

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Coagulation factor X (Homo sapiens (Human)) | BDBM17654 (5-(5-chlorothiophen-2-yl)-N-[(3S)-1-[(2S)-1-(morph...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2 | -49.2 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
GSK | Assay Description The ability of test compounds to inhibit human fXa in vitro was determined in a fluorescence assay using rhodamime 110, bis-(Boc-L-glycylglycyl-L-arg... | J Med Chem 50: 1546-57 (2007) Article DOI: 10.1021/jm060870c BindingDB Entry DOI: 10.7270/Q2F18X06 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM17643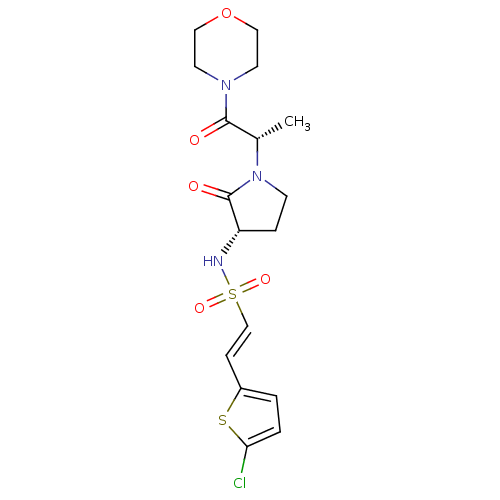 ((E)-2-(5-chlorothiophen-2-yl)-N-[(3S)-1-[(2S)-1-(m...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL DrugBank MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | 4 | -47.5 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
GSK | Assay Description The ability of test compounds to inhibit human fXa in vitro was determined in a fluorescence assay using rhodamime 110, bis-(Boc-L-glycylglycyl-L-arg... | J Med Chem 50: 1546-57 (2007) Article DOI: 10.1021/jm060870c BindingDB Entry DOI: 10.7270/Q2F18X06 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM17641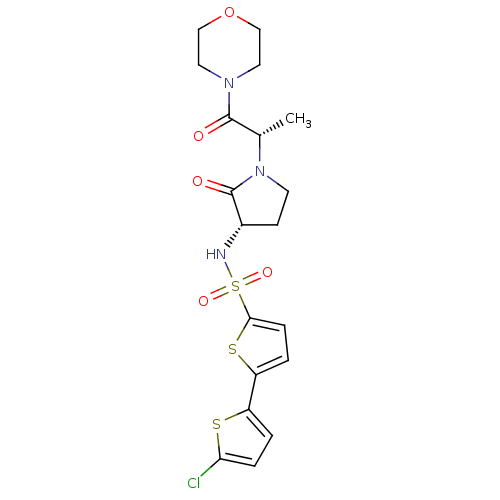 (5-(5-chlorothiophen-2-yl)-N-[(3S)-1-[(2S)-1-(morph...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | MMDB PC cid PC sid PDB UniChem Similars | MMDB PDB Article PubMed | 4 | -47.5 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
GSK | Assay Description The ability of test compounds to inhibit human fXa in vitro was determined in a fluorescence assay using rhodamime 110, bis-(Boc-L-glycylglycyl-L-arg... | J Med Chem 50: 1546-57 (2007) Article DOI: 10.1021/jm060870c BindingDB Entry DOI: 10.7270/Q2F18X06 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM17653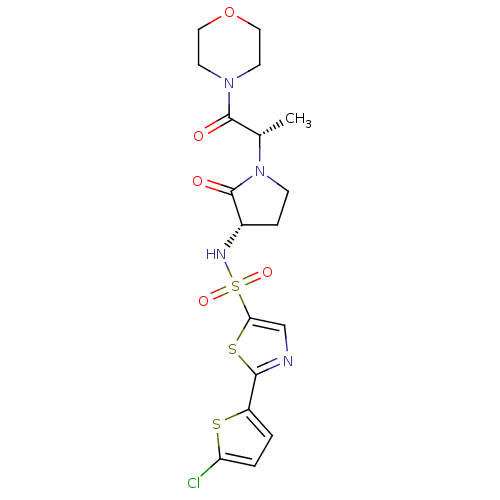 (2-(5-chlorothiophen-2-yl)-N-[(3S)-1-[(2S)-1-(morph...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 5 | -46.9 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
GSK | Assay Description The ability of test compounds to inhibit human fXa in vitro was determined in a fluorescence assay using rhodamime 110, bis-(Boc-L-glycylglycyl-L-arg... | J Med Chem 50: 1546-57 (2007) Article DOI: 10.1021/jm060870c BindingDB Entry DOI: 10.7270/Q2F18X06 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM12538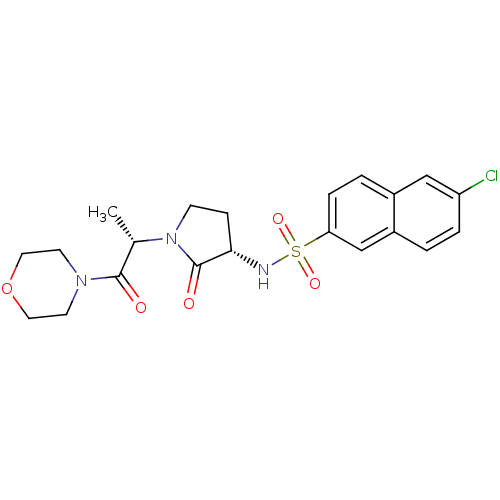 (6-chloro-N-[(3S)-1-[(2S)-1-(morpholin-4-yl)-1-oxop...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | DrugBank MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | 6 | -46.5 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
GSK | Assay Description The ability of test compounds to inhibit human fXa in vitro was determined in a fluorescence assay using rhodamime 110, bis-(Boc-L-glycylglycyl-L-arg... | J Med Chem 50: 1546-57 (2007) Article DOI: 10.1021/jm060870c BindingDB Entry DOI: 10.7270/Q2F18X06 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM17642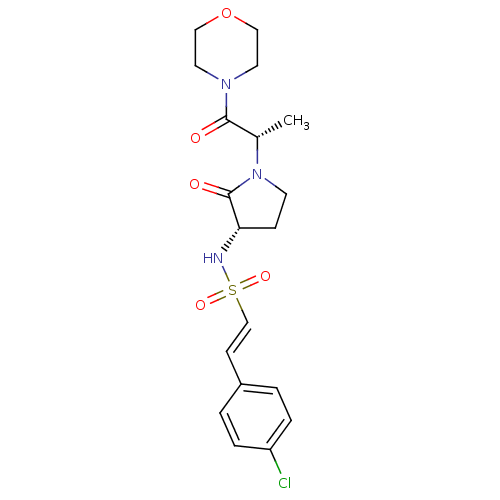 ((E)-2-(4-chlorophenyl)-N-[(3S)-1-[(2S)-1-(morpholi...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | PDB Article PubMed | 11 | -45.0 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
GSK | Assay Description The ability of test compounds to inhibit human fXa in vitro was determined in a fluorescence assay using rhodamime 110, bis-(Boc-L-glycylglycyl-L-arg... | J Med Chem 50: 1546-57 (2007) Article DOI: 10.1021/jm060870c BindingDB Entry DOI: 10.7270/Q2F18X06 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM12547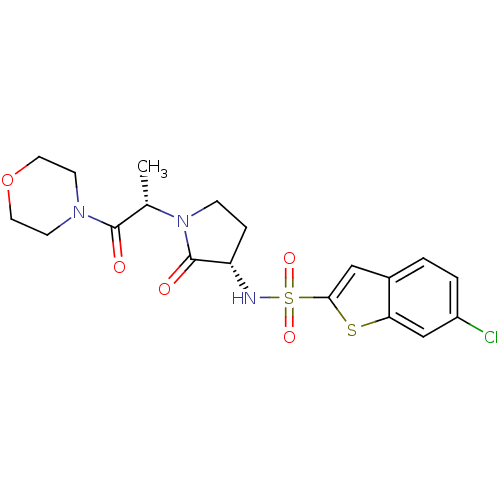 (6-chloro-N-[(3S)-1-[(2S)-1-(morpholin-4-yl)-1-oxop...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | DrugBank MMDB PC cid PC sid PDB UniChem Similars | MMDB PDB Article PubMed | 15 | -44.2 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
GSK | Assay Description The ability of test compounds to inhibit human fXa in vitro was determined in a fluorescence assay using rhodamime 110, bis-(Boc-L-glycylglycyl-L-arg... | J Med Chem 50: 1546-57 (2007) Article DOI: 10.1021/jm060870c BindingDB Entry DOI: 10.7270/Q2F18X06 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM17656 (6-(5-chlorothiophen-2-yl)-N-[(3S)-1-[(2S)-1-(morph...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 24 | -43.1 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
GSK | Assay Description The ability of test compounds to inhibit human fXa in vitro was determined in a fluorescence assay using rhodamime 110, bis-(Boc-L-glycylglycyl-L-arg... | J Med Chem 50: 1546-57 (2007) Article DOI: 10.1021/jm060870c BindingDB Entry DOI: 10.7270/Q2F18X06 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM12546 (5-chloro-N-[(3S)-1-[(2S)-1-(morpholin-4-yl)-1-oxop...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | DrugBank MMDB PC cid PC sid PDB UniChem Similars | MMDB PDB Article PubMed | 47 | -41.4 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
GSK | Assay Description The ability of test compounds to inhibit human fXa in vitro was determined in a fluorescence assay using rhodamime 110, bis-(Boc-L-glycylglycyl-L-arg... | J Med Chem 50: 1546-57 (2007) Article DOI: 10.1021/jm060870c BindingDB Entry DOI: 10.7270/Q2F18X06 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM12550 (5-chloro-N-[(3S)-1-[(2S)-1-(morpholin-4-yl)-1-oxop...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | DrugBank MMDB PC cid PC sid PDB UniChem Similars | MMDB PDB Article PubMed | 90 | -39.8 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
GSK | Assay Description The ability of test compounds to inhibit human fXa in vitro was determined in a fluorescence assay using rhodamime 110, bis-(Boc-L-glycylglycyl-L-arg... | J Med Chem 50: 1546-57 (2007) Article DOI: 10.1021/jm060870c BindingDB Entry DOI: 10.7270/Q2F18X06 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM17644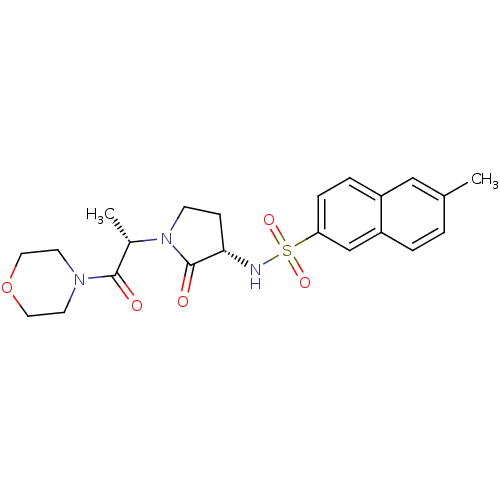 (6-methyl-N-[(3S)-1-[(2S)-1-(morpholin-4-yl)-1-oxop...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 109 | -39.3 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
GSK | Assay Description The ability of test compounds to inhibit human fXa in vitro was determined in a fluorescence assay using rhodamime 110, bis-(Boc-L-glycylglycyl-L-arg... | J Med Chem 50: 1546-57 (2007) Article DOI: 10.1021/jm060870c BindingDB Entry DOI: 10.7270/Q2F18X06 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM12548 (5-chloro-N-[(3S)-1-[(2S)-1-(morpholin-4-yl)-1-oxop...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 112 | -39.3 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
GSK | Assay Description The ability of test compounds to inhibit human fXa in vitro was determined in a fluorescence assay using rhodamime 110, bis-(Boc-L-glycylglycyl-L-arg... | J Med Chem 50: 1546-57 (2007) Article DOI: 10.1021/jm060870c BindingDB Entry DOI: 10.7270/Q2F18X06 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM17647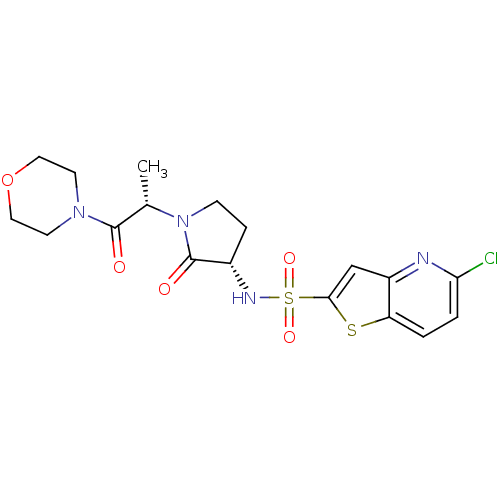 (5-chloro-N-[(3S)-1-[(2S)-1-(morpholin-4-yl)-1-oxop...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 163 | -38.4 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
GSK | Assay Description The ability of test compounds to inhibit human fXa in vitro was determined in a fluorescence assay using rhodamime 110, bis-(Boc-L-glycylglycyl-L-arg... | J Med Chem 50: 1546-57 (2007) Article DOI: 10.1021/jm060870c BindingDB Entry DOI: 10.7270/Q2F18X06 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM17645 (5-chloro-N-[(3S)-1-[(2S)-1-(morpholin-4-yl)-1-oxop...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 165 | -38.3 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
GSK | Assay Description The ability of test compounds to inhibit human fXa in vitro was determined in a fluorescence assay using rhodamime 110, bis-(Boc-L-glycylglycyl-L-arg... | J Med Chem 50: 1546-57 (2007) Article DOI: 10.1021/jm060870c BindingDB Entry DOI: 10.7270/Q2F18X06 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM12551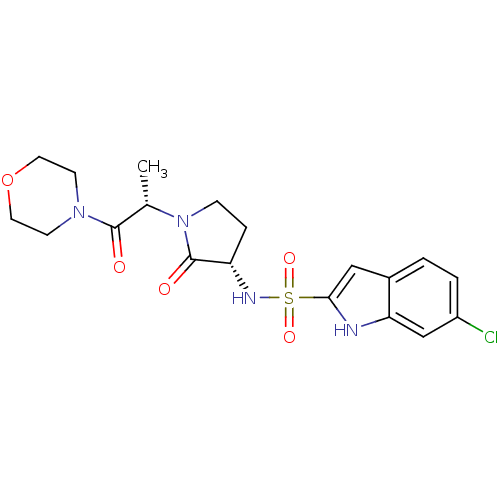 (6-chloro-N-[(3S)-1-[(2S)-1-(morpholin-4-yl)-1-oxop...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 170 | -38.2 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
GSK | Assay Description The ability of test compounds to inhibit human fXa in vitro was determined in a fluorescence assay using rhodamime 110, bis-(Boc-L-glycylglycyl-L-arg... | J Med Chem 50: 1546-57 (2007) Article DOI: 10.1021/jm060870c BindingDB Entry DOI: 10.7270/Q2F18X06 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM12553 (6-chloro-N-[(3S)-1-[(2S)-1-(morpholin-4-yl)-1-oxop...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 285 | -37.0 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
GSK | Assay Description The ability of test compounds to inhibit human fXa in vitro was determined in a fluorescence assay using rhodamime 110, bis-(Boc-L-glycylglycyl-L-arg... | J Med Chem 50: 1546-57 (2007) Article DOI: 10.1021/jm060870c BindingDB Entry DOI: 10.7270/Q2F18X06 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM17646 (4-[(E)-2-chloroethenyl]-N-[(3S)-1-[(2S)-1-(morphol...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 314 | -36.7 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
GSK | Assay Description The ability of test compounds to inhibit human fXa in vitro was determined in a fluorescence assay using rhodamime 110, bis-(Boc-L-glycylglycyl-L-arg... | J Med Chem 50: 1546-57 (2007) Article DOI: 10.1021/jm060870c BindingDB Entry DOI: 10.7270/Q2F18X06 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM17655 (5-(5-chlorothiophen-2-yl)-N-[(3S)-1-[(2S)-1-(morph...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | MMDB PC cid PC sid PDB UniChem | MMDB PDB Article PubMed | 534 | -35.4 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
GSK | Assay Description The ability of test compounds to inhibit human fXa in vitro was determined in a fluorescence assay using rhodamime 110, bis-(Boc-L-glycylglycyl-L-arg... | J Med Chem 50: 1546-57 (2007) Article DOI: 10.1021/jm060870c BindingDB Entry DOI: 10.7270/Q2F18X06 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM12552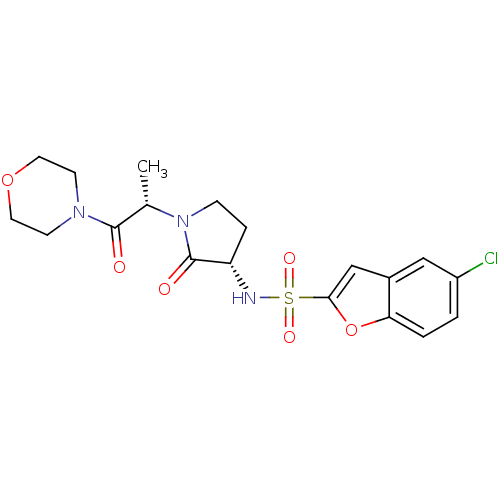 (5-chloro-N-[(3S)-1-[(2S)-1-(morpholin-4-yl)-1-oxop...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 782 | -34.5 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
GSK | Assay Description The ability of test compounds to inhibit human fXa in vitro was determined in a fluorescence assay using rhodamime 110, bis-(Boc-L-glycylglycyl-L-arg... | J Med Chem 50: 1546-57 (2007) Article DOI: 10.1021/jm060870c BindingDB Entry DOI: 10.7270/Q2F18X06 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM17652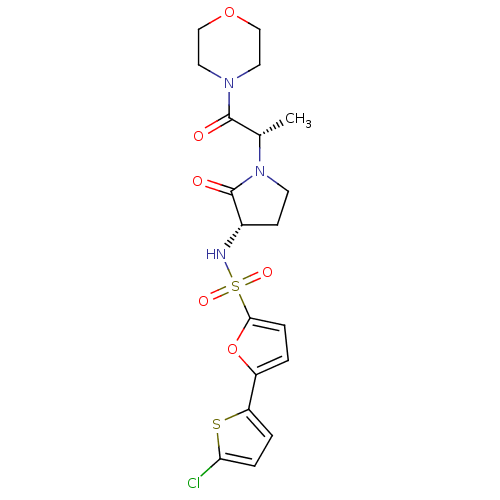 (5-(5-chlorothiophen-2-yl)-N-[(3S)-1-[(2S)-1-(morph...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.20E+3 | -33.5 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
GSK | Assay Description The ability of test compounds to inhibit human fXa in vitro was determined in a fluorescence assay using rhodamime 110, bis-(Boc-L-glycylglycyl-L-arg... | J Med Chem 50: 1546-57 (2007) Article DOI: 10.1021/jm060870c BindingDB Entry DOI: 10.7270/Q2F18X06 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM17648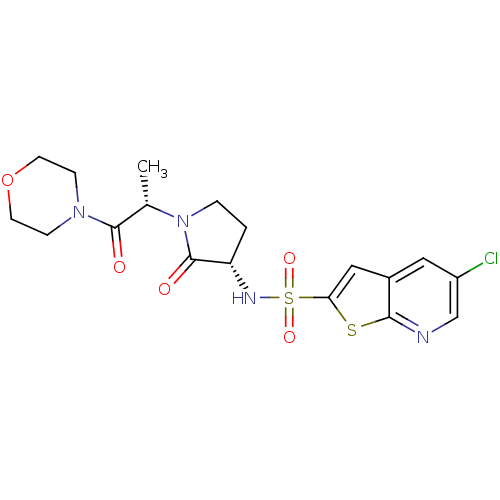 (5-chloro-N-[(3S)-1-[(2S)-1-(morpholin-4-yl)-1-oxop...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2.90E+3 | -31.3 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
GSK | Assay Description The ability of test compounds to inhibit human fXa in vitro was determined in a fluorescence assay using rhodamime 110, bis-(Boc-L-glycylglycyl-L-arg... | J Med Chem 50: 1546-57 (2007) Article DOI: 10.1021/jm060870c BindingDB Entry DOI: 10.7270/Q2F18X06 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM17651 (5-(5-chloro-1,3-thiazol-2-yl)-N-[(3S)-1-[(2S)-1-(m...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 3.23E+3 | -31.0 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
GSK | Assay Description The ability of test compounds to inhibit human fXa in vitro was determined in a fluorescence assay using rhodamime 110, bis-(Boc-L-glycylglycyl-L-arg... | J Med Chem 50: 1546-57 (2007) Article DOI: 10.1021/jm060870c BindingDB Entry DOI: 10.7270/Q2F18X06 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM17649 (6-chloro-N-[(3S)-1-[(2S)-1-(morpholin-4-yl)-1-oxop...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 3.67E+3 | -30.7 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
GSK | Assay Description The ability of test compounds to inhibit human fXa in vitro was determined in a fluorescence assay using rhodamime 110, bis-(Boc-L-glycylglycyl-L-arg... | J Med Chem 50: 1546-57 (2007) Article DOI: 10.1021/jm060870c BindingDB Entry DOI: 10.7270/Q2F18X06 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM12549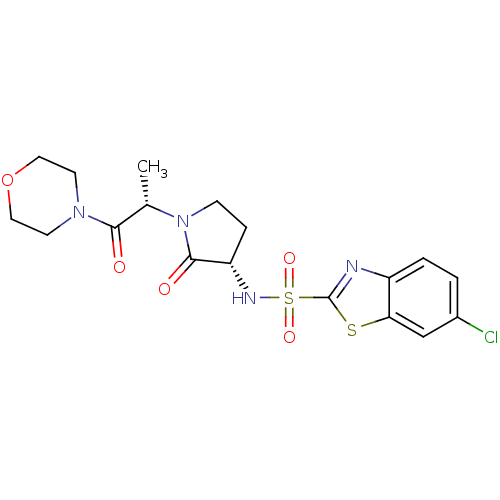 (6-chloro-N-[(3S)-1-[(2S)-1-(morpholin-4-yl)-1-oxop...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 4.16E+3 | -30.4 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
GSK | Assay Description The ability of test compounds to inhibit human fXa in vitro was determined in a fluorescence assay using rhodamime 110, bis-(Boc-L-glycylglycyl-L-arg... | J Med Chem 50: 1546-57 (2007) Article DOI: 10.1021/jm060870c BindingDB Entry DOI: 10.7270/Q2F18X06 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM17650 (5-(5-chloro-1,3,4-thiadiazol-2-yl)-N-[(3S)-1-[(2S)...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | >1.58E+4 | >-27.1 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
GSK | Assay Description The ability of test compounds to inhibit human fXa in vitro was determined in a fluorescence assay using rhodamime 110, bis-(Boc-L-glycylglycyl-L-arg... | J Med Chem 50: 1546-57 (2007) Article DOI: 10.1021/jm060870c BindingDB Entry DOI: 10.7270/Q2F18X06 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||