

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Fatty acid synthase (Homo sapiens (Human)) | BDBM24567 ((2S)-1-[(2S,3S)-3-hexyl-4-oxooxetan-2-yl]tridecan-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid UniChem Patents Similars | DrugBank Article PubMed | 280 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wayne State University Curated by ChEMBL | Assay Description Antagonist activity at recombinant FASN thioesterase domain by fluorigenic assay | J Med Chem 54: 5615-38 (2011) Article DOI: 10.1021/jm2005805 BindingDB Entry DOI: 10.7270/Q21N8261 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fatty acid synthase [2202-2509] (Homo sapiens (Human)) | BDBM24988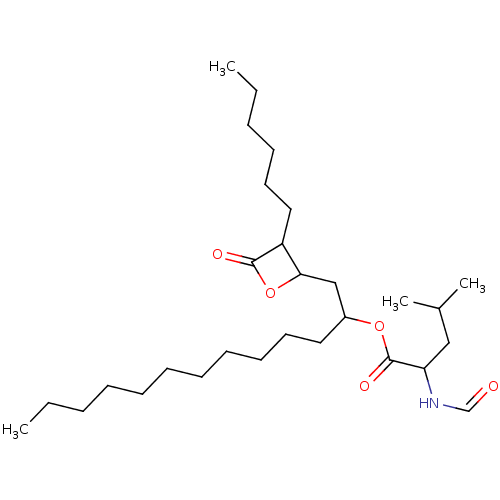 (1-(3-hexyl-4-oxooxetan-2-yl)tridecan-2-yl 2-formam...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | 300 | -9.25 | n/a | n/a | n/a | n/a | n/a | 7.5 | 37 |
Burnham Institute for Medical Research | Assay Description The reaction mixture consisted of FAS thioesterase domain (FASTE) in buffer, which was preincubated with test compounds for 30 min. The reaction was ... | Mol Cancer Ther 6: 2120-6 (2007) Article DOI: 10.1158/1535-7163.MCT-07-0187 BindingDB Entry DOI: 10.7270/Q2F769V8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fatty acid synthase (Homo sapiens (Human)) | BDBM50124408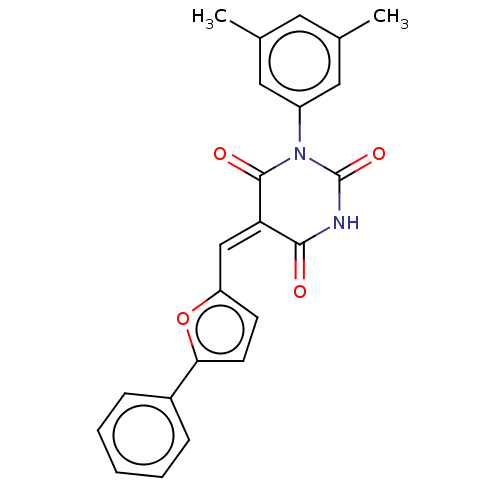 (CHEMBL2165266) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 380 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of South Alabama Curated by ChEMBL | Assay Description Inhibition of fatty acid synthase KE domain (unknown origin) | Bioorg Med Chem Lett 25: 4363-9 (2015) Article DOI: 10.1016/j.bmcl.2015.08.087 BindingDB Entry DOI: 10.7270/Q2057HR2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fatty acid synthase [2202-2509] (Homo sapiens (Human)) | BDBM24984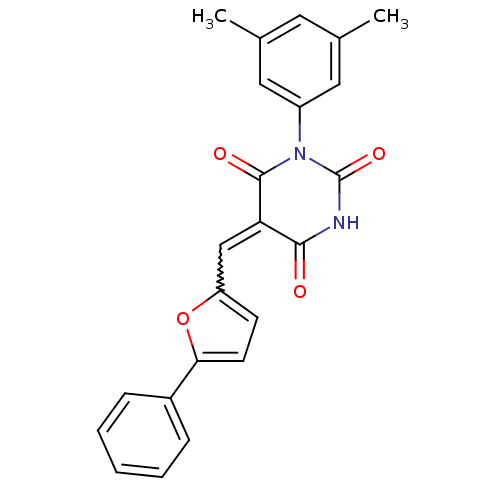 ((5E)-1-(3,5-dimethylphenyl)-5-[(5-phenylfuran-2-yl...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | 380 | -9.10 | n/a | n/a | n/a | n/a | n/a | 7.5 | 37 |
Burnham Institute for Medical Research | Assay Description The reaction mixture consisted of FAS thioesterase domain (FASTE) in buffer, which was preincubated with test compounds for 30 min. The reaction was ... | Mol Cancer Ther 6: 2120-6 (2007) Article DOI: 10.1158/1535-7163.MCT-07-0187 BindingDB Entry DOI: 10.7270/Q2F769V8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fatty acid synthase (Homo sapiens (Human)) | BDBM24984 ((5E)-1-(3,5-dimethylphenyl)-5-[(5-phenylfuran-2-yl...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | 380 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wayne State University Curated by ChEMBL | Assay Description Antagonist activity at human recombinant FASN thioesterase domain by fluorigenic assay | J Med Chem 54: 5615-38 (2011) Article DOI: 10.1021/jm2005805 BindingDB Entry DOI: 10.7270/Q21N8261 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fatty acid synthase [2202-2509] (Homo sapiens (Human)) | BDBM24987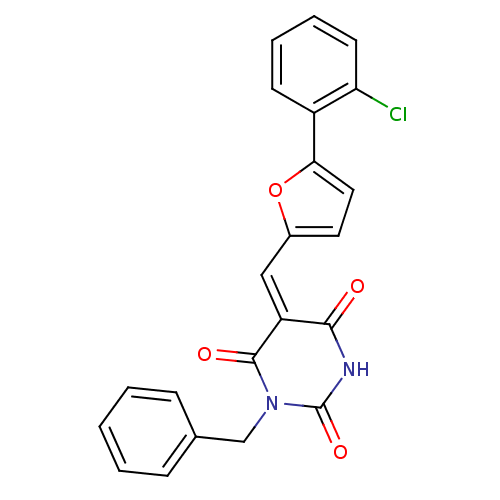 ((5E)-1-benzyl-5-{[5-(2-chlorophenyl)furan-2-yl]met...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | 850 | -8.61 | n/a | n/a | n/a | n/a | n/a | 7.5 | 37 |
Burnham Institute for Medical Research | Assay Description The reaction mixture consisted of FAS thioesterase domain (FASTE) in buffer, which was preincubated with test compounds for 30 min. The reaction was ... | Mol Cancer Ther 6: 2120-6 (2007) Article DOI: 10.1158/1535-7163.MCT-07-0187 BindingDB Entry DOI: 10.7270/Q2F769V8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fatty acid synthase [2202-2509] (Homo sapiens (Human)) | BDBM24986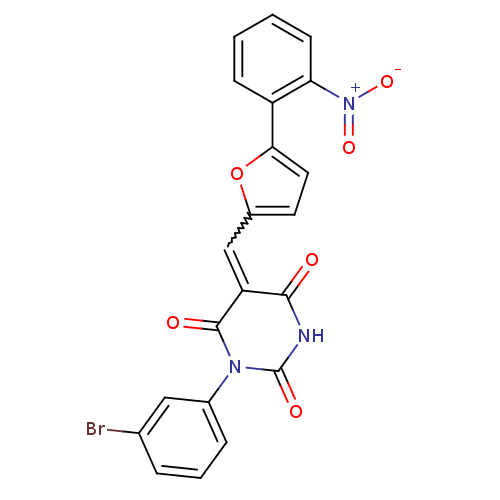 ((5E)-1-(3-bromophenyl)-5-{[5-(2-nitrophenyl)furan-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 880 | -8.59 | n/a | n/a | n/a | n/a | n/a | 7.5 | 37 |
Burnham Institute for Medical Research | Assay Description The reaction mixture consisted of FAS thioesterase domain (FASTE) in buffer, which was preincubated with test compounds for 30 min. The reaction was ... | Mol Cancer Ther 6: 2120-6 (2007) Article DOI: 10.1158/1535-7163.MCT-07-0187 BindingDB Entry DOI: 10.7270/Q2F769V8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fatty acid synthase [2202-2509] (Homo sapiens (Human)) | BDBM24985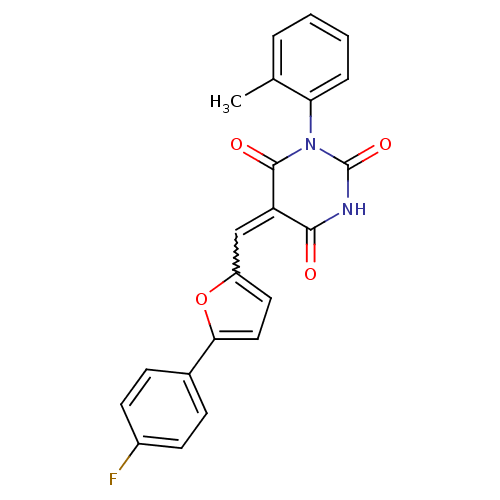 ((5E)-5-{[5-(4-fluorophenyl)furan-2-yl]methylidene}...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 910 | -8.57 | n/a | n/a | n/a | n/a | n/a | 7.5 | 37 |
Burnham Institute for Medical Research | Assay Description The reaction mixture consisted of FAS thioesterase domain (FASTE) in buffer, which was preincubated with test compounds for 30 min. The reaction was ... | Mol Cancer Ther 6: 2120-6 (2007) Article DOI: 10.1158/1535-7163.MCT-07-0187 BindingDB Entry DOI: 10.7270/Q2F769V8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fatty acid synthase (Homo sapiens (Human)) | BDBM50070208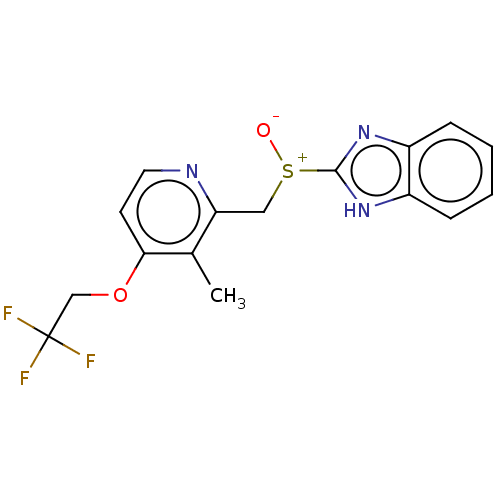 (A-65006 | AG-1749 | CHEBI:6375 | Lansoprazole | Pr...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 1.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1021/acs.jmedchem.2c00642 BindingDB Entry DOI: 10.7270/Q24J0K3R | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fatty acid synthase (Homo sapiens (Human)) | BDBM50394664 (CHEMBL2165413) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wayne State University Curated by ChEMBL | Assay Description Antagonist activity at recombinant FASN thioesterase domain by fluorigenic assay | J Med Chem 54: 5615-38 (2011) Article DOI: 10.1021/jm2005805 BindingDB Entry DOI: 10.7270/Q21N8261 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fatty acid synthase (Homo sapiens (Human)) | BDBM50241343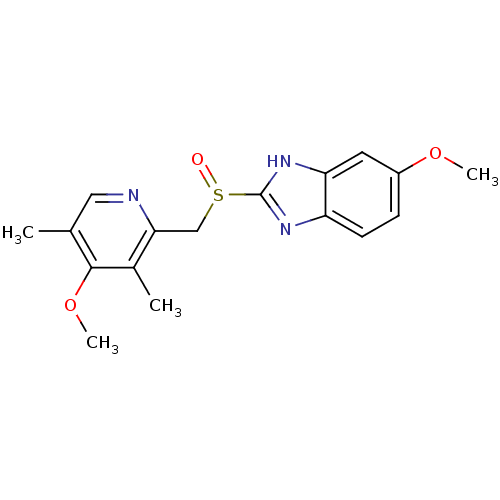 ((RS)-6-methoxy-2-((4-methoxy-3,5-dimethylpyridin-2...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 3.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Indiana University School of Medicine Curated by ChEMBL | Assay Description Inhibition of purified recombinant FASN TE activity (unknown origin) using 4-MUH as substrate preincubated for 30 mins before substrate addition meas... | J Med Chem 58: 778-84 (2015) Article DOI: 10.1021/jm501543u BindingDB Entry DOI: 10.7270/Q20K2B7H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fatty acid synthase (Homo sapiens (Human)) | BDBM50241342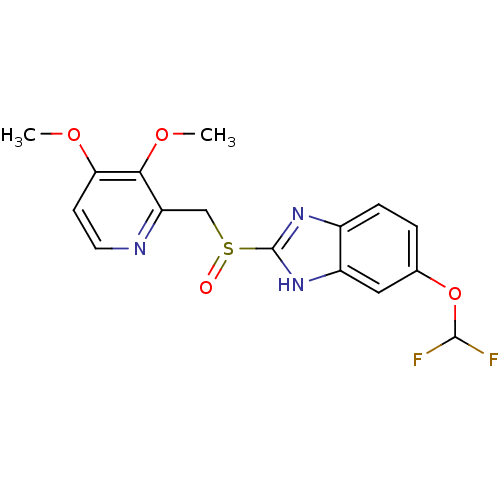 (5-(difluoromethoxy)-2-((3,4-dimethoxypyridin-2-yl)...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 4.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Indiana University School of Medicine Curated by ChEMBL | Assay Description Inhibition of purified recombinant FASN TE activity (unknown origin) using 4-MUH as substrate preincubated for 30 mins before substrate addition meas... | J Med Chem 58: 778-84 (2015) Article DOI: 10.1021/jm501543u BindingDB Entry DOI: 10.7270/Q20K2B7H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fatty acid synthase (Homo sapiens (Human)) | BDBM50070208 (A-65006 | AG-1749 | CHEBI:6375 | Lansoprazole | Pr...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 5.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Indiana University School of Medicine Curated by ChEMBL | Assay Description Inhibition of purified recombinant FASN TE activity (unknown origin) using 4-MUH as substrate preincubated for 30 mins before substrate addition meas... | J Med Chem 58: 778-84 (2015) Article DOI: 10.1021/jm501543u BindingDB Entry DOI: 10.7270/Q20K2B7H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fatty acid synthase (Homo sapiens (Human)) | BDBM50070209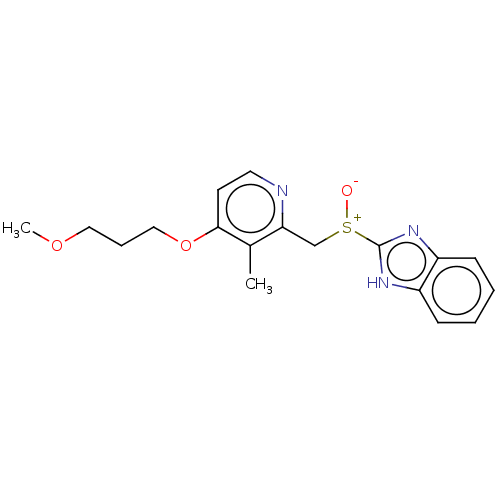 (Aciphex | CHEBI:8768 | LY-307640 | Rabeprazole) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 5.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Indiana University School of Medicine Curated by ChEMBL | Assay Description Inhibition of purified recombinant FASN TE activity (unknown origin) using 4-MUH as substrate preincubated for 30 mins before substrate addition meas... | J Med Chem 58: 778-84 (2015) Article DOI: 10.1021/jm501543u BindingDB Entry DOI: 10.7270/Q20K2B7H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fatty acid synthase (Homo sapiens (Human)) | BDBM50142501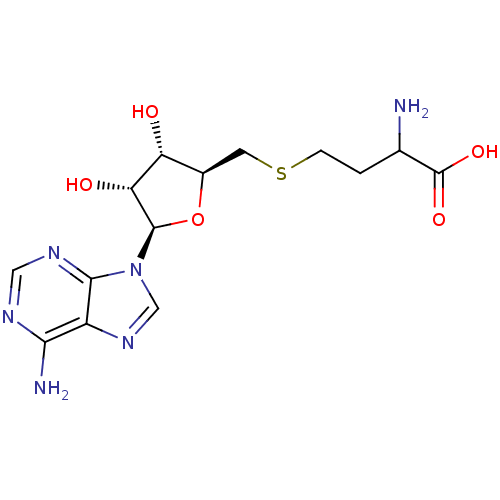 ((+/-)-2-amino-4-(((2S,3S,4R,5R)-5-(6-amino-9H-puri...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ Pierre et Marie Curie Curated by ChEMBL | Assay Description Inhibition constant against Escherichia coli cyclopropane fatty acid synthase | Bioorg Med Chem Lett 14: 1661-4 (2004) Article DOI: 10.1016/j.bmcl.2004.01.051 BindingDB Entry DOI: 10.7270/Q26H4HZX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fatty acid synthase (Homo sapiens (Human)) | BDBM50142502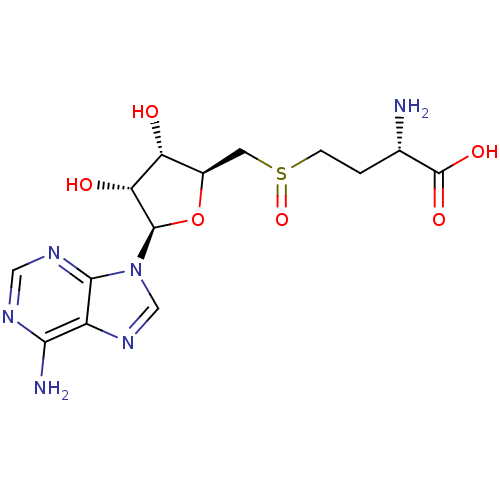 (2-Amino-4-[(2S,3S,4R,5R)-5-(6-amino-purin-9-yl)-3,...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ Pierre et Marie Curie Curated by ChEMBL | Assay Description Inhibition constant against Escherichia coli cyclopropane fatty acid synthase | Bioorg Med Chem Lett 14: 1661-4 (2004) Article DOI: 10.1016/j.bmcl.2004.01.051 BindingDB Entry DOI: 10.7270/Q26H4HZX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fatty acid synthase (Homo sapiens (Human)) | BDBM50142502 (2-Amino-4-[(2S,3S,4R,5R)-5-(6-amino-purin-9-yl)-3,...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ Pierre et Marie Curie Curated by ChEMBL | Assay Description Inhibition constant against Escherichia coli cyclopropane fatty acid synthase | Bioorg Med Chem Lett 14: 1661-4 (2004) Article DOI: 10.1016/j.bmcl.2004.01.051 BindingDB Entry DOI: 10.7270/Q26H4HZX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fatty acid synthase (Homo sapiens (Human)) | BDBM50142502 (2-Amino-4-[(2S,3S,4R,5R)-5-(6-amino-purin-9-yl)-3,...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ Pierre et Marie Curie Curated by ChEMBL | Assay Description Inhibition constant against Escherichia coli cyclopropane fatty acid synthase | Bioorg Med Chem Lett 14: 1661-4 (2004) Article DOI: 10.1016/j.bmcl.2004.01.051 BindingDB Entry DOI: 10.7270/Q26H4HZX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fatty acid synthase (Homo sapiens (Human)) | BDBM50142505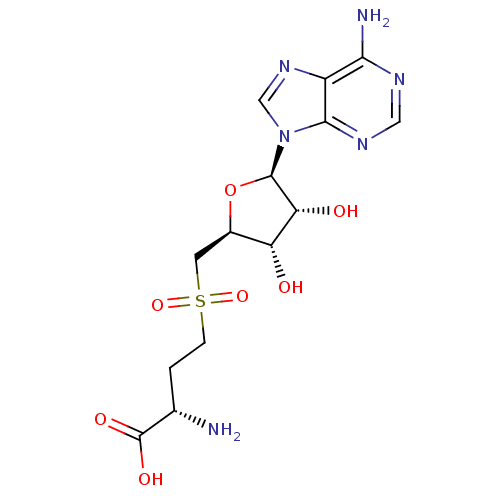 (2-Amino-4-[(2S,3S,4R,5R)-5-(6-amino-purin-9-yl)-3,...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ Pierre et Marie Curie Curated by ChEMBL | Assay Description Inhibition constant against Escherichia coli cyclopropane fatty acid synthase | Bioorg Med Chem Lett 14: 1661-4 (2004) Article DOI: 10.1016/j.bmcl.2004.01.051 BindingDB Entry DOI: 10.7270/Q26H4HZX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fatty acid synthase (Homo sapiens (Human)) | BDBM50009672 (AdoHcy | CHEMBL418052 | S-(5'-adenosyl)-L-homocyst...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 1.50E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ Pierre et Marie Curie Curated by ChEMBL | Assay Description Inhibition constant against Escherichia coli cyclopropane fatty acid synthase | Bioorg Med Chem Lett 14: 1661-4 (2004) Article DOI: 10.1016/j.bmcl.2004.01.051 BindingDB Entry DOI: 10.7270/Q26H4HZX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fatty acid synthase (Homo sapiens (Human)) | BDBM50142506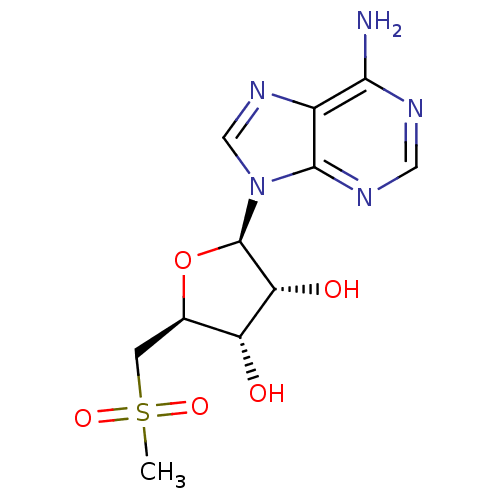 ((2R,3R,4S,5S)-2-(6-Amino-purin-9-yl)-5-methanesulf...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.20E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ Pierre et Marie Curie Curated by ChEMBL | Assay Description Inhibition constant against Escherichia coli cyclopropane fatty acid synthase | Bioorg Med Chem Lett 14: 1661-4 (2004) Article DOI: 10.1016/j.bmcl.2004.01.051 BindingDB Entry DOI: 10.7270/Q26H4HZX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fatty acid synthase (Homo sapiens (Human)) | BDBM50142504 ((2R,3R,4S,5S)-2-(6-Amino-purin-9-yl)-5-methanesulf...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 5.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ Pierre et Marie Curie Curated by ChEMBL | Assay Description Inhibition constant against Escherichia coli cyclopropane fatty acid synthase | Bioorg Med Chem Lett 14: 1661-4 (2004) Article DOI: 10.1016/j.bmcl.2004.01.051 BindingDB Entry DOI: 10.7270/Q26H4HZX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fatty acid synthase (Homo sapiens (Human)) | BDBM22111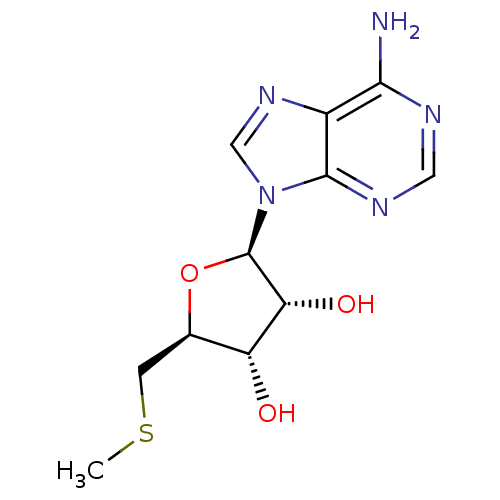 ((2R,3R,4S,5S)-2-(6-amino-9H-purin-9-yl)-5-[(methyl...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 1.50E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ Pierre et Marie Curie Curated by ChEMBL | Assay Description Inhibition constant against Escherichia coli cyclopropane fatty acid synthase | Bioorg Med Chem Lett 14: 1661-4 (2004) Article DOI: 10.1016/j.bmcl.2004.01.051 BindingDB Entry DOI: 10.7270/Q26H4HZX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fatty acid synthase (Homo sapiens (Human)) | BDBM50366942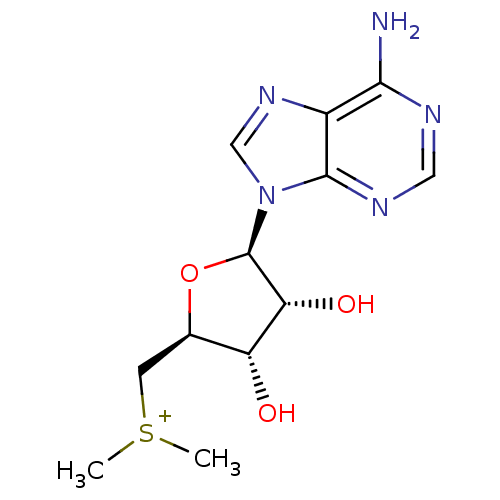 (CHEMBL540135) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.50E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ Pierre et Marie Curie Curated by ChEMBL | Assay Description Inhibition constant against Escherichia coli cyclopropane fatty acid synthase | Bioorg Med Chem Lett 14: 1661-4 (2004) Article DOI: 10.1016/j.bmcl.2004.01.051 BindingDB Entry DOI: 10.7270/Q26H4HZX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fatty acid synthase (Homo sapiens (Human)) | BDBM50291713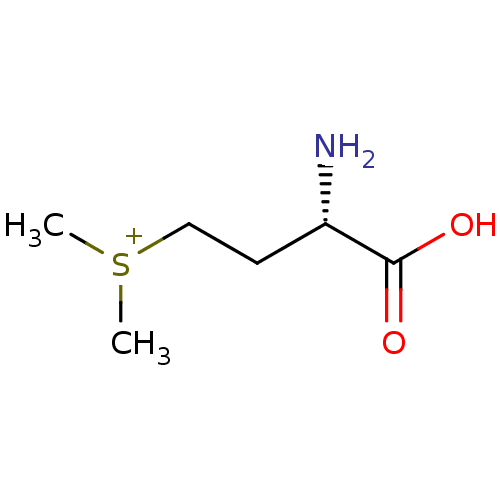 (((S)-3-Amino-3-carboxy-propyl)-dimethyl-sulfonium ...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid UniChem Similars | Article PubMed | 1.40E+8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ Pierre et Marie Curie Curated by ChEMBL | Assay Description Inhibition constant against Escherichia coli cyclopropane fatty acid synthase | Bioorg Med Chem Lett 14: 1661-4 (2004) Article DOI: 10.1016/j.bmcl.2004.01.051 BindingDB Entry DOI: 10.7270/Q26H4HZX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fatty acid synthase (Homo sapiens (Human)) | BDBM50142500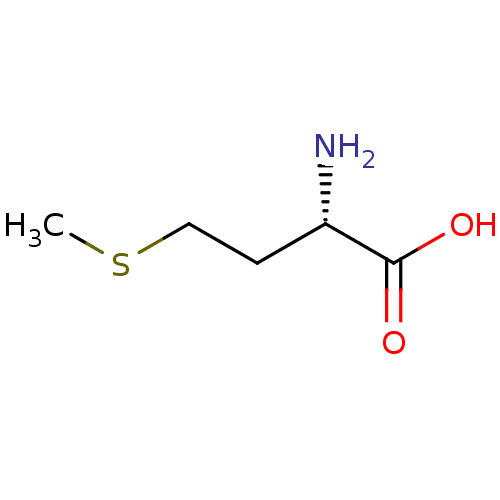 ((2S)-2-amino-4-(methylsulfanyl)butanoic acid | (S)...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | >1.50E+8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ Pierre et Marie Curie Curated by ChEMBL | Assay Description Inhibition constant against Escherichia coli cyclopropane fatty acid synthase | Bioorg Med Chem Lett 14: 1661-4 (2004) Article DOI: 10.1016/j.bmcl.2004.01.051 BindingDB Entry DOI: 10.7270/Q26H4HZX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||