

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Aromatase (Homo sapiens (Human)) | BDBM9955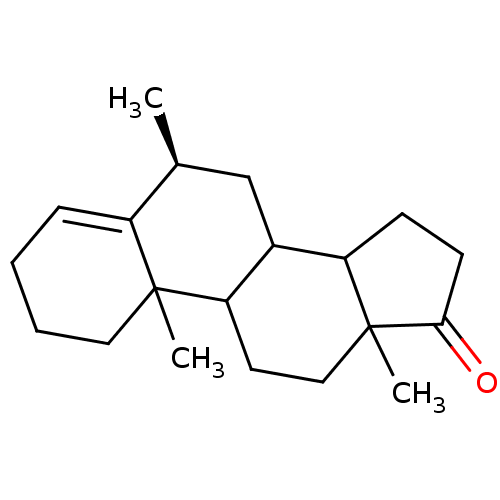 ((8S)-2,8,15-trimethyltetracyclo[8.7.0.0^{2,7}.0^{1...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 3.10 | -50.5 | 37 | n/a | n/a | n/a | n/a | 7.5 | 37 |
Tohoku College of Pharmacy | Assay Description The enzyme activity was assayed by measuring the 3H-labeled H2O formed from [1beta-3H] Androstenedione during aromatization. After incubation, the re... | J Med Chem 39: 2245-52 (1996) Article DOI: 10.1021/jm960047o BindingDB Entry DOI: 10.7270/Q2X34VPV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM9960 ((8R)-2,8,15-trimethyltetracyclo[8.7.0.0^{2,7}.0^{1...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 5.30 | -49.1 | 49 | n/a | n/a | n/a | n/a | 7.5 | 37 |
Tohoku College of Pharmacy | Assay Description The enzyme activity was assayed by measuring the 3H-labeled H2O formed from [1beta-3H] Androstenedione during aromatization. After incubation, the re... | J Med Chem 39: 2245-52 (1996) Article DOI: 10.1021/jm960047o BindingDB Entry DOI: 10.7270/Q2X34VPV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM9968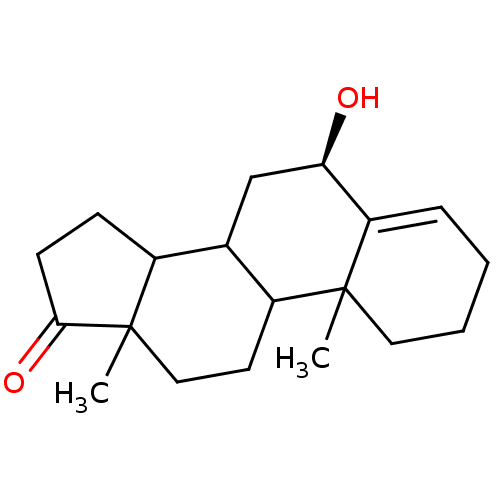 ((8R)-8-hydroxy-2,15-dimethyltetracyclo[8.7.0.0^{2,...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 6 | -48.8 | 50 | n/a | n/a | n/a | n/a | 7.5 | 37 |
Tohoku College of Pharmacy | Assay Description The enzyme activity was assayed by measuring the 3H-labeled H2O formed from [1beta-3H] Androstenedione during aromatization. After incubation, the re... | J Med Chem 39: 2245-52 (1996) Article DOI: 10.1021/jm960047o BindingDB Entry DOI: 10.7270/Q2X34VPV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM9981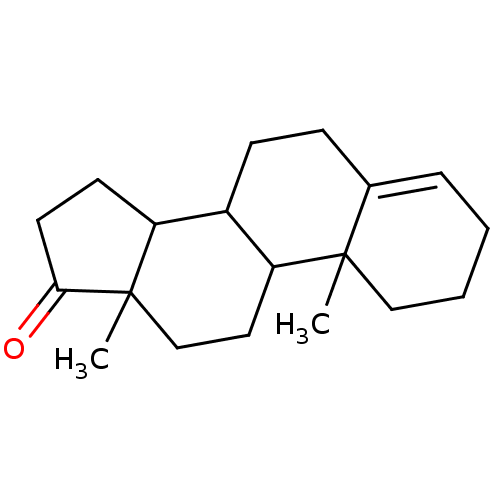 (2,15-dimethyltetracyclo[8.7.0.0^{2,7}.0^{11,15}]he...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 6.80 | n/a | 60 | n/a | n/a | n/a | n/a | n/a | n/a |
Tohoku College of Pharmacy | Assay Description The enzyme activity was assayed by measuring the 3H-labeled H2O formed from [1beta-3H] Androstenedione during aromatization. After incubation, the re... | J Med Chem 39: 2245-52 (1996) Article DOI: 10.1021/jm960047o BindingDB Entry DOI: 10.7270/Q2X34VPV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM9967 ((8S)-8-methoxy-2,15-dimethyltetracyclo[8.7.0.0^{2,...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 12 | -47.0 | 120 | n/a | n/a | n/a | n/a | 7.5 | 37 |
Tohoku College of Pharmacy | Assay Description The enzyme activity was assayed by measuring the 3H-labeled H2O formed from [1beta-3H] Androstenedione during aromatization. After incubation, the re... | J Med Chem 39: 2245-52 (1996) Article DOI: 10.1021/jm960047o BindingDB Entry DOI: 10.7270/Q2X34VPV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM9970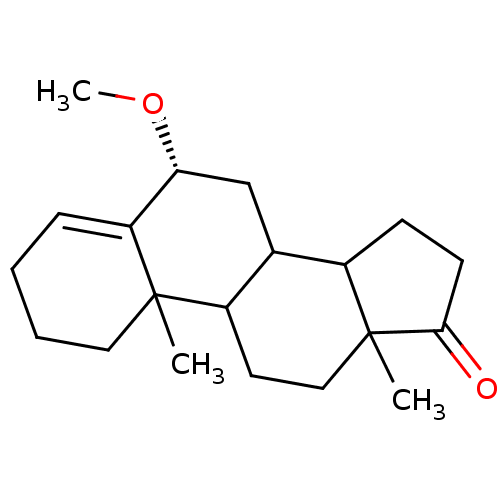 ((8R)-8-methoxy-2,15-dimethyltetracyclo[8.7.0.0^{2,...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 12 | n/a | 130 | n/a | n/a | n/a | n/a | n/a | n/a |
Tohoku College of Pharmacy | Assay Description The enzyme activity was assayed by measuring the 3H-labeled H2O formed from [1beta-3H] Androstenedione during aromatization. After incubation, the re... | J Med Chem 39: 2245-52 (1996) Article DOI: 10.1021/jm960047o BindingDB Entry DOI: 10.7270/Q2X34VPV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM9956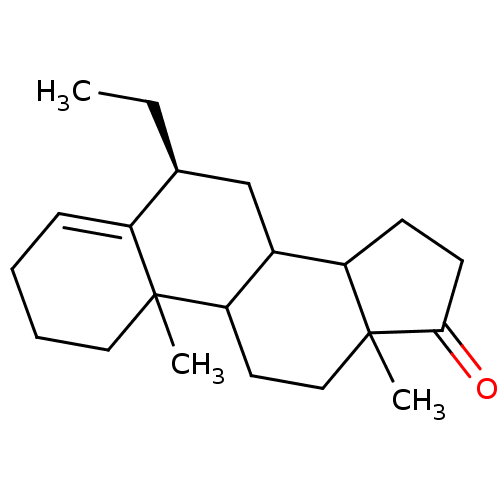 ((8S)-8-ethyl-2,15-dimethyltetracyclo[8.7.0.0^{2,7}...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 14 | -46.6 | 110 | n/a | n/a | n/a | n/a | 7.5 | 37 |
Tohoku College of Pharmacy | Assay Description The enzyme activity was assayed by measuring the 3H-labeled H2O formed from [1beta-3H] Androstenedione during aromatization. After incubation, the re... | J Med Chem 39: 2245-52 (1996) Article DOI: 10.1021/jm960047o BindingDB Entry DOI: 10.7270/Q2X34VPV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM9966 ((8S)-2,15-dimethyl-14-oxotetracyclo[8.7.0.0^{2,7}....) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 18 | -46.0 | 160 | n/a | n/a | n/a | n/a | 7.5 | 37 |
Tohoku College of Pharmacy | Assay Description The enzyme activity was assayed by measuring the 3H-labeled H2O formed from [1beta-3H] Androstenedione during aromatization. After incubation, the re... | J Med Chem 39: 2245-52 (1996) Article DOI: 10.1021/jm960047o BindingDB Entry DOI: 10.7270/Q2X34VPV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM9965 ((8S)-8-hydroxy-2,15-dimethyltetracyclo[8.7.0.0^{2,...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 21 | -45.6 | 190 | n/a | n/a | n/a | n/a | 7.5 | 37 |
Tohoku College of Pharmacy | Assay Description The enzyme activity was assayed by measuring the 3H-labeled H2O formed from [1beta-3H] Androstenedione during aromatization. After incubation, the re... | J Med Chem 39: 2245-52 (1996) Article DOI: 10.1021/jm960047o BindingDB Entry DOI: 10.7270/Q2X34VPV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM9969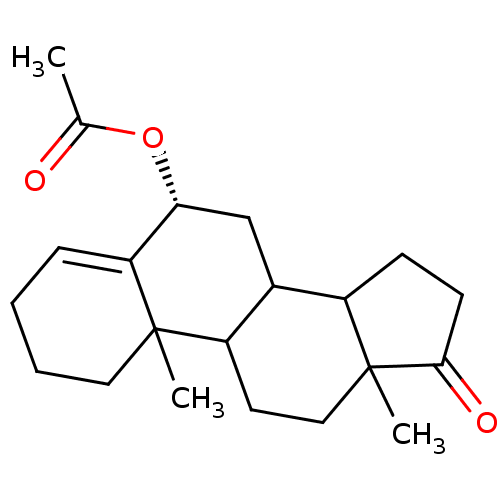 ((8R)-2,15-dimethyl-14-oxotetracyclo[8.7.0.0^{2,7}....) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 24 | n/a | 260 | n/a | n/a | n/a | n/a | n/a | n/a |
Tohoku College of Pharmacy | Assay Description The enzyme activity was assayed by measuring the 3H-labeled H2O formed from [1beta-3H] Androstenedione during aromatization. After incubation, the re... | J Med Chem 39: 2245-52 (1996) Article DOI: 10.1021/jm960047o BindingDB Entry DOI: 10.7270/Q2X34VPV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM9957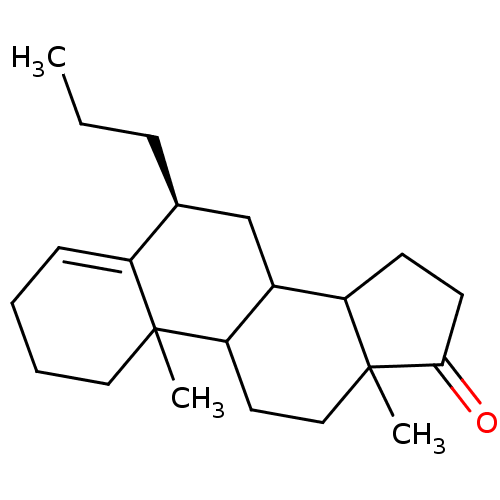 ((8S)-2,15-dimethyl-8-propyltetracyclo[8.7.0.0^{2,7...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 30 | -44.7 | 230 | n/a | n/a | n/a | n/a | 7.5 | 37 |
Tohoku College of Pharmacy | Assay Description The enzyme activity was assayed by measuring the 3H-labeled H2O formed from [1beta-3H] Androstenedione during aromatization. After incubation, the re... | J Med Chem 39: 2245-52 (1996) Article DOI: 10.1021/jm960047o BindingDB Entry DOI: 10.7270/Q2X34VPV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM9961 ((8R)-8-ethyl-2,15-dimethyltetracyclo[8.7.0.0^{2,7}...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 43 | -43.7 | 320 | n/a | n/a | n/a | n/a | 7.5 | 37 |
Tohoku College of Pharmacy | Assay Description The enzyme activity was assayed by measuring the 3H-labeled H2O formed from [1beta-3H] Androstenedione during aromatization. After incubation, the re... | J Med Chem 39: 2245-52 (1996) Article DOI: 10.1021/jm960047o BindingDB Entry DOI: 10.7270/Q2X34VPV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM9982 ((14S)-2,15-dimethyltetracyclo[8.7.0.0^{2,7}.0^{11,...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 45 | n/a | 360 | n/a | n/a | n/a | n/a | n/a | n/a |
Tohoku College of Pharmacy | Assay Description The enzyme activity was assayed by measuring the 3H-labeled H2O formed from [1beta-3H] Androstenedione during aromatization. After incubation, the re... | J Med Chem 39: 2245-52 (1996) Article DOI: 10.1021/jm960047o BindingDB Entry DOI: 10.7270/Q2X34VPV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM9962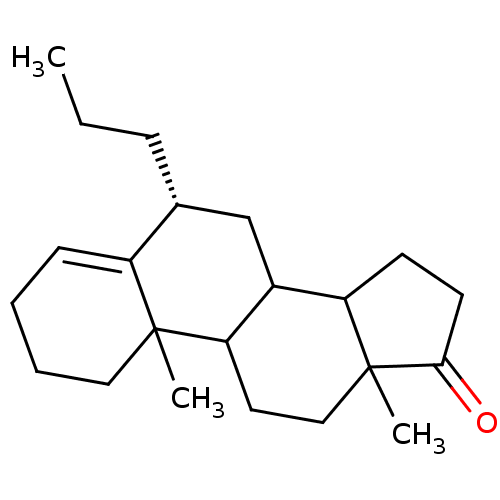 ((8R)-2,15-dimethyl-8-propyltetracyclo[8.7.0.0^{2,7...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 51 | -43.3 | 440 | n/a | n/a | n/a | n/a | 7.5 | 37 |
Tohoku College of Pharmacy | Assay Description The enzyme activity was assayed by measuring the 3H-labeled H2O formed from [1beta-3H] Androstenedione during aromatization. After incubation, the re... | J Med Chem 39: 2245-52 (1996) Article DOI: 10.1021/jm960047o BindingDB Entry DOI: 10.7270/Q2X34VPV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM9976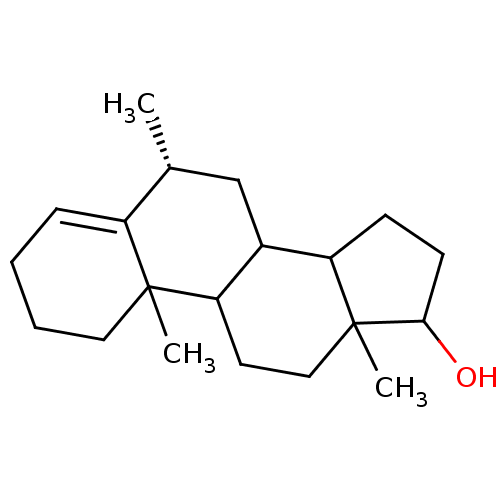 ((8R)-2,8,15-trimethyltetracyclo[8.7.0.0^{2,7}.0^{1...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 140 | n/a | 1.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Tohoku College of Pharmacy | Assay Description The enzyme activity was assayed by measuring the 3H-labeled H2O formed from [1beta-3H] Androstenedione during aromatization. After incubation, the re... | J Med Chem 39: 2245-52 (1996) Article DOI: 10.1021/jm960047o BindingDB Entry DOI: 10.7270/Q2X34VPV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM9972 ((8S)-8-ethyl-2,15-dimethyltetracyclo[8.7.0.0^{2,7}...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 180 | n/a | 1.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Tohoku College of Pharmacy | Assay Description The enzyme activity was assayed by measuring the 3H-labeled H2O formed from [1beta-3H] Androstenedione during aromatization. After incubation, the re... | J Med Chem 39: 2245-52 (1996) Article DOI: 10.1021/jm960047o BindingDB Entry DOI: 10.7270/Q2X34VPV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM9977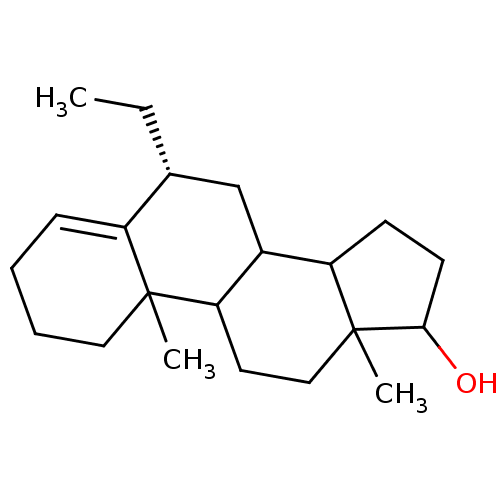 ((8R)-8-ethyl-2,15-dimethyltetracyclo[8.7.0.0^{2,7}...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 340 | n/a | 3.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Tohoku College of Pharmacy | Assay Description The enzyme activity was assayed by measuring the 3H-labeled H2O formed from [1beta-3H] Androstenedione during aromatization. After incubation, the re... | J Med Chem 39: 2245-52 (1996) Article DOI: 10.1021/jm960047o BindingDB Entry DOI: 10.7270/Q2X34VPV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM9971 ((8S)-2,8,15-trimethyltetracyclo[8.7.0.0^{2,7}.0^{1...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 380 | n/a | 3.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Tohoku College of Pharmacy | Assay Description The enzyme activity was assayed by measuring the 3H-labeled H2O formed from [1beta-3H] Androstenedione during aromatization. After incubation, the re... | J Med Chem 39: 2245-52 (1996) Article DOI: 10.1021/jm960047o BindingDB Entry DOI: 10.7270/Q2X34VPV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM9958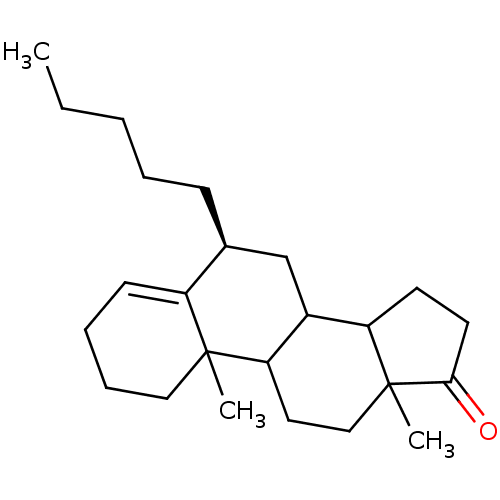 ((8S)-2,15-dimethyl-8-pentyltetracyclo[8.7.0.0^{2,7...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 400 | -38.0 | 9.00E+3 | n/a | n/a | n/a | n/a | 7.5 | 37 |
Tohoku College of Pharmacy | Assay Description The enzyme activity was assayed by measuring the 3H-labeled H2O formed from [1beta-3H] Androstenedione during aromatization. After incubation, the re... | J Med Chem 39: 2245-52 (1996) Article DOI: 10.1021/jm960047o BindingDB Entry DOI: 10.7270/Q2X34VPV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM9963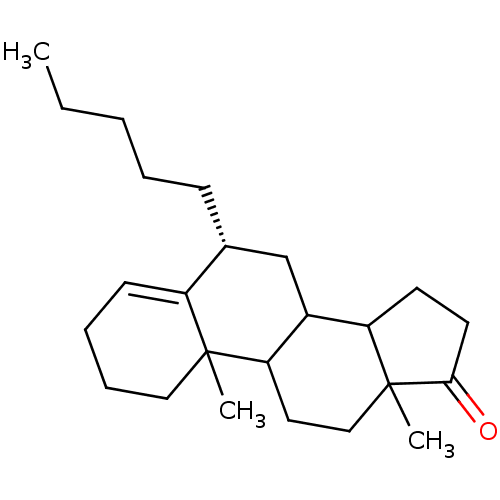 ((8R)-2,15-dimethyl-8-pentyltetracyclo[8.7.0.0^{2,7...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 1.20E+3 | -35.2 | 1.20E+4 | n/a | n/a | n/a | n/a | 7.5 | 37 |
Tohoku College of Pharmacy | Assay Description The enzyme activity was assayed by measuring the 3H-labeled H2O formed from [1beta-3H] Androstenedione during aromatization. After incubation, the re... | J Med Chem 39: 2245-52 (1996) Article DOI: 10.1021/jm960047o BindingDB Entry DOI: 10.7270/Q2X34VPV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM9964 ((8R)-2,15-dimethyl-8-octyltetracyclo[8.7.0.0^{2,7}...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 2.60E+3 | -33.2 | >5.00E+4 | n/a | n/a | n/a | n/a | 7.5 | 37 |
Tohoku College of Pharmacy | Assay Description The enzyme activity was assayed by measuring the 3H-labeled H2O formed from [1beta-3H] Androstenedione during aromatization. After incubation, the re... | J Med Chem 39: 2245-52 (1996) Article DOI: 10.1021/jm960047o BindingDB Entry DOI: 10.7270/Q2X34VPV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM9973 ((8S)-2,15-dimethyl-8-propyltetracyclo[8.7.0.0^{2,7...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 2.90E+3 | n/a | 1.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Tohoku College of Pharmacy | Assay Description The enzyme activity was assayed by measuring the 3H-labeled H2O formed from [1beta-3H] Androstenedione during aromatization. After incubation, the re... | J Med Chem 39: 2245-52 (1996) Article DOI: 10.1021/jm960047o BindingDB Entry DOI: 10.7270/Q2X34VPV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM9959 ((8S)-2,15-dimethyl-8-octyltetracyclo[8.7.0.0^{2,7}...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 4.10E+3 | -32.0 | >5.00E+4 | n/a | n/a | n/a | n/a | 7.5 | 37 |
Tohoku College of Pharmacy | Assay Description The enzyme activity was assayed by measuring the 3H-labeled H2O formed from [1beta-3H] Androstenedione during aromatization. After incubation, the re... | J Med Chem 39: 2245-52 (1996) Article DOI: 10.1021/jm960047o BindingDB Entry DOI: 10.7270/Q2X34VPV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM9978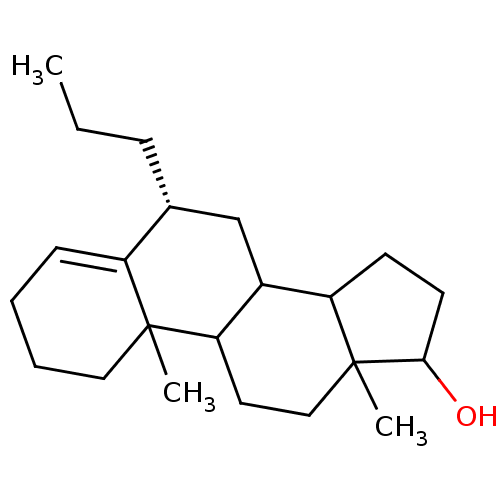 ((8R)-2,15-dimethyl-8-propyltetracyclo[8.7.0.0^{2,7...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 5.50E+3 | n/a | 4.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Tohoku College of Pharmacy | Assay Description The enzyme activity was assayed by measuring the 3H-labeled H2O formed from [1beta-3H] Androstenedione during aromatization. After incubation, the re... | J Med Chem 39: 2245-52 (1996) Article DOI: 10.1021/jm960047o BindingDB Entry DOI: 10.7270/Q2X34VPV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM9974 ((8S)-2,15-dimethyl-8-pentyltetracyclo[8.7.0.0^{2,7...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 4.40E+4 | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Tohoku College of Pharmacy | Assay Description The enzyme activity was assayed by measuring the 3H-labeled H2O formed from [1beta-3H] Androstenedione during aromatization. After incubation, the re... | J Med Chem 39: 2245-52 (1996) Article DOI: 10.1021/jm960047o BindingDB Entry DOI: 10.7270/Q2X34VPV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM9979 ((8R)-2,15-dimethyl-8-pentyltetracyclo[8.7.0.0^{2,7...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 1.00E+5 | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Tohoku College of Pharmacy | Assay Description The enzyme activity was assayed by measuring the 3H-labeled H2O formed from [1beta-3H] Androstenedione during aromatization. After incubation, the re... | J Med Chem 39: 2245-52 (1996) Article DOI: 10.1021/jm960047o BindingDB Entry DOI: 10.7270/Q2X34VPV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM9980 ((8R)-2,15-dimethyl-8-octyltetracyclo[8.7.0.0^{2,7}...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Tohoku College of Pharmacy | Assay Description The enzyme activity was assayed by measuring the 3H-labeled H2O formed from [1beta-3H] Androstenedione during aromatization. After incubation, the re... | J Med Chem 39: 2245-52 (1996) Article DOI: 10.1021/jm960047o BindingDB Entry DOI: 10.7270/Q2X34VPV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM9975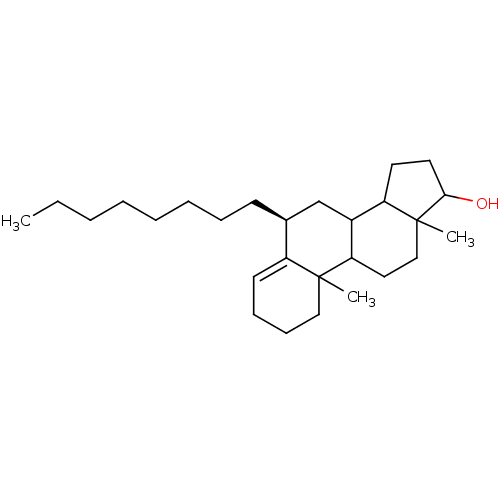 ((8S)-2,15-dimethyl-8-octyltetracyclo[8.7.0.0^{2,7}...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Tohoku College of Pharmacy | Assay Description The enzyme activity was assayed by measuring the 3H-labeled H2O formed from [1beta-3H] Androstenedione during aromatization. After incubation, the re... | J Med Chem 39: 2245-52 (1996) Article DOI: 10.1021/jm960047o BindingDB Entry DOI: 10.7270/Q2X34VPV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||