

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Beta-lactamase (Pseudomonas aeruginosa (PAO1)) | BDBM50347189 (CHEMBL1795572) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck& Co. Curated by ChEMBL | Assay Description Inhibition of Pseudomonas aeruginosa beta lactamase AmpC | Bioorg Med Chem Lett 21: 4363-5 (2011) Article DOI: 10.1016/j.bmcl.2011.04.122 BindingDB Entry DOI: 10.7270/Q24Q7V94 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Pseudomonas aeruginosa (PAO1)) | BDBM50347184 (CHEMBL1795567) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck& Co. Curated by ChEMBL | Assay Description Inhibition of Pseudomonas aeruginosa beta lactamase AmpC | Bioorg Med Chem Lett 21: 4363-5 (2011) Article DOI: 10.1016/j.bmcl.2011.04.122 BindingDB Entry DOI: 10.7270/Q24Q7V94 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Pseudomonas aeruginosa (PAO1)) | BDBM50347182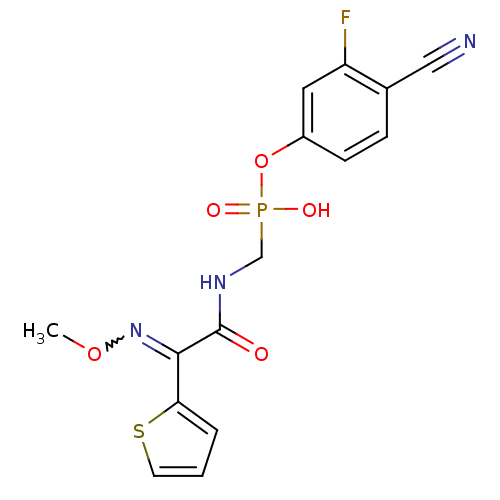 (CHEMBL1795565) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck& Co. Curated by ChEMBL | Assay Description Inhibition of Pseudomonas aeruginosa beta lactamase AmpC | Bioorg Med Chem Lett 21: 4363-5 (2011) Article DOI: 10.1016/j.bmcl.2011.04.122 BindingDB Entry DOI: 10.7270/Q24Q7V94 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Pseudomonas aeruginosa (PAO1)) | BDBM50067060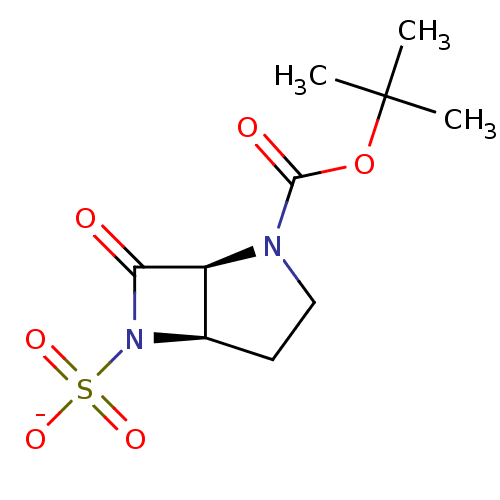 (CHEMBL128523 | Sodium; (1S,5R)-2-tert-butoxycarbon...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd. Curated by ChEMBL | Assay Description Inhibition of P. aeruginosa 18SH Beta-lactamase. | J Med Chem 41: 3961-71 (1998) Article DOI: 10.1021/jm980023c BindingDB Entry DOI: 10.7270/Q2HT2NF0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Pseudomonas aeruginosa (PAO1)) | BDBM50347188 (CHEMBL1795571) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck& Co. Curated by ChEMBL | Assay Description Inhibition of Pseudomonas aeruginosa beta lactamase AmpC | Bioorg Med Chem Lett 21: 4363-5 (2011) Article DOI: 10.1016/j.bmcl.2011.04.122 BindingDB Entry DOI: 10.7270/Q24Q7V94 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Pseudomonas aeruginosa (PAO1)) | BDBM50347188 (CHEMBL1795571) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck& Co. Curated by ChEMBL | Assay Description Inhibition of Pseudomonas aeruginosa beta lactamase AmpC | Bioorg Med Chem Lett 21: 4363-5 (2011) Article DOI: 10.1016/j.bmcl.2011.04.122 BindingDB Entry DOI: 10.7270/Q24Q7V94 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Pseudomonas aeruginosa (PAO1)) | BDBM50067058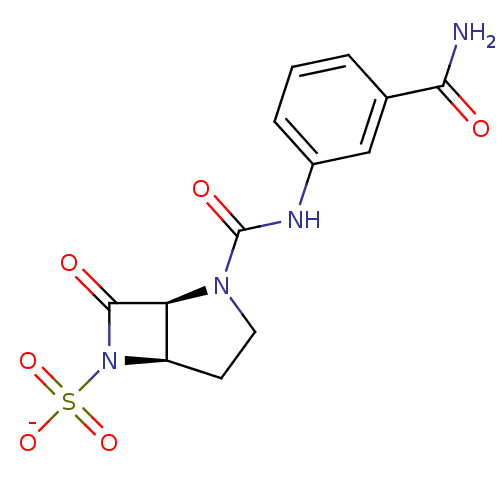 (CHEMBL338157 | Sodium; (1S,5R)-2-(3-carbamoyl-phen...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 24 | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd. Curated by ChEMBL | Assay Description Inhibition of P. aeruginosa 18SH Beta-lactamase. | J Med Chem 41: 3961-71 (1998) Article DOI: 10.1021/jm980023c BindingDB Entry DOI: 10.7270/Q2HT2NF0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Pseudomonas aeruginosa (PAO1)) | BDBM50067055 (CHEMBL338933 | Sodium; (1S,5R)-2-[2-(4-hydroxy-phe...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd. Curated by ChEMBL | Assay Description Inhibition of P. aeruginosa 18SH Beta-lactamase. | J Med Chem 41: 3961-71 (1998) Article DOI: 10.1021/jm980023c BindingDB Entry DOI: 10.7270/Q2HT2NF0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Pseudomonas aeruginosa (PAO1)) | BDBM50408523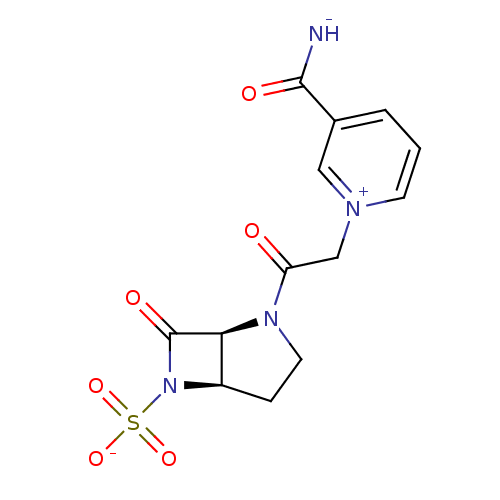 (CHEMBL2079714) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 27 | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd. Curated by ChEMBL | Assay Description Inhibition of P. aeruginosa 18SH Beta-lactamase. | J Med Chem 41: 3961-71 (1998) Article DOI: 10.1021/jm980023c BindingDB Entry DOI: 10.7270/Q2HT2NF0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Pseudomonas aeruginosa (PAO1)) | BDBM50067050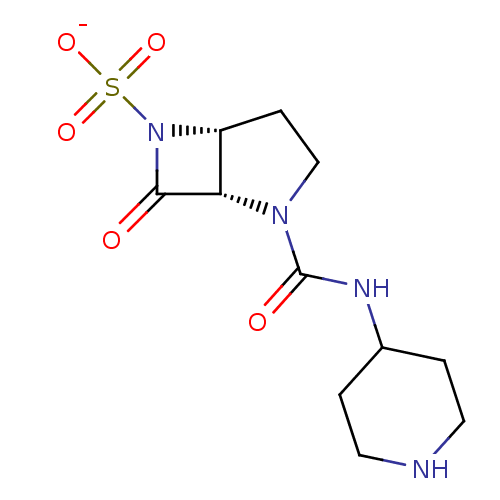 (CHEMBL336630 | Sodium; (1S,5R)-7-oxo-2-(piperidin-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 39 | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd. Curated by ChEMBL | Assay Description Inhibition of P. aeruginosa 18SH Beta-lactamase. | J Med Chem 41: 3961-71 (1998) Article DOI: 10.1021/jm980023c BindingDB Entry DOI: 10.7270/Q2HT2NF0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Pseudomonas aeruginosa (PAO1)) | BDBM50347183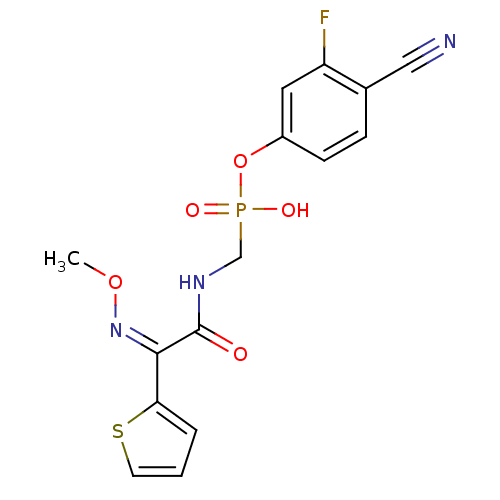 (CHEMBL1795566) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 46 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck& Co. Curated by ChEMBL | Assay Description Inhibition of Pseudomonas aeruginosa beta lactamase AmpC | Bioorg Med Chem Lett 21: 4363-5 (2011) Article DOI: 10.1016/j.bmcl.2011.04.122 BindingDB Entry DOI: 10.7270/Q24Q7V94 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Pseudomonas aeruginosa (PAO1)) | BDBM50067051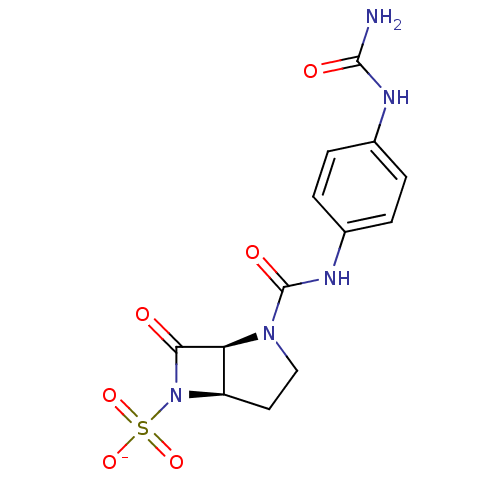 (CHEMBL130187 | Sodium; (1S,5R)-7-oxo-2-(4-ureido-p...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 48 | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd. Curated by ChEMBL | Assay Description Inhibition of P. aeruginosa 18SH Beta-lactamase. | J Med Chem 41: 3961-71 (1998) Article DOI: 10.1021/jm980023c BindingDB Entry DOI: 10.7270/Q2HT2NF0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Pseudomonas aeruginosa (PAO1)) | BDBM50067047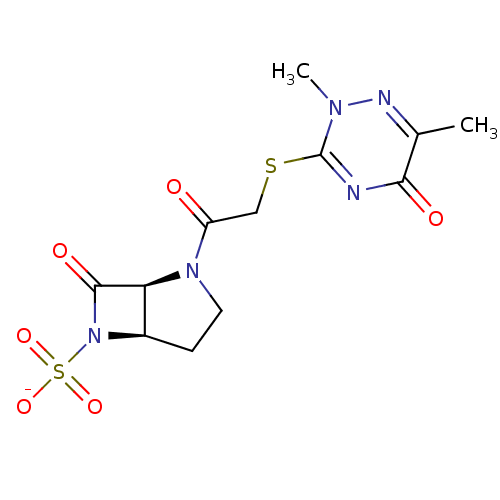 (CHEMBL341484 | Sodium; (1S,5R)-2-[2-(2,6-dimethyl-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 55 | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd. Curated by ChEMBL | Assay Description Inhibition of P. aeruginosa 18SH Beta-lactamase. | J Med Chem 41: 3961-71 (1998) Article DOI: 10.1021/jm980023c BindingDB Entry DOI: 10.7270/Q2HT2NF0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Pseudomonas aeruginosa (PAO1)) | BDBM50067065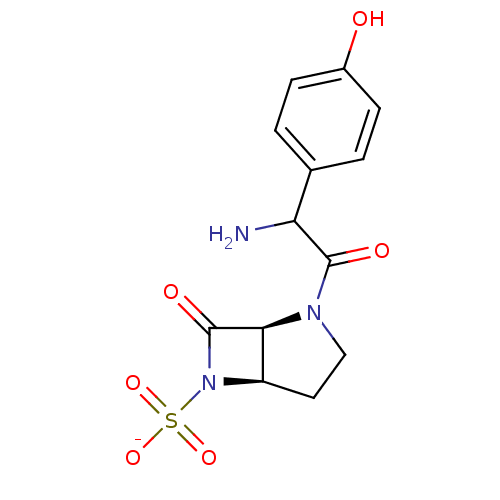 (CHEMBL130854 | Sodium; (1S,5R)-2-[2-amino-2-(4-hyd...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 90 | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd. Curated by ChEMBL | Assay Description Inhibition of P. aeruginosa 18SH Beta-lactamase. | J Med Chem 41: 3961-71 (1998) Article DOI: 10.1021/jm980023c BindingDB Entry DOI: 10.7270/Q2HT2NF0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Pseudomonas aeruginosa (PAO1)) | BDBM50067057 (CHEMBL340937 | Sodium; (1S,5R)-2-((E)-3-1H-imidazo...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd. Curated by ChEMBL | Assay Description Inhibition of P. aeruginosa 18SH Beta-lactamase. | J Med Chem 41: 3961-71 (1998) Article DOI: 10.1021/jm980023c BindingDB Entry DOI: 10.7270/Q2HT2NF0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Pseudomonas aeruginosa (PAO1)) | BDBM50339145 (CHEMBL1689063 | trans-7-oxo-6-(sulfooxy)-1,6-diaza...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 128 | n/a | n/a | n/a | n/a | n/a | n/a |
Novexel SA Curated by ChEMBL | Assay Description Inhibition of Pseudomonas aeruginosa beta-lactamase AmpC assessed as nitrocefin hydrolysis after 5 mins enzyme-compound preincubation | Antimicrob Agents Chemother 54: 5132-8 (2010) Article DOI: 10.1128/AAC.00568-10 BindingDB Entry DOI: 10.7270/Q2CJ8DSV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Pseudomonas aeruginosa (PAO1)) | BDBM50067062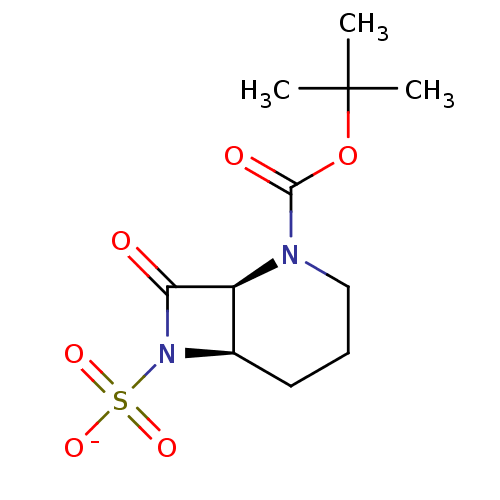 (CHEMBL415234 | Sodium; (1S,6R)-2-tert-butoxycarbon...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 145 | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd. Curated by ChEMBL | Assay Description Inhibition of P. aeruginosa 18SH Beta-lactamase. | J Med Chem 41: 3961-71 (1998) Article DOI: 10.1021/jm980023c BindingDB Entry DOI: 10.7270/Q2HT2NF0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Pseudomonas aeruginosa (PAO1)) | BDBM50067056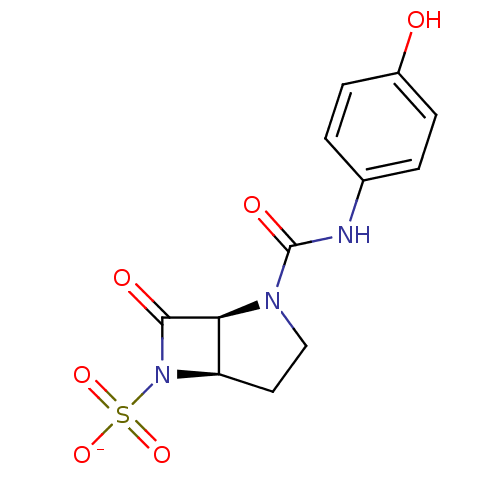 (CHEMBL338351 | Sodium; (1S,5R)-2-(4-hydroxy-phenyl...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 155 | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd. Curated by ChEMBL | Assay Description Inhibition of P. aeruginosa 18SH Beta-lactamase. | J Med Chem 41: 3961-71 (1998) Article DOI: 10.1021/jm980023c BindingDB Entry DOI: 10.7270/Q2HT2NF0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Pseudomonas aeruginosa (PAO1)) | BDBM50067046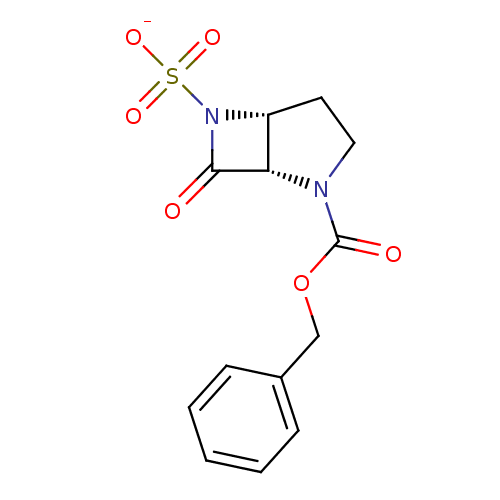 (CHEMBL340423 | Sodium; (1S,5R)-2-benzyloxycarbonyl...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd. Curated by ChEMBL | Assay Description Inhibition of P. aeruginosa 18SH Beta-lactamase. | J Med Chem 41: 3961-71 (1998) Article DOI: 10.1021/jm980023c BindingDB Entry DOI: 10.7270/Q2HT2NF0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Pseudomonas aeruginosa (PAO1)) | BDBM50347185 (CHEMBL1795568) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck& Co. Curated by ChEMBL | Assay Description Inhibition of Pseudomonas aeruginosa beta lactamase AmpC | Bioorg Med Chem Lett 21: 4363-5 (2011) Article DOI: 10.1016/j.bmcl.2011.04.122 BindingDB Entry DOI: 10.7270/Q24Q7V94 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Pseudomonas aeruginosa (PAO1)) | BDBM50067061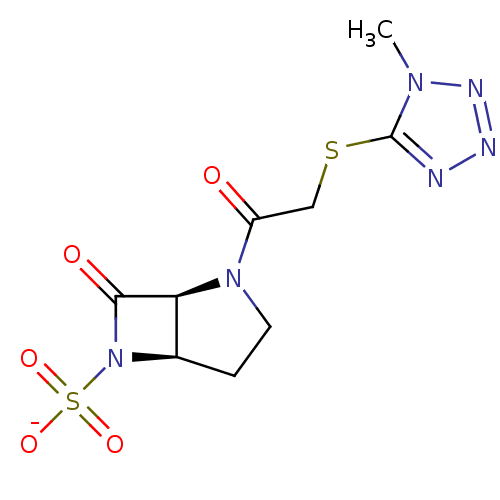 (CHEMBL341496 | Sodium; (1S,5R)-2-[2-(1-methyl-1H-t...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd. Curated by ChEMBL | Assay Description Inhibition of P. aeruginosa 18SH Beta-lactamase. | J Med Chem 41: 3961-71 (1998) Article DOI: 10.1021/jm980023c BindingDB Entry DOI: 10.7270/Q2HT2NF0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Pseudomonas aeruginosa (PAO1)) | BDBM50067063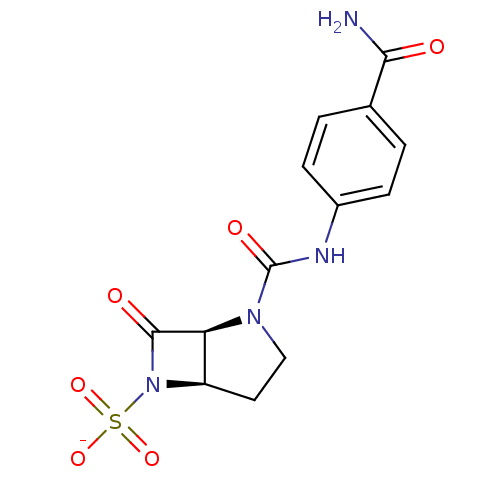 (CHEMBL339600 | Sodium; (1S,5R)-2-(4-carbamoyl-phen...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 217 | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd. Curated by ChEMBL | Assay Description Inhibition of P. aeruginosa 18SH Beta-lactamase. | J Med Chem 41: 3961-71 (1998) Article DOI: 10.1021/jm980023c BindingDB Entry DOI: 10.7270/Q2HT2NF0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Pseudomonas aeruginosa (PAO1)) | BDBM50067049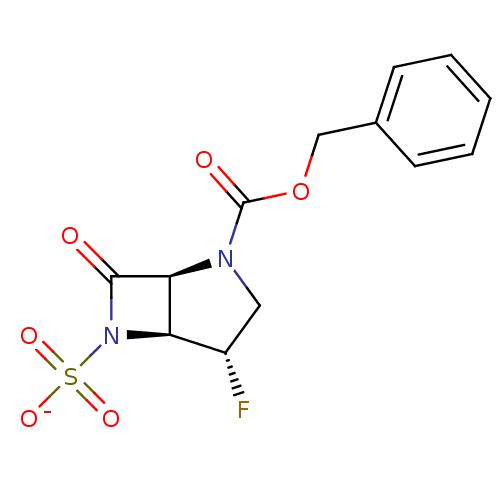 (CHEMBL340351 | Sodium; (1S,4S,5S)-2-benzyloxycarbo...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 380 | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd. Curated by ChEMBL | Assay Description Inhibition of P. aeruginosa 18SH Beta-lactamase. | J Med Chem 41: 3961-71 (1998) Article DOI: 10.1021/jm980023c BindingDB Entry DOI: 10.7270/Q2HT2NF0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Pseudomonas aeruginosa (PAO1)) | BDBM50076680 (CHEMBL6461 | Ro-48-1220 | Sodium; (2S,3R,5R)-3-((Z...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 380 | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-LaRoche Ltd Curated by ChEMBL | Assay Description Compound was tested for inhibition of Beta-lactamase from Pseudomonas aeruginosa 18SH | J Med Chem 39: 3712-22 (1996) Article DOI: 10.1021/jm9601967 BindingDB Entry DOI: 10.7270/Q2ZK5H99 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Pseudomonas aeruginosa (PAO1)) | BDBM50076680 (CHEMBL6461 | Ro-48-1220 | Sodium; (2S,3R,5R)-3-((Z...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 380 | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-LaRoche Ltd Curated by ChEMBL | Assay Description Compound was tested for inhibition of Beta-lactamase from Pseudomonas aeruginosa 18SH | J Med Chem 39: 3712-22 (1996) Article DOI: 10.1021/jm9601967 BindingDB Entry DOI: 10.7270/Q2ZK5H99 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Pseudomonas aeruginosa (PAO1)) | BDBM50309906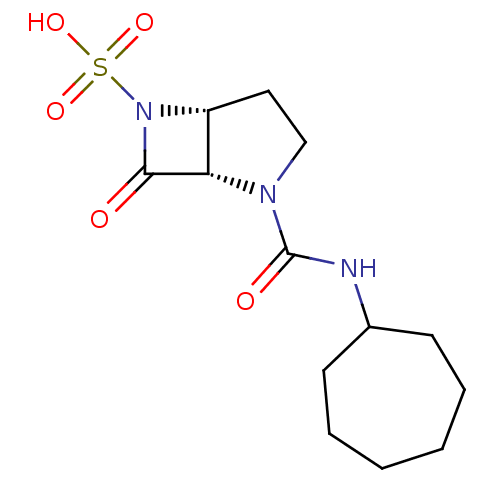 ((1S,5R)-2-(cycloheptylcarbamoyl)-7-oxo-2,6-diazabi...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 400 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Labs Curated by ChEMBL | Assay Description Inhibition of Pseudomonas aeruginosa beta lactamase AmpC by spectrophotometry | Bioorg Med Chem Lett 20: 918-21 (2010) Article DOI: 10.1016/j.bmcl.2009.12.069 BindingDB Entry DOI: 10.7270/Q2KW5G5S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Pseudomonas aeruginosa (PAO1)) | BDBM50067059 (CHEMBL129690 | Sodium; (1S,5R)-7-oxo-2-phenylcarba...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 420 | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd. Curated by ChEMBL | Assay Description Inhibition of P. aeruginosa 18SH Beta-lactamase. | J Med Chem 41: 3961-71 (1998) Article DOI: 10.1021/jm980023c BindingDB Entry DOI: 10.7270/Q2HT2NF0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Pseudomonas aeruginosa (PAO1)) | BDBM50053175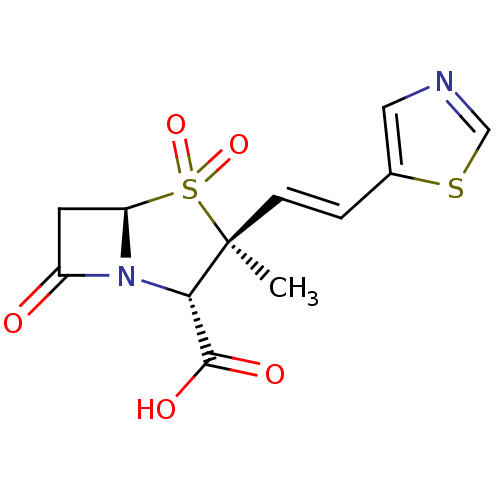 ((2S,3S,5R)-3-Methyl-4,4,7-trioxo-3-((E)-2-thiazol-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 540 | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-LaRoche Ltd Curated by ChEMBL | Assay Description Compound was tested for inhibition of Pseudomonas aeruginosa 18SH ,class C of Beta-lactamase | J Med Chem 39: 3712-22 (1996) Article DOI: 10.1021/jm9601967 BindingDB Entry DOI: 10.7270/Q2ZK5H99 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Pseudomonas aeruginosa (PAO1)) | BDBM50309903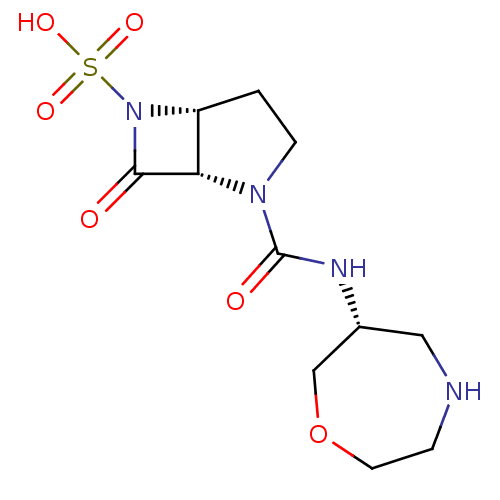 ((1S,5R)-2-((R)-1,4-oxazepan-6-ylcarbamoyl)-7-oxo-2...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 600 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Labs Curated by ChEMBL | Assay Description Inhibition of Pseudomonas aeruginosa beta lactamase AmpC by spectrophotometry | Bioorg Med Chem Lett 20: 918-21 (2010) Article DOI: 10.1016/j.bmcl.2009.12.069 BindingDB Entry DOI: 10.7270/Q2KW5G5S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Pseudomonas aeruginosa (PAO1)) | BDBM50348181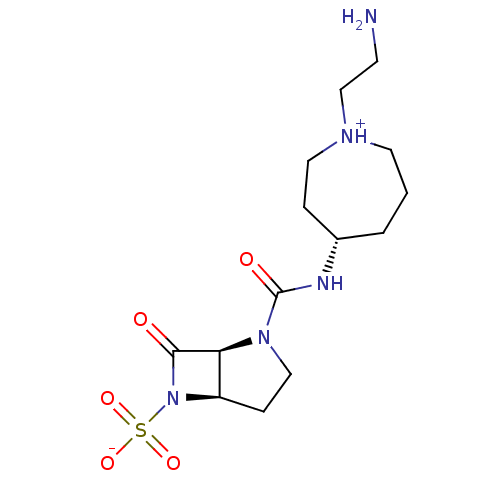 (CHEMBL1800870) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 600 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Labs Curated by ChEMBL | Assay Description Inhibition of Pseudomonas aeruginosa CL5701 AmpC by spectrophotometric assay | Bioorg Med Chem Lett 21: 4267-70 (2011) Article DOI: 10.1016/j.bmcl.2011.05.065 BindingDB Entry DOI: 10.7270/Q2WW7J1C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Pseudomonas aeruginosa (PAO1)) | BDBM50067052 (CHEMBL129226 | Sodium; (1S,5R)-2-[2-(1-methyl-1H-i...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 612 | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd. Curated by ChEMBL | Assay Description Inhibition of P. aeruginosa 18SH Beta-lactamase. | J Med Chem 41: 3961-71 (1998) Article DOI: 10.1021/jm980023c BindingDB Entry DOI: 10.7270/Q2HT2NF0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Pseudomonas aeruginosa (PAO1)) | BDBM50067066 (CHEMBL340707 | Sodium; (1S,4R,5S)-2-benzyloxycarbo...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 613 | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd. Curated by ChEMBL | Assay Description Inhibition of P. aeruginosa 18SH Beta-lactamase. | J Med Chem 41: 3961-71 (1998) Article DOI: 10.1021/jm980023c BindingDB Entry DOI: 10.7270/Q2HT2NF0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Pseudomonas aeruginosa (PAO1)) | BDBM212440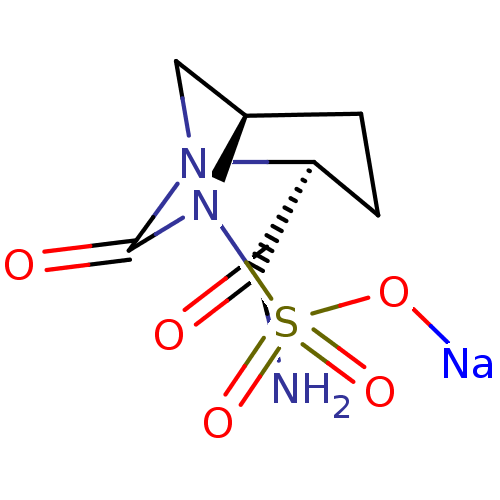 (US8772490, H) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 650 | n/a | n/a | n/a | n/a | 7.0 | 30 |
Meiji Seika Pharma Co., Ltd. US Patent | Assay Description For the measurement of β-lactamase inhibitory activity, 100 uM (final concentration) nitrocefin (Oxoid) was used as a substrate, and 2.5% DMSO, ... | US Patent US8772490 (2014) BindingDB Entry DOI: 10.7270/Q2NK3CWQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Pseudomonas aeruginosa (PAO1)) | BDBM159600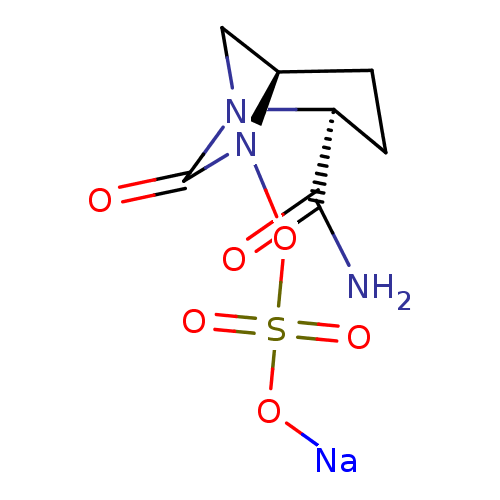 (US8772490, Example 23 | US9035062, 23) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 650 | n/a | n/a | n/a | n/a | 7.0 | 30 |
Meiji Seika Pharma Co., Ltd. US Patent | Assay Description For the measurement of β-lactamase inhibitory activity, 100 uM (final concentration) nitrocefin (Oxoid) was used as a substrate, and 2.5% DMSO, ... | US Patent US8772490 (2014) BindingDB Entry DOI: 10.7270/Q2NK3CWQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Pseudomonas aeruginosa (PAO1)) | BDBM191563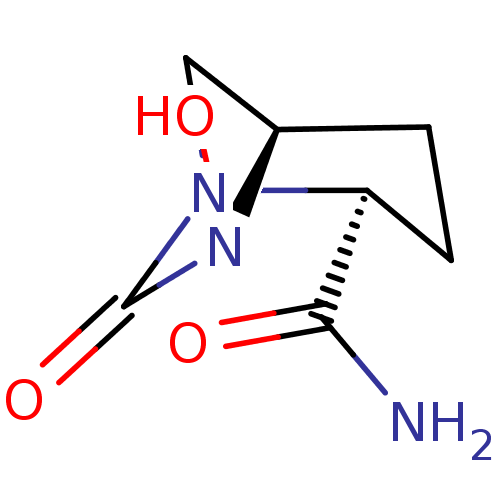 (US9676777, example 23) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | US Patent | n/a | n/a | 650 | n/a | n/a | n/a | n/a | 7.0 | n/a |
MEIJI SEIKA PHARMA CO., LTD. US Patent | Assay Description For the measurement of β-lactamase inhibitory activity, 100 μM (final concentration) nitrocefin (Oxoid) was used as a substrate, and 2.5% D... | US Patent US9676777 (2017) BindingDB Entry DOI: 10.7270/Q2G15Z17 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Pseudomonas aeruginosa (PAO1)) | BDBM50021959 (CHEMBL777 | MM 14151 | US9120808, Clavulanic acid ...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 800 | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd. Curated by ChEMBL | Assay Description Deacylation of 18SH Beta-lactamase of Pseudomonas aeruginosa | J Med Chem 41: 3961-71 (1998) Article DOI: 10.1021/jm980023c BindingDB Entry DOI: 10.7270/Q2HT2NF0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Pseudomonas aeruginosa (PAO1)) | BDBM50309905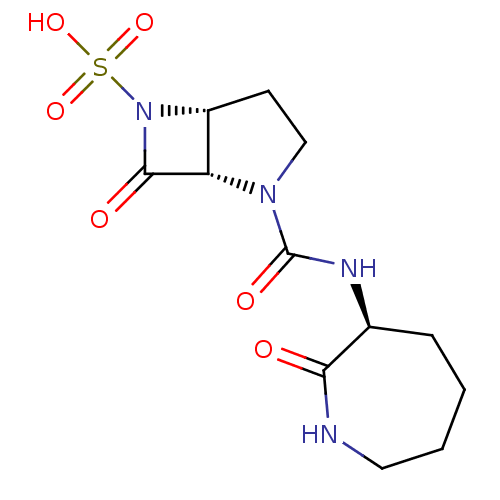 ((1S,5R)-7-oxo-2-((S)-2-oxoazepan-3-ylcarbamoyl)-2,...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 800 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Labs Curated by ChEMBL | Assay Description Inhibition of Pseudomonas aeruginosa beta lactamase AmpC by spectrophotometry | Bioorg Med Chem Lett 20: 918-21 (2010) Article DOI: 10.1016/j.bmcl.2009.12.069 BindingDB Entry DOI: 10.7270/Q2KW5G5S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Pseudomonas aeruginosa (PAO1)) | BDBM50053173 ((2S,3S,5R)-3-Methyl-4,4,7-trioxo-3-[1,2,3]triazol-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 820 | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-LaRoche Ltd Curated by ChEMBL | Assay Description Compound was tested for inhibition of Beta-lactamase from Pseudomonas aeruginosa 18SH | J Med Chem 39: 3712-22 (1996) Article DOI: 10.1021/jm9601967 BindingDB Entry DOI: 10.7270/Q2ZK5H99 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Pseudomonas aeruginosa (PAO1)) | BDBM50067048 (CHEMBL129651 | Sodium; (1S,5R)-2-[2-(4-methyl-4H-[...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 853 | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd. Curated by ChEMBL | Assay Description Inhibition of P. aeruginosa 18SH Beta-lactamase. | J Med Chem 41: 3961-71 (1998) Article DOI: 10.1021/jm980023c BindingDB Entry DOI: 10.7270/Q2HT2NF0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Pseudomonas aeruginosa (PAO1)) | BDBM50348179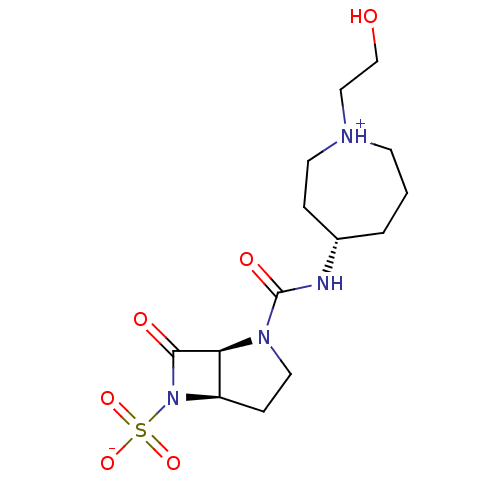 (CHEMBL1800868) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 900 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Labs Curated by ChEMBL | Assay Description Inhibition of Pseudomonas aeruginosa CL5701 AmpC by spectrophotometric assay | Bioorg Med Chem Lett 21: 4267-70 (2011) Article DOI: 10.1016/j.bmcl.2011.05.065 BindingDB Entry DOI: 10.7270/Q2WW7J1C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Pseudomonas aeruginosa (PAO1)) | BDBM50348182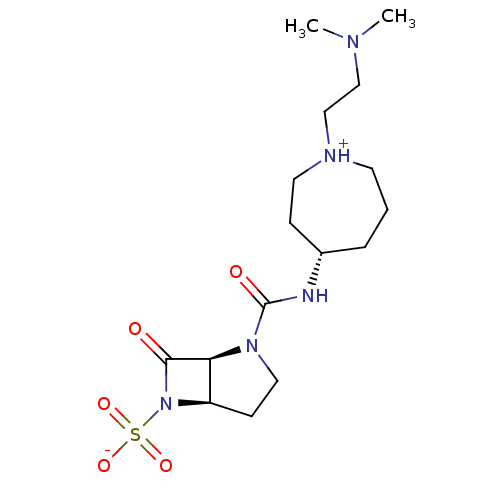 (CHEMBL1800871) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 900 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Labs Curated by ChEMBL | Assay Description Inhibition of Pseudomonas aeruginosa CL5701 AmpC by spectrophotometric assay | Bioorg Med Chem Lett 21: 4267-70 (2011) Article DOI: 10.1016/j.bmcl.2011.05.065 BindingDB Entry DOI: 10.7270/Q2WW7J1C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Pseudomonas aeruginosa (PAO1)) | BDBM50053173 ((2S,3S,5R)-3-Methyl-4,4,7-trioxo-3-[1,2,3]triazol-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | US Patent | n/a | n/a | 950 | n/a | n/a | n/a | n/a | 7.0 | n/a |
MEIJI SEIKA PHARMA CO., LTD. US Patent | Assay Description For the measurement of β-lactamase inhibitory activity, 100 μM (final concentration) nitrocefin (Oxoid) was used as a substrate, and 2.5% D... | US Patent US9676777 (2017) BindingDB Entry DOI: 10.7270/Q2G15Z17 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Pseudomonas aeruginosa (PAO1)) | BDBM212441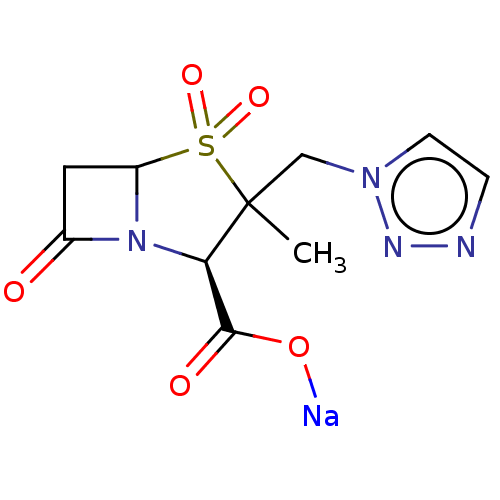 (US8772490, TAZ) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 950 | n/a | n/a | n/a | n/a | 7.0 | 30 |
Meiji Seika Pharma Co., Ltd. US Patent | Assay Description For the measurement of β-lactamase inhibitory activity, 100 uM (final concentration) nitrocefin (Oxoid) was used as a substrate, and 2.5% DMSO, ... | US Patent US8772490 (2014) BindingDB Entry DOI: 10.7270/Q2NK3CWQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Pseudomonas aeruginosa (PAO1)) | BDBM50309899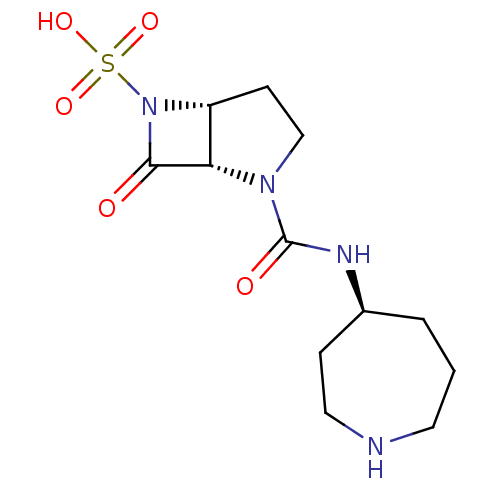 ((1S,5R)-2-((S)-azepan-4-ylcarbamoyl)-7-oxo-2,6-dia...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Labs Curated by ChEMBL | Assay Description Inhibition of Pseudomonas aeruginosa beta lactamase AmpC by spectrophotometry | Bioorg Med Chem Lett 20: 918-21 (2010) Article DOI: 10.1016/j.bmcl.2009.12.069 BindingDB Entry DOI: 10.7270/Q2KW5G5S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Pseudomonas aeruginosa (PAO1)) | BDBM50309899 ((1S,5R)-2-((S)-azepan-4-ylcarbamoyl)-7-oxo-2,6-dia...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Labs Curated by ChEMBL | Assay Description Inhibition of Pseudomonas aeruginosa CL5701 AmpC by spectrophotometric assay | Bioorg Med Chem Lett 21: 4267-70 (2011) Article DOI: 10.1016/j.bmcl.2011.05.065 BindingDB Entry DOI: 10.7270/Q2WW7J1C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Pseudomonas aeruginosa (PAO1)) | BDBM50067053 (CHEMBL127782 | Sodium; (1S,5R)-2-benzyloxycarbonyl...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.04E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd. Curated by ChEMBL | Assay Description Inhibition of P. aeruginosa 18SH Beta-lactamase. | J Med Chem 41: 3961-71 (1998) Article DOI: 10.1021/jm980023c BindingDB Entry DOI: 10.7270/Q2HT2NF0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Pseudomonas aeruginosa (PAO1)) | BDBM50309904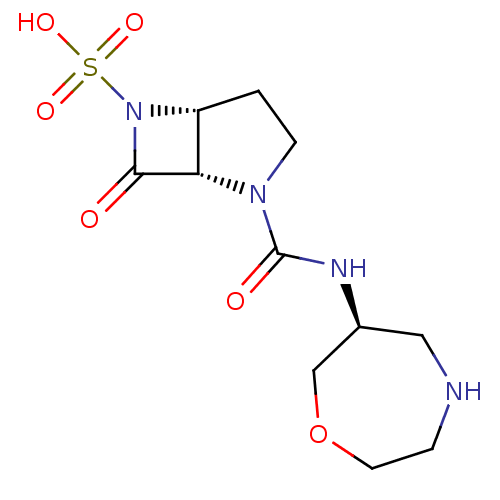 ((1S,5R)-2-((S)-1,4-oxazepan-6-ylcarbamoyl)-7-oxo-2...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Labs Curated by ChEMBL | Assay Description Inhibition of Pseudomonas aeruginosa beta lactamase AmpC by spectrophotometry | Bioorg Med Chem Lett 20: 918-21 (2010) Article DOI: 10.1016/j.bmcl.2009.12.069 BindingDB Entry DOI: 10.7270/Q2KW5G5S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Pseudomonas aeruginosa (PAO1)) | BDBM50348180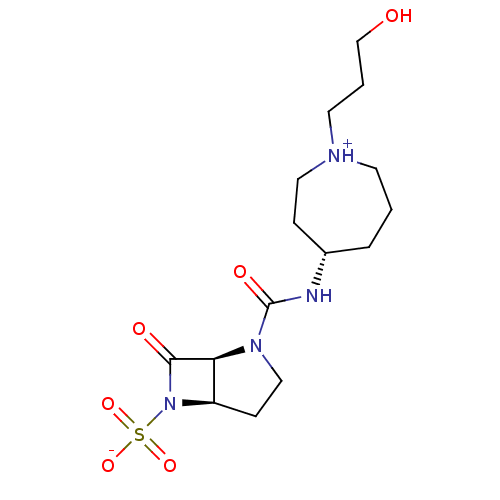 (CHEMBL1800869) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Labs Curated by ChEMBL | Assay Description Inhibition of Pseudomonas aeruginosa CL5701 AmpC by spectrophotometric assay | Bioorg Med Chem Lett 21: 4267-70 (2011) Article DOI: 10.1016/j.bmcl.2011.05.065 BindingDB Entry DOI: 10.7270/Q2WW7J1C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Pseudomonas aeruginosa (PAO1)) | BDBM50315411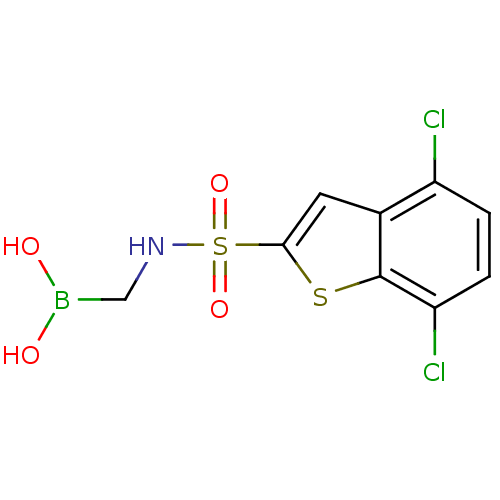 (4,7-Dichloro-1-benzothien-2-yl sulfonylaminomethyl...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck& Co. Curated by ChEMBL | Assay Description Inhibition of Pseudomonas aeruginosa beta-lactamase AmpC | Bioorg Med Chem Lett 20: 2622-4 (2010) Article DOI: 10.1016/j.bmcl.2010.02.065 BindingDB Entry DOI: 10.7270/Q2ZS2WN8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Pseudomonas aeruginosa (PAO1)) | BDBM50309908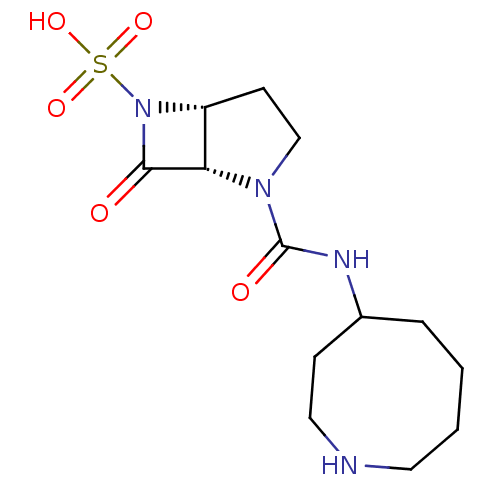 ((1S,5R)-2-(azocan-4-ylcarbamoyl)-7-oxo-2,6-diazabi...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Labs Curated by ChEMBL | Assay Description Inhibition of Pseudomonas aeruginosa beta lactamase AmpC by spectrophotometry | Bioorg Med Chem Lett 20: 918-21 (2010) Article DOI: 10.1016/j.bmcl.2009.12.069 BindingDB Entry DOI: 10.7270/Q2KW5G5S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 104 total ) | Next | Last >> |