

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Aurora kinase A (Homo sapiens (Human)) | BDBM92800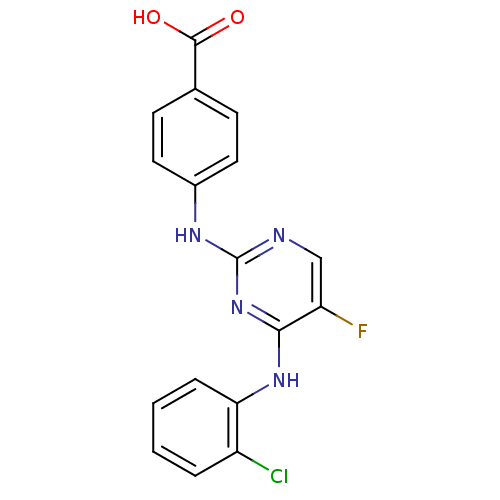 (Bisanilinopyrimidine, 3o | US9249124, 16) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | MMDB PC cid PC sid PDB UniChem Similars | PDB US Patent | n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | 7.4 | 18 |
H. Lee Moffitt Cancer Center and Research Institute, Inc. US Patent | Assay Description Aurora A was exchanged into 50 mM phosphate buffer (pH 7.4) including 1 mM DTT via PD-10 columns and was concentrated to 20 mg mL-1 using Amicon Ultr... | US Patent US9249124 (2016) BindingDB Entry DOI: 10.7270/Q2J1020R | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Aurora kinase A (Homo sapiens (Human)) | BDBM92800 (Bisanilinopyrimidine, 3o | US9249124, 16) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | MMDB PC cid PC sid PDB UniChem Similars | PDB US Patent | n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | 7.4 | 25 |
H. Lee Moffitt Cancer Center and Research Institute, Inc. US Patent | Assay Description Reactions were carried out at room temperature in 15 mM HEPES buffer (pH 7.4) containing 20 mM NaCl, 1 mM EGTA, 0.02% Tween 20, 10 mM MgCl2, 5% (v/v)... | US Patent US9249124 (2016) BindingDB Entry DOI: 10.7270/Q2J1020R | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Aurora kinase A (Homo sapiens (Human)) | BDBM92835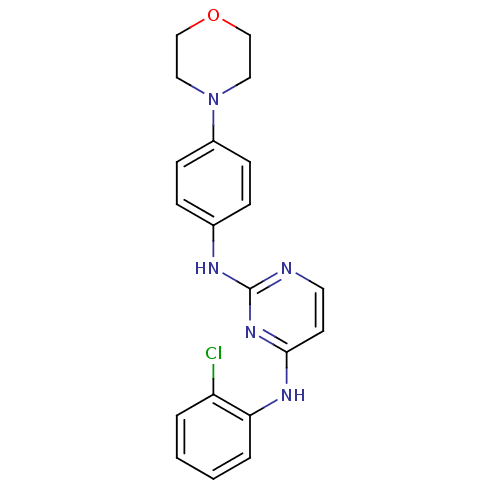 (Bisanilinopyrimidine, 9h | US9249124, 51) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | <1 | n/a | n/a | n/a | n/a | n/a | 37 |
H. Lee Moffitt Cancer Center and Research Institute, Inc. US Patent | Assay Description Specifically, MDA-MB-468 cells (American Type Culture Collection) were maintained in Dulbecco's modified Eagle's medium (DMEM) plus 10% fetus bovine ... | US Patent US9249124 (2016) BindingDB Entry DOI: 10.7270/Q2J1020R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aurora kinase A (Homo sapiens (Human)) | BDBM92840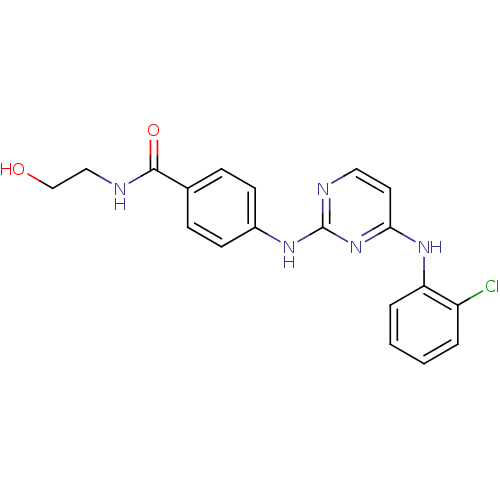 (Bisanilinopyrimidine, 9l | US9249124, 56) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Similars | US Patent | n/a | n/a | <1 | n/a | n/a | n/a | n/a | n/a | 37 |
H. Lee Moffitt Cancer Center and Research Institute, Inc. US Patent | Assay Description Specifically, MDA-MB-468 cells (American Type Culture Collection) were maintained in Dulbecco's modified Eagle's medium (DMEM) plus 10% fetus bovine ... | US Patent US9249124 (2016) BindingDB Entry DOI: 10.7270/Q2J1020R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aurora kinase A (Homo sapiens (Human)) | BDBM92836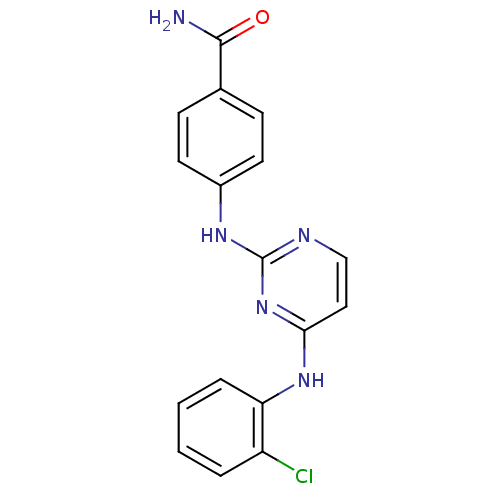 (Bisanilinopyrimidine, 6p | US9249124, 52) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | <1 | n/a | n/a | n/a | n/a | n/a | 37 |
H. Lee Moffitt Cancer Center and Research Institute, Inc. US Patent | Assay Description Specifically, MDA-MB-468 cells (American Type Culture Collection) were maintained in Dulbecco's modified Eagle's medium (DMEM) plus 10% fetus bovine ... | US Patent US9249124 (2016) BindingDB Entry DOI: 10.7270/Q2J1020R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aurora kinase A (Homo sapiens (Human)) | BDBM92837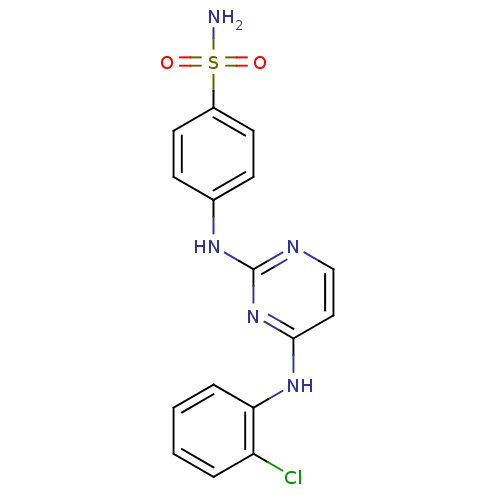 (Bisanilinopyrimidine, 9i | US9249124, 53) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | <1 | n/a | n/a | n/a | n/a | n/a | 37 |
H. Lee Moffitt Cancer Center and Research Institute, Inc. US Patent | Assay Description Specifically, MDA-MB-468 cells (American Type Culture Collection) were maintained in Dulbecco's modified Eagle's medium (DMEM) plus 10% fetus bovine ... | US Patent US9249124 (2016) BindingDB Entry DOI: 10.7270/Q2J1020R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aurora kinase A (Homo sapiens (Human)) | BDBM92841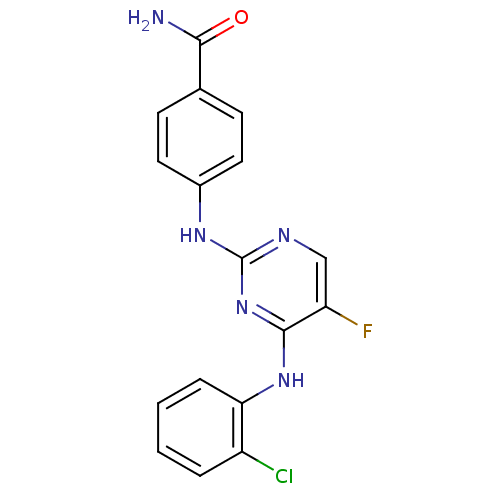 (Bisanilinopyrimidine, 9m | US9249124, 57) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | <1 | n/a | n/a | n/a | n/a | n/a | 37 |
H. Lee Moffitt Cancer Center and Research Institute, Inc. US Patent | Assay Description Specifically, MDA-MB-468 cells (American Type Culture Collection) were maintained in Dulbecco's modified Eagle's medium (DMEM) plus 10% fetus bovine ... | US Patent US9249124 (2016) BindingDB Entry DOI: 10.7270/Q2J1020R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aurora kinase A (Homo sapiens (Human)) | BDBM92838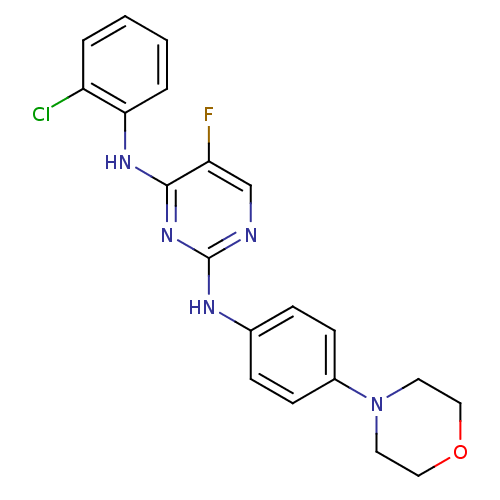 (Bisanilinopyrimidine, 9j | US9249124, 54) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | <1 | n/a | n/a | n/a | n/a | n/a | 37 |
H. Lee Moffitt Cancer Center and Research Institute, Inc. US Patent | Assay Description Specifically, MDA-MB-468 cells (American Type Culture Collection) were maintained in Dulbecco's modified Eagle's medium (DMEM) plus 10% fetus bovine ... | US Patent US9249124 (2016) BindingDB Entry DOI: 10.7270/Q2J1020R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aurora kinase A (Homo sapiens (Human)) | BDBM92839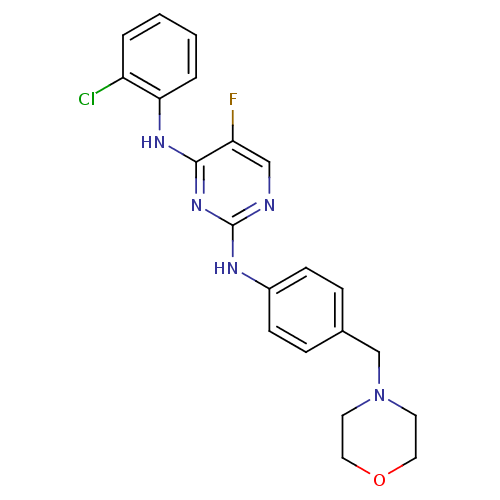 (Bisanilinopyrimidine, 9k | US9249124, 55) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <1 | n/a | n/a | n/a | n/a | n/a | 37 |
H. Lee Moffitt Cancer Center and Research Institute, Inc. US Patent | Assay Description Specifically, MDA-MB-468 cells (American Type Culture Collection) were maintained in Dulbecco's modified Eagle's medium (DMEM) plus 10% fetus bovine ... | US Patent US9249124 (2016) BindingDB Entry DOI: 10.7270/Q2J1020R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aurora kinase A (Homo sapiens (Human)) | BDBM92810 (Bisanilinopyrimidine, 6c | US9249124, 26) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 2 | n/a | n/a | n/a | n/a | 7.4 | 25 |
H. Lee Moffitt Cancer Center and Research Institute, Inc. US Patent | Assay Description Reactions were carried out at room temperature in 15 mM HEPES buffer (pH 7.4) containing 20 mM NaCl, 1 mM EGTA, 0.02% Tween 20, 10 mM MgCl2, 5% (v/v)... | US Patent US9249124 (2016) BindingDB Entry DOI: 10.7270/Q2J1020R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aurora kinase A (Homo sapiens (Human)) | BDBM87054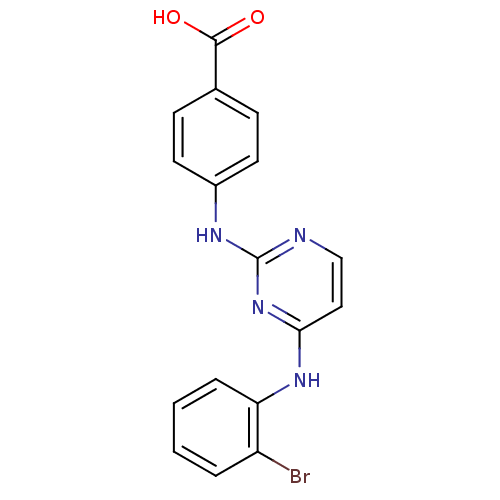 (Bisanilinopyrimidine inhibitor, 8 | Bisanilinopyri...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem Similars | PDB US Patent | n/a | n/a | 2.10 | 13 | n/a | n/a | n/a | 7.4 | n/a |
H. Lee Moffitt Cancer Center and Research Institute, Inc. US Patent | Assay Description The binding of inhibitors to Aurora A kinase was analyzed with a MicroCal iTC200 titration calorimeter (GE Healthcare, Piscataway, N.J.). The protein... | US Patent US9249124 (2016) BindingDB Entry DOI: 10.7270/Q2J1020R | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Aurora kinase A (Homo sapiens (Human)) | BDBM87054 (Bisanilinopyrimidine inhibitor, 8 | Bisanilinopyri...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem Similars | PDB US Patent | n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | 7.4 | 25 |
H. Lee Moffitt Cancer Center and Research Institute, Inc. US Patent | Assay Description Reactions were carried out at room temperature in 15 mM HEPES buffer (pH 7.4) containing 20 mM NaCl, 1 mM EGTA, 0.02% Tween 20, 10 mM MgCl2, 5% (v/v)... | US Patent US9249124 (2016) BindingDB Entry DOI: 10.7270/Q2J1020R | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Aurora kinase A (Homo sapiens (Human)) | BDBM87054 (Bisanilinopyrimidine inhibitor, 8 | Bisanilinopyri...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem Similars | PDB US Patent | n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | 7.4 | 18 |
H. Lee Moffitt Cancer Center and Research Institute, Inc. US Patent | Assay Description Aurora A was exchanged into 50 mM phosphate buffer (pH 7.4) including 1 mM DTT via PD-10 columns and was concentrated to 20 mg mL-1 using Amicon Ultr... | US Patent US9249124 (2016) BindingDB Entry DOI: 10.7270/Q2J1020R | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Aurora kinase A (Homo sapiens (Human)) | BDBM87053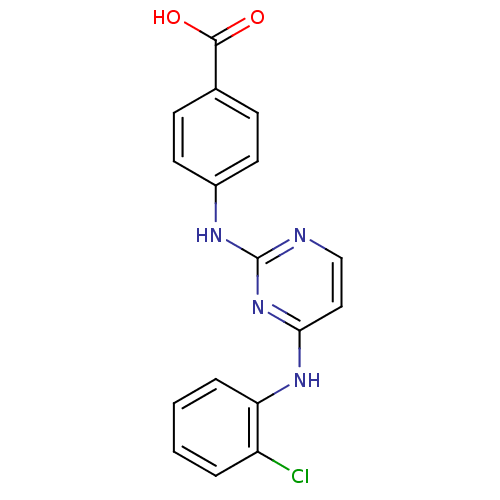 (Bisanilinopyrimidine inhibitor, 7 | Bisanilinopyri...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem Similars | PDB US Patent | n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | 7.4 | 25 |
H. Lee Moffitt Cancer Center and Research Institute, Inc. US Patent | Assay Description Reactions were carried out at room temperature in 15 mM HEPES buffer (pH 7.4) containing 20 mM NaCl, 1 mM EGTA, 0.02% Tween 20, 10 mM MgCl2, 5% (v/v)... | US Patent US9249124 (2016) BindingDB Entry DOI: 10.7270/Q2J1020R | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Aurora kinase A (Homo sapiens (Human)) | BDBM87053 (Bisanilinopyrimidine inhibitor, 7 | Bisanilinopyri...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem Similars | PDB US Patent | n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | 7.4 | 18 |
H. Lee Moffitt Cancer Center and Research Institute, Inc. US Patent | Assay Description Aurora A was exchanged into 50 mM phosphate buffer (pH 7.4) including 1 mM DTT via PD-10 columns and was concentrated to 20 mg mL-1 using Amicon Ultr... | US Patent US9249124 (2016) BindingDB Entry DOI: 10.7270/Q2J1020R | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Aurora kinase A (Homo sapiens (Human)) | BDBM87053 (Bisanilinopyrimidine inhibitor, 7 | Bisanilinopyri...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem Similars | PDB US Patent | n/a | n/a | 2.5 | 15 | n/a | n/a | n/a | 7.4 | n/a |
H. Lee Moffitt Cancer Center and Research Institute, Inc. US Patent | Assay Description The binding of inhibitors to Aurora A kinase was analyzed with a MicroCal iTC200 titration calorimeter (GE Healthcare, Piscataway, N.J.). The protein... | US Patent US9249124 (2016) BindingDB Entry DOI: 10.7270/Q2J1020R | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Aurora kinase A (Homo sapiens (Human)) | BDBM50004205 (MK-045 | MK-0457 | TOZASERTIB | US9249124, VX680 |...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 2.80 | 17 | n/a | n/a | n/a | 7.4 | n/a |
H. Lee Moffitt Cancer Center and Research Institute, Inc. US Patent | Assay Description The binding of inhibitors to Aurora A kinase was analyzed with a MicroCal iTC200 titration calorimeter (GE Healthcare, Piscataway, N.J.). The protein... | US Patent US9249124 (2016) BindingDB Entry DOI: 10.7270/Q2J1020R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aurora kinase A (Homo sapiens (Human)) | BDBM50004205 (MK-045 | MK-0457 | TOZASERTIB | US9249124, VX680 |...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 2.80 | n/a | n/a | n/a | n/a | 7.4 | 18 |
H. Lee Moffitt Cancer Center and Research Institute, Inc. US Patent | Assay Description Aurora A was exchanged into 50 mM phosphate buffer (pH 7.4) including 1 mM DTT via PD-10 columns and was concentrated to 20 mg mL-1 using Amicon Ultr... | US Patent US9249124 (2016) BindingDB Entry DOI: 10.7270/Q2J1020R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aurora kinase A (Homo sapiens (Human)) | BDBM92844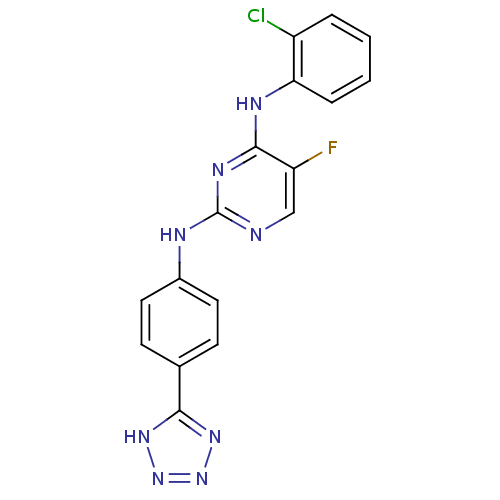 (Bisanilinopyrimidine, 12b | US9249124, 60) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 2.90 | n/a | n/a | n/a | n/a | 7.4 | 25 |
H. Lee Moffitt Cancer Center and Research Institute, Inc. US Patent | Assay Description Reactions were carried out at room temperature in 15 mM HEPES buffer (pH 7.4) containing 20 mM NaCl, 1 mM EGTA, 0.02% Tween 20, 10 mM MgCl2, 5% (v/v)... | US Patent US9249124 (2016) BindingDB Entry DOI: 10.7270/Q2J1020R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aurora kinase A (Homo sapiens (Human)) | BDBM92846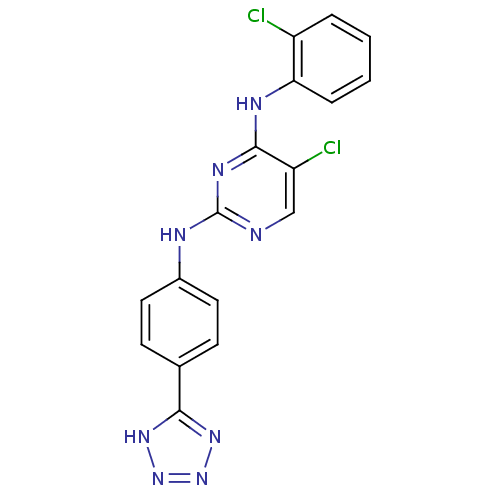 (Bisanilinopyrimidine, 12d | US9249124, 62) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 3 | n/a | n/a | n/a | n/a | 7.4 | 25 |
H. Lee Moffitt Cancer Center and Research Institute, Inc. US Patent | Assay Description Reactions were carried out at room temperature in 15 mM HEPES buffer (pH 7.4) containing 20 mM NaCl, 1 mM EGTA, 0.02% Tween 20, 10 mM MgCl2, 5% (v/v)... | US Patent US9249124 (2016) BindingDB Entry DOI: 10.7270/Q2J1020R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aurora kinase A (Homo sapiens (Human)) | BDBM92843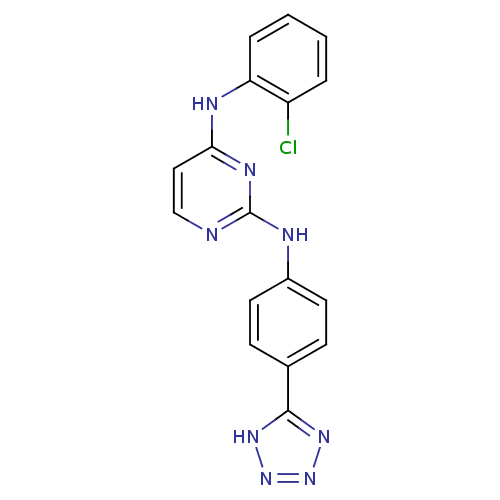 (Bisanilinopyrimidine, 12a | US9249124, 59) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | MMDB PC cid PC sid PDB UniChem Similars | PDB US Patent | n/a | n/a | 3.10 | n/a | n/a | n/a | n/a | 7.4 | 25 |
H. Lee Moffitt Cancer Center and Research Institute, Inc. US Patent | Assay Description Reactions were carried out at room temperature in 15 mM HEPES buffer (pH 7.4) containing 20 mM NaCl, 1 mM EGTA, 0.02% Tween 20, 10 mM MgCl2, 5% (v/v)... | US Patent US9249124 (2016) BindingDB Entry DOI: 10.7270/Q2J1020R | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Aurora kinase A (Homo sapiens (Human)) | BDBM92843 (Bisanilinopyrimidine, 12a | US9249124, 59) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | MMDB PC cid PC sid PDB UniChem Similars | PDB US Patent | n/a | n/a | 3.10 | n/a | n/a | n/a | n/a | 7.4 | 18 |
H. Lee Moffitt Cancer Center and Research Institute, Inc. US Patent | Assay Description Aurora A was exchanged into 50 mM phosphate buffer (pH 7.4) including 1 mM DTT via PD-10 columns and was concentrated to 20 mg mL-1 using Amicon Ultr... | US Patent US9249124 (2016) BindingDB Entry DOI: 10.7270/Q2J1020R | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Aurora kinase A (Homo sapiens (Human)) | BDBM92843 (Bisanilinopyrimidine, 12a | US9249124, 59) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | MMDB PC cid PC sid PDB UniChem Similars | PDB US Patent | n/a | n/a | 3.10 | 18 | n/a | n/a | n/a | 7.4 | n/a |
H. Lee Moffitt Cancer Center and Research Institute, Inc. US Patent | Assay Description The binding of inhibitors to Aurora A kinase was analyzed with a MicroCal iTC200 titration calorimeter (GE Healthcare, Piscataway, N.J.). The protein... | US Patent US9249124 (2016) BindingDB Entry DOI: 10.7270/Q2J1020R | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Aurora kinase A (Homo sapiens (Human)) | BDBM92805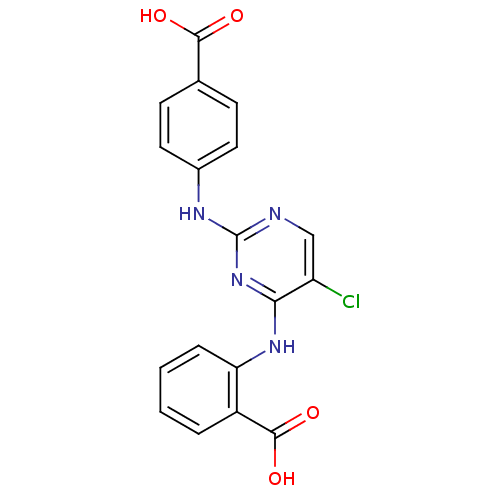 (Bisanilinopyrimidine, 4d | US9249124, 21) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 3.17 | n/a | n/a | n/a | n/a | 7.4 | 25 |
H. Lee Moffitt Cancer Center and Research Institute, Inc. US Patent | Assay Description Reactions were carried out at room temperature in 15 mM HEPES buffer (pH 7.4) containing 20 mM NaCl, 1 mM EGTA, 0.02% Tween 20, 10 mM MgCl2, 5% (v/v)... | US Patent US9249124 (2016) BindingDB Entry DOI: 10.7270/Q2J1020R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aurora kinase A (Homo sapiens (Human)) | BDBM92817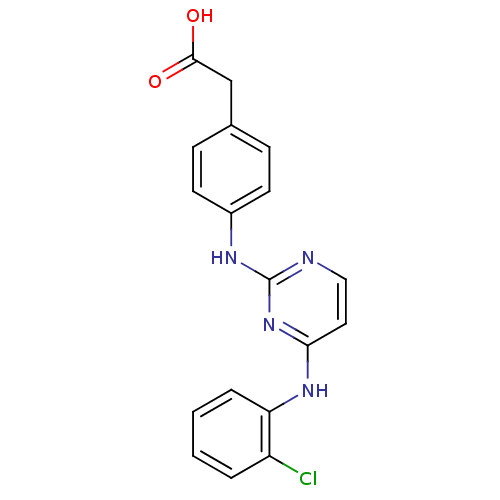 (Bisanilinopyrimidine, 6j | US9249124, 33) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | US Patent | n/a | n/a | 3.30 | n/a | n/a | n/a | n/a | 7.4 | 25 |
H. Lee Moffitt Cancer Center and Research Institute, Inc. US Patent | Assay Description Reactions were carried out at room temperature in 15 mM HEPES buffer (pH 7.4) containing 20 mM NaCl, 1 mM EGTA, 0.02% Tween 20, 10 mM MgCl2, 5% (v/v)... | US Patent US9249124 (2016) BindingDB Entry DOI: 10.7270/Q2J1020R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aurora kinase A (Homo sapiens (Human)) | BDBM87052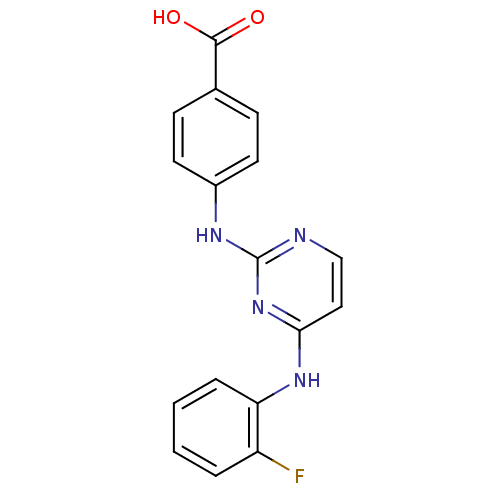 (Bisanilinopyrimidine inhibitor, 6 | Bisanilinopyri...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem Patents Similars | PDB US Patent | n/a | n/a | 3.70 | n/a | n/a | n/a | n/a | 7.4 | 18 |
H. Lee Moffitt Cancer Center and Research Institute, Inc. US Patent | Assay Description Aurora A was exchanged into 50 mM phosphate buffer (pH 7.4) including 1 mM DTT via PD-10 columns and was concentrated to 20 mg mL-1 using Amicon Ultr... | US Patent US9249124 (2016) BindingDB Entry DOI: 10.7270/Q2J1020R | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Aurora kinase A (Homo sapiens (Human)) | BDBM87052 (Bisanilinopyrimidine inhibitor, 6 | Bisanilinopyri...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem Patents Similars | PDB US Patent | n/a | n/a | 3.70 | 16 | n/a | n/a | n/a | 7.4 | n/a |
H. Lee Moffitt Cancer Center and Research Institute, Inc. US Patent | Assay Description The binding of inhibitors to Aurora A kinase was analyzed with a MicroCal iTC200 titration calorimeter (GE Healthcare, Piscataway, N.J.). The protein... | US Patent US9249124 (2016) BindingDB Entry DOI: 10.7270/Q2J1020R | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Aurora kinase A (Homo sapiens (Human)) | BDBM87052 (Bisanilinopyrimidine inhibitor, 6 | Bisanilinopyri...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem Patents Similars | PDB US Patent | n/a | n/a | 3.70 | n/a | n/a | n/a | n/a | 7.4 | 25 |
H. Lee Moffitt Cancer Center and Research Institute, Inc. US Patent | Assay Description Reactions were carried out at room temperature in 15 mM HEPES buffer (pH 7.4) containing 20 mM NaCl, 1 mM EGTA, 0.02% Tween 20, 10 mM MgCl2, 5% (v/v)... | US Patent US9249124 (2016) BindingDB Entry DOI: 10.7270/Q2J1020R | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Aurora kinase A (Homo sapiens (Human)) | BDBM92799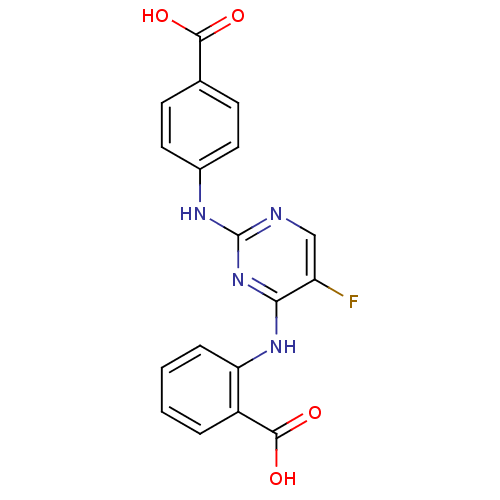 (Bisanilinopyrimidine, 3n | US9249124, 15) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 3.90 | n/a | n/a | n/a | n/a | 7.4 | 25 |
H. Lee Moffitt Cancer Center and Research Institute, Inc. US Patent | Assay Description Reactions were carried out at room temperature in 15 mM HEPES buffer (pH 7.4) containing 20 mM NaCl, 1 mM EGTA, 0.02% Tween 20, 10 mM MgCl2, 5% (v/v)... | US Patent US9249124 (2016) BindingDB Entry DOI: 10.7270/Q2J1020R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aurora kinase A (Homo sapiens (Human)) | BDBM92812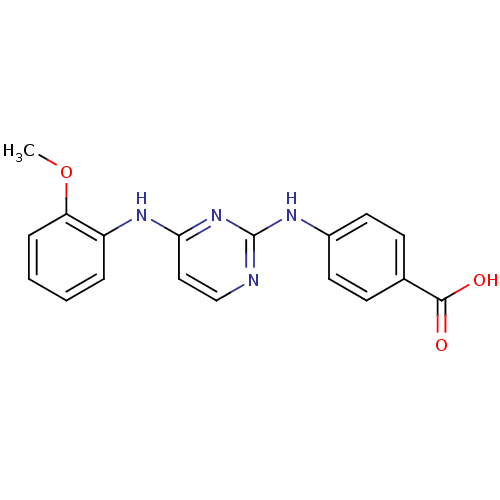 (Bisanilinopyrimidine, 6e | US9249124, 28) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 4 | n/a | n/a | n/a | n/a | 7.4 | 25 |
H. Lee Moffitt Cancer Center and Research Institute, Inc. US Patent | Assay Description Reactions were carried out at room temperature in 15 mM HEPES buffer (pH 7.4) containing 20 mM NaCl, 1 mM EGTA, 0.02% Tween 20, 10 mM MgCl2, 5% (v/v)... | US Patent US9249124 (2016) BindingDB Entry DOI: 10.7270/Q2J1020R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aurora kinase A (Homo sapiens (Human)) | BDBM209949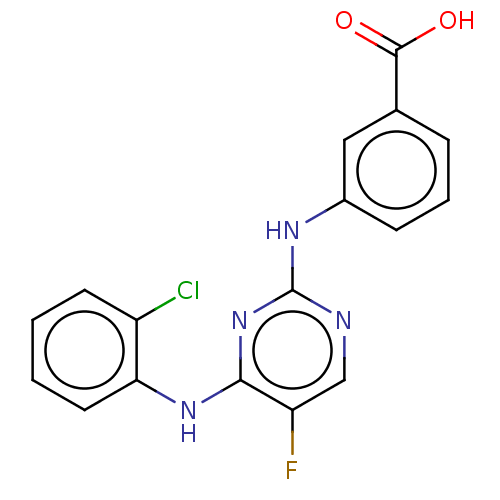 (US9249124, 43) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 4.5 | n/a | n/a | n/a | n/a | 7.4 | 25 |
H. Lee Moffitt Cancer Center and Research Institute, Inc. US Patent | Assay Description Reactions were carried out at room temperature in 15 mM HEPES buffer (pH 7.4) containing 20 mM NaCl, 1 mM EGTA, 0.02% Tween 20, 10 mM MgCl2, 5% (v/v)... | US Patent US9249124 (2016) BindingDB Entry DOI: 10.7270/Q2J1020R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aurora kinase A (Homo sapiens (Human)) | BDBM92803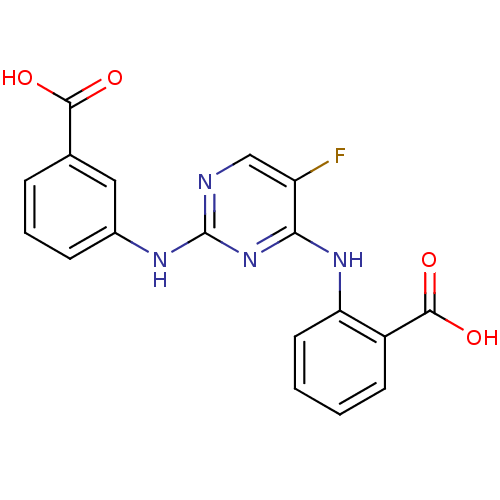 (Bisanilinopyrimidine, 3r | US9249124, 19) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 5.10 | n/a | n/a | n/a | n/a | 7.4 | 25 |
H. Lee Moffitt Cancer Center and Research Institute, Inc. US Patent | Assay Description Reactions were carried out at room temperature in 15 mM HEPES buffer (pH 7.4) containing 20 mM NaCl, 1 mM EGTA, 0.02% Tween 20, 10 mM MgCl2, 5% (v/v)... | US Patent US9249124 (2016) BindingDB Entry DOI: 10.7270/Q2J1020R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aurora kinase A (Homo sapiens (Human)) | BDBM87048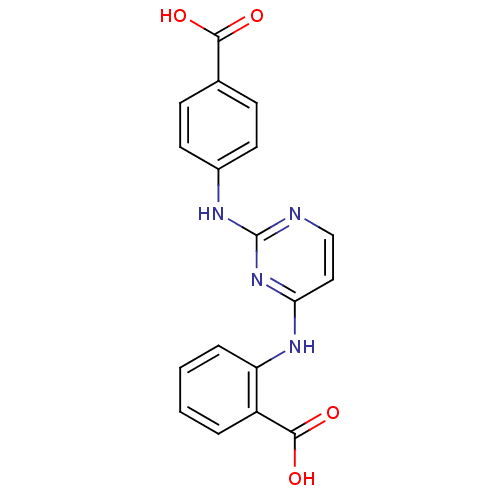 (Bisanilinopyrimidine inhibitor, 2 | Bisanilinopyri...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem Similars | PDB US Patent | n/a | n/a | 6.10 | n/a | n/a | n/a | n/a | 7.4 | 18 |
H. Lee Moffitt Cancer Center and Research Institute, Inc. US Patent | Assay Description Aurora A was exchanged into 50 mM phosphate buffer (pH 7.4) including 1 mM DTT via PD-10 columns and was concentrated to 20 mg mL-1 using Amicon Ultr... | US Patent US9249124 (2016) BindingDB Entry DOI: 10.7270/Q2J1020R | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Aurora kinase A (Homo sapiens (Human)) | BDBM87048 (Bisanilinopyrimidine inhibitor, 2 | Bisanilinopyri...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem Similars | PDB US Patent | n/a | n/a | 6.10 | n/a | n/a | n/a | n/a | 7.4 | 25 |
H. Lee Moffitt Cancer Center and Research Institute, Inc. US Patent | Assay Description Reactions were carried out at room temperature in 15 mM HEPES buffer (pH 7.4) containing 20 mM NaCl, 1 mM EGTA, 0.02% Tween 20, 10 mM MgCl2, 5% (v/v)... | US Patent US9249124 (2016) BindingDB Entry DOI: 10.7270/Q2J1020R | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Aurora kinase A (Homo sapiens (Human)) | BDBM87048 (Bisanilinopyrimidine inhibitor, 2 | Bisanilinopyri...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem Similars | PDB US Patent | n/a | n/a | 6.10 | 34 | n/a | n/a | n/a | 7.4 | n/a |
H. Lee Moffitt Cancer Center and Research Institute, Inc. US Patent | Assay Description The binding of inhibitors to Aurora A kinase was analyzed with a MicroCal iTC200 titration calorimeter (GE Healthcare, Piscataway, N.J.). The protein... | US Patent US9249124 (2016) BindingDB Entry DOI: 10.7270/Q2J1020R | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Aurora kinase A (Homo sapiens (Human)) | BDBM209946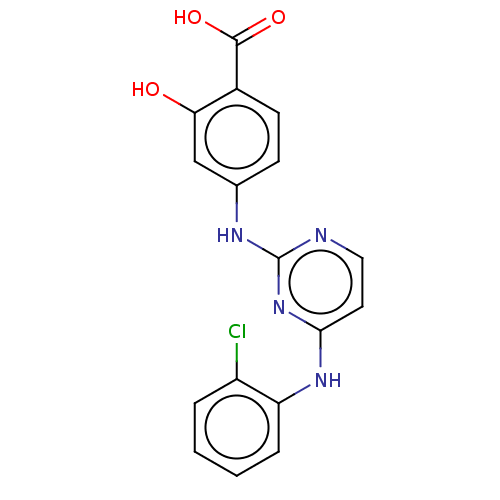 (US9249124, 34) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 6.60 | n/a | n/a | n/a | n/a | 7.4 | 25 |
H. Lee Moffitt Cancer Center and Research Institute, Inc. US Patent | Assay Description Reactions were carried out at room temperature in 15 mM HEPES buffer (pH 7.4) containing 20 mM NaCl, 1 mM EGTA, 0.02% Tween 20, 10 mM MgCl2, 5% (v/v)... | US Patent US9249124 (2016) BindingDB Entry DOI: 10.7270/Q2J1020R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aurora kinase A (Homo sapiens (Human)) | BDBM92825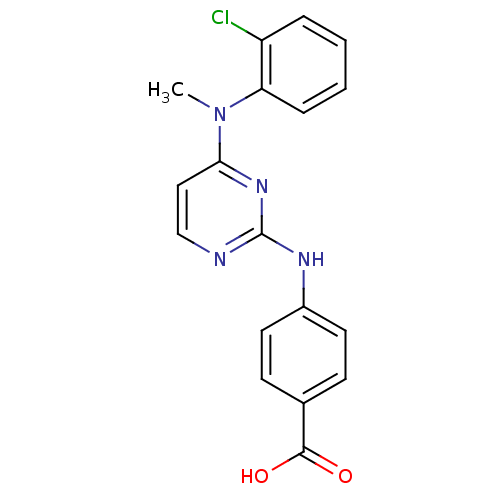 (Bisanilinopyrimidine, 6r | US9249124, 41) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 8.5 | n/a | n/a | n/a | n/a | 7.4 | 25 |
H. Lee Moffitt Cancer Center and Research Institute, Inc. US Patent | Assay Description Reactions were carried out at room temperature in 15 mM HEPES buffer (pH 7.4) containing 20 mM NaCl, 1 mM EGTA, 0.02% Tween 20, 10 mM MgCl2, 5% (v/v)... | US Patent US9249124 (2016) BindingDB Entry DOI: 10.7270/Q2J1020R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aurora kinase A (Homo sapiens (Human)) | BDBM87047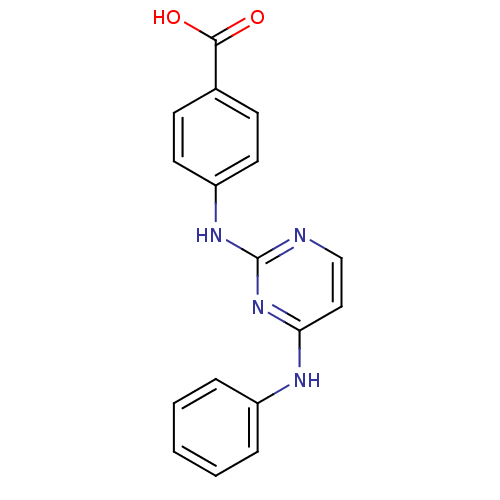 (Bisanilinopyrimidine inhibitor, 1 | Bisanilinopyri...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid PDB UniChem Similars | PDB US Patent | n/a | n/a | 10 | n/a | n/a | n/a | n/a | 7.4 | 25 |
H. Lee Moffitt Cancer Center and Research Institute, Inc. US Patent | Assay Description Reactions were carried out at room temperature in 15 mM HEPES buffer (pH 7.4) containing 20 mM NaCl, 1 mM EGTA, 0.02% Tween 20, 10 mM MgCl2, 5% (v/v)... | US Patent US9249124 (2016) BindingDB Entry DOI: 10.7270/Q2J1020R | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Aurora kinase A (Homo sapiens (Human)) | BDBM87047 (Bisanilinopyrimidine inhibitor, 1 | Bisanilinopyri...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid PDB UniChem Similars | PDB US Patent | n/a | n/a | 10 | 39 | n/a | n/a | n/a | 7.4 | n/a |
H. Lee Moffitt Cancer Center and Research Institute, Inc. US Patent | Assay Description The binding of inhibitors to Aurora A kinase was analyzed with a MicroCal iTC200 titration calorimeter (GE Healthcare, Piscataway, N.J.). The protein... | US Patent US9249124 (2016) BindingDB Entry DOI: 10.7270/Q2J1020R | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Aurora kinase A (Homo sapiens (Human)) | BDBM87047 (Bisanilinopyrimidine inhibitor, 1 | Bisanilinopyri...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid PDB UniChem Similars | PDB US Patent | n/a | n/a | 10 | n/a | n/a | n/a | n/a | 7.4 | 18 |
H. Lee Moffitt Cancer Center and Research Institute, Inc. US Patent | Assay Description Aurora A was exchanged into 50 mM phosphate buffer (pH 7.4) including 1 mM DTT via PD-10 columns and was concentrated to 20 mg mL-1 using Amicon Ultr... | US Patent US9249124 (2016) BindingDB Entry DOI: 10.7270/Q2J1020R | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Aurora kinase A (Homo sapiens (Human)) | BDBM16011 (2-{[5-fluoro-2-(phenylamino)pyrimidin-4-yl]amino}b...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 11.3 | n/a | n/a | n/a | n/a | 7.4 | 25 |
H. Lee Moffitt Cancer Center and Research Institute, Inc. US Patent | Assay Description Reactions were carried out at room temperature in 15 mM HEPES buffer (pH 7.4) containing 20 mM NaCl, 1 mM EGTA, 0.02% Tween 20, 10 mM MgCl2, 5% (v/v)... | US Patent US9249124 (2016) BindingDB Entry DOI: 10.7270/Q2J1020R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aurora kinase A (Homo sapiens (Human)) | BDBM92840 (Bisanilinopyrimidine, 9l | US9249124, 56) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 12.3 | n/a | n/a | n/a | n/a | 7.4 | 25 |
H. Lee Moffitt Cancer Center and Research Institute, Inc. US Patent | Assay Description Reactions were carried out at room temperature in 15 mM HEPES buffer (pH 7.4) containing 20 mM NaCl, 1 mM EGTA, 0.02% Tween 20, 10 mM MgCl2, 5% (v/v)... | US Patent US9249124 (2016) BindingDB Entry DOI: 10.7270/Q2J1020R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aurora kinase A (Homo sapiens (Human)) | BDBM92841 (Bisanilinopyrimidine, 9m | US9249124, 57) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 14.4 | n/a | n/a | n/a | n/a | 7.4 | 25 |
H. Lee Moffitt Cancer Center and Research Institute, Inc. US Patent | Assay Description Reactions were carried out at room temperature in 15 mM HEPES buffer (pH 7.4) containing 20 mM NaCl, 1 mM EGTA, 0.02% Tween 20, 10 mM MgCl2, 5% (v/v)... | US Patent US9249124 (2016) BindingDB Entry DOI: 10.7270/Q2J1020R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aurora kinase A (Homo sapiens (Human)) | BDBM92845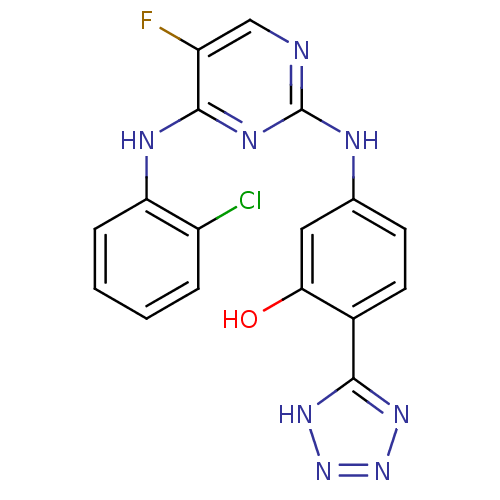 (Bisanilinopyrimidine, 12c | US9249124, 61) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 17.3 | n/a | n/a | n/a | n/a | 7.4 | 25 |
H. Lee Moffitt Cancer Center and Research Institute, Inc. US Patent | Assay Description Reactions were carried out at room temperature in 15 mM HEPES buffer (pH 7.4) containing 20 mM NaCl, 1 mM EGTA, 0.02% Tween 20, 10 mM MgCl2, 5% (v/v)... | US Patent US9249124 (2016) BindingDB Entry DOI: 10.7270/Q2J1020R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aurora kinase A (Homo sapiens (Human)) | BDBM92830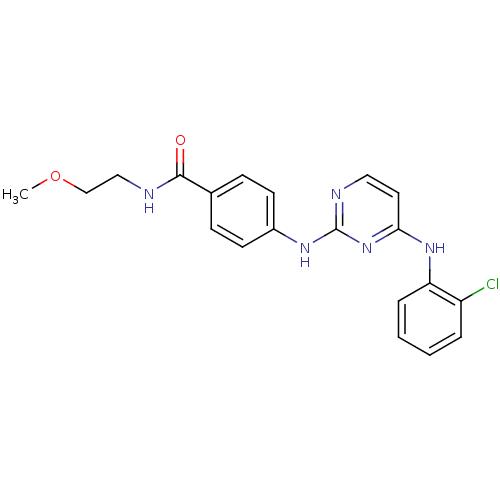 (Bisanilinopyrimidine, 9c | US9249124, 46) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 18 | n/a | n/a | n/a | n/a | 7.4 | 25 |
H. Lee Moffitt Cancer Center and Research Institute, Inc. US Patent | Assay Description Reactions were carried out at room temperature in 15 mM HEPES buffer (pH 7.4) containing 20 mM NaCl, 1 mM EGTA, 0.02% Tween 20, 10 mM MgCl2, 5% (v/v)... | US Patent US9249124 (2016) BindingDB Entry DOI: 10.7270/Q2J1020R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aurora kinase A (Homo sapiens (Human)) | BDBM92802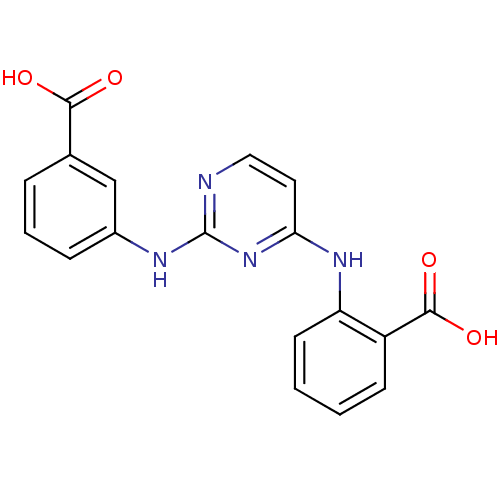 (Bisanilinopyrimidine, 3q | US9249124, 18) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 18.3 | n/a | n/a | n/a | n/a | 7.4 | 25 |
H. Lee Moffitt Cancer Center and Research Institute, Inc. US Patent | Assay Description Reactions were carried out at room temperature in 15 mM HEPES buffer (pH 7.4) containing 20 mM NaCl, 1 mM EGTA, 0.02% Tween 20, 10 mM MgCl2, 5% (v/v)... | US Patent US9249124 (2016) BindingDB Entry DOI: 10.7270/Q2J1020R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aurora kinase A (Homo sapiens (Human)) | BDBM209947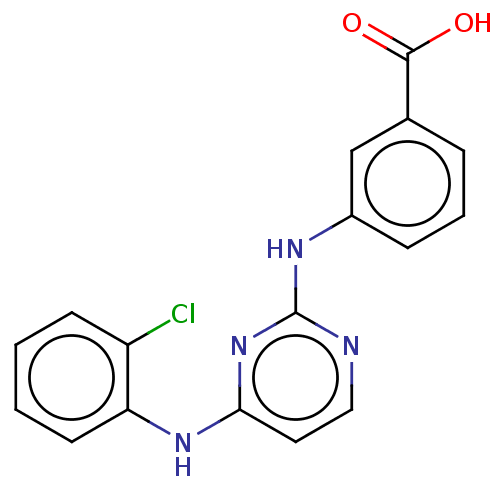 (US9249124, 38) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 18.7 | n/a | n/a | n/a | n/a | 7.4 | 25 |
H. Lee Moffitt Cancer Center and Research Institute, Inc. US Patent | Assay Description Reactions were carried out at room temperature in 15 mM HEPES buffer (pH 7.4) containing 20 mM NaCl, 1 mM EGTA, 0.02% Tween 20, 10 mM MgCl2, 5% (v/v)... | US Patent US9249124 (2016) BindingDB Entry DOI: 10.7270/Q2J1020R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aurora kinase A (Homo sapiens (Human)) | BDBM92801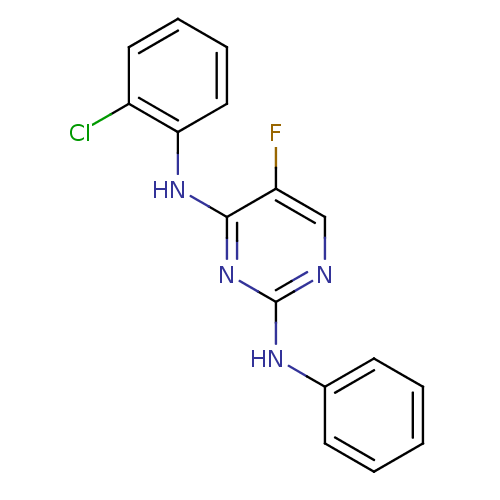 (Bisanilinopyrimidine, 3p | US9249124, 17) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 19.9 | n/a | n/a | n/a | n/a | 7.4 | 25 |
H. Lee Moffitt Cancer Center and Research Institute, Inc. US Patent | Assay Description Reactions were carried out at room temperature in 15 mM HEPES buffer (pH 7.4) containing 20 mM NaCl, 1 mM EGTA, 0.02% Tween 20, 10 mM MgCl2, 5% (v/v)... | US Patent US9249124 (2016) BindingDB Entry DOI: 10.7270/Q2J1020R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aurora kinase A (Homo sapiens (Human)) | BDBM92842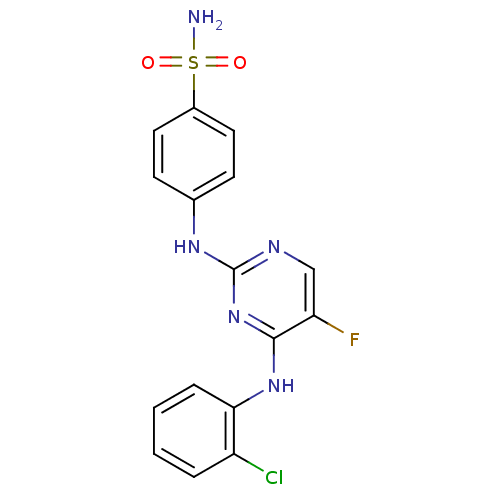 (Bisanilinopyrimidine, 9n | US9249124, 58) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 21.2 | n/a | n/a | n/a | n/a | 7.4 | 25 |
H. Lee Moffitt Cancer Center and Research Institute, Inc. US Patent | Assay Description Reactions were carried out at room temperature in 15 mM HEPES buffer (pH 7.4) containing 20 mM NaCl, 1 mM EGTA, 0.02% Tween 20, 10 mM MgCl2, 5% (v/v)... | US Patent US9249124 (2016) BindingDB Entry DOI: 10.7270/Q2J1020R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aurora kinase A (Homo sapiens (Human)) | BDBM92838 (Bisanilinopyrimidine, 9j | US9249124, 54) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 21.4 | n/a | n/a | n/a | n/a | 7.4 | 25 |
H. Lee Moffitt Cancer Center and Research Institute, Inc. US Patent | Assay Description Reactions were carried out at room temperature in 15 mM HEPES buffer (pH 7.4) containing 20 mM NaCl, 1 mM EGTA, 0.02% Tween 20, 10 mM MgCl2, 5% (v/v)... | US Patent US9249124 (2016) BindingDB Entry DOI: 10.7270/Q2J1020R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 141 total ) | Next | Last >> |