2018.12.012
BindingDB Entry DOI: 10.7270/Q29P353V |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Lyn
(Homo sapiens (Human)) | BDBM2579

((2S,3R,4R,6R)-3-methoxy-2-methyl-4-(methylamino)-2...)Show SMILES CN[C@@H]1C[C@H]2O[C@@](C)([C@@H]1OC)n1c3ccccc3c3c4CNC(=O)c4c4c5ccccc5n2c4c13 |r| Show InChI InChI=1S/C28H26N4O3/c1-28-26(34-3)17(29-2)12-20(35-28)31-18-10-6-4-8-14(18)22-23-16(13-30-27(23)33)21-15-9-5-7-11-19(15)32(28)25(21)24(22)31/h4-11,17,20,26,29H,12-13H2,1-3H3,(H,30,33)/t17-,20-,26-,28+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Southeast University
Curated by ChEMBL
| Assay Description
Inhibition of LYNA (unknown origin) incubated for 1 hr by spectrophotometric analysis |
Bioorg Med Chem 24: 3483-93 (2016)
Article DOI: 10.1016/j.bmc.2016.05.057
BindingDB Entry DOI: 10.7270/Q29G5PQT |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Lyn
(Homo sapiens (Human)) | BDBM50430875

(CHEMBL2336005 | US9062066, LCB 03-0110)Show SMILES Oc1cccc(Nc2ccnc3cc(sc23)-c2cccc(CN3CCOCC3)c2)c1 Show InChI InChI=1S/C24H23N3O2S/c28-20-6-2-5-19(14-20)26-21-7-8-25-22-15-23(30-24(21)22)18-4-1-3-17(13-18)16-27-9-11-29-12-10-27/h1-8,13-15,28H,9-12,16H2,(H,25,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 2.30 | n/a | n/a | n/a | n/a | 8.0 | n/a |
KOREA INSTITUTE OF SCIENCE AND TECHNOLOGY
US Patent
| Assay Description
The inhibitory activity measurement against the kinases mentioned above was measured in the kinase inhibition reaction mixture containing 2 ul purifi... |
US Patent US9062066 (2015)
BindingDB Entry DOI: 10.7270/Q21G0K17 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Lyn
(Homo sapiens (Human)) | BDBM2579

((2S,3R,4R,6R)-3-methoxy-2-methyl-4-(methylamino)-2...)Show SMILES CN[C@@H]1C[C@H]2O[C@@](C)([C@@H]1OC)n1c3ccccc3c3c4CNC(=O)c4c4c5ccccc5n2c4c13 |r| Show InChI InChI=1S/C28H26N4O3/c1-28-26(34-3)17(29-2)12-20(35-28)31-18-10-6-4-8-14(18)22-23-16(13-30-27(23)33)21-15-9-5-7-11-19(15)32(28)25(21)24(22)31/h4-11,17,20,26,29H,12-13H2,1-3H3,(H,30,33)/t17-,20-,26-,28+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.70 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Florida
Curated by ChEMBL
| Assay Description
Inhibition of human LYN-B using poly[Glu:Tyr] (4:1) as substrate by [gamma-33P]-ATP assay |
Eur J Med Chem 161: 456-467 (2019)
Article DOI: 10.1016/j.ejmech.2018.10.052
BindingDB Entry DOI: 10.7270/Q2W380MT |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Lyn
(Homo sapiens (Human)) | BDBM8793
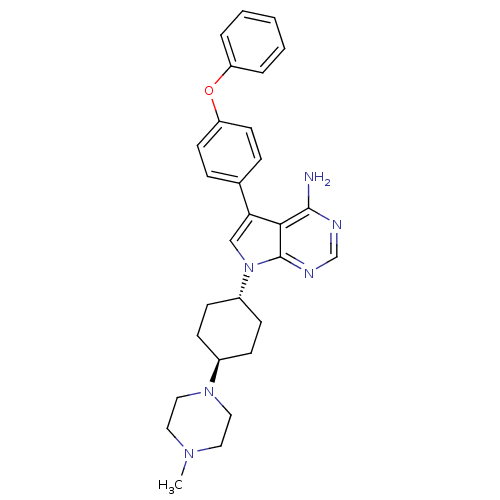
(7-[4-(4-methylpiperazin-1-yl)cyclohexyl]-5-(4-phen...)Show SMILES CN1CCN(CC1)[C@H]1CC[C@@H](CC1)n1cc(-c2ccc(Oc3ccccc3)cc2)c2c(N)ncnc12 |r,wU:10.14,wD:7.7,(-.91,-10.51,;-1.37,-9.04,;-2.87,-8.71,;-3.34,-7.24,;-2.3,-6.11,;-.8,-6.44,;-.33,-7.91,;-2.72,-4.63,;-1.64,-3.52,;-2.06,-2.04,;-3.55,-1.66,;-4.63,-2.76,;-4.21,-4.25,;-4.03,-.2,;-3.12,1.05,;-4.03,2.3,;-3.55,3.76,;-4.69,4.8,;-4.36,6.3,;-2.89,6.77,;-2.56,8.27,;-1.1,8.74,;-.48,10.15,;1.05,10.33,;1.96,9.09,;1.35,7.68,;-.18,7.5,;-1.75,5.73,;-2.08,4.23,;-5.49,1.82,;-6.82,2.59,;-6.82,4.13,;-8.16,1.82,;-8.16,.28,;-6.82,-.49,;-5.49,.28,)| Show InChI InChI=1S/C29H34N6O/c1-33-15-17-34(18-16-33)22-9-11-23(12-10-22)35-19-26(27-28(30)31-20-32-29(27)35)21-7-13-25(14-8-21)36-24-5-3-2-4-6-24/h2-8,13-14,19-20,22-23H,9-12,15-18H2,1H3,(H2,30,31,32)/t22-,23- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| | n/a | n/a | <3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Lyn
(Homo sapiens (Human)) | BDBM50178611
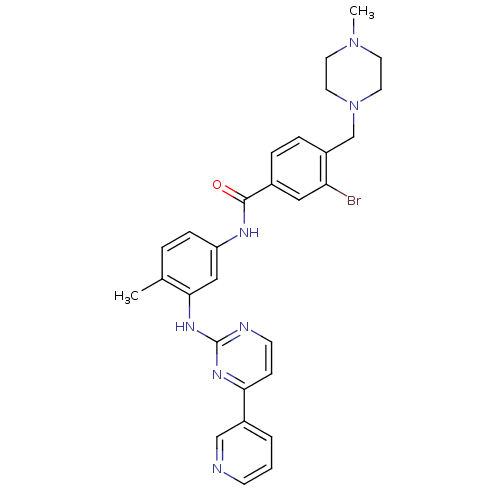
(3-bromo-N-(4-methyl-3-(4-(pyridin-3-yl)pyrimidin-2...)Show SMILES CN1CCN(Cc2ccc(cc2Br)C(=O)Nc2ccc(C)c(Nc3nccc(n3)-c3cccnc3)c2)CC1 Show InChI InChI=1S/C29H30BrN7O/c1-20-5-8-24(17-27(20)35-29-32-11-9-26(34-29)22-4-3-10-31-18-22)33-28(38)21-6-7-23(25(30)16-21)19-37-14-12-36(2)13-15-37/h3-11,16-18H,12-15,19H2,1-2H3,(H,33,38)(H,32,34,35) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Nippon Shinyaku Co. Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of human Lyn kinase expressed in Sf9 cells |
Bioorg Med Chem Lett 17: 2712-7 (2007)
Article DOI: 10.1016/j.bmcl.2007.03.002
BindingDB Entry DOI: 10.7270/Q20C4WK9 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Lyn
(Homo sapiens (Human)) | BDBM50208747

((R)-4-((3-(dimethylamino)pyrrolidin-1-yl)methyl)-N...)Show SMILES CN(C)[C@@H]1CCN(Cc2ccc(cc2C(F)(F)F)C(=O)Nc2ccc(C)c(Nc3nccc(n3)-c3cncnc3)c2)C1 Show InChI InChI=1S/C30H31F3N8O/c1-19-4-7-23(13-27(19)39-29-36-10-8-26(38-29)22-14-34-18-35-15-22)37-28(42)20-5-6-21(25(12-20)30(31,32)33)16-41-11-9-24(17-41)40(2)3/h4-8,10,12-15,18,24H,9,11,16-17H2,1-3H3,(H,37,42)(H,36,38,39)/t24-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Nippon Shinyaku Co. Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of human Lyn kinase expressed in Sf9 cells |
Bioorg Med Chem Lett 17: 2712-7 (2007)
Article DOI: 10.1016/j.bmcl.2007.03.002
BindingDB Entry DOI: 10.7270/Q20C4WK9 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Lyn
(Homo sapiens (Human)) | BDBM378885

(US10266537, Compound 93)Show SMILES Cc1ccc(NC(=O)c2ccc(C)c(c2)C(F)(F)F)cc1C#Cc1nn([C@H]2CC[C@H](O)CC2)c2ncnc(N)c12 |r,wU:26.27,wD:29.31,(-5.46,5.4,;-3.98,5.8,;-3.58,7.29,;-2.09,7.69,;-1,6.6,;.49,7,;1.57,5.91,;1.18,4.42,;3.06,6.31,;3.46,7.79,;4.95,8.19,;6.04,7.1,;7.53,7.5,;5.64,5.62,;4.15,5.22,;6.73,4.53,;8.22,4.93,;6.33,3.04,;7.82,3.44,;-1.4,5.11,;-2.89,4.71,;-3.29,3.22,;-3.68,1.74,;-4.08,.25,;-3.18,-1,;-4.08,-2.24,;-3.68,-3.73,;-2.2,-4.13,;-1.8,-5.62,;-2.89,-6.7,;-2.49,-8.19,;-4.38,-6.31,;-4.77,-4.82,;-5.55,-1.77,;-6.88,-2.54,;-8.22,-1.77,;-8.22,-.23,;-6.88,.54,;-6.88,2.08,;-5.55,-.23,)| Show InChI InChI=1S/C29H27F3N6O2/c1-16-4-7-20(36-28(40)19-5-3-17(2)23(14-19)29(30,31)32)13-18(16)6-12-24-25-26(33)34-15-35-27(25)38(37-24)21-8-10-22(39)11-9-21/h3-5,7,13-15,21-22,39H,8-11H2,1-2H3,(H,36,40)(H2,33,34,35)/t21-,22- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human full length recombinant LYN using poly(Glu,Tyr)4:1 as substrate incubated for 40 mins in presence of [gamma33P-ATP] by radiometri... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2M90DC6 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Lyn
(Homo sapiens (Human)) | BDBM378885

(US10266537, Compound 93)Show SMILES Cc1ccc(NC(=O)c2ccc(C)c(c2)C(F)(F)F)cc1C#Cc1nn([C@H]2CC[C@H](O)CC2)c2ncnc(N)c12 |r,wU:26.27,wD:29.31,(-5.46,5.4,;-3.98,5.8,;-3.58,7.29,;-2.09,7.69,;-1,6.6,;.49,7,;1.57,5.91,;1.18,4.42,;3.06,6.31,;3.46,7.79,;4.95,8.19,;6.04,7.1,;7.53,7.5,;5.64,5.62,;4.15,5.22,;6.73,4.53,;8.22,4.93,;6.33,3.04,;7.82,3.44,;-1.4,5.11,;-2.89,4.71,;-3.29,3.22,;-3.68,1.74,;-4.08,.25,;-3.18,-1,;-4.08,-2.24,;-3.68,-3.73,;-2.2,-4.13,;-1.8,-5.62,;-2.89,-6.7,;-2.49,-8.19,;-4.38,-6.31,;-4.77,-4.82,;-5.55,-1.77,;-6.88,-2.54,;-8.22,-1.77,;-8.22,-.23,;-6.88,.54,;-6.88,2.08,;-5.55,-.23,)| Show InChI InChI=1S/C29H27F3N6O2/c1-16-4-7-20(36-28(40)19-5-3-17(2)23(14-19)29(30,31)32)13-18(16)6-12-24-25-26(33)34-15-35-27(25)38(37-24)21-8-10-22(39)11-9-21/h3-5,7,13-15,21-22,39H,8-11H2,1-2H3,(H,36,40)(H2,33,34,35)/t21-,22- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Kalypsys, Inc.
| Assay Description
The aim of this experiment is to detect the inhibitory activity of the compounds of the present invention against in vitro protein kinases using isot... |
Bioorg Med Chem Lett 17: 3562-9 (2007)
BindingDB Entry DOI: 10.7270/Q21C2065 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Lyn
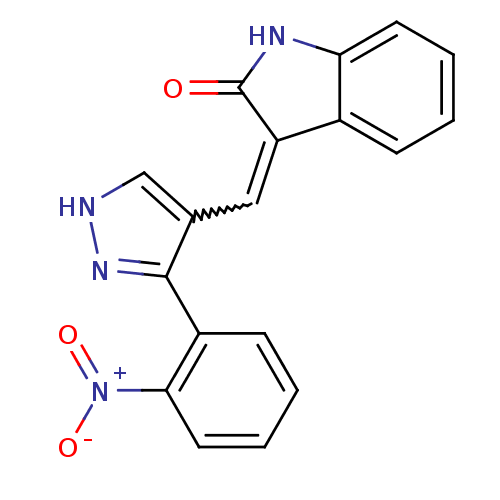
(Homo sapiens (Human)) | BDBM50431812
(CHEMBL2347053)Show SMILES [O-][N+](=O)c1ccccc1-c1n[nH]cc1C=C1C(=O)Nc2ccccc12 |w:14.15| Show InChI InChI=1S/C18H12N4O3/c23-18-14(12-5-1-3-7-15(12)20-18)9-11-10-19-21-17(11)13-6-2-4-8-16(13)22(24)25/h1-10H,(H,19,21)(H,20,23) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
East China University of Science and Technology
Curated by ChEMBL
| Assay Description
Inhibition of LYNA (unknown origin) after 10 mins by mobility shift assay |
Bioorg Med Chem 21: 1724-34 (2013)
Article DOI: 10.1016/j.bmc.2013.01.047
BindingDB Entry DOI: 10.7270/Q2GQ704N |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Lyn
(Homo sapiens (Human)) | BDBM50430875

(CHEMBL2336005 | US9062066, LCB 03-0110)Show SMILES Oc1cccc(Nc2ccnc3cc(sc23)-c2cccc(CN3CCOCC3)c2)c1 Show InChI InChI=1S/C24H23N3O2S/c28-20-6-2-5-19(14-20)26-21-7-8-25-22-15-23(30-24(21)22)18-4-1-3-17(13-18)16-27-9-11-29-12-10-27/h1-8,13-15,28H,9-12,16H2,(H,25,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 4.30 | n/a | n/a | n/a | n/a | 8.0 | n/a |
KOREA INSTITUTE OF SCIENCE AND TECHNOLOGY
US Patent
| Assay Description
The inhibitory activity measurement against the kinases mentioned above was measured in the kinase inhibition reaction mixture containing 2 ul purifi... |
US Patent US9062066 (2015)
BindingDB Entry DOI: 10.7270/Q21G0K17 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Lyn
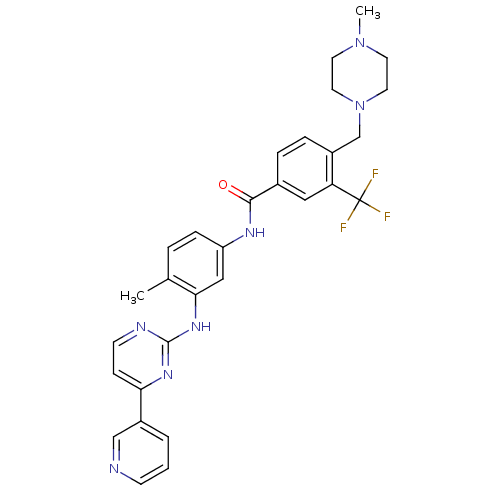
(Homo sapiens (Human)) | BDBM50178615
(CHEMBL203343 | N-(4-methyl-3-(4-(pyridin-3-yl)pyri...)Show SMILES CN1CCN(Cc2ccc(cc2C(F)(F)F)C(=O)Nc2ccc(C)c(Nc3nccc(n3)-c3cccnc3)c2)CC1 Show InChI InChI=1S/C30H30F3N7O/c1-20-5-8-24(17-27(20)38-29-35-11-9-26(37-29)22-4-3-10-34-18-22)36-28(41)21-6-7-23(25(16-21)30(31,32)33)19-40-14-12-39(2)13-15-40/h3-11,16-18H,12-15,19H2,1-2H3,(H,36,41)(H,35,37,38) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 4.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Nippon Shinyaku Co. Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of human Lyn kinase expressed in Sf9 cells |
Bioorg Med Chem Lett 17: 2712-7 (2007)
Article DOI: 10.1016/j.bmcl.2007.03.002
BindingDB Entry DOI: 10.7270/Q20C4WK9 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Lyn
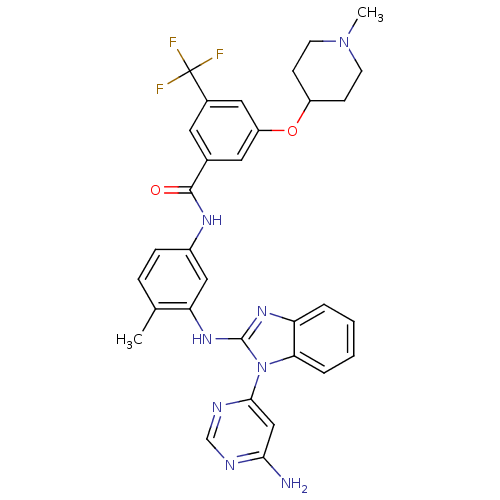
(Homo sapiens (Human)) | BDBM50311920
(CHEMBL1076432 | N-(3-(1-(6-aminopyrimidin-4-yl)-1H...)Show SMILES CN1CCC(CC1)Oc1cc(cc(c1)C(F)(F)F)C(=O)Nc1ccc(C)c(Nc2nc3ccccc3n2-c2cc(N)ncn2)c1 Show InChI InChI=1S/C32H31F3N8O2/c1-19-7-8-22(16-26(19)41-31-40-25-5-3-4-6-27(25)43(31)29-17-28(36)37-18-38-29)39-30(44)20-13-21(32(33,34)35)15-24(14-20)45-23-9-11-42(2)12-10-23/h3-8,13-18,23H,9-12H2,1-2H3,(H,39,44)(H,40,41)(H2,36,37,38) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Genomics Institute of the Novartis Research Foundation (GNF)
Curated by ChEMBL
| Assay Description
Inhibition of Tel-fused LYN expressed in mouse BAF3 cells |
Bioorg Med Chem Lett 19: 6691-5 (2009)
Article DOI: 10.1016/j.bmcl.2009.09.123
BindingDB Entry DOI: 10.7270/Q2Q52PRR |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Lyn
(Homo sapiens (Human)) | BDBM50311921

(CHEMBL1086731 | N-(3-(1-(6-aminopyrimidin-4-yl)-1H...)Show SMILES Cc1ccc(NC(=O)c2ccc(s2)C(C)(C)C)cc1Nc1nc2ccccc2n1-c1cc(N)ncn1 Show InChI InChI=1S/C27H27N7OS/c1-16-9-10-17(31-25(35)21-11-12-22(36-21)27(2,3)4)13-19(16)33-26-32-18-7-5-6-8-20(18)34(26)24-14-23(28)29-15-30-24/h5-15H,1-4H3,(H,31,35)(H,32,33)(H2,28,29,30) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Genomics Institute of the Novartis Research Foundation (GNF)
Curated by ChEMBL
| Assay Description
Inhibition of Tel-fused LYN expressed in mouse BAF3 cells |
Bioorg Med Chem Lett 19: 6691-5 (2009)
Article DOI: 10.1016/j.bmcl.2009.09.123
BindingDB Entry DOI: 10.7270/Q2Q52PRR |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Lyn
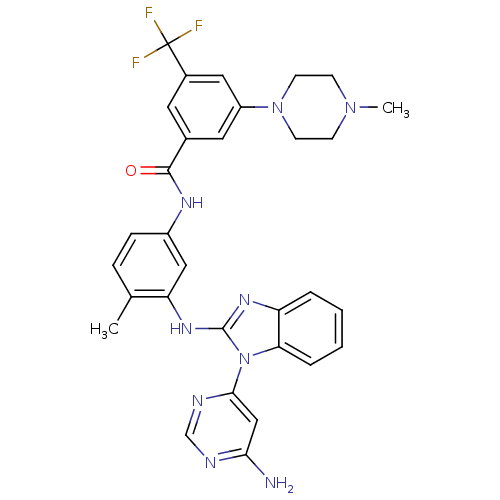
(Homo sapiens (Human)) | BDBM50311926
(CHEMBL1076436 | N-(3-(1-(6-aminopyrimidin-4-yl)-1H...)Show SMILES CN1CCN(CC1)c1cc(cc(c1)C(F)(F)F)C(=O)Nc1ccc(C)c(Nc2nc3ccccc3n2-c2cc(N)ncn2)c1 Show InChI InChI=1S/C31H30F3N9O/c1-19-7-8-22(38-29(44)20-13-21(31(32,33)34)15-23(14-20)42-11-9-41(2)10-12-42)16-25(19)40-30-39-24-5-3-4-6-26(24)43(30)28-17-27(35)36-18-37-28/h3-8,13-18H,9-12H2,1-2H3,(H,38,44)(H,39,40)(H2,35,36,37) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Genomics Institute of the Novartis Research Foundation (GNF)
Curated by ChEMBL
| Assay Description
Inhibition of Tel-fused LYN expressed in mouse BAF3 cells |
Bioorg Med Chem Lett 19: 6691-5 (2009)
Article DOI: 10.1016/j.bmcl.2009.09.123
BindingDB Entry DOI: 10.7270/Q2Q52PRR |
More data for this
Ligand-Target Pair | |
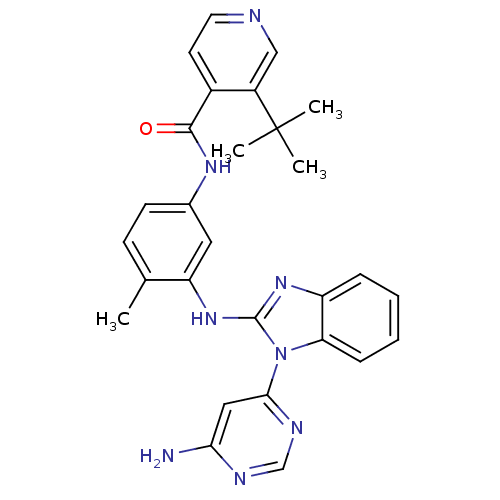
Tyrosine-protein kinase Lyn
(Homo sapiens (Human)) | BDBM50311940
(CHEMBL1080475 | N-(3-(1-(6-aminopyrimidin-4-yl)-1H...)Show SMILES Cc1ccc(NC(=O)c2ccncc2C(C)(C)C)cc1Nc1nc2ccccc2n1-c1cc(N)ncn1 Show InChI InChI=1S/C28H28N8O/c1-17-9-10-18(33-26(37)19-11-12-30-15-20(19)28(2,3)4)13-22(17)35-27-34-21-7-5-6-8-23(21)36(27)25-14-24(29)31-16-32-25/h5-16H,1-4H3,(H,33,37)(H,34,35)(H2,29,31,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Genomics Institute of the Novartis Research Foundation (GNF)
Curated by ChEMBL
| Assay Description
Inhibition of Tel-fused LYN expressed in mouse BAF3 cells |
Bioorg Med Chem Lett 19: 6691-5 (2009)
Article DOI: 10.1016/j.bmcl.2009.09.123
BindingDB Entry DOI: 10.7270/Q2Q52PRR |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Lyn
(Homo sapiens (Human)) | BDBM50506460

(CHEMBL4467948)Show SMILES Cc1ccc(cc1C#Cc1cc(cnc1N)-c1cnn(C)c1)C(=O)Nc1ccc(Cl)cc1Cl Show InChI InChI=1S/C25H19Cl2N5O/c1-15-3-4-18(25(33)31-23-8-7-21(26)11-22(23)27)9-16(15)5-6-17-10-19(12-29-24(17)28)20-13-30-32(2)14-20/h3-4,7-14H,1-2H3,(H2,28,29)(H,31,33) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human full length recombinant LYN by radiometric scintillation counting analysis |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00976
BindingDB Entry DOI: 10.7270/Q2J96B6S |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Lyn
(Homo sapiens (Human)) | BDBM50208748

((4-(((S)-3-((dimethylamino)methyl)pyrrolidin-1-yl)...)Show SMILES CN(C)C[C@@H]1CCN(Cc2ccc(cc2C(F)(F)F)C(O)Nc2ccc(C)c(Nc3nccc(n3)-c3cncnc3)c2)C1 |w:19.20| Show InChI InChI=1S/C31H35F3N8O/c1-20-4-7-25(13-28(20)40-30-37-10-8-27(39-30)24-14-35-19-36-15-24)38-29(43)22-5-6-23(26(12-22)31(32,33)34)18-42-11-9-21(17-42)16-41(2)3/h4-8,10,12-15,19,21,29,38,43H,9,11,16-18H2,1-3H3,(H,37,39,40)/t21-,29?/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Nippon Shinyaku Co. Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of human Lyn kinase expressed in Sf9 cells |
Bioorg Med Chem Lett 17: 2712-7 (2007)
Article DOI: 10.1016/j.bmcl.2007.03.002
BindingDB Entry DOI: 10.7270/Q20C4WK9 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Lyn
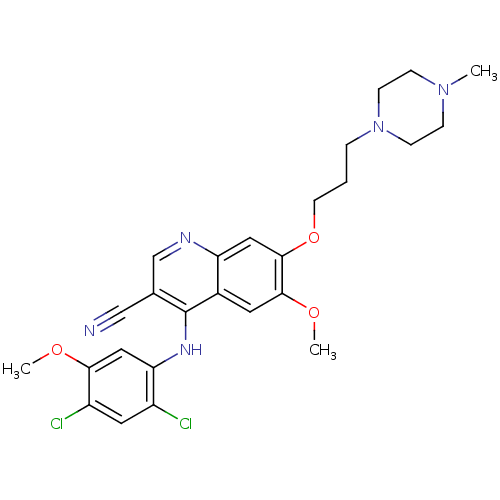
(Homo sapiens (Human)) | BDBM4552
(4-[(2,4-Dichloro-5-methoxyphenyl)amino]-6-methoxy-...)Show SMILES COc1cc(Nc2c(cnc3cc(OCCCN4CCN(C)CC4)c(OC)cc23)C#N)c(Cl)cc1Cl Show InChI InChI=1S/C26H29Cl2N5O3/c1-32-6-8-33(9-7-32)5-4-10-36-25-13-21-18(11-24(25)35-3)26(17(15-29)16-30-21)31-22-14-23(34-2)20(28)12-19(22)27/h11-14,16H,4-10H2,1-3H3,(H,30,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Center for Molecular Medicine of the Austrian Academy of Sciences
Curated by ChEMBL
| Assay Description
Inhibition of LYNB |
Leukemia 23: 477-85 (2009)
Article DOI: 10.1038/leu.2008.334
BindingDB Entry DOI: 10.7270/Q22Z15R6 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Lyn
(Homo sapiens (Human)) | BDBM4552

(4-[(2,4-Dichloro-5-methoxyphenyl)amino]-6-methoxy-...)Show SMILES COc1cc(Nc2c(cnc3cc(OCCCN4CCN(C)CC4)c(OC)cc23)C#N)c(Cl)cc1Cl Show InChI InChI=1S/C26H29Cl2N5O3/c1-32-6-8-33(9-7-32)5-4-10-36-25-13-21-18(11-24(25)35-3)26(17(15-29)16-30-21)31-22-14-23(34-2)20(28)12-19(22)27/h11-14,16H,4-10H2,1-3H3,(H,30,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Center for Molecular Medicine of the Austrian Academy of Sciences
Curated by ChEMBL
| Assay Description
Inhibition of LYNA |
Leukemia 23: 477-85 (2009)
Article DOI: 10.1038/leu.2008.334
BindingDB Entry DOI: 10.7270/Q22Z15R6 |
More data for this
Ligand-Target Pair | |
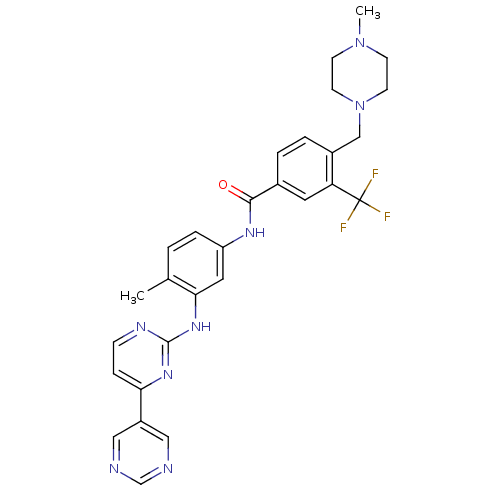
Tyrosine-protein kinase Lyn
(Homo sapiens (Human)) | BDBM50178614
(CHEMBL205723 | N-(4-methyl-3-(4-(pyrimidin-5-yl)py...)Show SMILES CN1CCN(Cc2ccc(cc2C(F)(F)F)C(=O)Nc2ccc(C)c(Nc3nccc(n3)-c3cncnc3)c2)CC1 Show InChI InChI=1S/C29H29F3N8O/c1-19-3-6-23(14-26(19)38-28-35-8-7-25(37-28)22-15-33-18-34-16-22)36-27(41)20-4-5-21(24(13-20)29(30,31)32)17-40-11-9-39(2)10-12-40/h3-8,13-16,18H,9-12,17H2,1-2H3,(H,36,41)(H,35,37,38) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 8.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Nippon Shinyaku Co. Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of human Lyn kinase expressed in Sf9 cells |
Bioorg Med Chem Lett 17: 2712-7 (2007)
Article DOI: 10.1016/j.bmcl.2007.03.002
BindingDB Entry DOI: 10.7270/Q20C4WK9 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Lyn
(Homo sapiens (Human)) | BDBM50611364

(CHEMBL5280361) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| | n/a | n/a | 8.90 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Lyn
(Homo sapiens (Human)) | BDBM50311927
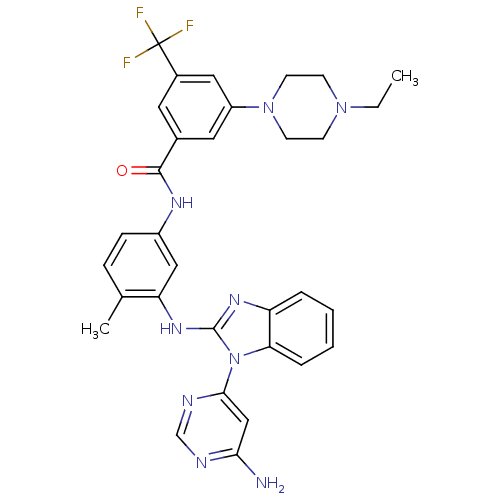
(CHEMBL1076437 | N-(3-(1-(6-aminopyrimidin-4-yl)-1H...)Show SMILES CCN1CCN(CC1)c1cc(cc(c1)C(F)(F)F)C(=O)Nc1ccc(C)c(Nc2nc3ccccc3n2-c2cc(N)ncn2)c1 Show InChI InChI=1S/C32H32F3N9O/c1-3-42-10-12-43(13-11-42)24-15-21(14-22(16-24)32(33,34)35)30(45)39-23-9-8-20(2)26(17-23)41-31-40-25-6-4-5-7-27(25)44(31)29-18-28(36)37-19-38-29/h4-9,14-19H,3,10-13H2,1-2H3,(H,39,45)(H,40,41)(H2,36,37,38) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Genomics Institute of the Novartis Research Foundation (GNF)
Curated by ChEMBL
| Assay Description
Inhibition of Tel-fused LYN expressed in mouse BAF3 cells |
Bioorg Med Chem Lett 19: 6691-5 (2009)
Article DOI: 10.1016/j.bmcl.2009.09.123
BindingDB Entry DOI: 10.7270/Q2Q52PRR |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Lyn
(Homo sapiens (Human)) | BDBM50577406
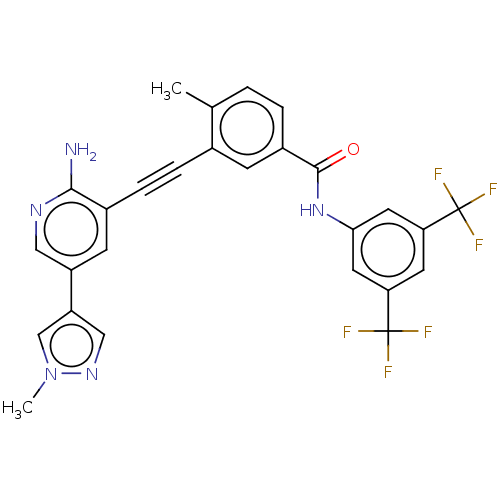
(CHEMBL4862806)Show SMILES Cc1ccc(cc1C#Cc1cc(cnc1N)-c1cnn(C)c1)C(=O)Nc1cc(cc(c1)C(F)(F)F)C(F)(F)F | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human full length recombinant LYN by radiometric scintillation counting analysis |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00976
BindingDB Entry DOI: 10.7270/Q2J96B6S |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Lyn
(Homo sapiens (Human)) | BDBM387846
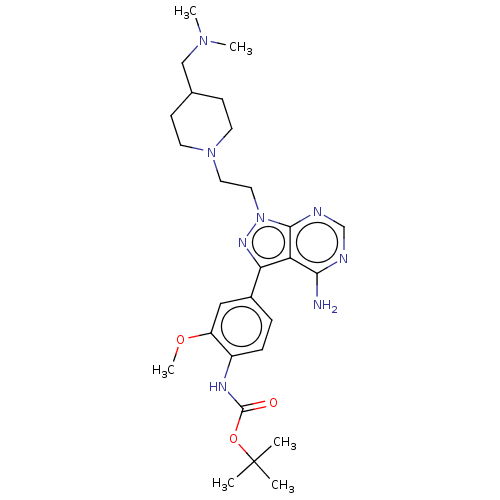
(US10294227, Code 503)Show SMILES COc1cc(ccc1NC(=O)OC(C)(C)C)-c1nn(CCN2CCC(CN(C)C)CC2)c2ncnc(N)c12 Show InChI InChI=1S/C27H40N8O3/c1-27(2,3)38-26(36)31-20-8-7-19(15-21(20)37-6)23-22-24(28)29-17-30-25(22)35(32-23)14-13-34-11-9-18(10-12-34)16-33(4)5/h7-8,15,17-18H,9-14,16H2,1-6H3,(H,31,36)(H2,28,29,30) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Inc.
| Assay Description
Compound IC50 values were determined from 10-point, 1:3 dilution curves starting at either 100 μM or 10 μM with 10 μM ATP, by Reaction... |
Bioorg Med Chem Lett 19: 773-7 (2009)
BindingDB Entry DOI: 10.7270/Q27D2XFP |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Lyn
(Homo sapiens (Human)) | BDBM50184767
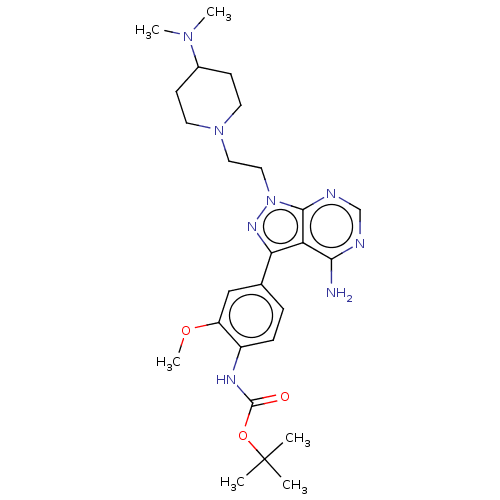
(CHEMBL3824089 | US10294227, Code 506)Show SMILES COc1cc(ccc1NC(=O)OC(C)(C)C)-c1nn(CCN2CCC(CC2)N(C)C)c2ncnc(N)c12 Show InChI InChI=1S/C26H38N8O3/c1-26(2,3)37-25(35)30-19-8-7-17(15-20(19)36-6)22-21-23(27)28-16-29-24(21)34(31-22)14-13-33-11-9-18(10-12-33)32(4)5/h7-8,15-16,18H,9-14H2,1-6H3,(H,30,35)(H2,27,28,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| US Patent
| n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Inc.
| Assay Description
Compound IC50 values were determined from 10-point, 1:3 dilution curves starting at either 100 μM or 10 μM with 10 μM ATP, by Reaction... |
Bioorg Med Chem Lett 19: 773-7 (2009)
BindingDB Entry DOI: 10.7270/Q27D2XFP |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data