

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Somatostatin receptor type 3 (Homo sapiens (Human)) | BDBM50352489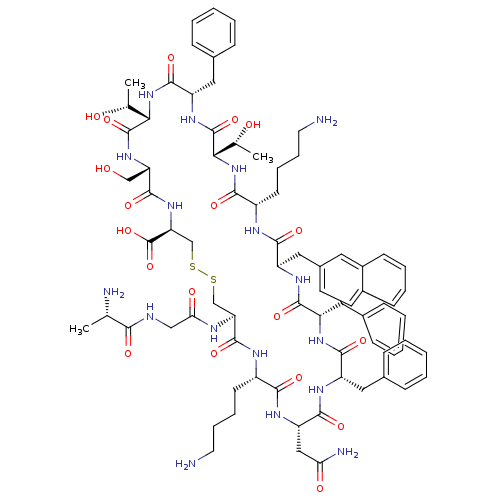 (CHEMBL1824052) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 0.230 | n/a | n/a | n/a | n/a |
Salk Institute Curated by ChEMBL | Assay Description Agonist activity at human sst3 receptor expressed in CCL39 cells after 5 hrs by luciferase reporter gene assay | J Med Chem 54: 5981-7 (2011) Article DOI: 10.1021/jm200307v BindingDB Entry DOI: 10.7270/Q2BV7H1N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 3 (Homo sapiens (Human)) | BDBM523757 (US11136312, Compound MM-I-87) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | n/a | 0.249 | n/a | n/a | n/a | n/a |
TBA | Assay Description Measurement of forskolin stimulated inhibition of cAMP was performed via time-resolved fluorescence resonance energy transfer (TR-FRET) LANCE assay (... | Citation and Details BindingDB Entry DOI: 10.7270/Q2DB851B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 3 (Homo sapiens (Human)) | BDBM523756 (US11136312, Compound MM-I-83) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | n/a | 0.276 | n/a | n/a | n/a | n/a |
TBA | Assay Description Measurement of forskolin stimulated inhibition of cAMP was performed via time-resolved fluorescence resonance energy transfer (TR-FRET) LANCE assay (... | Citation and Details BindingDB Entry DOI: 10.7270/Q2DB851B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 3 (Homo sapiens (Human)) | BDBM50352480 (CHEMBL1824051) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 0.350 | n/a | n/a | n/a | n/a |
Salk Institute Curated by ChEMBL | Assay Description Agonist activity at human sst3 receptor expressed in CCL39 cells after 5 hrs by luciferase reporter gene assay | J Med Chem 54: 5981-7 (2011) Article DOI: 10.1021/jm200307v BindingDB Entry DOI: 10.7270/Q2BV7H1N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 3 (Homo sapiens (Human)) | BDBM50154508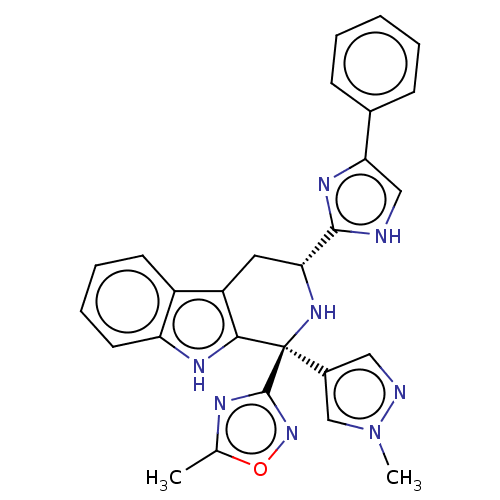 (CHEMBL3775042) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 0.460 | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Antagonist activity at human somatostatin receptor type 3 assessed as inhibition of cAMP levels | Bioorg Med Chem Lett 26: 1529-35 (2016) Article DOI: 10.1016/j.bmcl.2016.02.022 BindingDB Entry DOI: 10.7270/Q2ZG6V4J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 3 (Homo sapiens (Human)) | BDBM50021074 (CHEMBL3287628) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 0.516 | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Agonist activity at human SST3 expressed in CHO-K1 cells assessed as foreskin-stimulated cAMP accumulation after 45 mins by TR-FRET assay | ACS Med Chem Lett 5: 690-5 (2014) Article DOI: 10.1021/ml500079u BindingDB Entry DOI: 10.7270/Q22N53V4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 3 (Homo sapiens (Human)) | BDBM50352483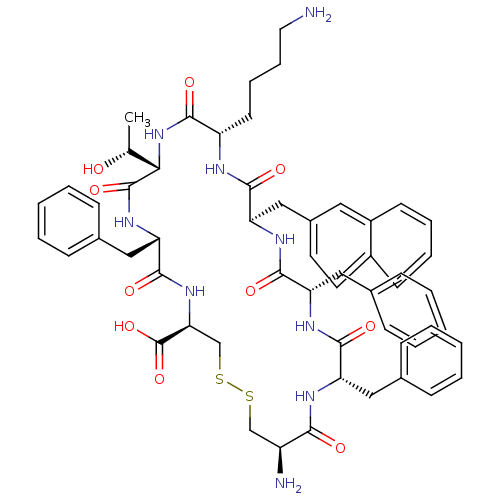 (CHEMBL1824056) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 0.530 | n/a | n/a | n/a | n/a |
Salk Institute Curated by ChEMBL | Assay Description Agonist activity at human sst3 receptor expressed in CCL39 cells after 5 hrs by luciferase reporter gene assay | J Med Chem 54: 5981-7 (2011) Article DOI: 10.1021/jm200307v BindingDB Entry DOI: 10.7270/Q2BV7H1N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 3 (Homo sapiens (Human)) | BDBM523754 (US11136312, Compound MM-I-66) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | n/a | 0.585 | n/a | n/a | n/a | n/a |
TBA | Assay Description Measurement of forskolin stimulated inhibition of cAMP was performed via time-resolved fluorescence resonance energy transfer (TR-FRET) LANCE assay (... | Citation and Details BindingDB Entry DOI: 10.7270/Q2DB851B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 3 (Homo sapiens (Human)) | BDBM523801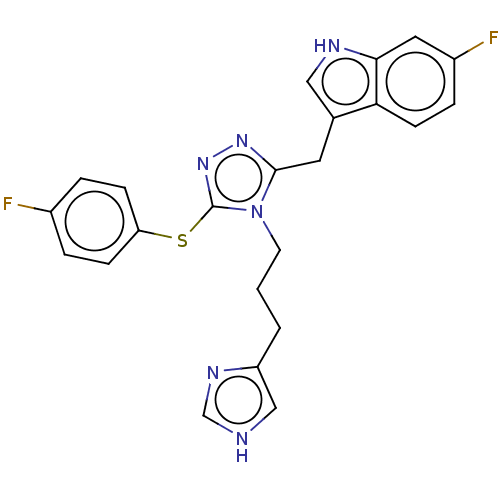 (US11136312, Compound SK-I-128) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | n/a | 0.619 | n/a | n/a | n/a | n/a |
TBA | Assay Description Measurement of forskolin stimulated inhibition of cAMP was performed via time-resolved fluorescence resonance energy transfer (TR-FRET) LANCE assay (... | Citation and Details BindingDB Entry DOI: 10.7270/Q2DB851B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 3 (Homo sapiens (Human)) | BDBM82547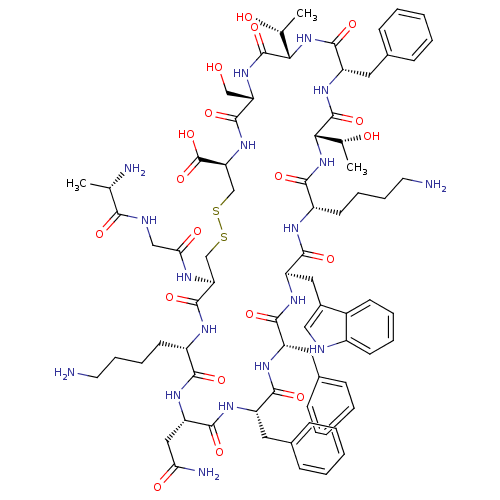 (SRIF-D-Trp8) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 0.630 | n/a | n/a | n/a | n/a |
Salk Institute Curated by ChEMBL | Assay Description Agonist activity at human sst3 receptor expressed in CCL39 cells after 5 hrs by luciferase reporter gene assay | J Med Chem 54: 5981-7 (2011) Article DOI: 10.1021/jm200307v BindingDB Entry DOI: 10.7270/Q2BV7H1N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 3 (Homo sapiens (Human)) | BDBM523770 (US11136312, Compound SK-I-124) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | n/a | 0.725 | n/a | n/a | n/a | n/a |
TBA | Assay Description Measurement of forskolin stimulated inhibition of cAMP was performed via time-resolved fluorescence resonance energy transfer (TR-FRET) LANCE assay (... | Citation and Details BindingDB Entry DOI: 10.7270/Q2DB851B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 3 (Homo sapiens (Human)) | BDBM81767 (15-28-Somatostatin-28 | CAS_38916-34-6 | CB6417646...) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 0.780 | n/a | n/a | n/a | n/a |
Salk Institute Curated by ChEMBL | Assay Description Agonist activity at human sst3 receptor expressed in CCL39 cells after 5 hrs by luciferase reporter gene assay | J Med Chem 54: 5981-7 (2011) Article DOI: 10.1021/jm200307v BindingDB Entry DOI: 10.7270/Q2BV7H1N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 3 (Homo sapiens (Human)) | BDBM50352481 (CHEMBL1824050) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 0.870 | n/a | n/a | n/a | n/a |
Salk Institute Curated by ChEMBL | Assay Description Agonist activity at human sst3 receptor expressed in CCL39 cells after 5 hrs by luciferase reporter gene assay | J Med Chem 54: 5981-7 (2011) Article DOI: 10.1021/jm200307v BindingDB Entry DOI: 10.7270/Q2BV7H1N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 3 (Homo sapiens (Human)) | BDBM50400517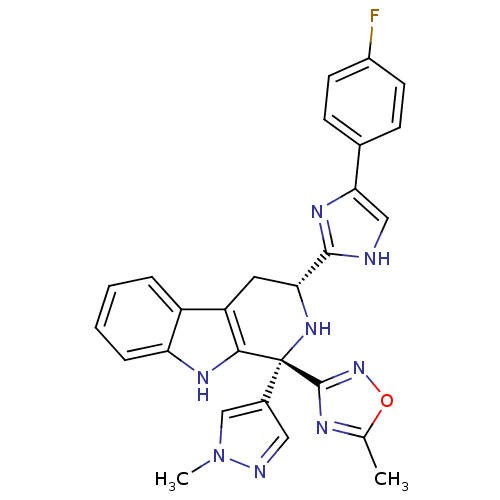 (CHEMBL2204935) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 0.950 | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Antagonist activity at human somatostatin receptor type 3 assessed as inhibition of cAMP levels | Bioorg Med Chem Lett 26: 1529-35 (2016) Article DOI: 10.1016/j.bmcl.2016.02.022 BindingDB Entry DOI: 10.7270/Q2ZG6V4J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 3 (Homo sapiens (Human)) | BDBM523803 (US11136312, Compound SK-I-132) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | n/a | 0.992 | n/a | n/a | n/a | n/a |
TBA | Assay Description Measurement of forskolin stimulated inhibition of cAMP was performed via time-resolved fluorescence resonance energy transfer (TR-FRET) LANCE assay (... | Citation and Details BindingDB Entry DOI: 10.7270/Q2DB851B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 3 (Homo sapiens (Human)) | BDBM523766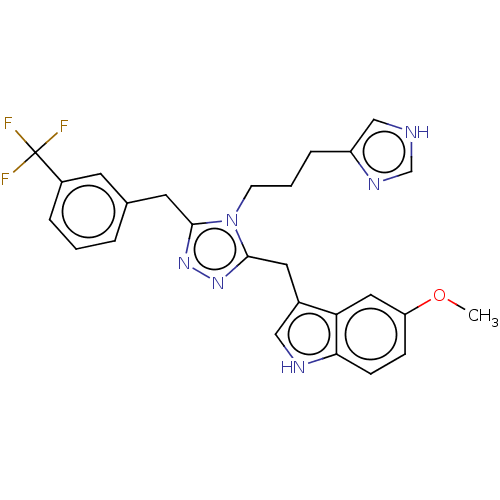 (US11136312, Compound SK-I-57) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | n/a | 1 | n/a | n/a | n/a | n/a |
TBA | Assay Description Measurement of forskolin stimulated inhibition of cAMP was performed via time-resolved fluorescence resonance energy transfer (TR-FRET) LANCE assay (... | Citation and Details BindingDB Entry DOI: 10.7270/Q2DB851B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 3 (Homo sapiens (Human)) | BDBM523760 (US11136312, Compound SK-I-22) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | n/a | 1.28 | n/a | n/a | n/a | n/a |
TBA | Assay Description Measurement of forskolin stimulated inhibition of cAMP was performed via time-resolved fluorescence resonance energy transfer (TR-FRET) LANCE assay (... | Citation and Details BindingDB Entry DOI: 10.7270/Q2DB851B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 3 (Homo sapiens (Human)) | BDBM50021075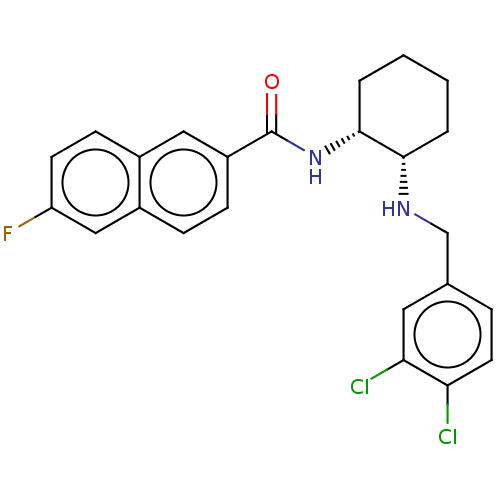 (CHEMBL3287629) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 1.60 | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Agonist activity at human SST3 expressed in CHO-K1 cells assessed as foreskin-stimulated cAMP accumulation after 45 mins by TR-FRET assay | ACS Med Chem Lett 5: 690-5 (2014) Article DOI: 10.1021/ml500079u BindingDB Entry DOI: 10.7270/Q22N53V4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 3 (Homo sapiens (Human)) | BDBM523759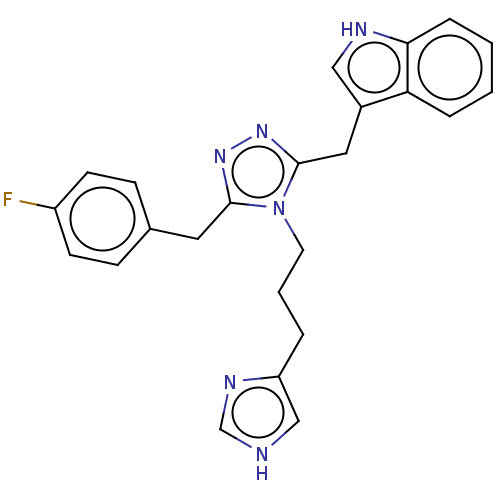 (US11136312, Compound SK-I-16) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | n/a | 1.76 | n/a | n/a | n/a | n/a |
TBA | Assay Description Measurement of forskolin stimulated inhibition of cAMP was performed via time-resolved fluorescence resonance energy transfer (TR-FRET) LANCE assay (... | Citation and Details BindingDB Entry DOI: 10.7270/Q2DB851B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 3 (Homo sapiens (Human)) | BDBM523762 (US11136312, Compound SK-I-25) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | n/a | 2.03 | n/a | n/a | n/a | n/a |
TBA | Assay Description Measurement of forskolin stimulated inhibition of cAMP was performed via time-resolved fluorescence resonance energy transfer (TR-FRET) LANCE assay (... | Citation and Details BindingDB Entry DOI: 10.7270/Q2DB851B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 3 (Homo sapiens (Human)) | BDBM50091557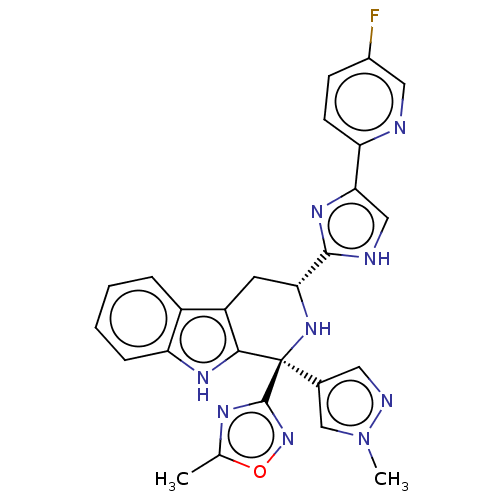 (CHEMBL3582317) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 2.5 | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Antagonist activity at human somatostatin receptor type 3 assessed as inhibition of cAMP levels | Bioorg Med Chem Lett 26: 1529-35 (2016) Article DOI: 10.1016/j.bmcl.2016.02.022 BindingDB Entry DOI: 10.7270/Q2ZG6V4J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 3 (Homo sapiens (Human)) | BDBM523758 (US11136312, Compound MM-I-89) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | n/a | 2.80 | n/a | n/a | n/a | n/a |
TBA | Assay Description Measurement of forskolin stimulated inhibition of cAMP was performed via time-resolved fluorescence resonance energy transfer (TR-FRET) LANCE assay (... | Citation and Details BindingDB Entry DOI: 10.7270/Q2DB851B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 3 (Homo sapiens (Human)) | BDBM50021063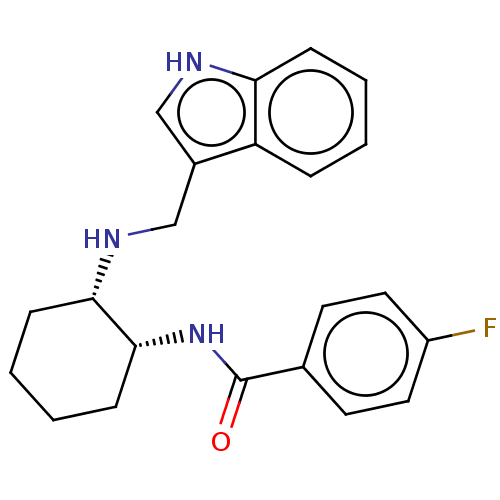 (CHEMBL3287613) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 2.90 | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Agonist activity at human SST3 expressed in CHO-K1 cells assessed as foreskin-stimulated cAMP accumulation after 45 mins by TR-FRET assay | ACS Med Chem Lett 5: 690-5 (2014) Article DOI: 10.1021/ml500079u BindingDB Entry DOI: 10.7270/Q22N53V4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 3 (Homo sapiens (Human)) | BDBM523802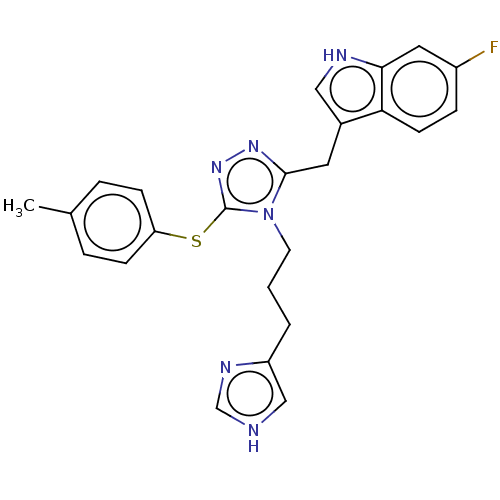 (US11136312, Compound SK-I-130) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | n/a | 3.09 | n/a | n/a | n/a | n/a |
TBA | Assay Description Measurement of forskolin stimulated inhibition of cAMP was performed via time-resolved fluorescence resonance energy transfer (TR-FRET) LANCE assay (... | Citation and Details BindingDB Entry DOI: 10.7270/Q2DB851B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 3 (Homo sapiens (Human)) | BDBM50154509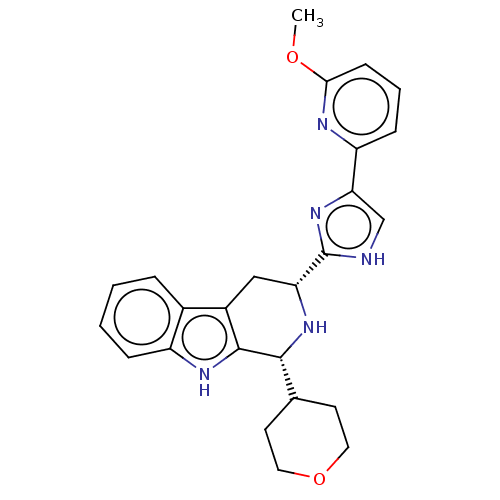 (CHEMBL3775387) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 3.10 | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Antagonist activity at human somatostatin receptor type 3 assessed as inhibition of cAMP levels | Bioorg Med Chem Lett 26: 1529-35 (2016) Article DOI: 10.1016/j.bmcl.2016.02.022 BindingDB Entry DOI: 10.7270/Q2ZG6V4J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 3 (Homo sapiens (Human)) | BDBM523767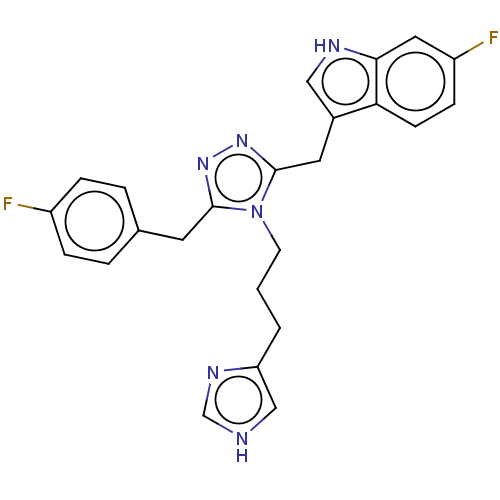 (US11136312, Compound SK-I-91) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | n/a | 3.17 | n/a | n/a | n/a | n/a |
TBA | Assay Description Measurement of forskolin stimulated inhibition of cAMP was performed via time-resolved fluorescence resonance energy transfer (TR-FRET) LANCE assay (... | Citation and Details BindingDB Entry DOI: 10.7270/Q2DB851B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 3 (Homo sapiens (Human)) | BDBM50154507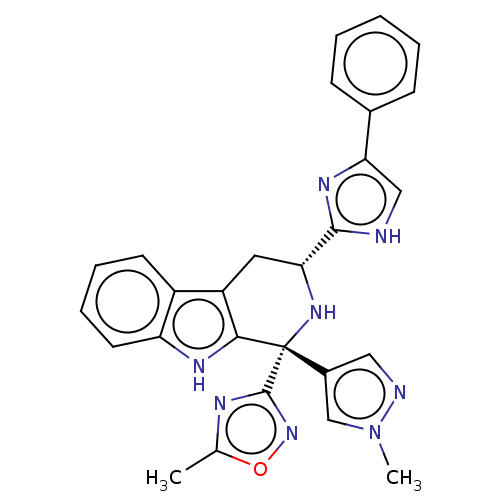 (CHEMBL3775630) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 3.30 | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Antagonist activity at human somatostatin receptor type 3 assessed as inhibition of cAMP levels | Bioorg Med Chem Lett 26: 1529-35 (2016) Article DOI: 10.1016/j.bmcl.2016.02.022 BindingDB Entry DOI: 10.7270/Q2ZG6V4J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 3 (Homo sapiens (Human)) | BDBM523755 (US11136312, Compound MM-I-72) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | n/a | 3.32 | n/a | n/a | n/a | n/a |
TBA | Assay Description Measurement of forskolin stimulated inhibition of cAMP was performed via time-resolved fluorescence resonance energy transfer (TR-FRET) LANCE assay (... | Citation and Details BindingDB Entry DOI: 10.7270/Q2DB851B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 3 (Homo sapiens (Human)) | BDBM50136824 ((4R,7S,10S,13S,16R,19S,22S,25R)-16-((1H-indol-3-yl...) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 3.33 | n/a | n/a | n/a | n/a |
Salk Institute Curated by ChEMBL | Assay Description Agonist activity at human sst3 receptor expressed in CCL39 cells after 5 hrs by luciferase reporter gene assay | J Med Chem 54: 5981-7 (2011) Article DOI: 10.1021/jm200307v BindingDB Entry DOI: 10.7270/Q2BV7H1N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 3 (Homo sapiens (Human)) | BDBM50021090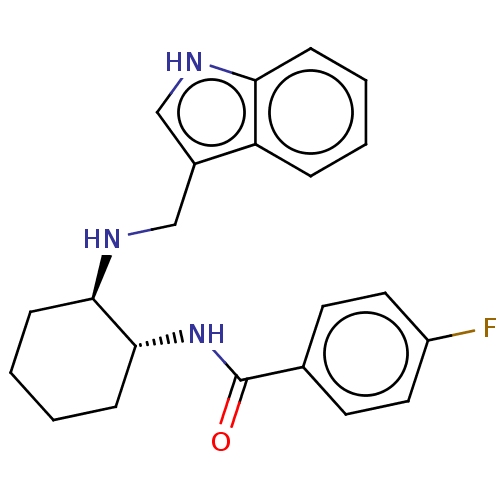 (CHEMBL3287632) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 3.70 | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Agonist activity at human SST3 expressed in CHO-K1 cells assessed as foreskin-stimulated cAMP accumulation after 45 mins by TR-FRET assay | ACS Med Chem Lett 5: 690-5 (2014) Article DOI: 10.1021/ml500079u BindingDB Entry DOI: 10.7270/Q22N53V4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 3 (Homo sapiens (Human)) | BDBM523742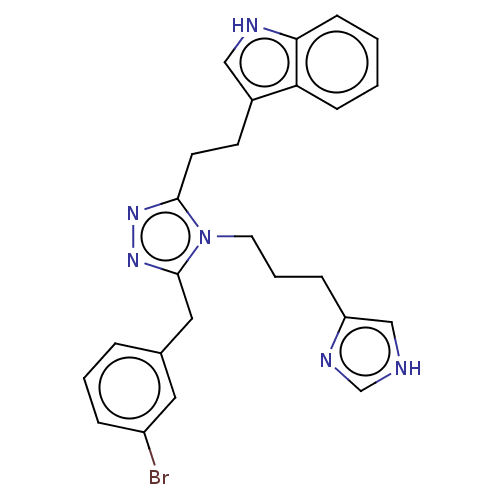 (US11136312, Compound SM-I-50) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | n/a | 4.35 | n/a | n/a | n/a | n/a |
TBA | Assay Description Measurement of forskolin stimulated inhibition of cAMP was performed via time-resolved fluorescence resonance energy transfer (TR-FRET) LANCE assay (... | Citation and Details BindingDB Entry DOI: 10.7270/Q2DB851B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 3 (Homo sapiens (Human)) | BDBM50021073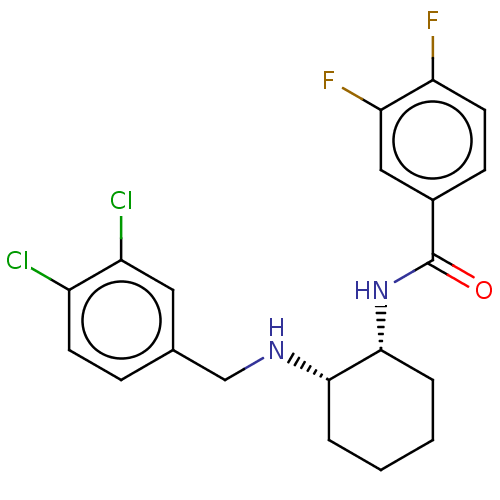 (CHEMBL3287627) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 4.5 | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Agonist activity at human SST3 expressed in CHO-K1 cells assessed as foreskin-stimulated cAMP accumulation after 45 mins by TR-FRET assay | ACS Med Chem Lett 5: 690-5 (2014) Article DOI: 10.1021/ml500079u BindingDB Entry DOI: 10.7270/Q22N53V4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 3 (Homo sapiens (Human)) | BDBM50352482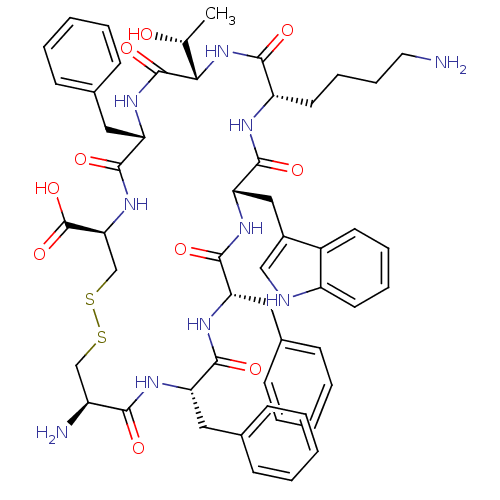 (CHEMBL1824055) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 4.70 | n/a | n/a | n/a | n/a |
Salk Institute Curated by ChEMBL | Assay Description Partial agonist activity at human sst3 receptor expressed in CCL39 cells after 5 hrs by luciferase reporter gene assay | J Med Chem 54: 5981-7 (2011) Article DOI: 10.1021/jm200307v BindingDB Entry DOI: 10.7270/Q2BV7H1N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 3 (Homo sapiens (Human)) | BDBM50154423 (CHEMBL3774684) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 4.80 | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Antagonist activity at human somatostatin receptor type 3 assessed as inhibition of cAMP levels | Bioorg Med Chem Lett 26: 1529-35 (2016) Article DOI: 10.1016/j.bmcl.2016.02.022 BindingDB Entry DOI: 10.7270/Q2ZG6V4J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 3 (Homo sapiens (Human)) | BDBM50110470 (CHEMBL3605798) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 5.20 | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Agonist activity at human SSTR3 expressed in CHO cells assessed as intracellular cAMP level after 45 mins by time-resolved fluorescence assay | Bioorg Med Chem Lett 25: 3520-5 (2015) Article DOI: 10.1016/j.bmcl.2015.06.087 BindingDB Entry DOI: 10.7270/Q29W0H9M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 3 (Homo sapiens (Human)) | BDBM50154465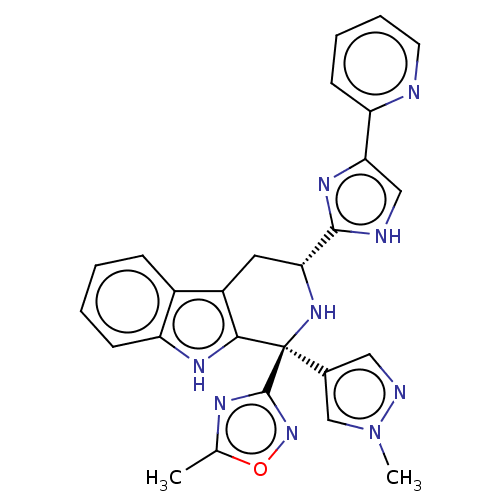 (CHEMBL3775760) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 5.5 | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Antagonist activity at human somatostatin receptor type 3 assessed as inhibition of cAMP levels | Bioorg Med Chem Lett 26: 1529-35 (2016) Article DOI: 10.1016/j.bmcl.2016.02.022 BindingDB Entry DOI: 10.7270/Q2ZG6V4J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 3 (Homo sapiens (Human)) | BDBM523765 (US11136312, Compound SK-I-56) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | n/a | 5.63 | n/a | n/a | n/a | n/a |
TBA | Assay Description Measurement of forskolin stimulated inhibition of cAMP was performed via time-resolved fluorescence resonance energy transfer (TR-FRET) LANCE assay (... | Citation and Details BindingDB Entry DOI: 10.7270/Q2DB851B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 3 (Homo sapiens (Human)) | BDBM50400519 (CHEMBL2204941) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 5.70 | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Antagonist activity at human somatostatin receptor type 3 assessed as inhibition of cAMP levels | Bioorg Med Chem Lett 26: 1529-35 (2016) Article DOI: 10.1016/j.bmcl.2016.02.022 BindingDB Entry DOI: 10.7270/Q2ZG6V4J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 3 (Homo sapiens (Human)) | BDBM523763 (US11136312, Compound SK-I-53) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | n/a | 5.97 | n/a | n/a | n/a | n/a |
TBA | Assay Description Measurement of forskolin stimulated inhibition of cAMP was performed via time-resolved fluorescence resonance energy transfer (TR-FRET) LANCE assay (... | Citation and Details BindingDB Entry DOI: 10.7270/Q2DB851B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 3 (Homo sapiens (Human)) | BDBM523764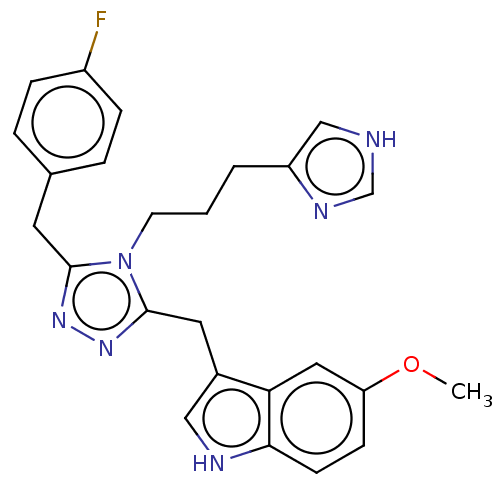 (US11136312, Compound SK-I-55) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | n/a | 6.02 | n/a | n/a | n/a | n/a |
TBA | Assay Description Measurement of forskolin stimulated inhibition of cAMP was performed via time-resolved fluorescence resonance energy transfer (TR-FRET) LANCE assay (... | Citation and Details BindingDB Entry DOI: 10.7270/Q2DB851B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 3 (Homo sapiens (Human)) | BDBM523738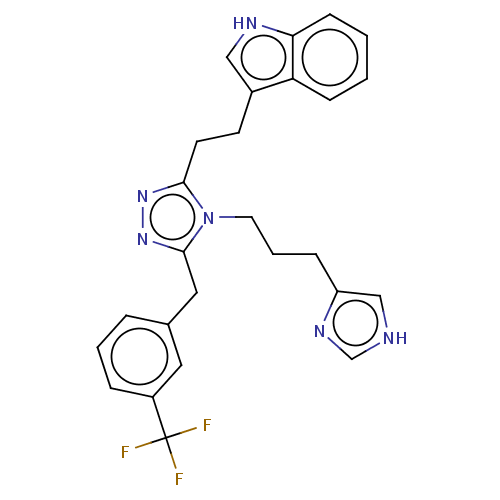 (US11136312, Compound BN-VI-97) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | n/a | 6.08 | n/a | n/a | n/a | n/a |
TBA | Assay Description Measurement of forskolin stimulated inhibition of cAMP was performed via time-resolved fluorescence resonance energy transfer (TR-FRET) LANCE assay (... | Citation and Details BindingDB Entry DOI: 10.7270/Q2DB851B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 3 (Homo sapiens (Human)) | BDBM50021064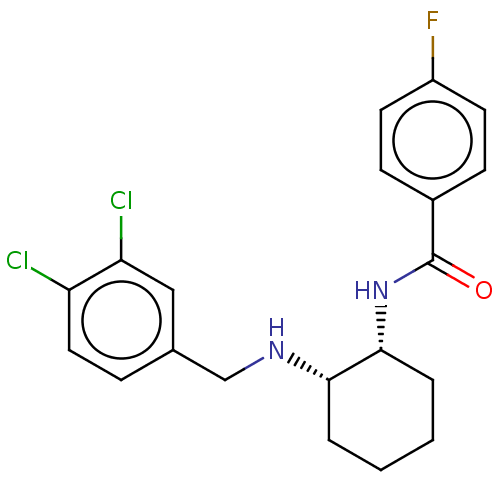 (CHEMBL3287614) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 6.10 | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Agonist activity at human SST3 expressed in CHO-K1 cells assessed as foreskin-stimulated cAMP accumulation after 45 mins by TR-FRET assay | ACS Med Chem Lett 5: 690-5 (2014) Article DOI: 10.1021/ml500079u BindingDB Entry DOI: 10.7270/Q22N53V4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 3 (Homo sapiens (Human)) | BDBM50154521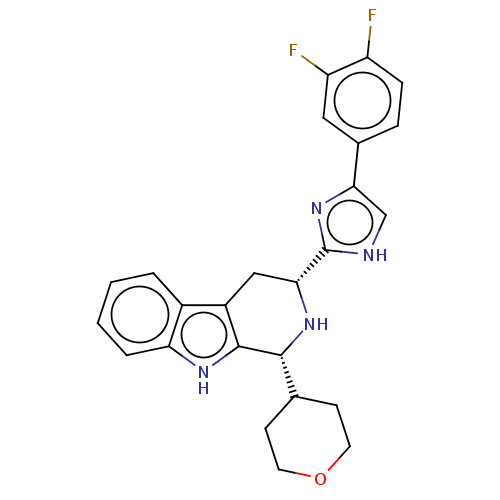 (CHEMBL3775532) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 6.40 | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Antagonist activity at human somatostatin receptor type 3 assessed as inhibition of cAMP levels | Bioorg Med Chem Lett 26: 1529-35 (2016) Article DOI: 10.1016/j.bmcl.2016.02.022 BindingDB Entry DOI: 10.7270/Q2ZG6V4J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 3 (Homo sapiens (Human)) | BDBM523768 (US11136312, Compound SK-I-105) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | n/a | 6.65 | n/a | n/a | n/a | n/a |
TBA | Assay Description Measurement of forskolin stimulated inhibition of cAMP was performed via time-resolved fluorescence resonance energy transfer (TR-FRET) LANCE assay (... | Citation and Details BindingDB Entry DOI: 10.7270/Q2DB851B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 3 (Homo sapiens (Human)) | BDBM523734 (US11136312, Compound BN-VI-56) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | n/a | 7.26 | n/a | n/a | n/a | n/a |
TBA | Assay Description Measurement of forskolin stimulated inhibition of cAMP was performed via time-resolved fluorescence resonance energy transfer (TR-FRET) LANCE assay (... | Citation and Details BindingDB Entry DOI: 10.7270/Q2DB851B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 3 (Homo sapiens (Human)) | BDBM523761 (US11136312, Compound SK-I-23) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | n/a | 7.27 | n/a | n/a | n/a | n/a |
TBA | Assay Description Measurement of forskolin stimulated inhibition of cAMP was performed via time-resolved fluorescence resonance energy transfer (TR-FRET) LANCE assay (... | Citation and Details BindingDB Entry DOI: 10.7270/Q2DB851B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 3 (Homo sapiens (Human)) | BDBM50154451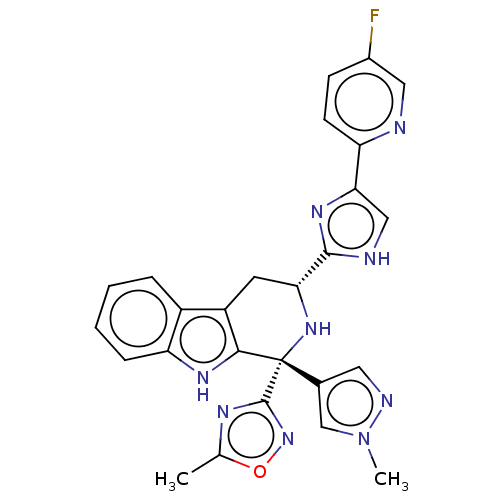 (CHEMBL3775061) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 7.40 | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Antagonist activity at human somatostatin receptor type 3 assessed as inhibition of cAMP levels | Bioorg Med Chem Lett 26: 1529-35 (2016) Article DOI: 10.1016/j.bmcl.2016.02.022 BindingDB Entry DOI: 10.7270/Q2ZG6V4J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 3 (Homo sapiens (Human)) | BDBM50546560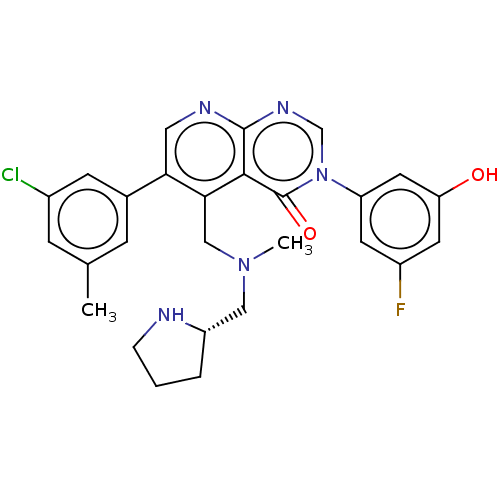 (CHEMBL4778342) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 7.90 | n/a | n/a | n/a | n/a |
TBA | Assay Description Agonist activity at human SST3 | Citation and Details Article DOI: 10.1016/j.bmcl.2020.127496 BindingDB Entry DOI: 10.7270/Q2183B4R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 3 (Homo sapiens (Human)) | BDBM50110461 (CHEMBL3605789) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 8.10 | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Agonist activity at human SSTR3 expressed in CHO cells assessed as intracellular cAMP level after 45 mins by time-resolved fluorescence assay | Bioorg Med Chem Lett 25: 3520-5 (2015) Article DOI: 10.1016/j.bmcl.2015.06.087 BindingDB Entry DOI: 10.7270/Q29W0H9M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 3 (Homo sapiens (Human)) | BDBM523793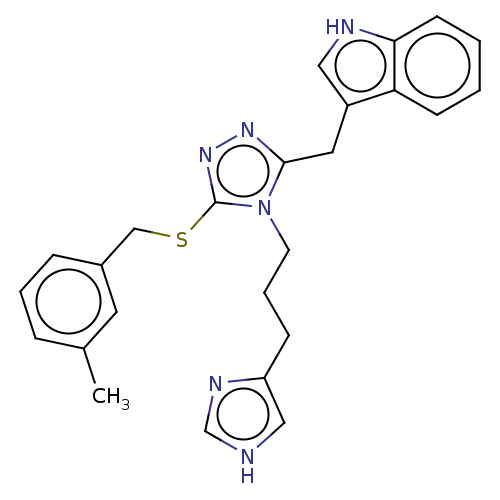 (US11136312, Compound AH.2.165) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | n/a | 8.41 | n/a | n/a | n/a | n/a |
TBA | Assay Description Measurement of forskolin stimulated inhibition of cAMP was performed via time-resolved fluorescence resonance energy transfer (TR-FRET) LANCE assay (... | Citation and Details BindingDB Entry DOI: 10.7270/Q2DB851B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 189 total ) | Next | Last >> |