

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM177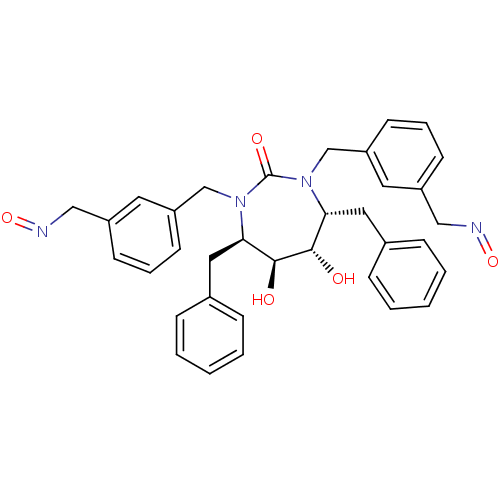 ((4R,5S,6S,7R)-4,7-dibenzyl-5,6-dihydroxy-1,3-bis({...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The DuPont Merck Pharmaceutical Company Curated by ChEMBL | Assay Description Inhibition of HIV protease, measured by assaying the cleavage of a fluorescent peptide substrate using HPLC | J Med Chem 41: 2019-28 (1998) Article DOI: 10.1021/jm9704199 BindingDB Entry DOI: 10.7270/Q29K49B0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM178 ((4R,5S,6S,7R)-4,7-dibenzyl-5,6-dihydroxy-1,3-bis({...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The DuPont Merck Pharmaceutical Company Curated by ChEMBL | Assay Description Inhibition of HIV protease, measured by assaying the cleavage of a fluorescent peptide substrate using HPLC | J Med Chem 41: 2019-28 (1998) Article DOI: 10.1021/jm9704199 BindingDB Entry DOI: 10.7270/Q29K49B0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50064592 ((4R,5S,6S,7R)-4,7-Dibenzyl-5,6-dihydroxy-1-(4-hydr...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0210 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The DuPont Merck Pharmaceutical Company Curated by ChEMBL | Assay Description Inhibition of HIV protease, measured by assaying the cleavage of a fluorescent peptide substrate using HPLC | J Med Chem 41: 2019-28 (1998) Article DOI: 10.1021/jm9704199 BindingDB Entry DOI: 10.7270/Q29K49B0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM182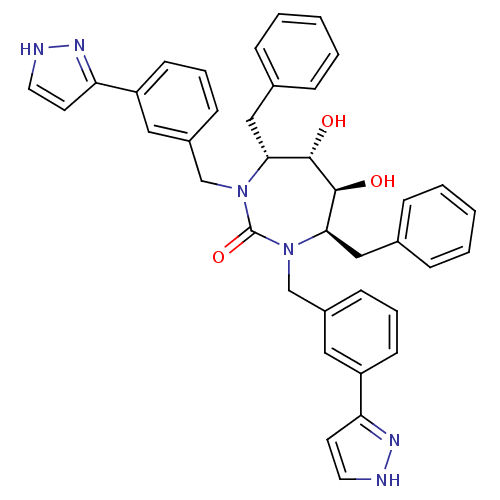 ((4R,5S,6S,7R)-4,7-dibenzyl-5,6-dihydroxy-1,3-bis({...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0270 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The DuPont Merck Pharmaceutical Company Curated by ChEMBL | Assay Description Inhibition of HIV protease, measured by assaying the cleavage of a fluorescent peptide substrate using HPLC | J Med Chem 41: 2019-28 (1998) Article DOI: 10.1021/jm9704199 BindingDB Entry DOI: 10.7270/Q29K49B0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM180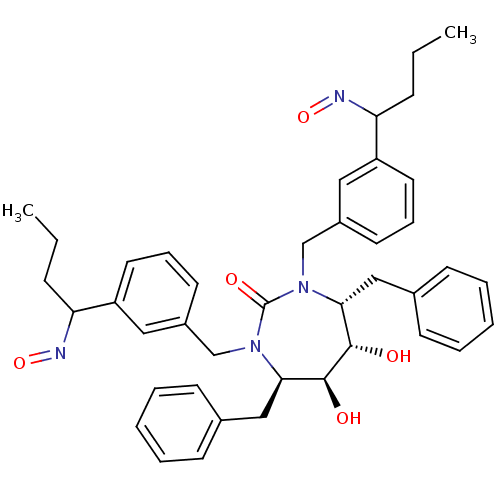 ((4R,5S,6S,7R)-4,7-dibenzyl-5,6-dihydroxy-1,3-bis({...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0310 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The DuPont Merck Pharmaceutical Company Curated by ChEMBL | Assay Description Inhibition of HIV protease, measured by assaying the cleavage of a fluorescent peptide substrate using HPLC | J Med Chem 41: 2019-28 (1998) Article DOI: 10.1021/jm9704199 BindingDB Entry DOI: 10.7270/Q29K49B0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM179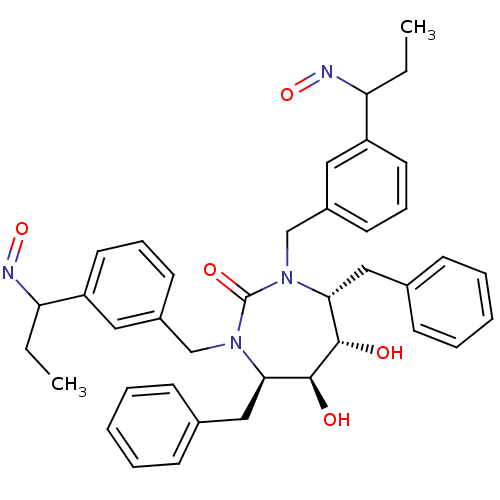 ((4R,5S,6S,7R)-4,7-dibenzyl-5,6-dihydroxy-1,3-bis({...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0310 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The DuPont Merck Pharmaceutical Company Curated by ChEMBL | Assay Description Inhibition of HIV protease, measured by assaying the cleavage of a fluorescent peptide substrate using HPLC | J Med Chem 41: 2019-28 (1998) Article DOI: 10.1021/jm9704199 BindingDB Entry DOI: 10.7270/Q29K49B0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50064599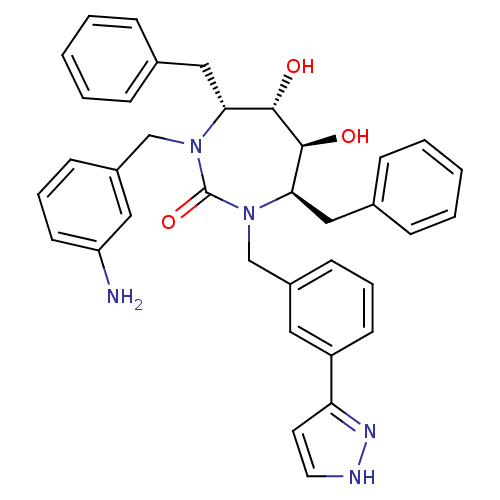 ((4R,5S,6S,7R)-1-(3-Amino-benzyl)-4,7-dibenzyl-5,6-...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0350 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The DuPont Merck Pharmaceutical Company Curated by ChEMBL | Assay Description Inhibition of HIV protease, measured by assaying the cleavage of a fluorescent peptide substrate using HPLC | J Med Chem 41: 2019-28 (1998) Article DOI: 10.1021/jm9704199 BindingDB Entry DOI: 10.7270/Q29K49B0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM175 ((4R,5S,6S,7R)-4,7-dibenzyl-5,6-dihydroxy-1,3-bis({...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0370 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The DuPont Merck Pharmaceutical Company Curated by ChEMBL | Assay Description Inhibition of HIV protease, measured by assaying the cleavage of a fluorescent peptide substrate using HPLC | J Med Chem 41: 2019-28 (1998) Article DOI: 10.1021/jm9704199 BindingDB Entry DOI: 10.7270/Q29K49B0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50064593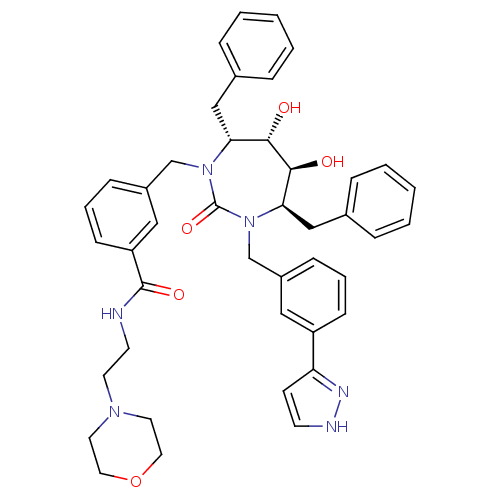 (3-{(4R,5S,6S,7R)-4,7-Dibenzyl-5,6-dihydroxy-2-oxo-...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0520 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The DuPont Merck Pharmaceutical Company Curated by ChEMBL | Assay Description Inhibition of HIV protease, measured by assaying the cleavage of a fluorescent peptide substrate using HPLC | J Med Chem 41: 2019-28 (1998) Article DOI: 10.1021/jm9704199 BindingDB Entry DOI: 10.7270/Q29K49B0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM187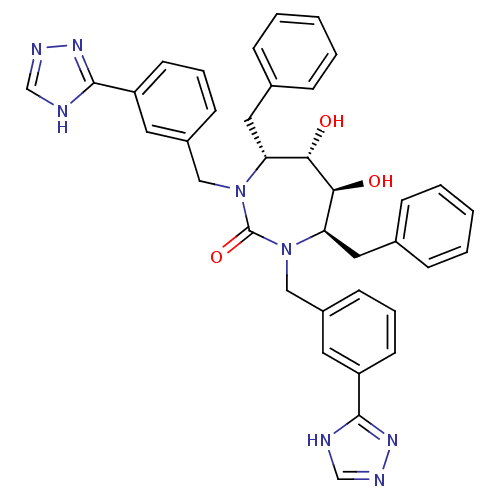 ((4R,5S,6S,7R)-4,7-dibenzyl-5,6-dihydroxy-1,3-bis({...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0520 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The DuPont Merck Pharmaceutical Company Curated by ChEMBL | Assay Description Inhibition of HIV protease, measured by assaying the cleavage of a fluorescent peptide substrate using HPLC | J Med Chem 41: 2019-28 (1998) Article DOI: 10.1021/jm9704199 BindingDB Entry DOI: 10.7270/Q29K49B0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM172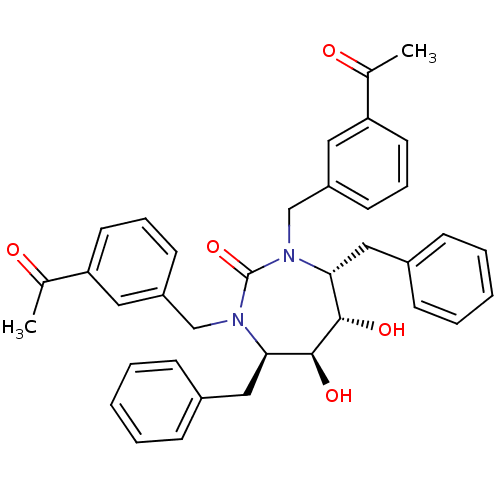 ((4R,5S,6S,7R)-4,7-dibenzyl-1,3-bis[(3-acetylphenyl...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The DuPont Merck Pharmaceutical Company Curated by ChEMBL | Assay Description Inhibition of HIV protease, measured by assaying the cleavage of a fluorescent peptide substrate using HPLC | J Med Chem 41: 2019-28 (1998) Article DOI: 10.1021/jm9704199 BindingDB Entry DOI: 10.7270/Q29K49B0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50064603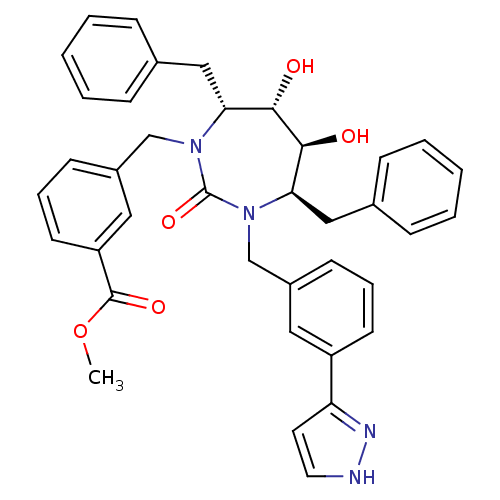 (3-{(4R,5S,6S,7R)-4,7-Dibenzyl-5,6-dihydroxy-2-oxo-...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The DuPont Merck Pharmaceutical Company Curated by ChEMBL | Assay Description Inhibition of HIV protease, measured by assaying the cleavage of a fluorescent peptide substrate using HPLC | J Med Chem 41: 2019-28 (1998) Article DOI: 10.1021/jm9704199 BindingDB Entry DOI: 10.7270/Q29K49B0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM184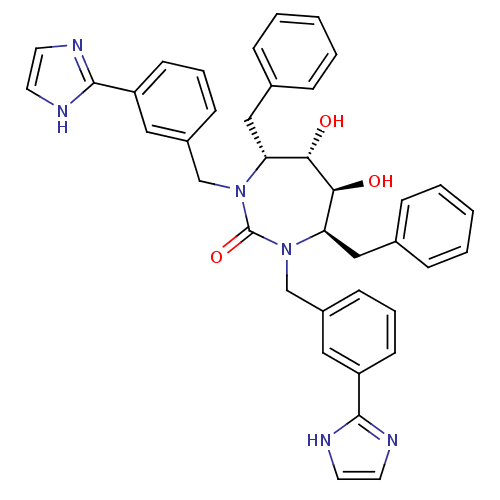 ((4R,5S,6S,7R)-4,7-dibenzyl-5,6-dihydroxy-1,3-bis({...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The DuPont Merck Pharmaceutical Company Curated by ChEMBL | Assay Description Inhibition of HIV protease, measured by assaying the cleavage of a fluorescent peptide substrate using HPLC | J Med Chem 41: 2019-28 (1998) Article DOI: 10.1021/jm9704199 BindingDB Entry DOI: 10.7270/Q29K49B0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50064600 ((4R,5S,6S,7R)-4,7-Dibenzyl-5,6-dihydroxy-1-(3-hydr...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The DuPont Merck Pharmaceutical Company Curated by ChEMBL | Assay Description Inhibition of HIV protease, measured by assaying the cleavage of a fluorescent peptide substrate using HPLC | J Med Chem 41: 2019-28 (1998) Article DOI: 10.1021/jm9704199 BindingDB Entry DOI: 10.7270/Q29K49B0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM186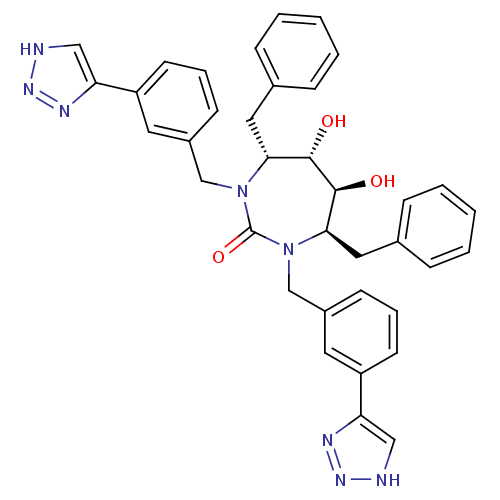 ((4R,5S,6S,7R)-4,7-dibenzyl-5,6-dihydroxy-1,3-bis({...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The DuPont Merck Pharmaceutical Company Curated by ChEMBL | Assay Description Inhibition of HIV protease, measured by assaying the cleavage of a fluorescent peptide substrate using HPLC | J Med Chem 41: 2019-28 (1998) Article DOI: 10.1021/jm9704199 BindingDB Entry DOI: 10.7270/Q29K49B0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50064591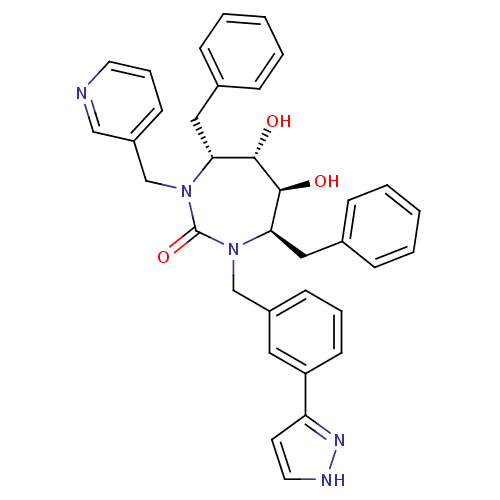 ((4R,5S,6S,7R)-4,7-Dibenzyl-5,6-dihydroxy-1-[3-(2H-...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The DuPont Merck Pharmaceutical Company Curated by ChEMBL | Assay Description Inhibition of HIV protease, measured by assaying the cleavage of a fluorescent peptide substrate using HPLC | J Med Chem 41: 2019-28 (1998) Article DOI: 10.1021/jm9704199 BindingDB Entry DOI: 10.7270/Q29K49B0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM185 ((4R,5S,6S,7R)-4,7-dibenzyl-5,6-dihydroxy-1,3-bis({...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.190 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The DuPont Merck Pharmaceutical Company Curated by ChEMBL | Assay Description Inhibition of HIV protease, measured by assaying the cleavage of a fluorescent peptide substrate using HPLC | J Med Chem 41: 2019-28 (1998) Article DOI: 10.1021/jm9704199 BindingDB Entry DOI: 10.7270/Q29K49B0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM173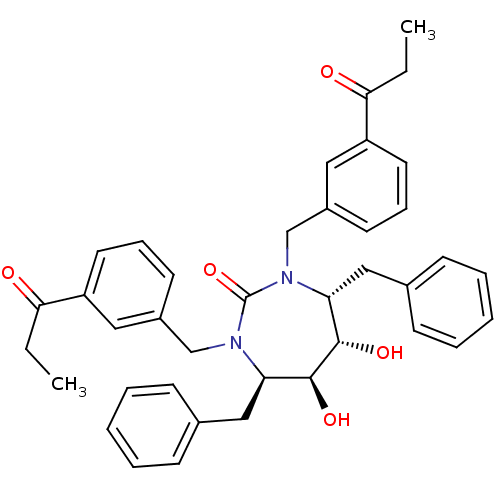 ((4R,5S,6S,7R)-4,7-dibenzyl-5,6-dihydroxy-1,3-bis[(...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.210 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The DuPont Merck Pharmaceutical Company Curated by ChEMBL | Assay Description Inhibition of HIV protease, measured by assaying the cleavage of a fluorescent peptide substrate using HPLC | J Med Chem 41: 2019-28 (1998) Article DOI: 10.1021/jm9704199 BindingDB Entry DOI: 10.7270/Q29K49B0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50064601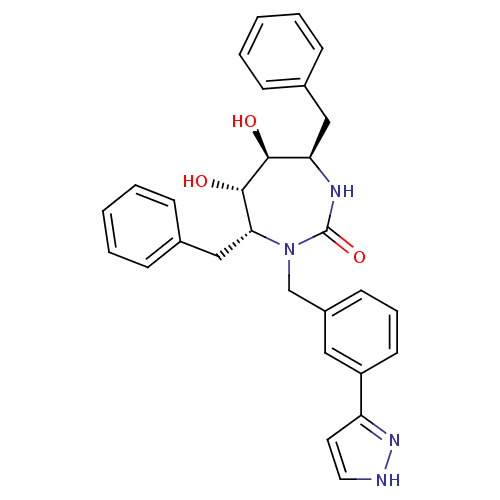 ((4R,5S,6S,7R)-4,7-Dibenzyl-5,6-dihydroxy-1-[3-(2H-...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.260 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The DuPont Merck Pharmaceutical Company Curated by ChEMBL | Assay Description Inhibition of HIV protease, measured by assaying the cleavage of a fluorescent peptide substrate using HPLC | J Med Chem 41: 2019-28 (1998) Article DOI: 10.1021/jm9704199 BindingDB Entry DOI: 10.7270/Q29K49B0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM151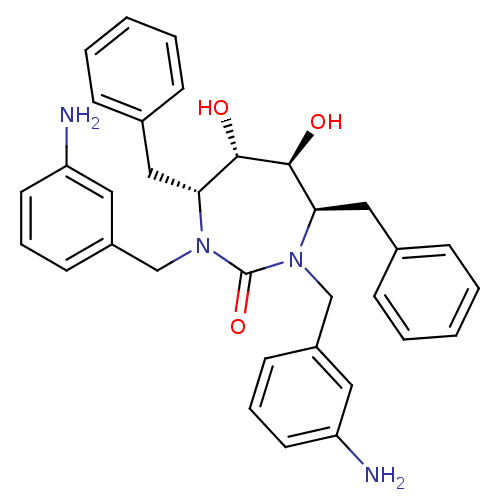 ((4R,5S,6S,7R)-1,3-bis[(3-aminophenyl)methyl]-4,7-d...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | 0.280 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The DuPont Merck Pharmaceutical Company Curated by ChEMBL | Assay Description Inhibition of HIV protease, measured by assaying the cleavage of a fluorescent peptide substrate using HPLC | J Med Chem 41: 2019-28 (1998) Article DOI: 10.1021/jm9704199 BindingDB Entry DOI: 10.7270/Q29K49B0 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM171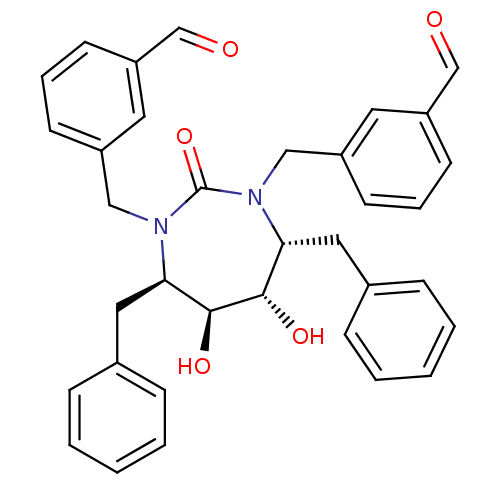 (3-{[(4R,5S,6S,7R)-4,7-dibenzyl-3-[(3-formylphenyl)...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.440 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The DuPont Merck Pharmaceutical Company Curated by ChEMBL | Assay Description Inhibition of HIV protease, measured by assaying the cleavage of a fluorescent peptide substrate using HPLC | J Med Chem 41: 2019-28 (1998) Article DOI: 10.1021/jm9704199 BindingDB Entry DOI: 10.7270/Q29K49B0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM183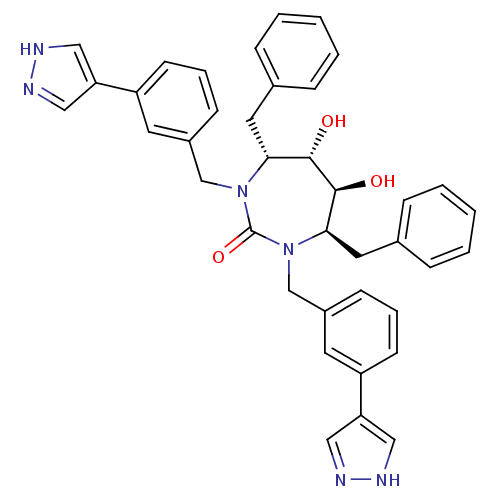 ((4R,5S,6S,7R)-4,7-dibenzyl-5,6-dihydroxy-1,3-bis({...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.620 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The DuPont Merck Pharmaceutical Company Curated by ChEMBL | Assay Description Inhibition of HIV protease, measured by assaying the cleavage of a fluorescent peptide substrate using HPLC | J Med Chem 41: 2019-28 (1998) Article DOI: 10.1021/jm9704199 BindingDB Entry DOI: 10.7270/Q29K49B0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM174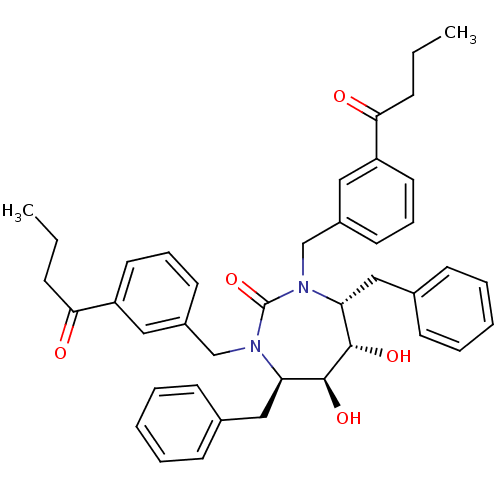 ((4R,5S,6S,7R)-4,7-dibenzyl-1,3-bis[(3-butanoylphen...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The DuPont Merck Pharmaceutical Company Curated by ChEMBL | Assay Description Inhibition of HIV protease, measured by assaying the cleavage of a fluorescent peptide substrate using HPLC | J Med Chem 41: 2019-28 (1998) Article DOI: 10.1021/jm9704199 BindingDB Entry DOI: 10.7270/Q29K49B0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM176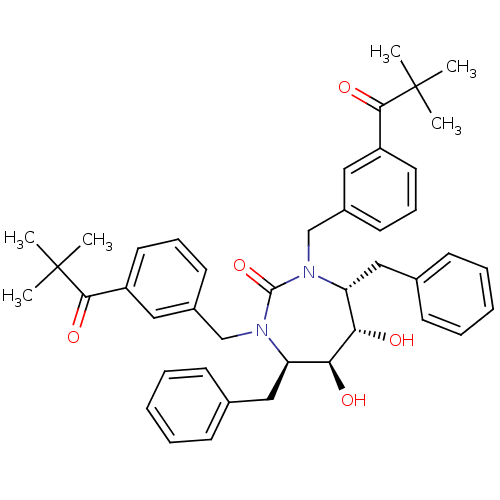 ((4R,5S,6S,7R)-4,7-dibenzyl-1,3-bis({[3-(2,2-dimeth...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The DuPont Merck Pharmaceutical Company Curated by ChEMBL | Assay Description Inhibition of HIV protease, measured by assaying the cleavage of a fluorescent peptide substrate using HPLC | J Med Chem 41: 2019-28 (1998) Article DOI: 10.1021/jm9704199 BindingDB Entry DOI: 10.7270/Q29K49B0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM181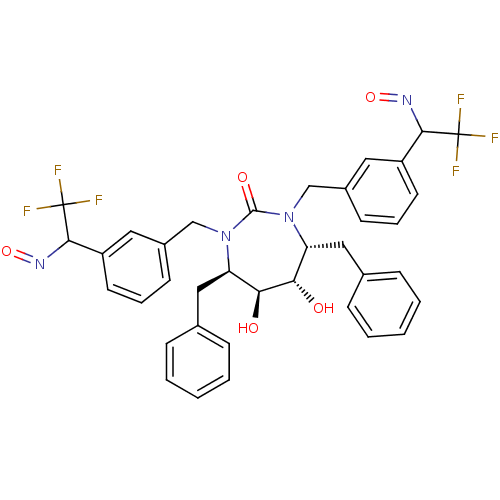 ((4R,5S,6S,7R)-4,7-dibenzyl-5,6-dihydroxy-1,3-bis({...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The DuPont Merck Pharmaceutical Company Curated by ChEMBL | Assay Description Inhibition of HIV protease, measured by assaying the cleavage of a fluorescent peptide substrate using HPLC | J Med Chem 41: 2019-28 (1998) Article DOI: 10.1021/jm9704199 BindingDB Entry DOI: 10.7270/Q29K49B0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM188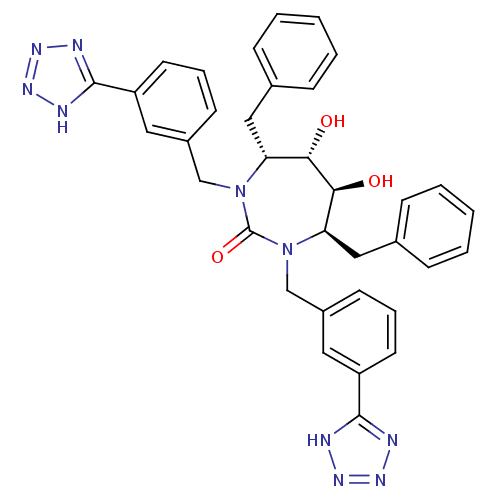 ((4R,5S,6S,7R)-4,7-dibenzyl-5,6-dihydroxy-1,3-bis({...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 6.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The DuPont Merck Pharmaceutical Company Curated by ChEMBL | Assay Description Inhibition of HIV protease, measured by assaying the cleavage of a fluorescent peptide substrate using HPLC | J Med Chem 41: 2019-28 (1998) Article DOI: 10.1021/jm9704199 BindingDB Entry DOI: 10.7270/Q29K49B0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||