

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Nuclear receptor ROR-gamma (Homo sapiens (Human)) | BDBM50543677 (CHEMBL4635823) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Phenex Pharmaceuticals AG Curated by ChEMBL | Assay Description Binding affinity to recombinant human RORgammat transfected in human HEK293T cells incubated for 24 hrs by dual-glo luciferase reporter gene assay | Bioorg Med Chem Lett 30: (2020) Article DOI: 10.1016/j.bmcl.2020.127174 BindingDB Entry DOI: 10.7270/Q2862M1T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor ROR-gamma (Homo sapiens (Human)) | BDBM392066 (US10301272, Example 6/64) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.370 | n/a | n/a | n/a | n/a | n/a | n/a |
Phenex Pharmaceuticals AG Curated by ChEMBL | Assay Description Inverse agonist activity at human GAL4 DBD-fused RORgammat LBD (237 to 497 residues) expressed in HEK293T cells assessed as reduction in ROR mediated... | Bioorg Med Chem Lett 28: 1446-1455 (2018) Article DOI: 10.1016/j.bmcl.2018.03.093 BindingDB Entry DOI: 10.7270/Q27W6FPC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor ROR-gamma (Homo sapiens (Human)) | BDBM359566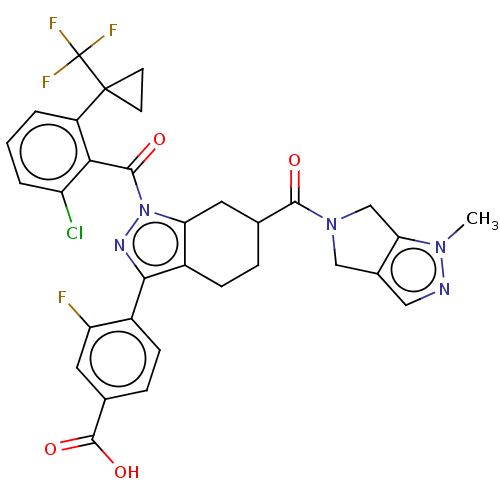 ((R or S)-4-(1-(2- chloro-6-(1- (trifluoromethyl)cy...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description The compounds of the invention inhibit RORgammaT activity. Activation of RORgammaT activity can be measured using, e.g., biochemical TR-FRET assay. I... | US Patent US10221142 (2019) BindingDB Entry DOI: 10.7270/Q2M90BZ0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor ROR-gamma (Homo sapiens (Human)) | BDBM359568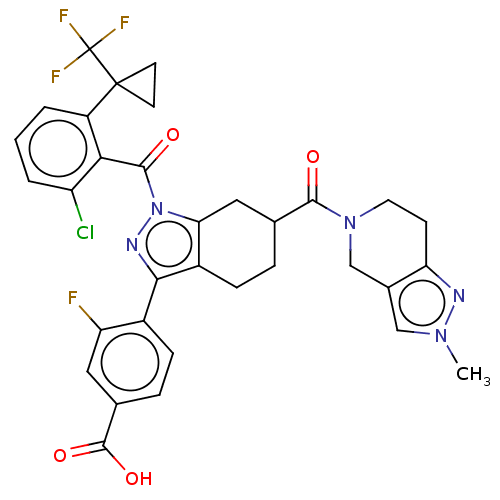 ((R or S)-4-(1-(2- chloro-6-(1- (trifluoromethyl)cy...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description The compounds of the invention inhibit RORgammaT activity. Activation of RORgammaT activity can be measured using, e.g., biochemical TR-FRET assay. I... | US Patent US10221142 (2019) BindingDB Entry DOI: 10.7270/Q2M90BZ0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor ROR-gamma (Homo sapiens (Human)) | BDBM359569 ((R or S)-4-(1-(2- chloro-6-(1- (trifluoromethyl)cy...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description The compounds of the invention inhibit RORgammaT activity. Activation of RORgammaT activity can be measured using, e.g., biochemical TR-FRET assay. I... | US Patent US10221142 (2019) BindingDB Entry DOI: 10.7270/Q2M90BZ0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor ROR-gamma (Homo sapiens (Human)) | BDBM359544 (4-{(6R or S)-1-({2- chloro-6-[1- (difluoromethyl)c...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description The compounds of the invention inhibit RORgammaT activity. Activation of RORgammaT activity can be measured using, e.g., biochemical TR-FRET assay. I... | US Patent US10221142 (2019) BindingDB Entry DOI: 10.7270/Q2M90BZ0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor ROR-gamma (Homo sapiens (Human)) | BDBM359570 ((R or S)-4-(1-(2- chloro-6-(1- (trifluoromethyl)cy...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description The compounds of the invention inhibit RORgammaT activity. Activation of RORgammaT activity can be measured using, e.g., biochemical TR-FRET assay. I... | US Patent US10221142 (2019) BindingDB Entry DOI: 10.7270/Q2M90BZ0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor ROR-gamma (Homo sapiens (Human)) | BDBM359543 (4-[(6R or S)-1-({2- chloro-6-[1- (trifluoromethyl)...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description The compounds of the invention inhibit RORgammaT activity. Activation of RORgammaT activity can be measured using, e.g., biochemical TR-FRET assay. I... | US Patent US10221142 (2019) BindingDB Entry DOI: 10.7270/Q2M90BZ0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor ROR-gamma (Homo sapiens (Human)) | BDBM359567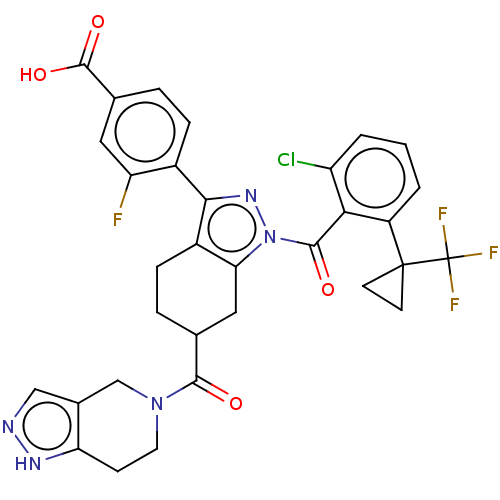 ((R or S)-4-(1-(2- chloro-6-(1- (trifluoromethyl)cy...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description The compounds of the invention inhibit RORgammaT activity. Activation of RORgammaT activity can be measured using, e.g., biochemical TR-FRET assay. I... | US Patent US10221142 (2019) BindingDB Entry DOI: 10.7270/Q2M90BZ0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor ROR-gamma (Homo sapiens (Human)) | BDBM359571 ((R or S)-4-(1-(2- chloro-6-(1- (trifluoromethyl)c...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description The compounds of the invention inhibit RORgammaT activity. Activation of RORgammaT activity can be measured using, e.g., biochemical TR-FRET assay. I... | US Patent US10221142 (2019) BindingDB Entry DOI: 10.7270/Q2M90BZ0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor ROR-gamma (Homo sapiens (Human)) | BDBM359545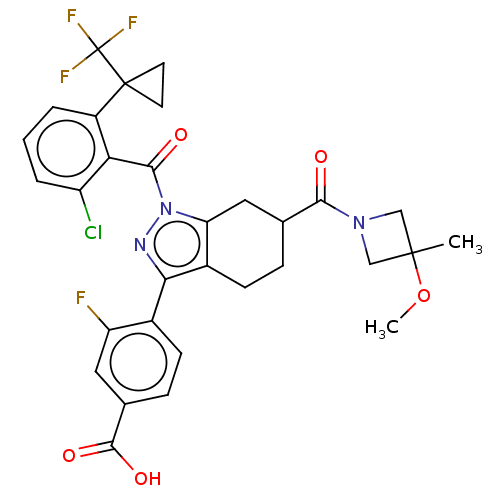 (4-{(6R or S)-1-({2- chloro-6-[1- (trifluoromethyl)...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description The compounds of the invention inhibit RORgammaT activity. Activation of RORgammaT activity can be measured using, e.g., biochemical TR-FRET assay. I... | US Patent US10221142 (2019) BindingDB Entry DOI: 10.7270/Q2M90BZ0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor ROR-gamma (Homo sapiens (Human)) | BDBM50281849 (CHEMBL4175305) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inverse agonist activity at human His6-tagged RORgammat LBD (264 to 518 residues) assessed as reduction in biotinylated RIP140 co-activator recruitme... | J Med Chem 61: 6724-6735 (2018) Article DOI: 10.1021/acs.jmedchem.8b00529 BindingDB Entry DOI: 10.7270/Q2Z60RK3 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Nuclear receptor ROR-gamma (Homo sapiens (Human)) | BDBM359548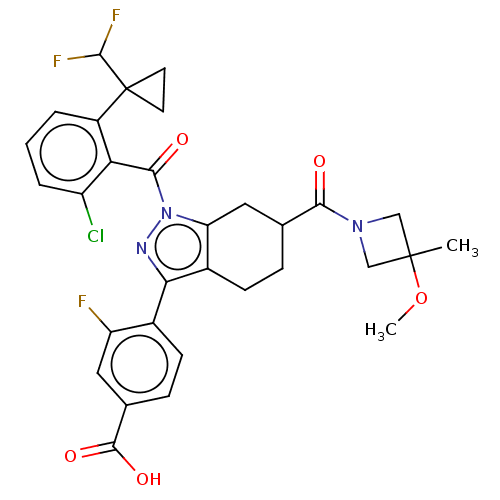 (4-{(6R or S)-1-({2- chloro-6-[1- (difluoromethyl)c...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description The compounds of the invention inhibit RORgammaT activity. Activation of RORgammaT activity can be measured using, e.g., biochemical TR-FRET assay. I... | US Patent US10221142 (2019) BindingDB Entry DOI: 10.7270/Q2M90BZ0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor ROR-gamma (Homo sapiens (Human)) | BDBM363753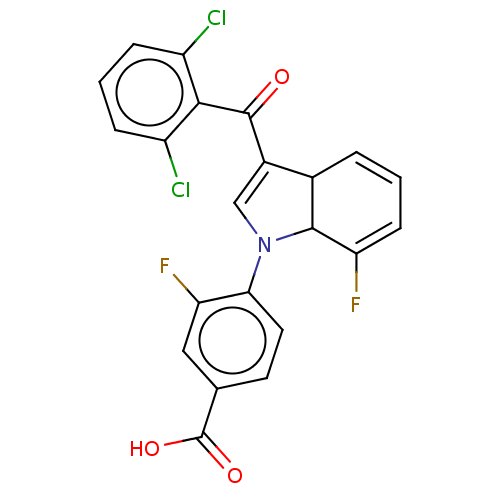 (US9855229, Compound 7) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.615 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research | Assay Description The assay is based on the principle that binding of the agonist to the RORγ causes a conformational change around helix 12 in the ligand binding... | J Med Chem 46: 3709-27 (2003) BindingDB Entry DOI: 10.7270/Q20867MM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor ROR-gamma (Homo sapiens (Human)) | BDBM392013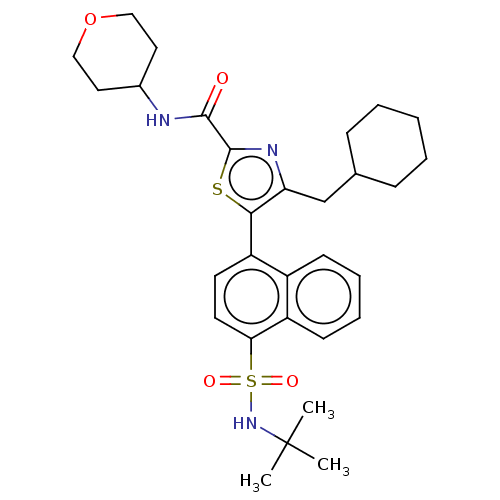 (US10301272, Example 6/11) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.690 | n/a | n/a | n/a | n/a | n/a | n/a |
Phenex Pharmaceuticals AG Curated by ChEMBL | Assay Description Inverse agonist activity at human GAL4 DBD-fused RORgammat LBD (237 to 497 residues) expressed in HEK293T cells assessed as reduction in ROR mediated... | Bioorg Med Chem Lett 28: 1446-1455 (2018) Article DOI: 10.1016/j.bmcl.2018.03.093 BindingDB Entry DOI: 10.7270/Q27W6FPC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor ROR-gamma (Homo sapiens (Human)) | BDBM359540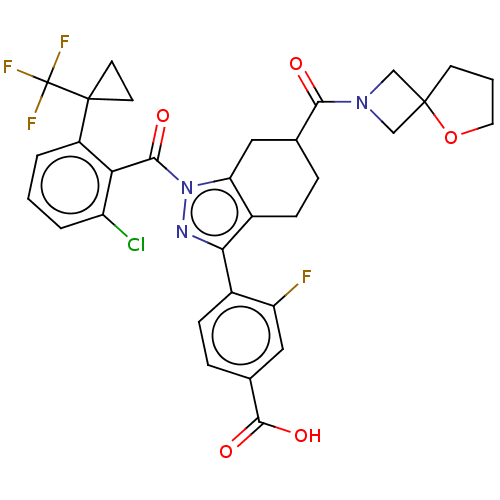 (4-[(6R or S)-1-({2- chloro-6-[1- (trifluoromethyl)...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description The compounds of the invention inhibit RORgammaT activity. Activation of RORgammaT activity can be measured using, e.g., biochemical TR-FRET assay. I... | US Patent US10221142 (2019) BindingDB Entry DOI: 10.7270/Q2M90BZ0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor ROR-gamma (Homo sapiens (Human)) | BDBM359547 (4-[(6R or S)-1-({2- chloro-6-[1- (trifluoromethyl)...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description The compounds of the invention inhibit RORgammaT activity. Activation of RORgammaT activity can be measured using, e.g., biochemical TR-FRET assay. I... | US Patent US10221142 (2019) BindingDB Entry DOI: 10.7270/Q2M90BZ0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor ROR-gamma (Homo sapiens (Human)) | BDBM359582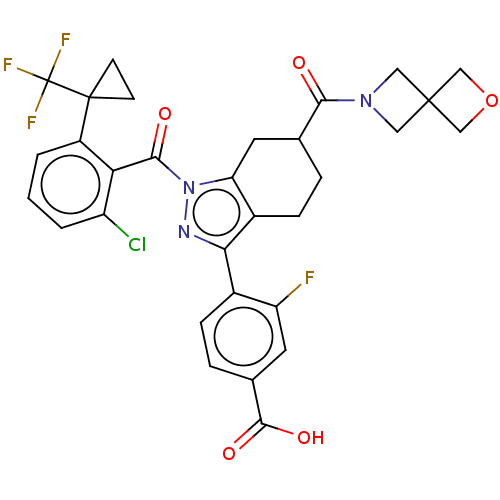 ((R or S)-4-(1-(2- chloro-6-(1- (trifluoromethyl)cy...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description The compounds of the invention inhibit RORgammaT activity. Activation of RORgammaT activity can be measured using, e.g., biochemical TR-FRET assay. I... | US Patent US10221142 (2019) BindingDB Entry DOI: 10.7270/Q2M90BZ0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor ROR-gamma (Homo sapiens (Human)) | BDBM359577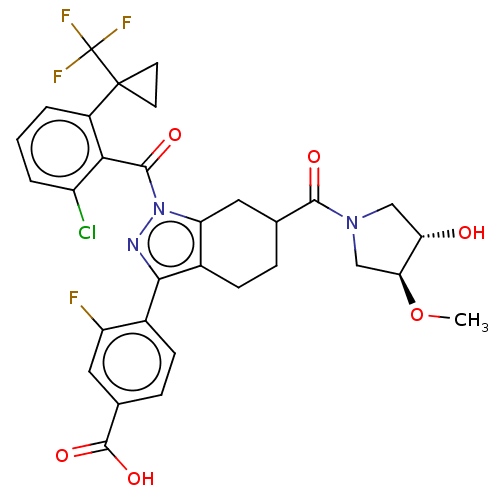 (4-[(6R or S)-1-({2- chloro-6-[1- (trifluoromethyl)...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description The compounds of the invention inhibit RORgammaT activity. Activation of RORgammaT activity can be measured using, e.g., biochemical TR-FRET assay. I... | US Patent US10221142 (2019) BindingDB Entry DOI: 10.7270/Q2M90BZ0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor ROR-gamma (Homo sapiens (Human)) | BDBM359535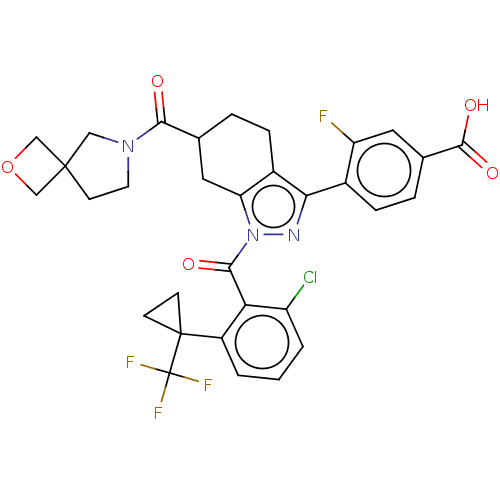 (US10221142, Example 21A) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description The compounds of the invention inhibit RORgammaT activity. Activation of RORgammaT activity can be measured using, e.g., biochemical TR-FRET assay. I... | US Patent US10221142 (2019) BindingDB Entry DOI: 10.7270/Q2M90BZ0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor ROR-gamma (Homo sapiens (Human)) | BDBM392136 (US10301272, Example 14/6) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.710 | n/a | n/a | n/a | n/a | n/a | n/a |
Phenex Pharmaceuticals AG Curated by ChEMBL | Assay Description Inverse agonist activity at human GAL4 DBD-fused RORgammat LBD (237 to 497 residues) expressed in HEK293T cells assessed as reduction in ROR mediated... | Bioorg Med Chem Lett 28: 1446-1455 (2018) Article DOI: 10.1016/j.bmcl.2018.03.093 BindingDB Entry DOI: 10.7270/Q27W6FPC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor ROR-gamma (Homo sapiens (Human)) | BDBM359551 (4-[(6R or S)-1-({2- chloro-6-[1- (difluoromethyl)c...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description The compounds of the invention inhibit RORgammaT activity. Activation of RORgammaT activity can be measured using, e.g., biochemical TR-FRET assay. I... | US Patent US10221142 (2019) BindingDB Entry DOI: 10.7270/Q2M90BZ0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor ROR-gamma (Homo sapiens (Human)) | BDBM359550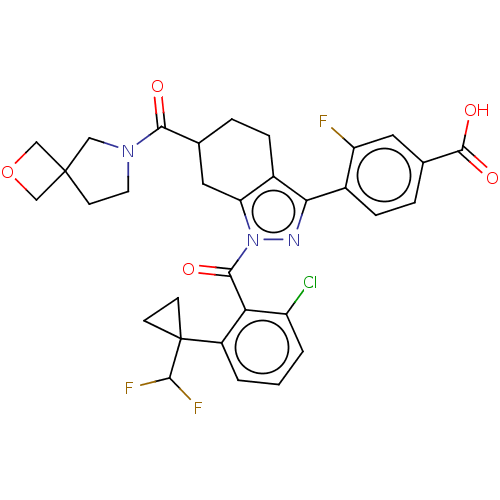 (4-[(6R or S)-1-({2- chloro-6-[1- (difluoromethyl)c...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description The compounds of the invention inhibit RORgammaT activity. Activation of RORgammaT activity can be measured using, e.g., biochemical TR-FRET assay. I... | US Patent US10221142 (2019) BindingDB Entry DOI: 10.7270/Q2M90BZ0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor ROR-gamma (Homo sapiens (Human)) | BDBM359586 (4-{(6R or S)-1-({2- chloro-6-[1- (trifluoromethyl)...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description The compounds of the invention inhibit RORgammaT activity. Activation of RORgammaT activity can be measured using, e.g., biochemical TR-FRET assay. I... | US Patent US10221142 (2019) BindingDB Entry DOI: 10.7270/Q2M90BZ0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor ROR-gamma (Homo sapiens (Human)) | BDBM359578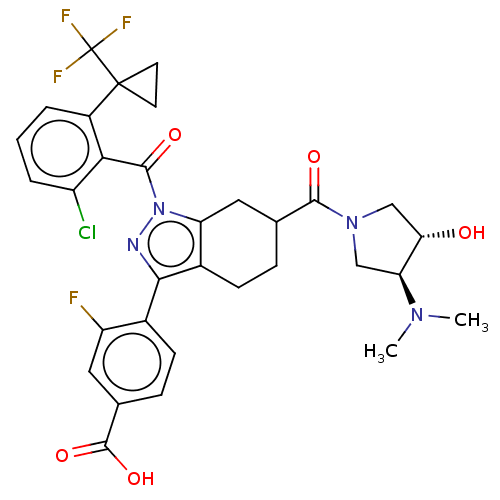 (4-[(6R or S)-1-({2- chloro-6-[1- (trifluoromethyl)...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description The compounds of the invention inhibit RORgammaT activity. Activation of RORgammaT activity can be measured using, e.g., biochemical TR-FRET assay. I... | US Patent US10221142 (2019) BindingDB Entry DOI: 10.7270/Q2M90BZ0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor ROR-gamma (Homo sapiens (Human)) | BDBM359539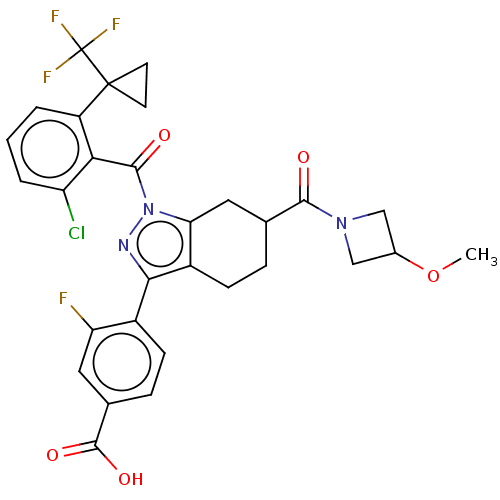 (4-{(6R or S)-1-({2- chloro-6-[1- (trifluoromethyl...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description The compounds of the invention inhibit RORgammaT activity. Activation of RORgammaT activity can be measured using, e.g., biochemical TR-FRET assay. I... | US Patent US10221142 (2019) BindingDB Entry DOI: 10.7270/Q2M90BZ0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor ROR-gamma (Homo sapiens (Human)) | BDBM359565 ((R or S)-4-(1-(2- chloro-6-(1- (trifluoromethyl)cy...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description The compounds of the invention inhibit RORgammaT activity. Activation of RORgammaT activity can be measured using, e.g., biochemical TR-FRET assay. I... | US Patent US10221142 (2019) BindingDB Entry DOI: 10.7270/Q2M90BZ0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor ROR-gamma (Homo sapiens (Human)) | BDBM189904 (US10227346, Example 29 | US10426135, Example 29 | ...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
Montana State University | J Med Chem 50: 4928-38 (2007) BindingDB Entry DOI: 10.7270/Q2GH9M91 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor ROR-gamma (Homo sapiens (Human)) | BDBM359584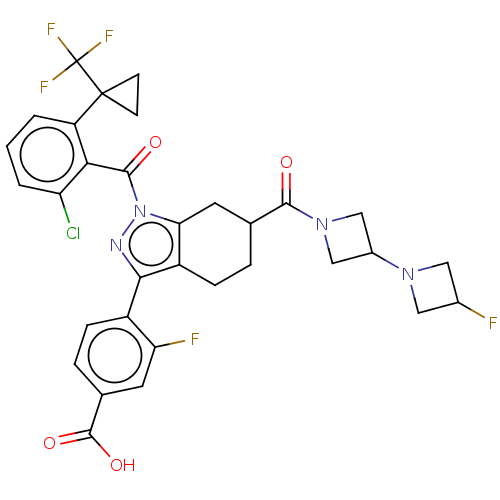 ((R or S)-4-(1-(2- chloro-6-(1- (trifluoromethyl)cy...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description The compounds of the invention inhibit RORgammaT activity. Activation of RORgammaT activity can be measured using, e.g., biochemical TR-FRET assay. I... | US Patent US10221142 (2019) BindingDB Entry DOI: 10.7270/Q2M90BZ0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor ROR-gamma (Homo sapiens (Human)) | BDBM359574 (4-[(6R or S)-1-({2- chloro-6-[1- (trifluoromethyl)...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description The compounds of the invention inhibit RORgammaT activity. Activation of RORgammaT activity can be measured using, e.g., biochemical TR-FRET assay. I... | US Patent US10221142 (2019) BindingDB Entry DOI: 10.7270/Q2M90BZ0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor ROR-gamma (Homo sapiens (Human)) | BDBM359564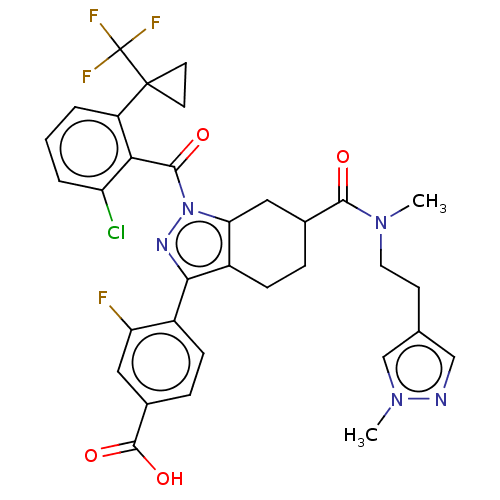 ((R or S)-4-(1-(2- chloro-6-(1- (trifluoromethyl)cy...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description The compounds of the invention inhibit RORgammaT activity. Activation of RORgammaT activity can be measured using, e.g., biochemical TR-FRET assay. I... | US Patent US10221142 (2019) BindingDB Entry DOI: 10.7270/Q2M90BZ0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor ROR-gamma (Homo sapiens (Human)) | BDBM359555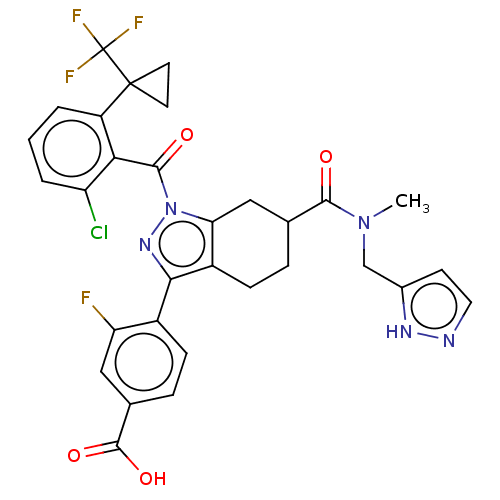 ((R or S)-4-(6-(((1H- pyrazol-5- yl)methyl)(methyl)...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description The compounds of the invention inhibit RORgammaT activity. Activation of RORgammaT activity can be measured using, e.g., biochemical TR-FRET assay. I... | US Patent US10221142 (2019) BindingDB Entry DOI: 10.7270/Q2M90BZ0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor ROR-gamma (Homo sapiens (Human)) | BDBM359552 ((R or S)-4-(1-(2- chloro-6-(1- (trifluoromethyl)cy...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description The compounds of the invention inhibit RORgammaT activity. Activation of RORgammaT activity can be measured using, e.g., biochemical TR-FRET assay. I... | US Patent US10221142 (2019) BindingDB Entry DOI: 10.7270/Q2M90BZ0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor ROR-gamma (Homo sapiens (Human)) | BDBM359541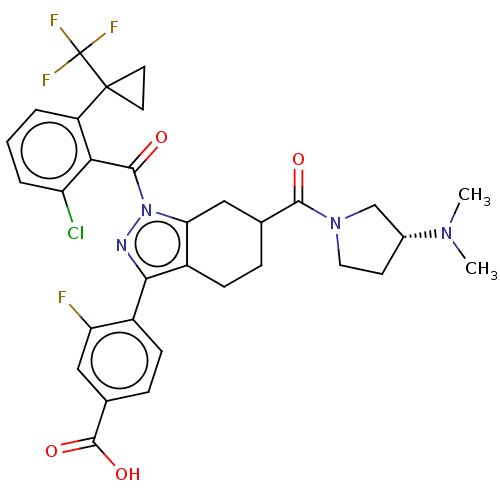 (4-[(6R or S)-1-({2- chloro-6-[1- (trifluoromethyl)...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description The compounds of the invention inhibit RORgammaT activity. Activation of RORgammaT activity can be measured using, e.g., biochemical TR-FRET assay. I... | US Patent US10221142 (2019) BindingDB Entry DOI: 10.7270/Q2M90BZ0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor ROR-gamma (Homo sapiens (Human)) | BDBM359541 (4-[(6R or S)-1-({2- chloro-6-[1- (trifluoromethyl)...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description The compounds of the invention inhibit RORgammaT activity. Activation of RORgammaT activity can be measured using, e.g., biochemical TR-FRET assay. I... | US Patent US10221142 (2019) BindingDB Entry DOI: 10.7270/Q2M90BZ0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor ROR-gamma (Homo sapiens (Human)) | BDBM189904 (US10227346, Example 29 | US10426135, Example 29 | ...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | 7.5 | 25 |
Pfizer Inc. US Patent | Assay Description Specifically, in one embodiment the aforementioned assay was performed as outlined below. The assay was carried out in black polystyrene, 384-well pl... | US Patent US9670201 (2017) BindingDB Entry DOI: 10.7270/Q28G8HWT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor ROR-gamma (Homo sapiens (Human)) | BDBM189904 (US10227346, Example 29 | US10426135, Example 29 | ...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
Universita di Napoli | Assay Description The activity of compound of the invention can be determined by a co-activator recruitment by TR-FRET (time-resolved fluorescence resonance energy tra... | J Med Chem 51: 1764-70 (2008) BindingDB Entry DOI: 10.7270/Q2SF2ZGV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor ROR-gamma (Homo sapiens (Human)) | BDBM189904 (US10227346, Example 29 | US10426135, Example 29 | ...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. US Patent | Assay Description The activity of compound of the invention can be determined by a co-activator recruitment by TR-FRET (time-resolved fluorescence resonance energy tra... | US Patent US10426135 (2019) BindingDB Entry DOI: 10.7270/Q23N25QB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor ROR-gamma (Homo sapiens (Human)) | BDBM411418 (N-(3-((3R,4R)-1-((R)-2-cyclopentylpropanoyl)- 3-me...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. US Patent | Assay Description In general, the assay is based on the interaction between N-terminally Six-Histidine-tagged-RORC2 ligand binding domain (6-His-RORC2 LBD), expressed ... | US Patent US10385036 (2019) BindingDB Entry DOI: 10.7270/Q24X5B40 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor ROR-gamma (Homo sapiens (Human)) | BDBM392076 (US10301272, Example 7/9) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.940 | n/a | n/a | n/a | n/a | n/a | n/a |
Phenex Pharmaceuticals AG Curated by ChEMBL | Assay Description Inverse agonist activity at human GAL4 DBD-fused RORgammat LBD (237 to 497 residues) expressed in HEK293T cells assessed as reduction in ROR mediated... | Bioorg Med Chem Lett 28: 1446-1455 (2018) Article DOI: 10.1016/j.bmcl.2018.03.093 BindingDB Entry DOI: 10.7270/Q27W6FPC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor ROR-gamma (Homo sapiens (Human)) | BDBM385484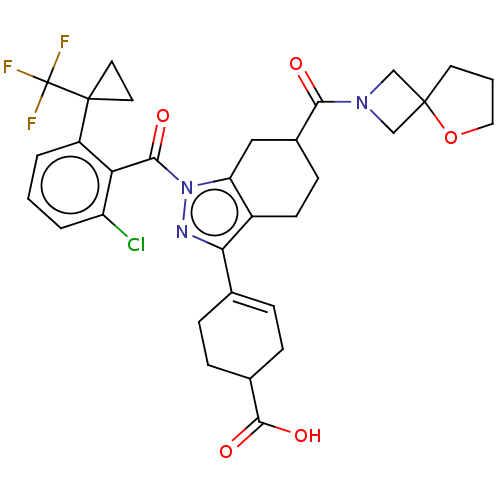 ((R or S)-4-((R or S)-1- (2-chloro-6-(1- (trifluoro...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
GSK | Assay Description To identify novel antagonists of RORgammaT, an assay was developed which employs the interaction of RORgammaT with its co-activator peptide SRC1_2. T... | Bioorg Med Chem Lett 17: 4363-8 (2007) BindingDB Entry DOI: 10.7270/Q27H1MXW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor ROR-gamma (Homo sapiens (Human)) | BDBM385486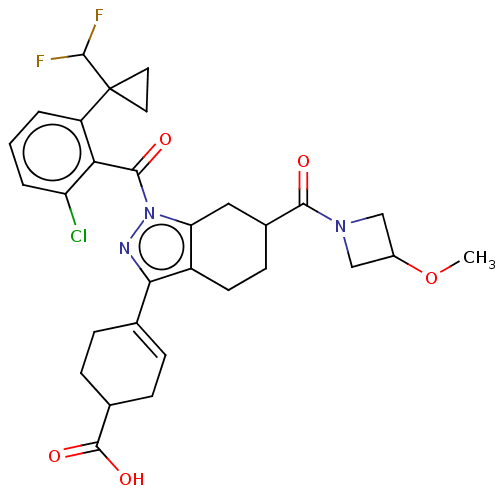 ((R or S)-4-((R or S)-1- (2-chloro-6-(1- (difluorom...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
GSK | Assay Description To identify novel antagonists of RORgammaT, an assay was developed which employs the interaction of RORgammaT with its co-activator peptide SRC1_2. T... | Bioorg Med Chem Lett 17: 4363-8 (2007) BindingDB Entry DOI: 10.7270/Q27H1MXW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor ROR-gamma (Homo sapiens (Human)) | BDBM385488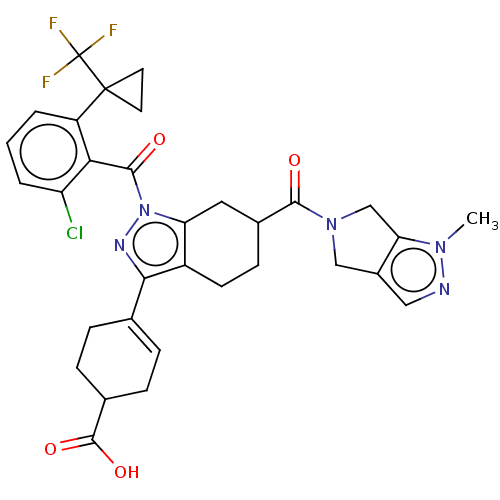 ((R or S)-4-((R or S)-1- (2-chloro-6-(1- (trifluoro...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
GSK | Assay Description To identify novel antagonists of RORgammaT, an assay was developed which employs the interaction of RORgammaT with its co-activator peptide SRC1_2. T... | Bioorg Med Chem Lett 17: 4363-8 (2007) BindingDB Entry DOI: 10.7270/Q27H1MXW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor ROR-gamma (Homo sapiens (Human)) | BDBM385490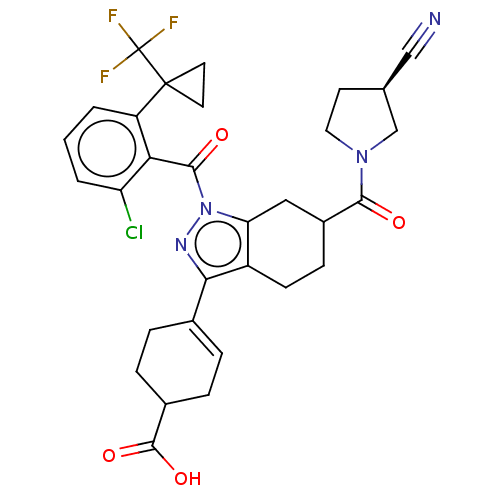 (4-((R or S)-1-(2-chloro-6-(1- (trifluoromethyl)cyc...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
GSK | Assay Description To identify novel antagonists of RORgammaT, an assay was developed which employs the interaction of RORgammaT with its co-activator peptide SRC1_2. T... | Bioorg Med Chem Lett 17: 4363-8 (2007) BindingDB Entry DOI: 10.7270/Q27H1MXW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor ROR-gamma (Homo sapiens (Human)) | BDBM385474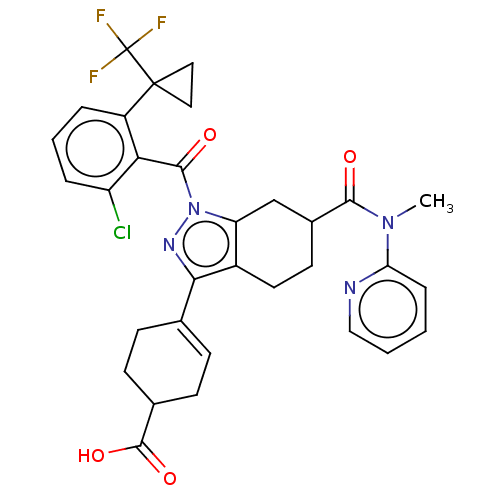 ((R or S)-4-((R or S)-1- (2-chloro-6-(1- (trifluoro...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
GSK | Assay Description To identify novel antagonists of RORgammaT, an assay was developed which employs the interaction of RORgammaT with its co-activator peptide SRC1_2. T... | Bioorg Med Chem Lett 17: 4363-8 (2007) BindingDB Entry DOI: 10.7270/Q27H1MXW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor ROR-gamma (Homo sapiens (Human)) | BDBM385476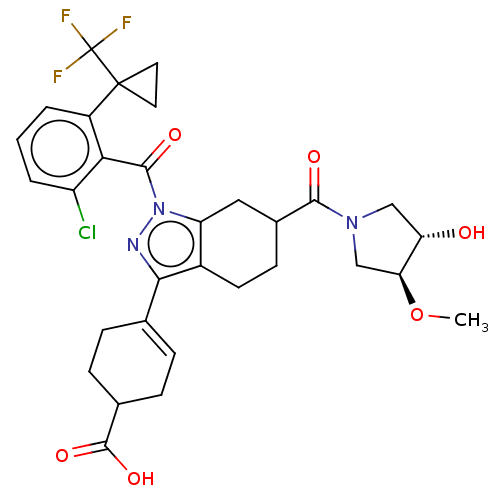 ((R or S)-4-((R or S)-1- (2-chloro-6-(1- (trifluoro...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
GSK | Assay Description To identify novel antagonists of RORgammaT, an assay was developed which employs the interaction of RORgammaT with its co-activator peptide SRC1_2. T... | Bioorg Med Chem Lett 17: 4363-8 (2007) BindingDB Entry DOI: 10.7270/Q27H1MXW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor ROR-gamma (Homo sapiens (Human)) | BDBM385434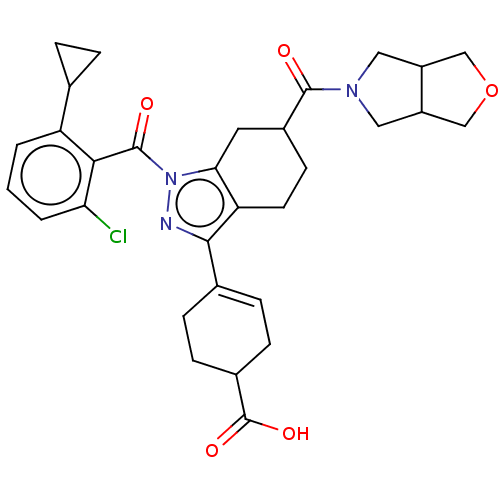 (4-((6R or S)-1-(2- chloro-6- cyclopropylbenzoyl)-6...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
GSK | Assay Description To identify novel antagonists of RORgammaT, an assay was developed which employs the interaction of RORgammaT with its co-activator peptide SRC1_2. T... | Bioorg Med Chem Lett 17: 4363-8 (2007) BindingDB Entry DOI: 10.7270/Q27H1MXW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor ROR-gamma (Homo sapiens (Human)) | BDBM385435 (4-((6R or S)-1-(2- chloro-6- cyclopropylbenzoyl)-6...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
GSK | Assay Description To identify novel antagonists of RORgammaT, an assay was developed which employs the interaction of RORgammaT with its co-activator peptide SRC1_2. T... | Bioorg Med Chem Lett 17: 4363-8 (2007) BindingDB Entry DOI: 10.7270/Q27H1MXW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor ROR-gamma (Homo sapiens (Human)) | BDBM385456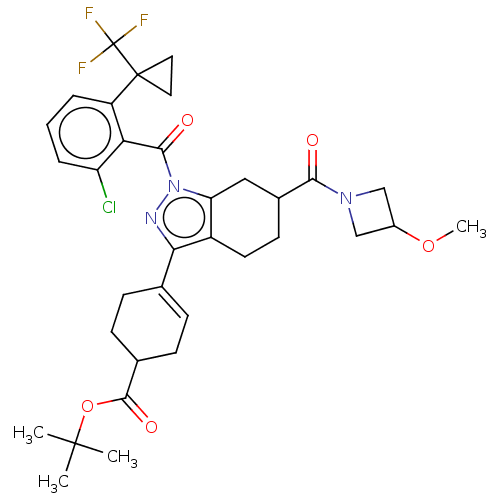 (US10287272, Example 5A | US10689369, Example 5A) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
GSK | Assay Description To identify novel antagonists of RORgammaT, an assay was developed which employs the interaction of RORgammaT with its co-activator peptide SRC1_2. T... | Bioorg Med Chem Lett 17: 4363-8 (2007) BindingDB Entry DOI: 10.7270/Q27H1MXW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor ROR-gamma (Homo sapiens (Human)) | BDBM338467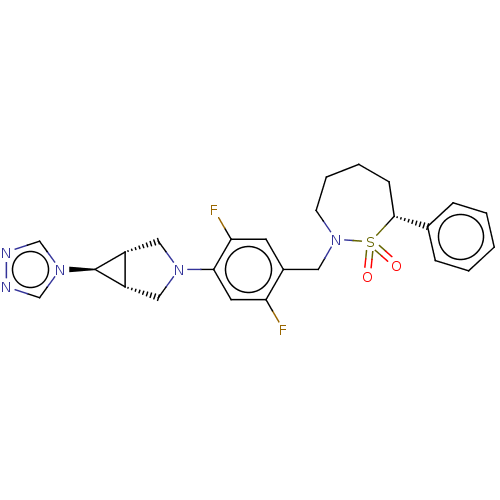 ((7R)-2-[[2,5-difluoro-4-[(1R,5S)- 6-(1,2,4-triazol...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech, Inc. US Patent | Assay Description Assays were carried out in 16-microL reaction volumes in black 384 Plus F Proxiplates (Perkin-Elmer 6008269). All assay components except test ligand... | US Patent US9751873 (2017) BindingDB Entry DOI: 10.7270/Q2BC41NZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 10067 total ) | Next | Last >> |