

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Prothrombin (Homo sapiens (Human)) | BDBM50070791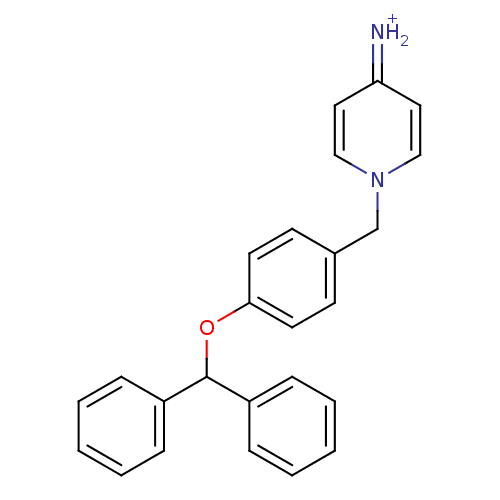 (4-Amino-1-(4-benzhydryloxy-benzyl)-pyridinium | CH...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 58 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Compound was evaluated for its inhibitory activity against human thrombin | Bioorg Med Chem Lett 8: 1697-702 (1999) BindingDB Entry DOI: 10.7270/Q26T0KRN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50070787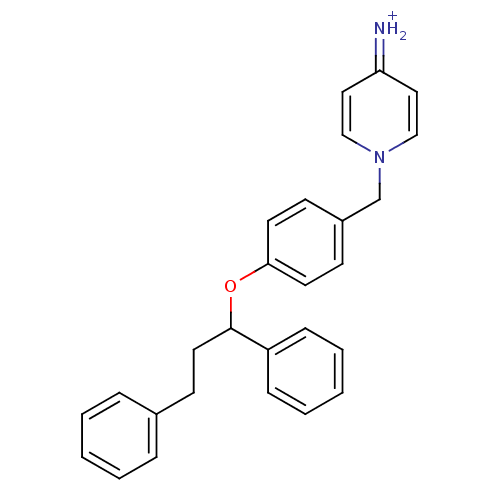 (4-Amino-1-[4-(1,3-diphenyl-propoxy)-benzyl]-pyridi...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | 160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Compound was evaluated for its inhibitory activity against human thrombin | Bioorg Med Chem Lett 8: 1697-702 (1999) BindingDB Entry DOI: 10.7270/Q26T0KRN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50070786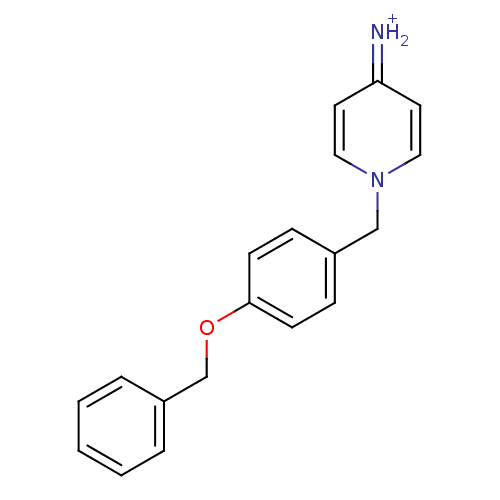 (4-Amino-1-(4-benzyloxy-benzyl)-pyridinium | CHEMBL...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Compound was evaluated for its inhibitory activity against human thrombin | Bioorg Med Chem Lett 8: 1697-702 (1999) BindingDB Entry DOI: 10.7270/Q26T0KRN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50070793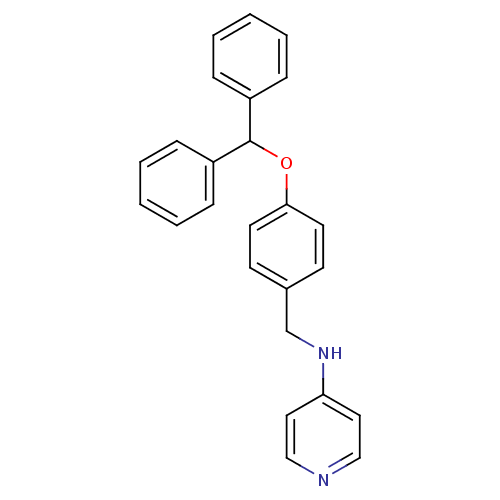 ((4-Benzhydryloxy-benzyl)-pyridin-4-yl-amine | CHEM...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Compound was evaluated for its inhibitory activity against human thrombin | Bioorg Med Chem Lett 8: 1697-702 (1999) BindingDB Entry DOI: 10.7270/Q26T0KRN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50070790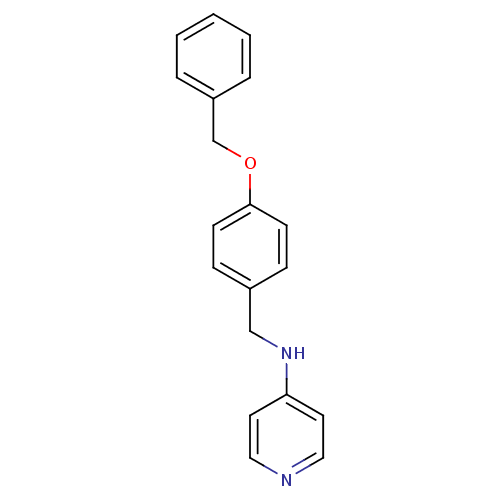 ((4-Benzyloxy-benzyl)-pyridin-4-yl-amine | CHEMBL47...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 4.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Compound was evaluated for its inhibitory activity against human thrombin | Bioorg Med Chem Lett 8: 1697-702 (1999) BindingDB Entry DOI: 10.7270/Q26T0KRN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50070788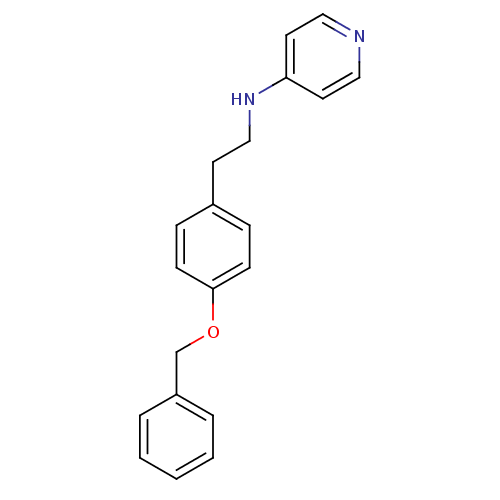 (CHEMBL48029 | N-(4-(benzyloxy)phenethyl)pyridin-4-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | 7.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Compound was evaluated for its inhibitory activity against human thrombin | Bioorg Med Chem Lett 8: 1697-702 (1999) BindingDB Entry DOI: 10.7270/Q26T0KRN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease 1 (Homo sapiens (Human)) | BDBM50070792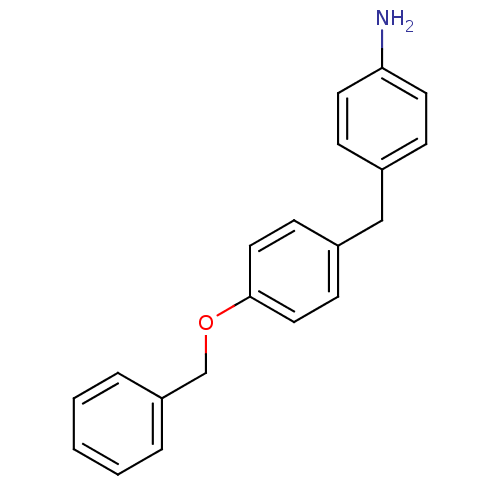 (4-(4-Benzyloxy-benzyl)-phenylamine | CHEMBL45648) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Compound was evaluated for its inhibitory activity against trypsin | Bioorg Med Chem Lett 8: 1697-702 (1999) BindingDB Entry DOI: 10.7270/Q26T0KRN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50070792 (4-(4-Benzyloxy-benzyl)-phenylamine | CHEMBL45648) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Compound was evaluated for its inhibitory activity against human thrombin | Bioorg Med Chem Lett 8: 1697-702 (1999) BindingDB Entry DOI: 10.7270/Q26T0KRN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease 1 (Homo sapiens (Human)) | BDBM50070787 (4-Amino-1-[4-(1,3-diphenyl-propoxy)-benzyl]-pyridi...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | 1.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Compound was evaluated for its inhibitory activity against trypsin | Bioorg Med Chem Lett 8: 1697-702 (1999) BindingDB Entry DOI: 10.7270/Q26T0KRN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease 1 (Homo sapiens (Human)) | BDBM50070786 (4-Amino-1-(4-benzyloxy-benzyl)-pyridinium | CHEMBL...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 7.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Compound was evaluated for its inhibitory activity against trypsin | Bioorg Med Chem Lett 8: 1697-702 (1999) BindingDB Entry DOI: 10.7270/Q26T0KRN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease 1 (Homo sapiens (Human)) | BDBM50070793 ((4-Benzhydryloxy-benzyl)-pyridin-4-yl-amine | CHEM...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 7.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Compound was evaluated for its inhibitory activity against trypsin | Bioorg Med Chem Lett 8: 1697-702 (1999) BindingDB Entry DOI: 10.7270/Q26T0KRN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease 1 (Homo sapiens (Human)) | BDBM50070790 ((4-Benzyloxy-benzyl)-pyridin-4-yl-amine | CHEMBL47...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 2.33E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Compound was evaluated for its inhibitory activity against trypsin | Bioorg Med Chem Lett 8: 1697-702 (1999) BindingDB Entry DOI: 10.7270/Q26T0KRN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50070789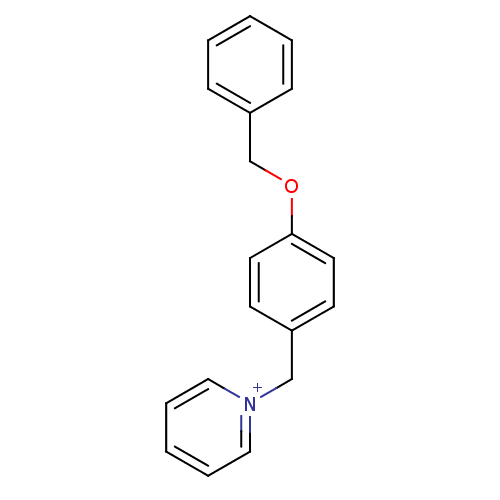 (1-(4-Benzyloxy-benzyl)-pyridinium | CHEMBL296280) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | >5.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Compound was evaluated for its inhibitory activity against human thrombin | Bioorg Med Chem Lett 8: 1697-702 (1999) BindingDB Entry DOI: 10.7270/Q26T0KRN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease 1 (Homo sapiens (Human)) | BDBM50070791 (4-Amino-1-(4-benzhydryloxy-benzyl)-pyridinium | CH...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 5.17E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Compound was evaluated for its inhibitory activity against trypsin | Bioorg Med Chem Lett 8: 1697-702 (1999) BindingDB Entry DOI: 10.7270/Q26T0KRN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease 1 (Homo sapiens (Human)) | BDBM50070788 (CHEMBL48029 | N-(4-(benzyloxy)phenethyl)pyridin-4-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | 5.68E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Compound was evaluated for its inhibitory activity against trypsin | Bioorg Med Chem Lett 8: 1697-702 (1999) BindingDB Entry DOI: 10.7270/Q26T0KRN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50070794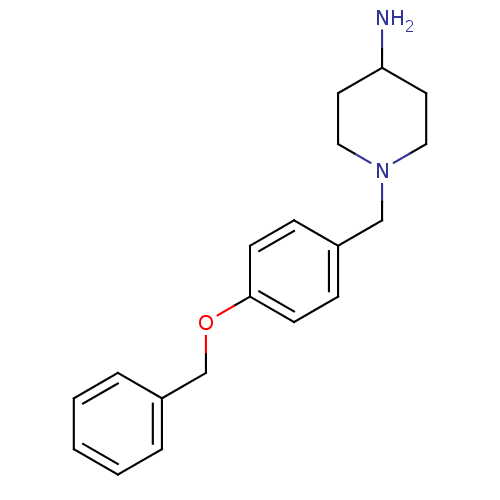 (1-(4-Benzyloxy-benzyl)-piperidin-4-ylamine | CHEMB...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | PubMed | 5.81E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Compound was evaluated for its inhibitory activity against human thrombin | Bioorg Med Chem Lett 8: 1697-702 (1999) BindingDB Entry DOI: 10.7270/Q26T0KRN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||