

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cystathionine gamma-lyase (Homo sapiens (Human)) | BDBM60996 (5-[(3-carboxy-4-hydroxy-phenyl)-(3-carboxy-4-keto-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 600 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human CSE using L-Cys as the substrate by tandem well based HTS assay | Citation and Details Article DOI: 10.1021/acs.jmedchem.8b01720 BindingDB Entry DOI: 10.7270/Q2W380Z9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cystathionine gamma-lyase (Homo sapiens (Human)) | BDBM50280040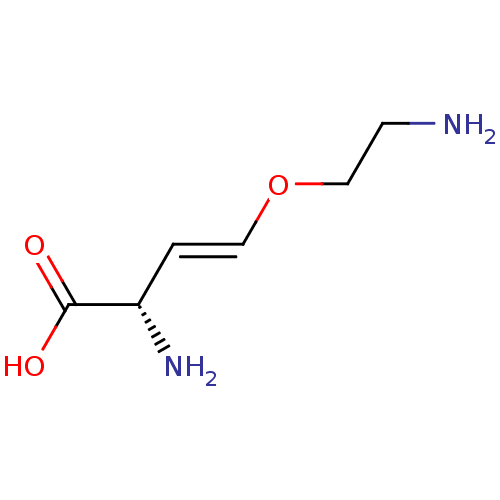 (CHEMBL1619821) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Chicago Curated by ChEMBL | Assay Description Inhibition of cystathionine gamma-lyase (unknown origin) | ACS Med Chem Lett 8: 1241-1245 (2017) Article DOI: 10.1021/acsmedchemlett.7b00313 BindingDB Entry DOI: 10.7270/Q2N300GT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cystathionine gamma-lyase (Homo sapiens (Human)) | BDBM50280013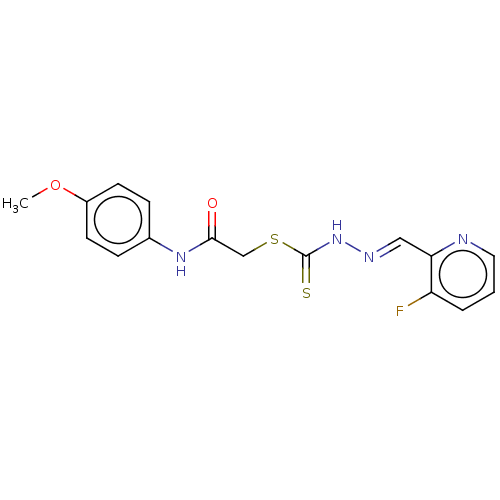 (CHEMBL4163832) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Chicago Curated by ChEMBL | Assay Description Inhibition of cystathionine gamma-lyase (unknown origin) assessed as reduction in cysteine formation from CPM using L-cystathionine pre-incubated for... | ACS Med Chem Lett 8: 1241-1245 (2017) Article DOI: 10.1021/acsmedchemlett.7b00313 BindingDB Entry DOI: 10.7270/Q2N300GT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cystathionine gamma-lyase (Homo sapiens (Human)) | BDBM60996 (5-[(3-carboxy-4-hydroxy-phenyl)-(3-carboxy-4-keto-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 1.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human CSE using H-Cys as the substrate by tandem well based HTS assay | Citation and Details Article DOI: 10.1021/acs.jmedchem.8b01720 BindingDB Entry DOI: 10.7270/Q2W380Z9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cystathionine gamma-lyase (Homo sapiens (Human)) | BDBM60996 (5-[(3-carboxy-4-hydroxy-phenyl)-(3-carboxy-4-keto-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 1.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human CSE using L-Cys as the substrate in presence of 0.025% Triton X-100 by tandem well based HTS assay | Citation and Details Article DOI: 10.1021/acs.jmedchem.8b01720 BindingDB Entry DOI: 10.7270/Q2W380Z9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cystathionine gamma-lyase (Homo sapiens (Human)) | BDBM60996 (5-[(3-carboxy-4-hydroxy-phenyl)-(3-carboxy-4-keto-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 1.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human CSE using L-Cys as the substrate in presence of 0.01% Triton X-100 by tandem well based HTS assay | Citation and Details Article DOI: 10.1021/acs.jmedchem.8b01720 BindingDB Entry DOI: 10.7270/Q2W380Z9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cystathionine gamma-lyase (Homo sapiens (Human)) | BDBM50280015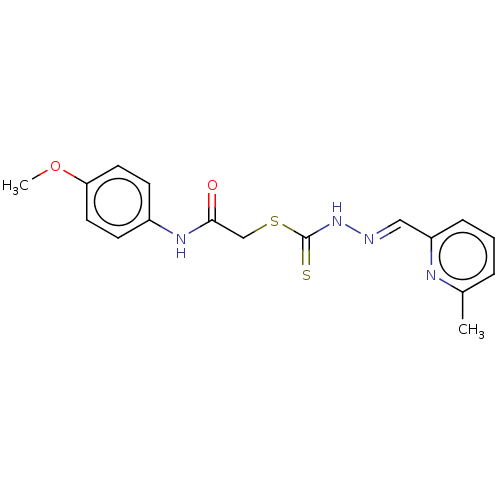 (CHEMBL4159854) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Chicago Curated by ChEMBL | Assay Description Inhibition of cystathionine gamma-lyase (unknown origin) assessed as reduction in cysteine formation from CPM using L-cystathionine pre-incubated for... | ACS Med Chem Lett 8: 1241-1245 (2017) Article DOI: 10.1021/acsmedchemlett.7b00313 BindingDB Entry DOI: 10.7270/Q2N300GT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cystathionine gamma-lyase (Homo sapiens (Human)) | BDBM50279931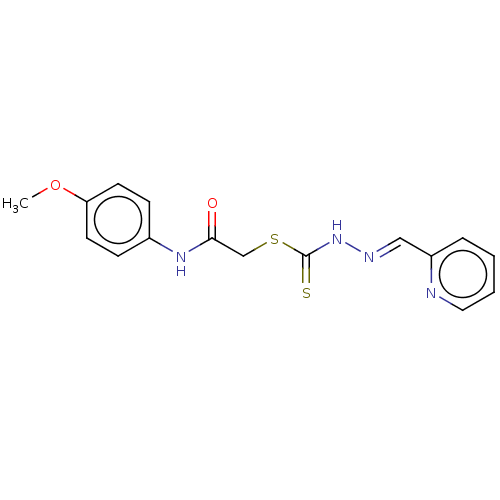 (CHEMBL4166278) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Chicago Curated by ChEMBL | Assay Description Inhibition of cystathionine gamma-lyase (unknown origin) assessed as reduction in cysteine formation from CPM using L-cystathionine pre-incubated for... | ACS Med Chem Lett 8: 1241-1245 (2017) Article DOI: 10.1021/acsmedchemlett.7b00313 BindingDB Entry DOI: 10.7270/Q2N300GT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cystathionine gamma-lyase (Homo sapiens (Human)) | BDBM50280022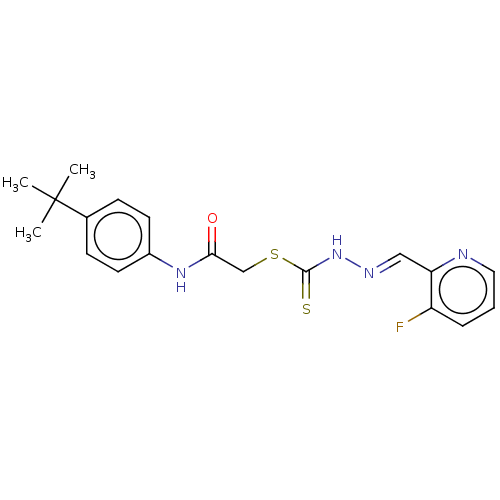 (CHEMBL4171052) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Chicago Curated by ChEMBL | Assay Description Inhibition of cystathionine gamma-lyase (unknown origin) assessed as reduction in cysteine formation from CPM using L-cystathionine pre-incubated for... | ACS Med Chem Lett 8: 1241-1245 (2017) Article DOI: 10.1021/acsmedchemlett.7b00313 BindingDB Entry DOI: 10.7270/Q2N300GT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cystathionine gamma-lyase (Homo sapiens (Human)) | BDBM50280014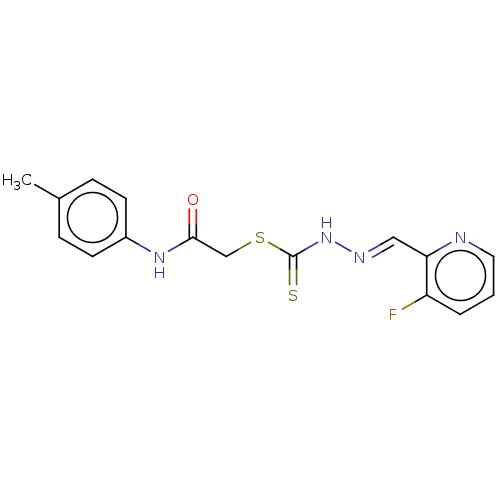 (CHEMBL4160453) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Chicago Curated by ChEMBL | Assay Description Inhibition of cystathionine gamma-lyase (unknown origin) assessed as reduction in cysteine formation from CPM using L-cystathionine pre-incubated for... | ACS Med Chem Lett 8: 1241-1245 (2017) Article DOI: 10.1021/acsmedchemlett.7b00313 BindingDB Entry DOI: 10.7270/Q2N300GT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cystathionine gamma-lyase (Homo sapiens (Human)) | BDBM50280026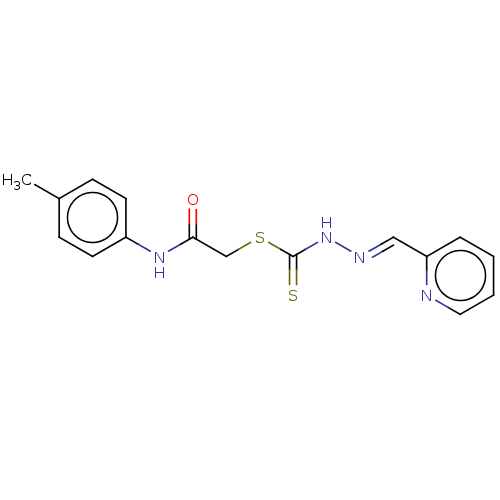 (CHEMBL4162824) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Chicago Curated by ChEMBL | Assay Description Inhibition of cystathionine gamma-lyase (unknown origin) assessed as reduction in cysteine formation from CPM using L-cystathionine pre-incubated for... | ACS Med Chem Lett 8: 1241-1245 (2017) Article DOI: 10.1021/acsmedchemlett.7b00313 BindingDB Entry DOI: 10.7270/Q2N300GT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cystathionine gamma-lyase (Homo sapiens (Human)) | BDBM50279935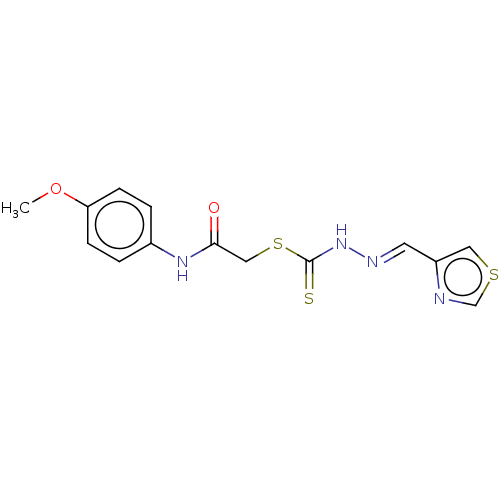 (CHEMBL4168463) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Chicago Curated by ChEMBL | Assay Description Inhibition of cystathionine gamma-lyase (unknown origin) assessed as reduction in cysteine formation from CPM using L-cystathionine pre-incubated for... | ACS Med Chem Lett 8: 1241-1245 (2017) Article DOI: 10.1021/acsmedchemlett.7b00313 BindingDB Entry DOI: 10.7270/Q2N300GT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cystathionine gamma-lyase (Homo sapiens (Human)) | BDBM50280019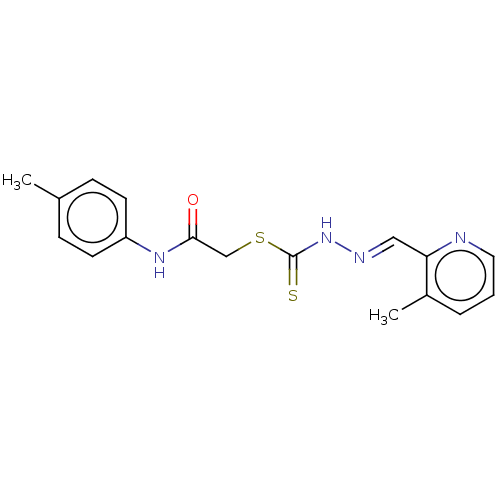 (CHEMBL4167775) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Chicago Curated by ChEMBL | Assay Description Inhibition of cystathionine gamma-lyase (unknown origin) assessed as reduction in cysteine formation from CPM using L-cystathionine pre-incubated for... | ACS Med Chem Lett 8: 1241-1245 (2017) Article DOI: 10.1021/acsmedchemlett.7b00313 BindingDB Entry DOI: 10.7270/Q2N300GT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cystathionine gamma-lyase (Homo sapiens (Human)) | BDBM60996 (5-[(3-carboxy-4-hydroxy-phenyl)-(3-carboxy-4-keto-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 2.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human CSE by methylene blue method | Citation and Details Article DOI: 10.1021/acs.jmedchem.8b01720 BindingDB Entry DOI: 10.7270/Q2W380Z9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cystathionine gamma-lyase (Homo sapiens (Human)) | BDBM50279998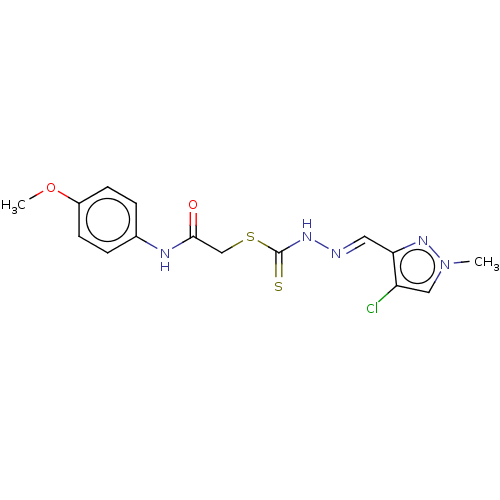 (CHEMBL4166122) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Chicago Curated by ChEMBL | Assay Description Inhibition of cystathionine gamma-lyase (unknown origin) assessed as reduction in cysteine formation from CPM using L-cystathionine pre-incubated for... | ACS Med Chem Lett 8: 1241-1245 (2017) Article DOI: 10.1021/acsmedchemlett.7b00313 BindingDB Entry DOI: 10.7270/Q2N300GT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cystathionine gamma-lyase (Homo sapiens (Human)) | BDBM50279999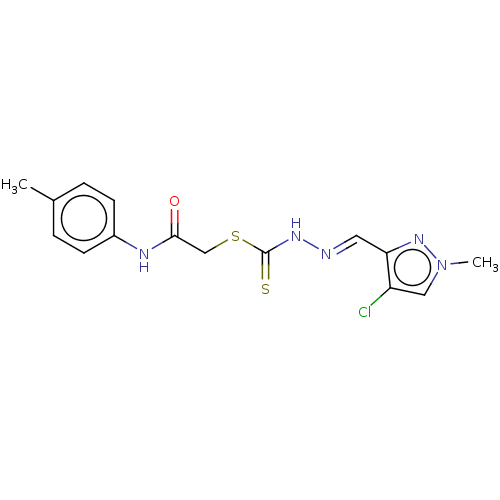 (CHEMBL4169541) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Chicago Curated by ChEMBL | Assay Description Inhibition of cystathionine gamma-lyase (unknown origin) assessed as reduction in cysteine formation from CPM using L-cystathionine pre-incubated for... | ACS Med Chem Lett 8: 1241-1245 (2017) Article DOI: 10.1021/acsmedchemlett.7b00313 BindingDB Entry DOI: 10.7270/Q2N300GT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cystathionine gamma-lyase (Homo sapiens (Human)) | BDBM50279979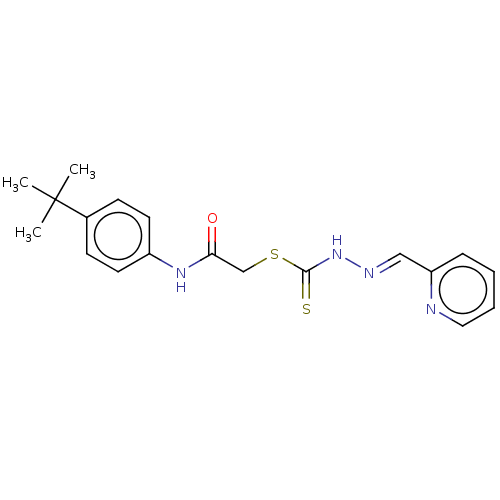 (CHEMBL4176894) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Chicago Curated by ChEMBL | Assay Description Inhibition of cystathionine gamma-lyase (unknown origin) assessed as reduction in cysteine formation from CPM using L-cystathionine pre-incubated for... | ACS Med Chem Lett 8: 1241-1245 (2017) Article DOI: 10.1021/acsmedchemlett.7b00313 BindingDB Entry DOI: 10.7270/Q2N300GT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cystathionine gamma-lyase (Homo sapiens (Human)) | BDBM60996 (5-[(3-carboxy-4-hydroxy-phenyl)-(3-carboxy-4-keto-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 3.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human CSE using H-Cys as the substrate by LC/MS/MS-based assay | Citation and Details Article DOI: 10.1021/acs.jmedchem.8b01720 BindingDB Entry DOI: 10.7270/Q2W380Z9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cystathionine gamma-lyase (Homo sapiens (Human)) | BDBM50280025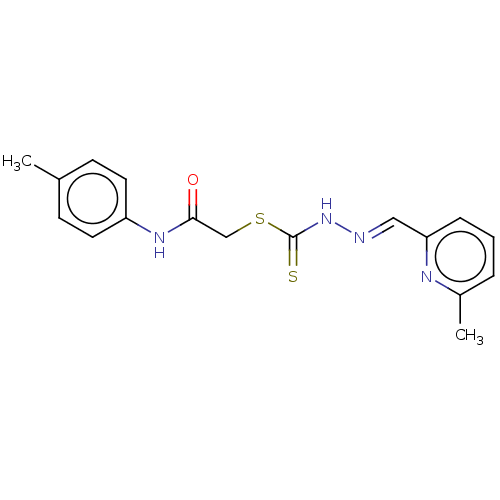 (CHEMBL4170080) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Chicago Curated by ChEMBL | Assay Description Inhibition of cystathionine gamma-lyase (unknown origin) assessed as reduction in cysteine formation from CPM using L-cystathionine pre-incubated for... | ACS Med Chem Lett 8: 1241-1245 (2017) Article DOI: 10.1021/acsmedchemlett.7b00313 BindingDB Entry DOI: 10.7270/Q2N300GT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cystathionine gamma-lyase (Homo sapiens (Human)) | BDBM50280023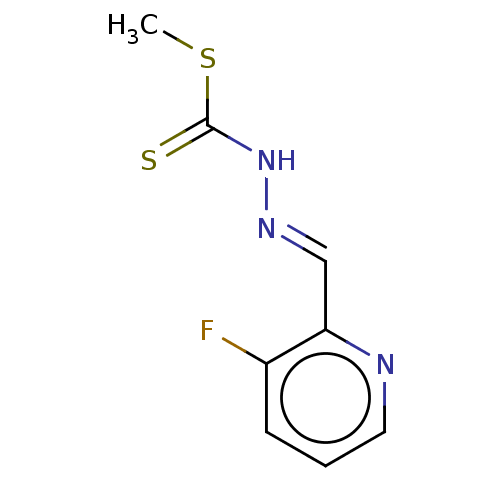 (CHEMBL4177072) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Chicago Curated by ChEMBL | Assay Description Inhibition of cystathionine gamma-lyase (unknown origin) assessed as reduction in cysteine formation from CPM using L-cystathionine pre-incubated for... | ACS Med Chem Lett 8: 1241-1245 (2017) Article DOI: 10.1021/acsmedchemlett.7b00313 BindingDB Entry DOI: 10.7270/Q2N300GT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cystathionine gamma-lyase (Homo sapiens (Human)) | BDBM50279975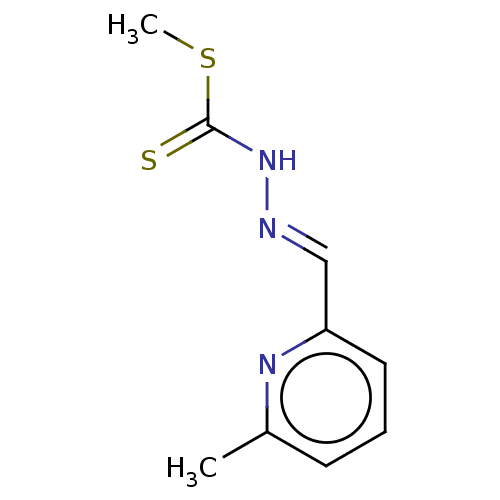 (CHEMBL4170257) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Chicago Curated by ChEMBL | Assay Description Inhibition of cystathionine gamma-lyase (unknown origin) assessed as reduction in cysteine formation from CPM using L-cystathionine pre-incubated for... | ACS Med Chem Lett 8: 1241-1245 (2017) Article DOI: 10.1021/acsmedchemlett.7b00313 BindingDB Entry DOI: 10.7270/Q2N300GT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cystathionine gamma-lyase (Homo sapiens (Human)) | BDBM50280027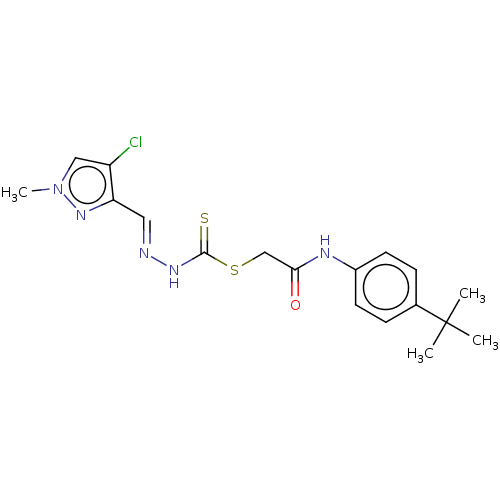 (CHEMBL4176749) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Chicago Curated by ChEMBL | Assay Description Inhibition of cystathionine gamma-lyase (unknown origin) assessed as reduction in cysteine formation from CPM using L-cystathionine pre-incubated for... | ACS Med Chem Lett 8: 1241-1245 (2017) Article DOI: 10.1021/acsmedchemlett.7b00313 BindingDB Entry DOI: 10.7270/Q2N300GT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cystathionine gamma-lyase (Homo sapiens (Human)) | BDBM50280012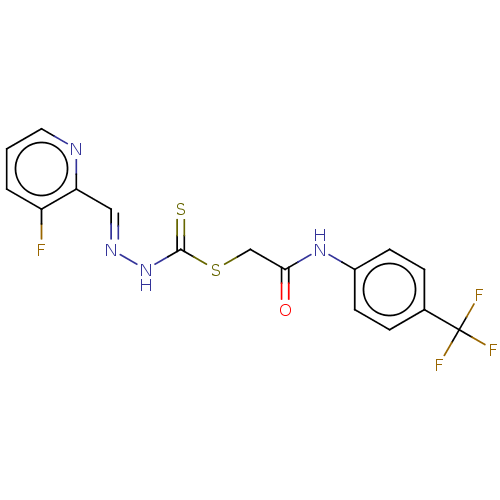 (CHEMBL4174491) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Chicago Curated by ChEMBL | Assay Description Inhibition of cystathionine gamma-lyase (unknown origin) assessed as reduction in cysteine formation from CPM using L-cystathionine pre-incubated for... | ACS Med Chem Lett 8: 1241-1245 (2017) Article DOI: 10.1021/acsmedchemlett.7b00313 BindingDB Entry DOI: 10.7270/Q2N300GT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cystathionine gamma-lyase (Homo sapiens (Human)) | BDBM50279978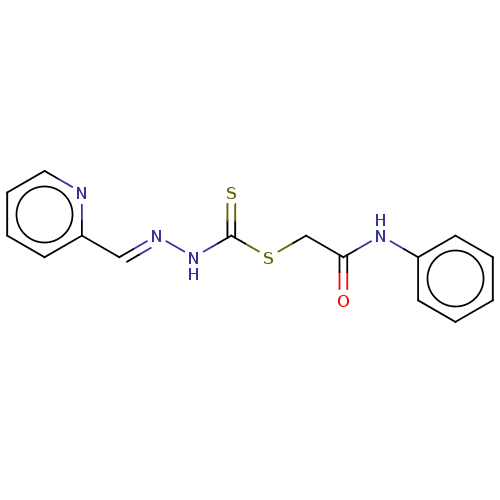 (CHEMBL4173530) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Chicago Curated by ChEMBL | Assay Description Inhibition of cystathionine gamma-lyase (unknown origin) assessed as reduction in cysteine formation from CPM using L-cystathionine pre-incubated for... | ACS Med Chem Lett 8: 1241-1245 (2017) Article DOI: 10.1021/acsmedchemlett.7b00313 BindingDB Entry DOI: 10.7270/Q2N300GT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cystathionine gamma-lyase (Homo sapiens (Human)) | BDBM50279974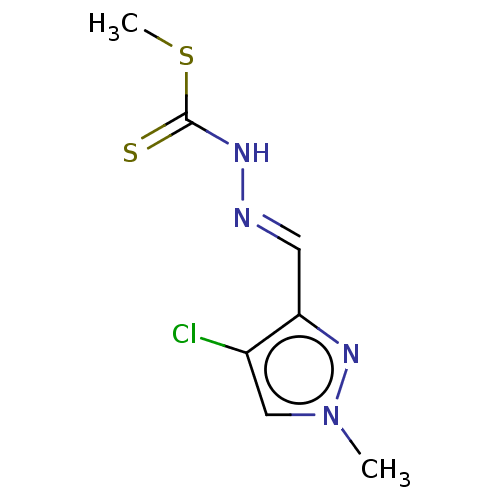 (CHEMBL4159631) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Chicago Curated by ChEMBL | Assay Description Inhibition of cystathionine gamma-lyase (unknown origin) assessed as reduction in cysteine formation from CPM using L-cystathionine pre-incubated for... | ACS Med Chem Lett 8: 1241-1245 (2017) Article DOI: 10.1021/acsmedchemlett.7b00313 BindingDB Entry DOI: 10.7270/Q2N300GT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cystathionine gamma-lyase (Homo sapiens (Human)) | BDBM50279943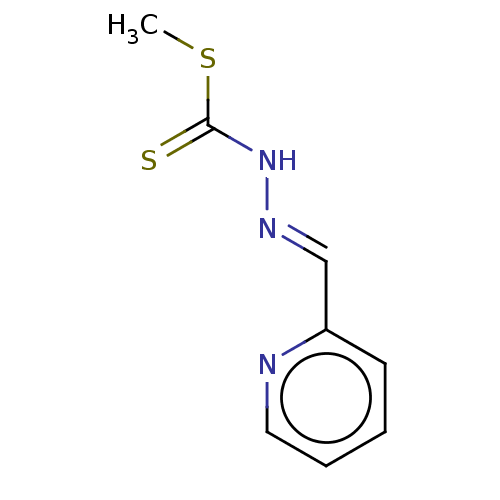 (CHEMBL4160479) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 6.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Chicago Curated by ChEMBL | Assay Description Inhibition of cystathionine gamma-lyase (unknown origin) assessed as reduction in cysteine formation from CPM using L-cystathionine pre-incubated for... | ACS Med Chem Lett 8: 1241-1245 (2017) Article DOI: 10.1021/acsmedchemlett.7b00313 BindingDB Entry DOI: 10.7270/Q2N300GT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cystathionine gamma-lyase (Homo sapiens (Human)) | BDBM50279940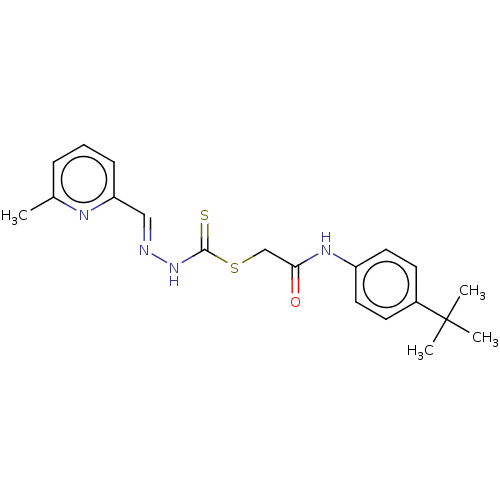 (CHEMBL4159463) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Chicago Curated by ChEMBL | Assay Description Inhibition of cystathionine gamma-lyase (unknown origin) assessed as reduction in cysteine formation from CPM using L-cystathionine pre-incubated for... | ACS Med Chem Lett 8: 1241-1245 (2017) Article DOI: 10.1021/acsmedchemlett.7b00313 BindingDB Entry DOI: 10.7270/Q2N300GT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cystathionine gamma-lyase (Homo sapiens (Human)) | BDBM50279933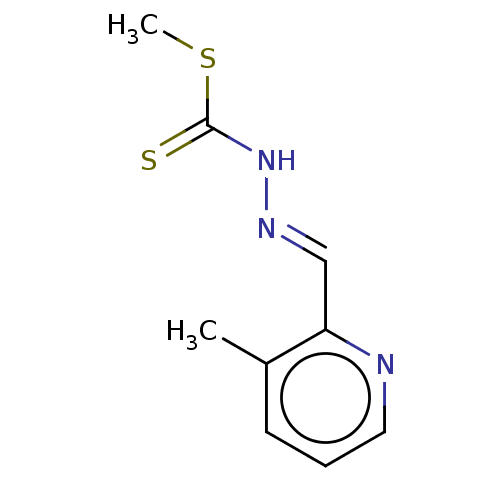 (CHEMBL4169868) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Chicago Curated by ChEMBL | Assay Description Inhibition of cystathionine gamma-lyase (unknown origin) assessed as reduction in cysteine formation from CPM using L-cystathionine pre-incubated for... | ACS Med Chem Lett 8: 1241-1245 (2017) Article DOI: 10.1021/acsmedchemlett.7b00313 BindingDB Entry DOI: 10.7270/Q2N300GT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cystathionine gamma-lyase (Homo sapiens (Human)) | BDBM50279932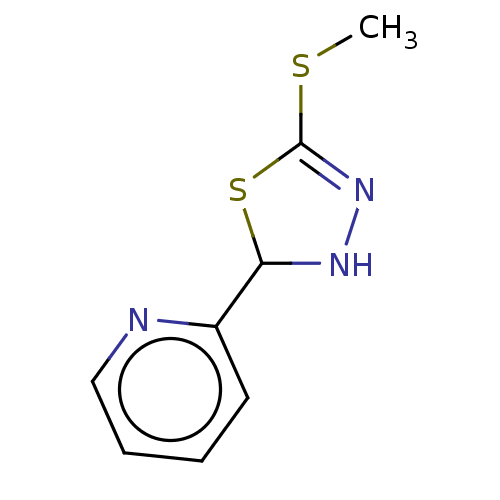 (CHEMBL3318351) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Chicago Curated by ChEMBL | Assay Description Inhibition of cystathionine gamma-lyase (unknown origin) assessed as reduction in cysteine formation from CPM using L-cystathionine pre-incubated for... | ACS Med Chem Lett 8: 1241-1245 (2017) Article DOI: 10.1021/acsmedchemlett.7b00313 BindingDB Entry DOI: 10.7270/Q2N300GT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cystathionine gamma-lyase (Homo sapiens (Human)) | BDBM64987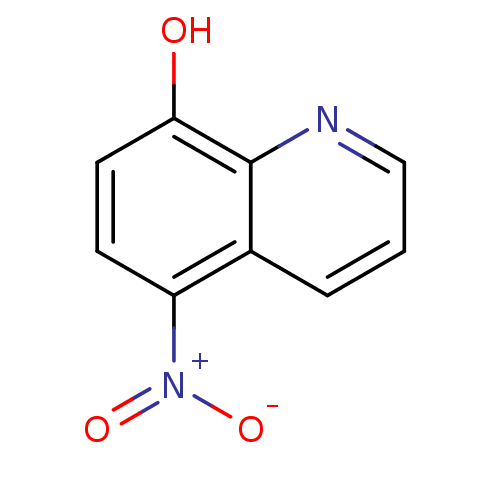 (5-nitro-8-quinolinol | 5-nitroquinolin-8-ol | 8-HY...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 6.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human CSE using H-Cys as the substrate by LC/MS/MS-based assay | Citation and Details Article DOI: 10.1021/acs.jmedchem.8b01720 BindingDB Entry DOI: 10.7270/Q2W380Z9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cystathionine gamma-lyase (Homo sapiens (Human)) | BDBM50548267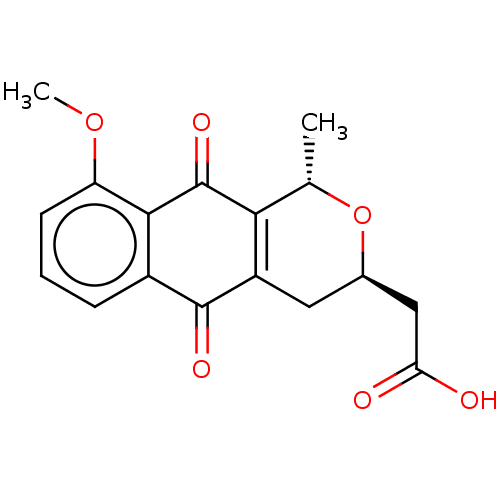 (CHEMBL4761159) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 6.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human CSE using L-Cys as the substrate by tandem well based HTS assay | Citation and Details Article DOI: 10.1021/acs.jmedchem.8b01720 BindingDB Entry DOI: 10.7270/Q2W380Z9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cystathionine gamma-lyase (Homo sapiens (Human)) | BDBM50548267 (CHEMBL4761159) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 6.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Non-covalent reversible inhibition of human CSE | Citation and Details Article DOI: 10.1021/acs.jmedchem.8b01720 BindingDB Entry DOI: 10.7270/Q2W380Z9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cystathionine gamma-lyase (Homo sapiens (Human)) | BDBM50279937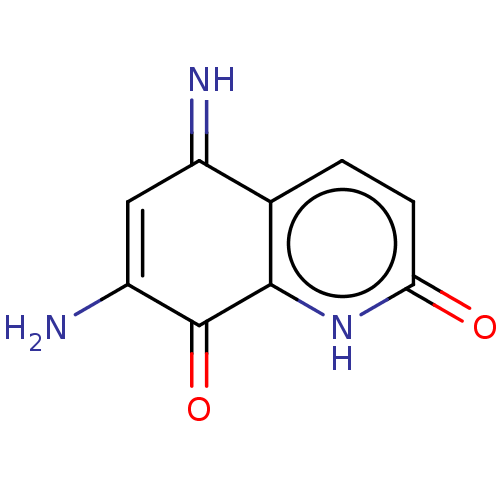 (CHEMBL1985550) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Chicago Curated by ChEMBL | Assay Description Inhibition of cystathionine gamma-lyase (unknown origin) | ACS Med Chem Lett 8: 1241-1245 (2017) Article DOI: 10.1021/acsmedchemlett.7b00313 BindingDB Entry DOI: 10.7270/Q2N300GT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cystathionine gamma-lyase (Homo sapiens (Human)) | BDBM50280028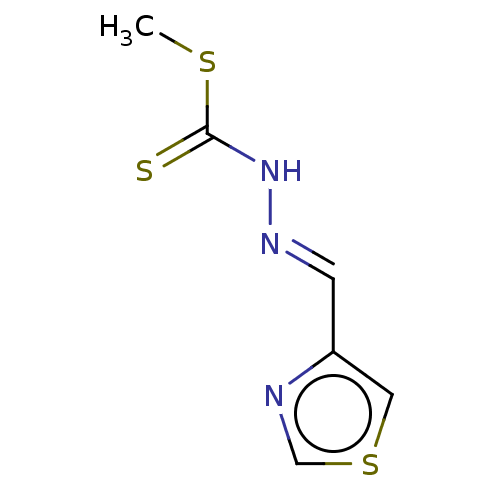 (CHEMBL4162997) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Chicago Curated by ChEMBL | Assay Description Inhibition of cystathionine gamma-lyase (unknown origin) assessed as reduction in cysteine formation from CPM using L-cystathionine pre-incubated for... | ACS Med Chem Lett 8: 1241-1245 (2017) Article DOI: 10.1021/acsmedchemlett.7b00313 BindingDB Entry DOI: 10.7270/Q2N300GT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cystathionine gamma-lyase (Homo sapiens (Human)) | BDBM50548267 (CHEMBL4761159) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 7.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human CSE using H-Cys as the substrate by tandem well based HTS assay | Citation and Details Article DOI: 10.1021/acs.jmedchem.8b01720 BindingDB Entry DOI: 10.7270/Q2W380Z9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cystathionine gamma-lyase (Homo sapiens (Human)) | BDBM64987 (5-nitro-8-quinolinol | 5-nitroquinolin-8-ol | 8-HY...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 8.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human CSE using L-Cys as the substrate by tandem well based HTS assay | Citation and Details Article DOI: 10.1021/acs.jmedchem.8b01720 BindingDB Entry DOI: 10.7270/Q2W380Z9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cystathionine gamma-lyase (Homo sapiens (Human)) | BDBM330317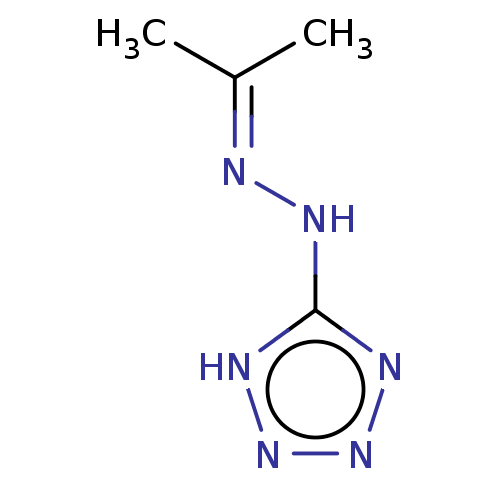 (US9725426, 21) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | US Patent | n/a | n/a | <1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
SOVA PHARMACEUTICALS, INC. US Patent | Assay Description Test compounds (from DMSO stock solutions) were added to (final concentrations) 20 ug/ml enzyme solution (human, mouse or rat recombinant CSE) plus 5... | US Patent US9725426 (2017) BindingDB Entry DOI: 10.7270/Q2765HG7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cystathionine gamma-lyase (Homo sapiens (Human)) | BDBM50279938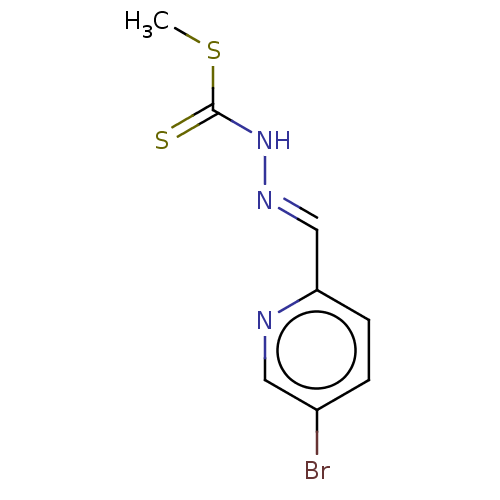 (CHEMBL4161944) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Chicago Curated by ChEMBL | Assay Description Inhibition of cystathionine gamma-lyase (unknown origin) assessed as reduction in cysteine formation from CPM using L-cystathionine pre-incubated for... | ACS Med Chem Lett 8: 1241-1245 (2017) Article DOI: 10.1021/acsmedchemlett.7b00313 BindingDB Entry DOI: 10.7270/Q2N300GT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cystathionine gamma-lyase (Homo sapiens (Human)) | BDBM50279936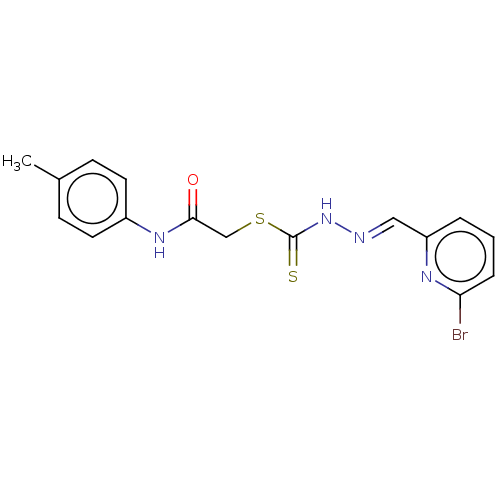 (CHEMBL4167285) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Chicago Curated by ChEMBL | Assay Description Binding affinity against sigma receptor | ACS Med Chem Lett 8: 1241-1245 (2017) Article DOI: 10.1021/acsmedchemlett.7b00313 BindingDB Entry DOI: 10.7270/Q2N300GT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cystathionine gamma-lyase (Homo sapiens (Human)) | BDBM330339 (US9725426, (E)-5-(2-(furan-2-ylmethylene)hydraziny...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | US Patent | n/a | n/a | <1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
SOVA PHARMACEUTICALS, INC. US Patent | Assay Description Test compounds (from DMSO stock solutions) were added to (final concentrations) 20 ug/ml enzyme solution (human, mouse or rat recombinant CSE) plus 5... | US Patent US9725426 (2017) BindingDB Entry DOI: 10.7270/Q2765HG7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cystathionine gamma-lyase (Homo sapiens (Human)) | BDBM330331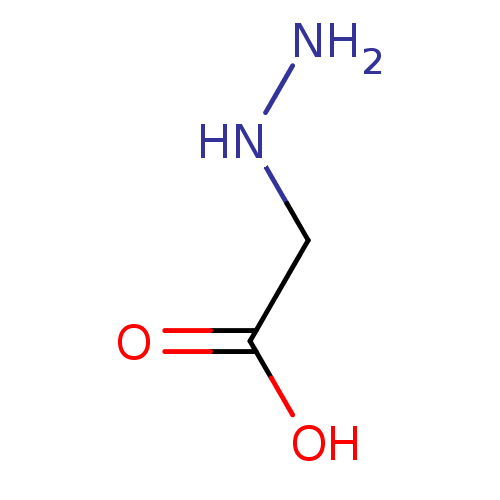 (US9725426, 2-hydrazinylacetic acid) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | US Patent | n/a | n/a | <1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
SOVA PHARMACEUTICALS, INC. US Patent | Assay Description Test compounds (from DMSO stock solutions) were added to (final concentrations) 20 ug/ml enzyme solution (human, mouse or rat recombinant CSE) plus 5... | US Patent US9725426 (2017) BindingDB Entry DOI: 10.7270/Q2765HG7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cystathionine gamma-lyase (Homo sapiens (Human)) | BDBM330299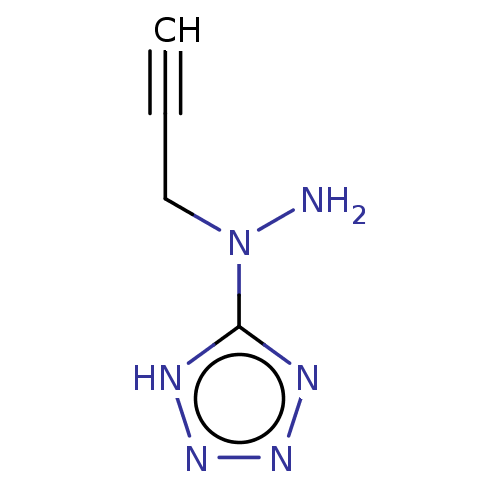 (US9725426, 3) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
SOVA PHARMACEUTICALS, INC. US Patent | Assay Description Test compounds (from DMSO stock solutions) were added to (final concentrations) 20 ug/ml enzyme solution (human, mouse or rat recombinant CSE) plus 5... | US Patent US9725426 (2017) BindingDB Entry DOI: 10.7270/Q2765HG7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cystathionine gamma-lyase (Homo sapiens (Human)) | BDBM330300 (US9725426, 4) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
SOVA PHARMACEUTICALS, INC. US Patent | Assay Description Test compounds (from DMSO stock solutions) were added to (final concentrations) 20 ug/ml enzyme solution (human, mouse or rat recombinant CSE) plus 5... | US Patent US9725426 (2017) BindingDB Entry DOI: 10.7270/Q2765HG7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cystathionine gamma-lyase (Homo sapiens (Human)) | BDBM330307 (US9725426, 11) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
SOVA PHARMACEUTICALS, INC. US Patent | Assay Description Test compounds (from DMSO stock solutions) were added to (final concentrations) 20 ug/ml enzyme solution (human, mouse or rat recombinant CSE) plus 5... | US Patent US9725426 (2017) BindingDB Entry DOI: 10.7270/Q2765HG7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cystathionine gamma-lyase (Homo sapiens (Human)) | BDBM330308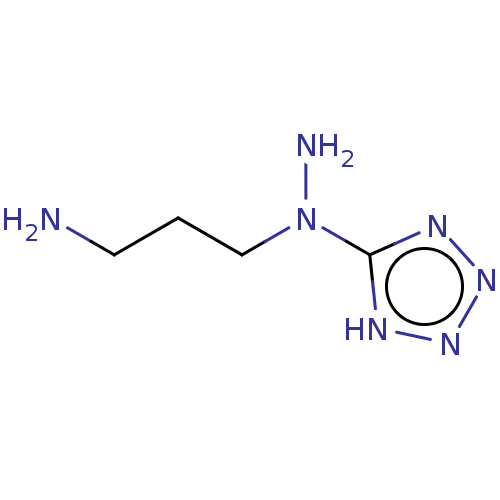 (US9725426, 12) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
SOVA PHARMACEUTICALS, INC. US Patent | Assay Description Test compounds (from DMSO stock solutions) were added to (final concentrations) 20 ug/ml enzyme solution (human, mouse or rat recombinant CSE) plus 5... | US Patent US9725426 (2017) BindingDB Entry DOI: 10.7270/Q2765HG7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cystathionine gamma-lyase (Homo sapiens (Human)) | BDBM330309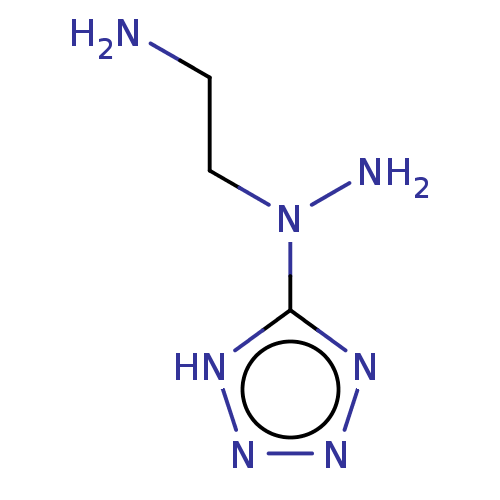 (US9725426, 13) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
SOVA PHARMACEUTICALS, INC. US Patent | Assay Description Test compounds (from DMSO stock solutions) were added to (final concentrations) 20 ug/ml enzyme solution (human, mouse or rat recombinant CSE) plus 5... | US Patent US9725426 (2017) BindingDB Entry DOI: 10.7270/Q2765HG7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cystathionine gamma-lyase (Homo sapiens (Human)) | BDBM330311 (US9725426, 15) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
SOVA PHARMACEUTICALS, INC. US Patent | Assay Description Test compounds (from DMSO stock solutions) were added to (final concentrations) 20 ug/ml enzyme solution (human, mouse or rat recombinant CSE) plus 5... | US Patent US9725426 (2017) BindingDB Entry DOI: 10.7270/Q2765HG7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cystathionine gamma-lyase (Homo sapiens (Human)) | BDBM330316 (US9725426, 20) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | US Patent | n/a | n/a | <1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
SOVA PHARMACEUTICALS, INC. US Patent | Assay Description Test compounds (from DMSO stock solutions) were added to (final concentrations) 20 ug/ml enzyme solution (human, mouse or rat recombinant CSE) plus 5... | US Patent US9725426 (2017) BindingDB Entry DOI: 10.7270/Q2765HG7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cystathionine gamma-lyase (Homo sapiens (Human)) | BDBM330318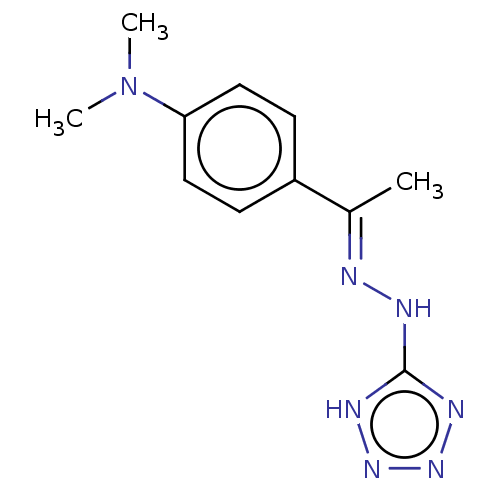 (US9725426, 22) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
SOVA PHARMACEUTICALS, INC. US Patent | Assay Description Test compounds (from DMSO stock solutions) were added to (final concentrations) 20 ug/ml enzyme solution (human, mouse or rat recombinant CSE) plus 5... | US Patent US9725426 (2017) BindingDB Entry DOI: 10.7270/Q2765HG7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cystathionine gamma-lyase (Homo sapiens (Human)) | BDBM330319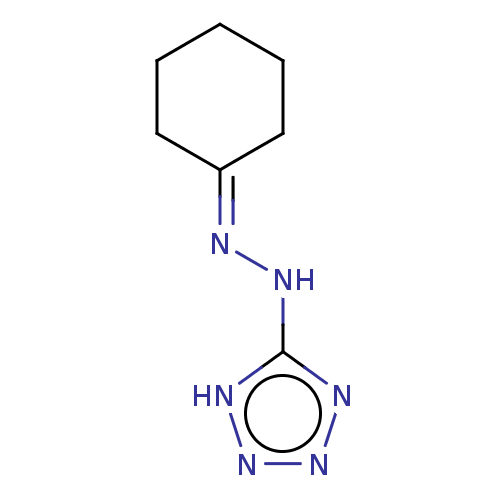 (US9725426, 23) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | US Patent | n/a | n/a | <1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
SOVA PHARMACEUTICALS, INC. US Patent | Assay Description Test compounds (from DMSO stock solutions) were added to (final concentrations) 20 ug/ml enzyme solution (human, mouse or rat recombinant CSE) plus 5... | US Patent US9725426 (2017) BindingDB Entry DOI: 10.7270/Q2765HG7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 160 total ) | Next | Last >> |