

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Genome polyprotein (Hepatitis C virus) | BDBM50120291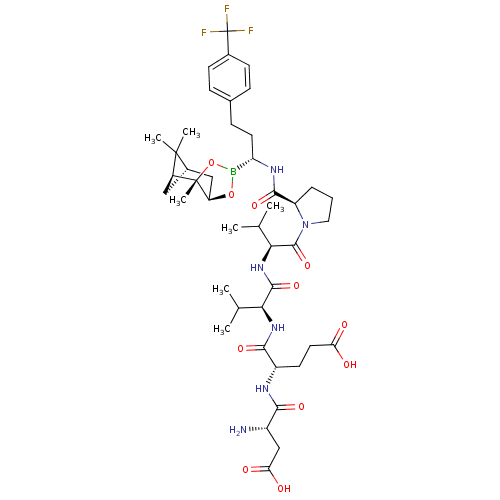 (CHEMBL107869 | Peptide Boronic Acid analogue) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Binding affinity against Hepatitis C virus NS3 protease | Bioorg Med Chem Lett 12: 3199-202 (2002) BindingDB Entry DOI: 10.7270/Q2R49Q38 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus) | BDBM50120311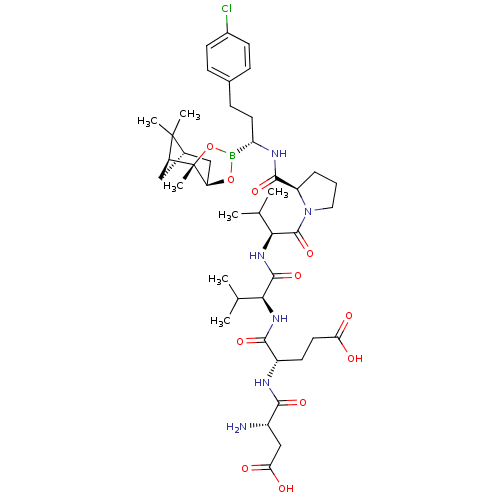 (CHEMBL320814 | Peptide Boronic Acid analogue) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Binding affinity against Hepatitis C virus NS3 protease | Bioorg Med Chem Lett 12: 3199-202 (2002) BindingDB Entry DOI: 10.7270/Q2R49Q38 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus) | BDBM50120290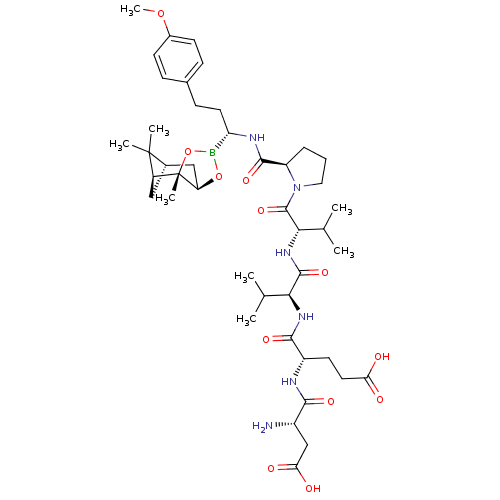 (CHEMBL107287 | Peptide Boronic Acid analogue) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Binding affinity against Hepatitis C virus NS3 protease | Bioorg Med Chem Lett 12: 3199-202 (2002) BindingDB Entry DOI: 10.7270/Q2R49Q38 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus) | BDBM50120295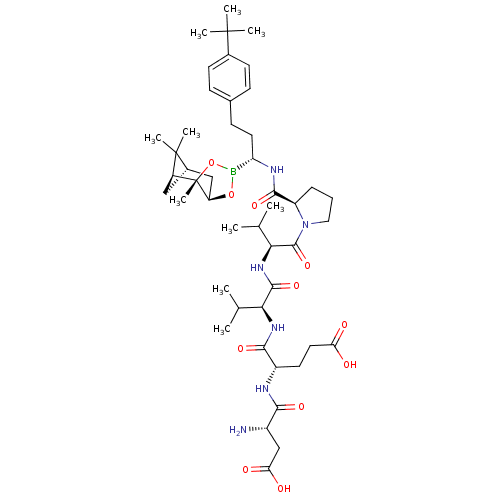 (CHEMBL324207 | Peptide Boronic Acid analogue) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Binding affinity against Hepatitis C virus NS3 protease | Bioorg Med Chem Lett 12: 3199-202 (2002) BindingDB Entry DOI: 10.7270/Q2R49Q38 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus) | BDBM50120293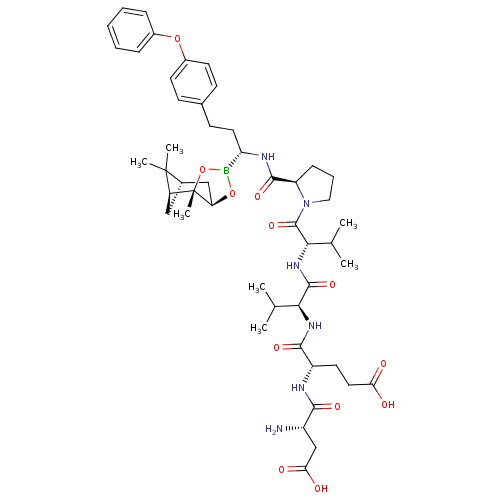 (CHEMBL413150 | Peptide Boronic Acid analogue) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Binding affinity against Hepatitis C virus NS3 protease | Bioorg Med Chem Lett 12: 3199-202 (2002) BindingDB Entry DOI: 10.7270/Q2R49Q38 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus) | BDBM50120285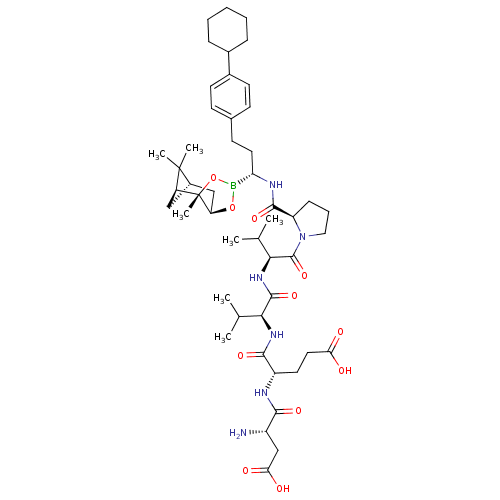 (CHEMBL108657 | Peptide Boronic Acid analogue) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Binding affinity against Hepatitis C virus NS3 protease | Bioorg Med Chem Lett 12: 3199-202 (2002) BindingDB Entry DOI: 10.7270/Q2R49Q38 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus) | BDBM50120304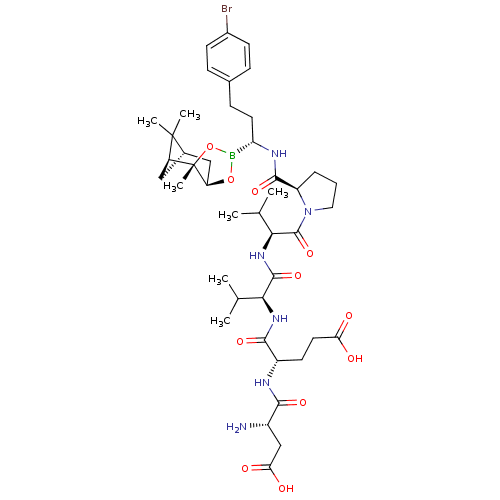 (CHEMBL108815 | Peptide Boronic Acid analogue) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Binding affinity against Hepatitis C virus NS3 protease | Bioorg Med Chem Lett 12: 3199-202 (2002) BindingDB Entry DOI: 10.7270/Q2R49Q38 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus) | BDBM50120306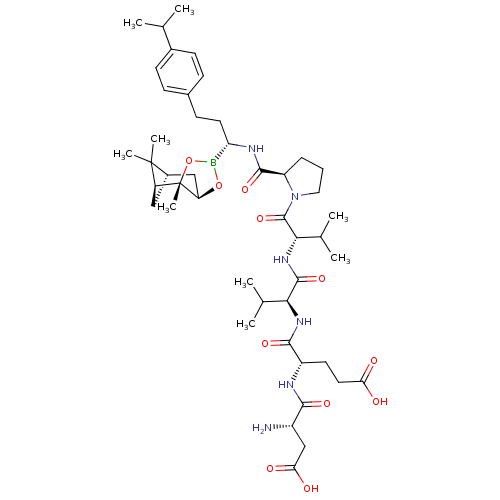 (CHEMBL262398 | Peptide Boronic Acid analogue) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Binding affinity against Hepatitis C virus NS3 protease | Bioorg Med Chem Lett 12: 3199-202 (2002) BindingDB Entry DOI: 10.7270/Q2R49Q38 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus) | BDBM50120284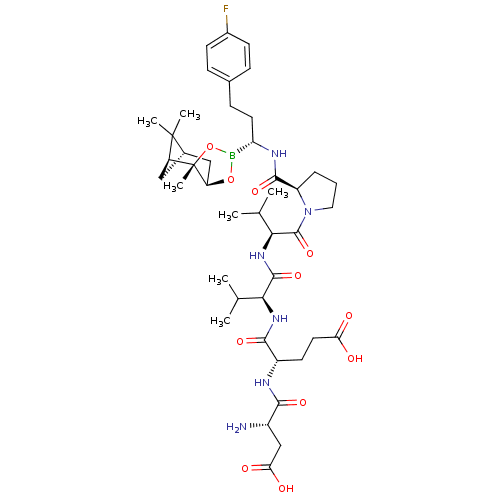 (CHEMBL107656 | Peptide Boronic Acid analogue) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Binding affinity against Hepatitis C virus NS3 protease | Bioorg Med Chem Lett 12: 3199-202 (2002) BindingDB Entry DOI: 10.7270/Q2R49Q38 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus) | BDBM50120303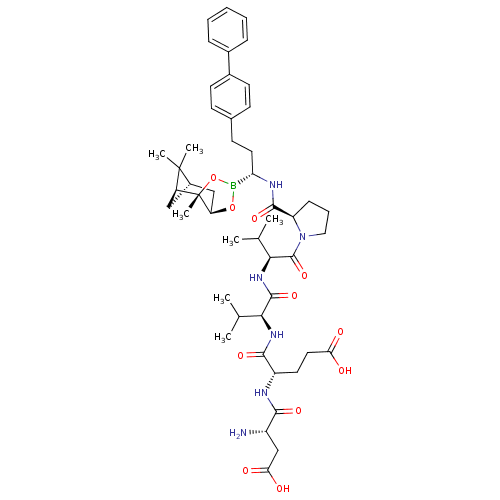 (CHEMBL432959 | Peptide Boronic Acid analogue) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Binding affinity against Hepatitis C virus NS3 protease | Bioorg Med Chem Lett 12: 3199-202 (2002) BindingDB Entry DOI: 10.7270/Q2R49Q38 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus) | BDBM50120286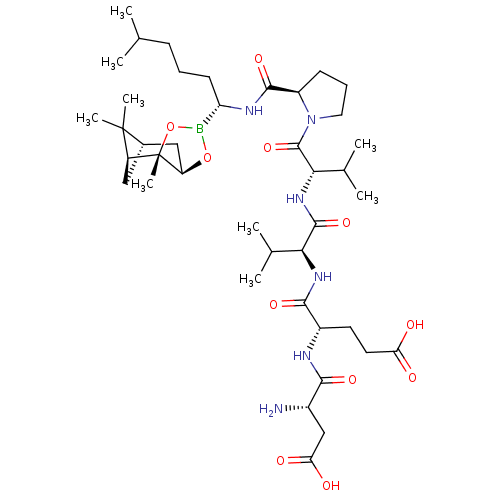 (CHEMBL322933 | Peptide Boronic Acid analogue) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Binding affinity against Hepatitis C virus NS3 protease | Bioorg Med Chem Lett 12: 3199-202 (2002) BindingDB Entry DOI: 10.7270/Q2R49Q38 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus) | BDBM50120299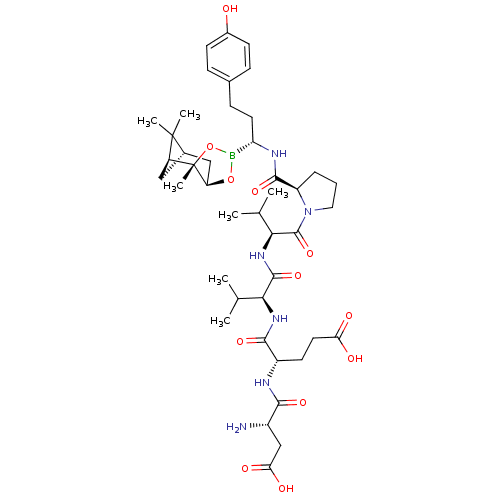 (CHEMBL110828 | Peptide Boronic Acid analogue) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Binding affinity against Hepatitis C virus NS3 protease | Bioorg Med Chem Lett 12: 3199-202 (2002) BindingDB Entry DOI: 10.7270/Q2R49Q38 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus) | BDBM50120298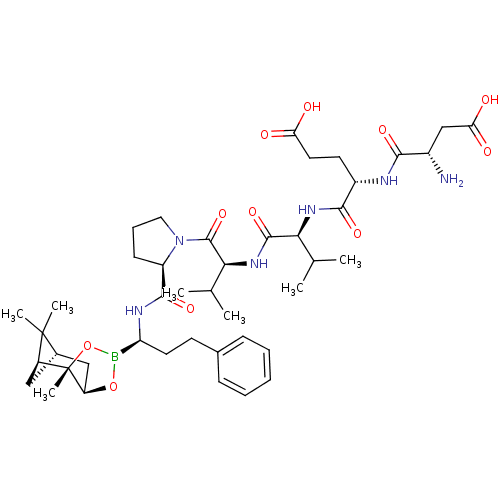 (CHEMBL109434 | Peptide Boronic Acid analogue) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Binding affinity against Hepatitis C virus NS3 protease | Bioorg Med Chem Lett 12: 3199-202 (2002) BindingDB Entry DOI: 10.7270/Q2R49Q38 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus) | BDBM50120296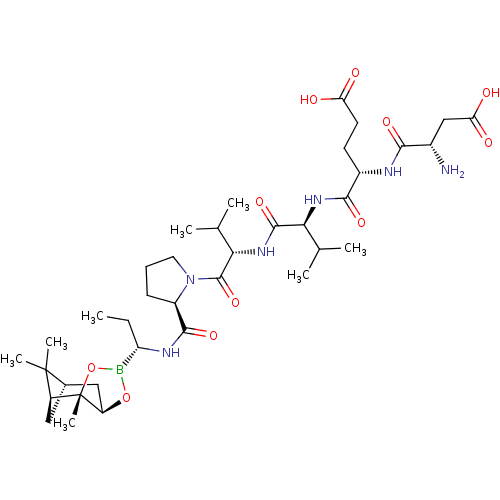 (CHEMBL419918 | Peptide Boronic Acid analogue) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Binding affinity against Hepatitis C virus NS3 protease | Bioorg Med Chem Lett 12: 3199-202 (2002) BindingDB Entry DOI: 10.7270/Q2R49Q38 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus) | BDBM50120308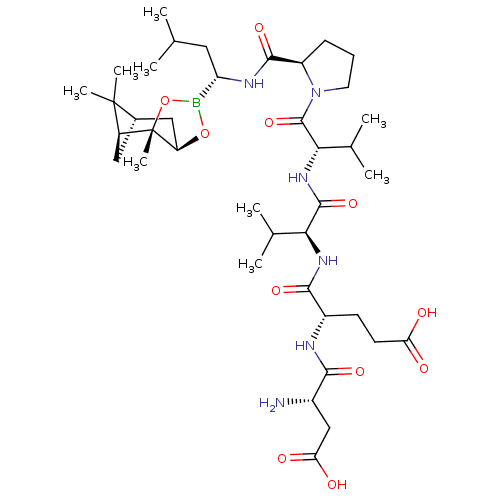 (CHEMBL443537 | Peptide Boronic Acid analogue) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Binding affinity against Hepatitis C virus NS3 protease | Bioorg Med Chem Lett 12: 3199-202 (2002) BindingDB Entry DOI: 10.7270/Q2R49Q38 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus) | BDBM50120302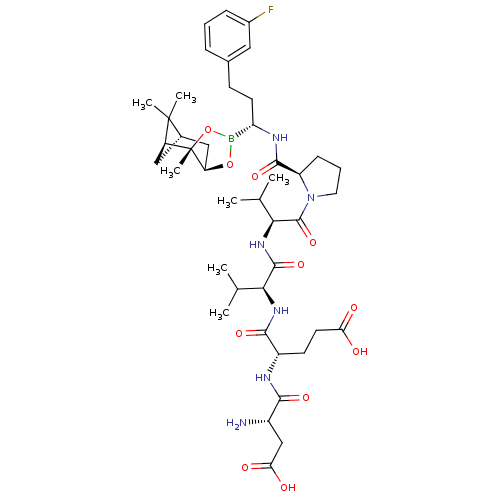 (CHEMBL108449 | Peptide Boronic Acid analogue) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Binding affinity against Hepatitis C virus NS3 protease | Bioorg Med Chem Lett 12: 3199-202 (2002) BindingDB Entry DOI: 10.7270/Q2R49Q38 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus) | BDBM50120289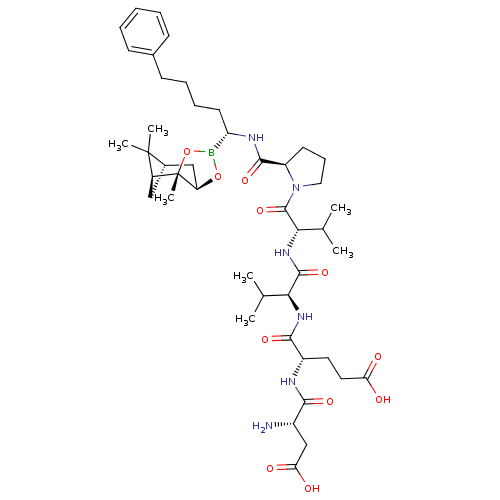 (CHEMBL419567 | Peptide Boronic Acid analogue) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Binding affinity against Hepatitis C virus NS3 protease | Bioorg Med Chem Lett 12: 3199-202 (2002) BindingDB Entry DOI: 10.7270/Q2R49Q38 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus) | BDBM50120307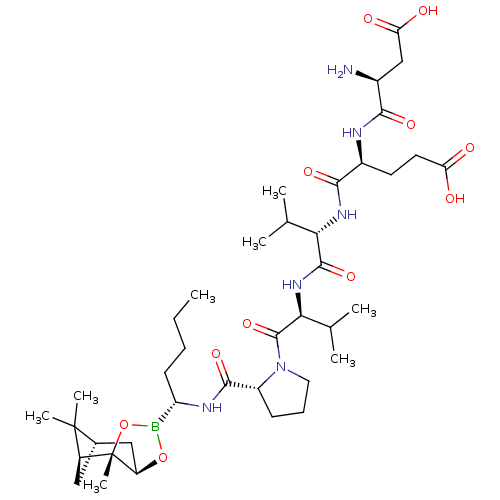 (CHEMBL263941 | Peptide Boronic Acid analogue) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Binding affinity against Hepatitis C virus NS3 protease | Bioorg Med Chem Lett 12: 3199-202 (2002) BindingDB Entry DOI: 10.7270/Q2R49Q38 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus) | BDBM50120288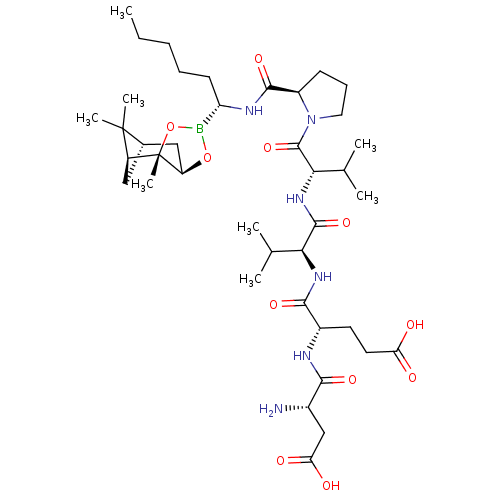 (CHEMBL432978 | Peptide Boronic Acid analogue) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Binding affinity against Hepatitis C virus NS3 protease | Bioorg Med Chem Lett 12: 3199-202 (2002) BindingDB Entry DOI: 10.7270/Q2R49Q38 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus) | BDBM50120305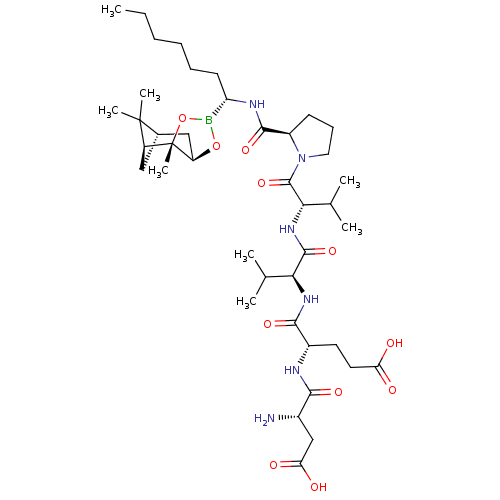 (CHEMBL111765 | Peptide Boronic Acid analogue) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Binding affinity against Hepatitis C virus NS3 protease | Bioorg Med Chem Lett 12: 3199-202 (2002) BindingDB Entry DOI: 10.7270/Q2R49Q38 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus) | BDBM50120300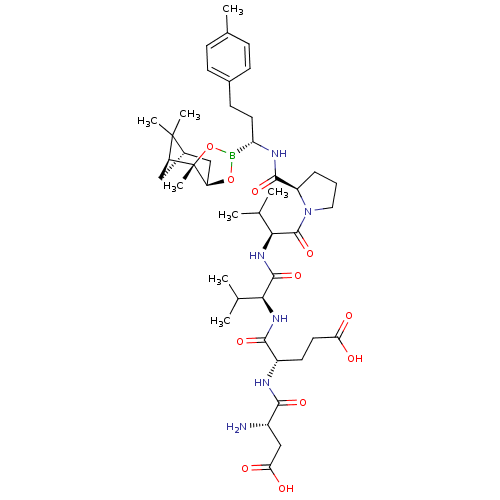 (CHEMBL431246 | Peptide Boronic Acid analogue) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 17 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Binding affinity against Hepatitis C virus NS3 protease | Bioorg Med Chem Lett 12: 3199-202 (2002) BindingDB Entry DOI: 10.7270/Q2R49Q38 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus) | BDBM50120283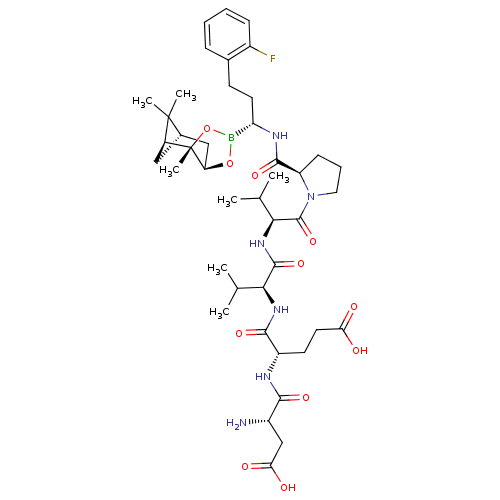 (CHEMBL322784 | Peptide Boronic Acid analogue) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 18 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Binding affinity against Hepatitis C virus NS3 protease | Bioorg Med Chem Lett 12: 3199-202 (2002) BindingDB Entry DOI: 10.7270/Q2R49Q38 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus) | BDBM50120301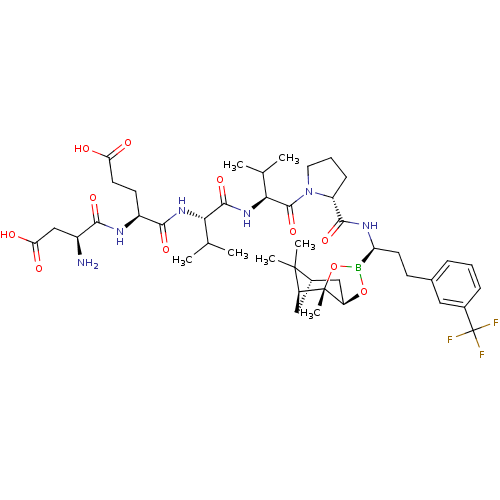 (CHEMBL322277 | Peptide Boronic Acid analogue) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Binding affinity against Hepatitis C virus NS3 protease | Bioorg Med Chem Lett 12: 3199-202 (2002) BindingDB Entry DOI: 10.7270/Q2R49Q38 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus) | BDBM50120287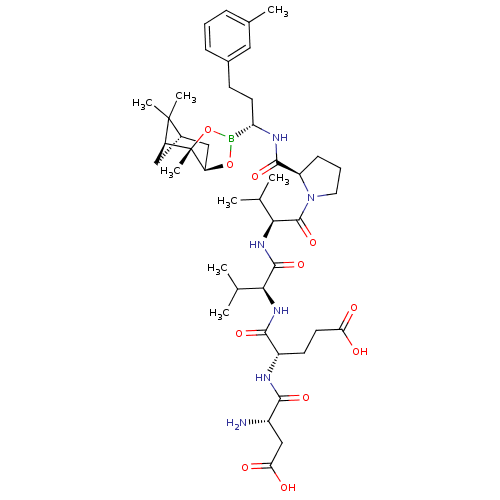 (CHEMBL109483 | Peptide Boronic Acid analogue) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 34 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Binding affinity against Hepatitis C virus NS3 protease | Bioorg Med Chem Lett 12: 3199-202 (2002) BindingDB Entry DOI: 10.7270/Q2R49Q38 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus) | BDBM50120292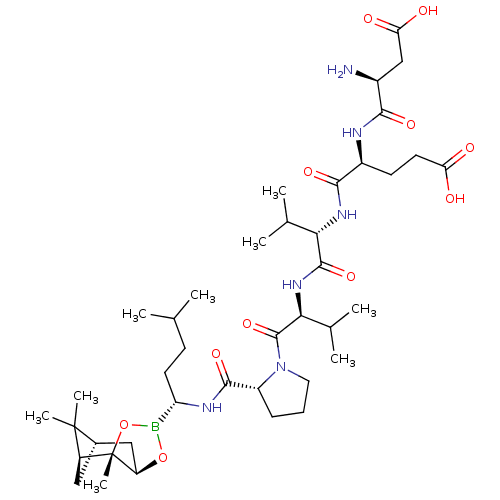 (CHEMBL322110 | Peptide Boronic Acid analogue) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 39 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Binding affinity against Hepatitis C virus NS3 protease | Bioorg Med Chem Lett 12: 3199-202 (2002) BindingDB Entry DOI: 10.7270/Q2R49Q38 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus) | BDBM50120314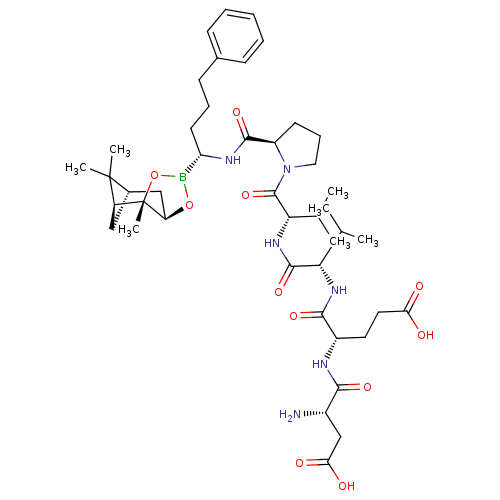 (CHEMBL321894 | Peptide Boronic Acid analogue) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Binding affinity against Hepatitis C virus NS3 protease | Bioorg Med Chem Lett 12: 3199-202 (2002) BindingDB Entry DOI: 10.7270/Q2R49Q38 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus) | BDBM50120309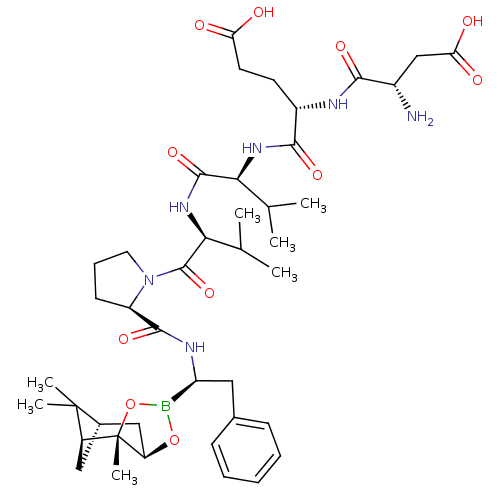 (CHEMBL108189 | Peptide Boronic Acid analogue) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Binding affinity against Hepatitis C virus NS3 protease | Bioorg Med Chem Lett 12: 3199-202 (2002) BindingDB Entry DOI: 10.7270/Q2R49Q38 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus) | BDBM50120313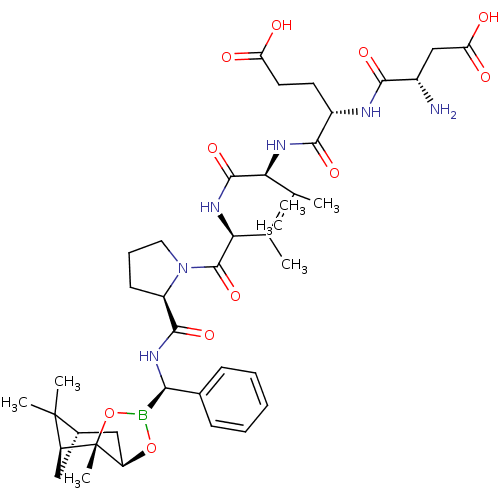 (CHEMBL320103 | Peptide Boronic Acid analogue) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Binding affinity against Hepatitis C virus NS3 protease | Bioorg Med Chem Lett 12: 3199-202 (2002) BindingDB Entry DOI: 10.7270/Q2R49Q38 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50120296 (CHEMBL419918 | Peptide Boronic Acid analogue) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibitory concentration against Human leukocyte Elastase | Bioorg Med Chem Lett 12: 3199-202 (2002) BindingDB Entry DOI: 10.7270/Q2R49Q38 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymotrypsin-C (Homo sapiens (Human)) | BDBM50120284 (CHEMBL107656 | Peptide Boronic Acid analogue) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibitory concentration against Human pancreatic Serine protease chymotrypsin | Bioorg Med Chem Lett 12: 3199-202 (2002) BindingDB Entry DOI: 10.7270/Q2R49Q38 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50120308 (CHEMBL443537 | Peptide Boronic Acid analogue) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 60 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibitory concentration against Human leukocyte Elastase | Bioorg Med Chem Lett 12: 3199-202 (2002) BindingDB Entry DOI: 10.7270/Q2R49Q38 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymotrypsin-C (Homo sapiens (Human)) | BDBM50120311 (CHEMBL320814 | Peptide Boronic Acid analogue) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 65 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibitory concentration against Human pancreatic Serine protease chymotrypsin | Bioorg Med Chem Lett 12: 3199-202 (2002) BindingDB Entry DOI: 10.7270/Q2R49Q38 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymotrypsin-C (Homo sapiens (Human)) | BDBM50120309 (CHEMBL108189 | Peptide Boronic Acid analogue) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 70 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibitory concentration against Human pancreatic Serine protease chymotrypsin | Bioorg Med Chem Lett 12: 3199-202 (2002) BindingDB Entry DOI: 10.7270/Q2R49Q38 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymotrypsin-C (Homo sapiens (Human)) | BDBM50120298 (CHEMBL109434 | Peptide Boronic Acid analogue) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 75 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibitory concentration against Human pancreatic Serine protease chymotrypsin | Bioorg Med Chem Lett 12: 3199-202 (2002) BindingDB Entry DOI: 10.7270/Q2R49Q38 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50120293 (CHEMBL413150 | Peptide Boronic Acid analogue) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 220 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibitory concentration against Human leukocyte Elastase | Bioorg Med Chem Lett 12: 3199-202 (2002) BindingDB Entry DOI: 10.7270/Q2R49Q38 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymotrypsin-C (Homo sapiens (Human)) | BDBM50120286 (CHEMBL322933 | Peptide Boronic Acid analogue) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 280 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibitory concentration against Human pancreatic Serine protease chymotrypsin | Bioorg Med Chem Lett 12: 3199-202 (2002) BindingDB Entry DOI: 10.7270/Q2R49Q38 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymotrypsin-C (Homo sapiens (Human)) | BDBM50120292 (CHEMBL322110 | Peptide Boronic Acid analogue) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibitory concentration against Human pancreatic Serine protease chymotrypsin | Bioorg Med Chem Lett 12: 3199-202 (2002) BindingDB Entry DOI: 10.7270/Q2R49Q38 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50120295 (CHEMBL324207 | Peptide Boronic Acid analogue) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 340 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibitory concentration against Human leukocyte Elastase | Bioorg Med Chem Lett 12: 3199-202 (2002) BindingDB Entry DOI: 10.7270/Q2R49Q38 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymotrypsin-C (Homo sapiens (Human)) | BDBM50120288 (CHEMBL432978 | Peptide Boronic Acid analogue) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 380 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibitory concentration against Human pancreatic Serine protease chymotrypsin | Bioorg Med Chem Lett 12: 3199-202 (2002) BindingDB Entry DOI: 10.7270/Q2R49Q38 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50120285 (CHEMBL108657 | Peptide Boronic Acid analogue) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 400 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibitory concentration against Human leukocyte Elastase | Bioorg Med Chem Lett 12: 3199-202 (2002) BindingDB Entry DOI: 10.7270/Q2R49Q38 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50120289 (CHEMBL419567 | Peptide Boronic Acid analogue) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 400 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibitory concentration against Human leukocyte Elastase | Bioorg Med Chem Lett 12: 3199-202 (2002) BindingDB Entry DOI: 10.7270/Q2R49Q38 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymotrypsin-C (Homo sapiens (Human)) | BDBM50120305 (CHEMBL111765 | Peptide Boronic Acid analogue) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 420 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibitory concentration against Human pancreatic Serine protease chymotrypsin | Bioorg Med Chem Lett 12: 3199-202 (2002) BindingDB Entry DOI: 10.7270/Q2R49Q38 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50120306 (CHEMBL262398 | Peptide Boronic Acid analogue) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 450 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibitory concentration against Human leukocyte Elastase | Bioorg Med Chem Lett 12: 3199-202 (2002) BindingDB Entry DOI: 10.7270/Q2R49Q38 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus) | BDBM50120294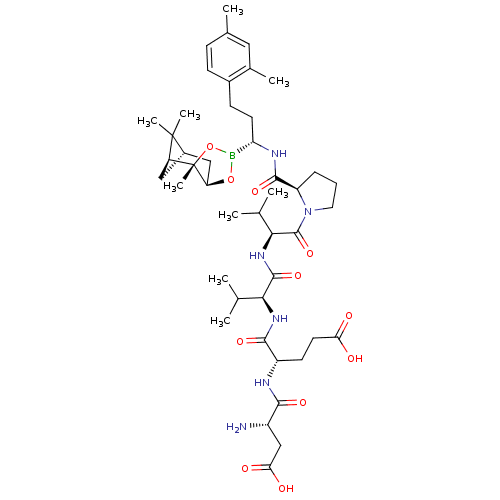 (CHEMBL326207 | Peptide Boronic Acid analogue) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 530 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibitory concentration of the compound against Hepatitis C virus NS3 protease | Bioorg Med Chem Lett 12: 3199-202 (2002) BindingDB Entry DOI: 10.7270/Q2R49Q38 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50120290 (CHEMBL107287 | Peptide Boronic Acid analogue) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 560 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibitory concentration against Human leukocyte Elastase | Bioorg Med Chem Lett 12: 3199-202 (2002) BindingDB Entry DOI: 10.7270/Q2R49Q38 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50120284 (CHEMBL107656 | Peptide Boronic Acid analogue) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 800 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibitory concentration against Human leukocyte Elastase | Bioorg Med Chem Lett 12: 3199-202 (2002) BindingDB Entry DOI: 10.7270/Q2R49Q38 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus) | BDBM50120310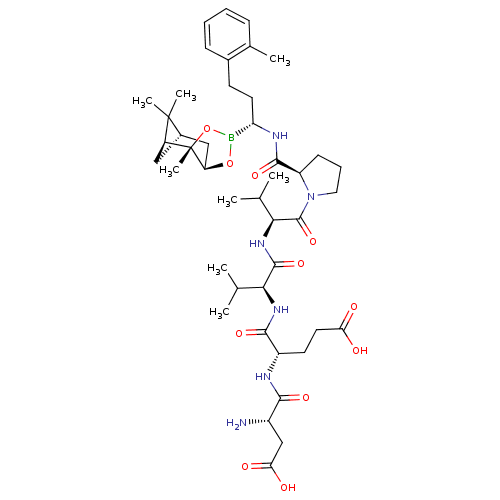 (CHEMBL109592 | Peptide Boronic Acid analogue) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 820 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibitory concentration of the compound against Hepatitis C virus NS3 protease | Bioorg Med Chem Lett 12: 3199-202 (2002) BindingDB Entry DOI: 10.7270/Q2R49Q38 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50120303 (CHEMBL432959 | Peptide Boronic Acid analogue) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 900 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibitory concentration against Human leukocyte Elastase | Bioorg Med Chem Lett 12: 3199-202 (2002) BindingDB Entry DOI: 10.7270/Q2R49Q38 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50120299 (CHEMBL110828 | Peptide Boronic Acid analogue) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 900 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibitory concentration against Human leukocyte Elastase | Bioorg Med Chem Lett 12: 3199-202 (2002) BindingDB Entry DOI: 10.7270/Q2R49Q38 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus) | BDBM50120297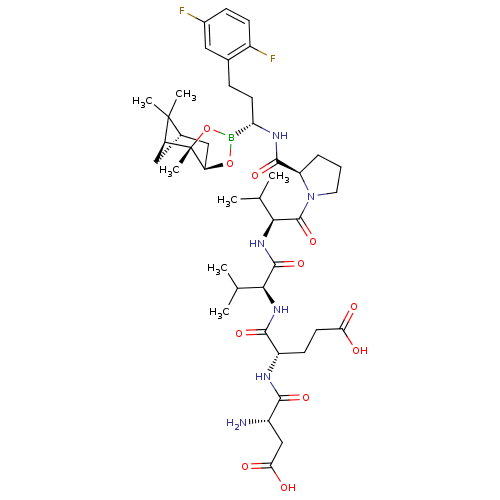 (CHEMBL278908 | Peptide Boronic Acid analogue) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 930 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibitory concentration of the compound against Hepatitis C virus NS3 protease | Bioorg Med Chem Lett 12: 3199-202 (2002) BindingDB Entry DOI: 10.7270/Q2R49Q38 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 69 total ) | Next | Last >> |