

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Urokinase-type plasminogen activator (Homo sapiens (Human)) | BDBM50102780 (2-(2-Hydroxy-3-phenyl-phenyl)-1H-indole-5-carboxam...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Axys Pharmaceuticals Inc. Curated by ChEMBL | Assay Description The compound was tested for inhibition of human urokinase type plasminogen activator (microPa) | J Med Chem 44: 3856-71 (2001) BindingDB Entry DOI: 10.7270/Q22R3QXR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urokinase-type plasminogen activator (Homo sapiens (Human)) | BDBM50106240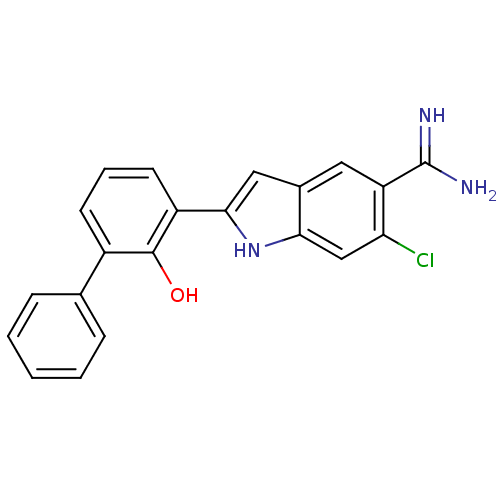 (6-Chloro-2-(2-hydroxy-biphenyl-3-yl)-1H-indole-5-c...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Axys Pharmaceuticals Inc. Curated by ChEMBL | Assay Description The compound was tested for inhibition of human urokinase type plasminogen activator (microPa) | J Med Chem 44: 3856-71 (2001) BindingDB Entry DOI: 10.7270/Q22R3QXR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urokinase-type plasminogen activator (Homo sapiens (Human)) | BDBM50106239 (6-Fluoro-2-(2-hydroxy-biphenyl-3-yl)-1H-indole-5-c...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Axys Pharmaceuticals Inc. Curated by ChEMBL | Assay Description The compound was tested for inhibition of human urokinase type plasminogen activator (microPa) | J Med Chem 44: 3856-71 (2001) BindingDB Entry DOI: 10.7270/Q22R3QXR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tissue-type plasminogen activator (Homo sapiens (Human)) | BDBM50102780 (2-(2-Hydroxy-3-phenyl-phenyl)-1H-indole-5-carboxam...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 35 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Axys Pharmaceuticals Inc. Curated by ChEMBL | Assay Description The compound was tested for inhibition of human tissue plasminogen activator | J Med Chem 44: 3856-71 (2001) BindingDB Entry DOI: 10.7270/Q22R3QXR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM50102780 (2-(2-Hydroxy-3-phenyl-phenyl)-1H-indole-5-carboxam...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 78 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Axys Pharmaceuticals Inc. Curated by ChEMBL | Assay Description Binding affinity against human coagulation factor X | J Med Chem 44: 3856-71 (2001) BindingDB Entry DOI: 10.7270/Q22R3QXR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tissue-type plasminogen activator (Homo sapiens (Human)) | BDBM50102780 (2-(2-Hydroxy-3-phenyl-phenyl)-1H-indole-5-carboxam...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Axys Pharmaceuticals Inc. Curated by ChEMBL | Assay Description The compound was tested for inhibition of human plasmin | J Med Chem 44: 3856-71 (2001) BindingDB Entry DOI: 10.7270/Q22R3QXR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urokinase-type plasminogen activator (Homo sapiens (Human)) | BDBM50106243 (2-(2-Hydroxy-biphenyl-3-yl)-6-methyl-1H-indole-5-c...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Axys Pharmaceuticals Inc. Curated by ChEMBL | Assay Description The compound was tested for inhibition of human urokinase type plasminogen activator (microPa) | J Med Chem 44: 3856-71 (2001) BindingDB Entry DOI: 10.7270/Q22R3QXR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tissue-type plasminogen activator (Homo sapiens (Human)) | BDBM50106240 (6-Chloro-2-(2-hydroxy-biphenyl-3-yl)-1H-indole-5-c...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Axys Pharmaceuticals Inc. Curated by ChEMBL | Assay Description The compound was tested for inhibition of human plasmin | J Med Chem 44: 3856-71 (2001) BindingDB Entry DOI: 10.7270/Q22R3QXR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease 1/Trypsin-2 (Homo sapiens (Human)) | BDBM50102780 (2-(2-Hydroxy-3-phenyl-phenyl)-1H-indole-5-carboxam...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Axys Pharmaceuticals Inc. Curated by ChEMBL | Assay Description The compound was tested for inhibition of human trypsin | J Med Chem 44: 3856-71 (2001) BindingDB Entry DOI: 10.7270/Q22R3QXR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor VII (Homo sapiens (Human)) | BDBM50102780 (2-(2-Hydroxy-3-phenyl-phenyl)-1H-indole-5-carboxam...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Axys Pharmaceuticals Inc. Curated by ChEMBL | Assay Description The compound was tested for inhibition of human coagulation factor VII | J Med Chem 44: 3856-71 (2001) BindingDB Entry DOI: 10.7270/Q22R3QXR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tissue-type plasminogen activator (Homo sapiens (Human)) | BDBM50106243 (2-(2-Hydroxy-biphenyl-3-yl)-6-methyl-1H-indole-5-c...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 210 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Axys Pharmaceuticals Inc. Curated by ChEMBL | Assay Description The compound was tested for inhibition of human plasmin | J Med Chem 44: 3856-71 (2001) BindingDB Entry DOI: 10.7270/Q22R3QXR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease 1/Trypsin-2 (Homo sapiens (Human)) | BDBM50106240 (6-Chloro-2-(2-hydroxy-biphenyl-3-yl)-1H-indole-5-c...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 230 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Axys Pharmaceuticals Inc. Curated by ChEMBL | Assay Description The compound was tested for inhibition of human trypsin | J Med Chem 44: 3856-71 (2001) BindingDB Entry DOI: 10.7270/Q22R3QXR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50102780 (2-(2-Hydroxy-3-phenyl-phenyl)-1H-indole-5-carboxam...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 320 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Axys Pharmaceuticals Inc. Curated by ChEMBL | Assay Description The compound was tested for inhibition of human thrombin | J Med Chem 44: 3856-71 (2001) BindingDB Entry DOI: 10.7270/Q22R3QXR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tissue-type plasminogen activator (Homo sapiens (Human)) | BDBM50106239 (6-Fluoro-2-(2-hydroxy-biphenyl-3-yl)-1H-indole-5-c...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 350 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Axys Pharmaceuticals Inc. Curated by ChEMBL | Assay Description The compound was tested for inhibition of human plasmin | J Med Chem 44: 3856-71 (2001) BindingDB Entry DOI: 10.7270/Q22R3QXR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urokinase-type plasminogen activator (Homo sapiens (Human)) | BDBM50106241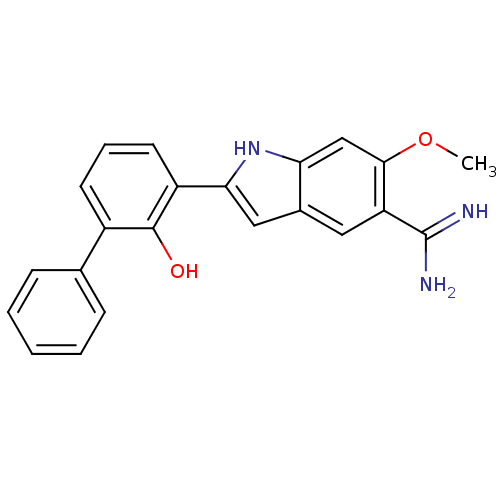 (2-(2-Hydroxy-biphenyl-3-yl)-6-methoxy-1H-indole-5-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 360 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Axys Pharmaceuticals Inc. Curated by ChEMBL | Assay Description The compound was tested for inhibition of human urokinase type plasminogen activator (microPa) | J Med Chem 44: 3856-71 (2001) BindingDB Entry DOI: 10.7270/Q22R3QXR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urokinase-type plasminogen activator (Homo sapiens (Human)) | BDBM50106238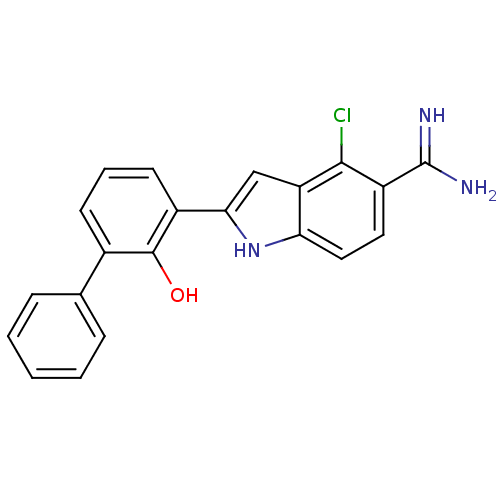 (4-Chloro-2-(2-hydroxy-biphenyl-3-yl)-1H-indole-5-c...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 490 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Axys Pharmaceuticals Inc. Curated by ChEMBL | Assay Description The compound was tested for inhibition of human urokinase type plasminogen activator (microPa) | J Med Chem 44: 3856-71 (2001) BindingDB Entry DOI: 10.7270/Q22R3QXR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor VII (Homo sapiens (Human)) | BDBM50106239 (6-Fluoro-2-(2-hydroxy-biphenyl-3-yl)-1H-indole-5-c...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 750 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Axys Pharmaceuticals Inc. Curated by ChEMBL | Assay Description The compound was tested for inhibition of human coagulation factor VII | J Med Chem 44: 3856-71 (2001) BindingDB Entry DOI: 10.7270/Q22R3QXR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease 1/Trypsin-2 (Homo sapiens (Human)) | BDBM50106239 (6-Fluoro-2-(2-hydroxy-biphenyl-3-yl)-1H-indole-5-c...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Axys Pharmaceuticals Inc. Curated by ChEMBL | Assay Description The compound was tested for inhibition of human trypsin | J Med Chem 44: 3856-71 (2001) BindingDB Entry DOI: 10.7270/Q22R3QXR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM50106239 (6-Fluoro-2-(2-hydroxy-biphenyl-3-yl)-1H-indole-5-c...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Axys Pharmaceuticals Inc. Curated by ChEMBL | Assay Description Binding affinity against human coagulation factor X | J Med Chem 44: 3856-71 (2001) BindingDB Entry DOI: 10.7270/Q22R3QXR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease 1/Trypsin-2 (Homo sapiens (Human)) | BDBM50106243 (2-(2-Hydroxy-biphenyl-3-yl)-6-methyl-1H-indole-5-c...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Axys Pharmaceuticals Inc. Curated by ChEMBL | Assay Description The compound was tested for inhibition of human trypsin | J Med Chem 44: 3856-71 (2001) BindingDB Entry DOI: 10.7270/Q22R3QXR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor VII (Homo sapiens (Human)) | BDBM50106240 (6-Chloro-2-(2-hydroxy-biphenyl-3-yl)-1H-indole-5-c...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 1.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Axys Pharmaceuticals Inc. Curated by ChEMBL | Assay Description The compound was tested for inhibition of human coagulation factor VII | J Med Chem 44: 3856-71 (2001) BindingDB Entry DOI: 10.7270/Q22R3QXR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urokinase-type plasminogen activator (Homo sapiens (Human)) | BDBM50106242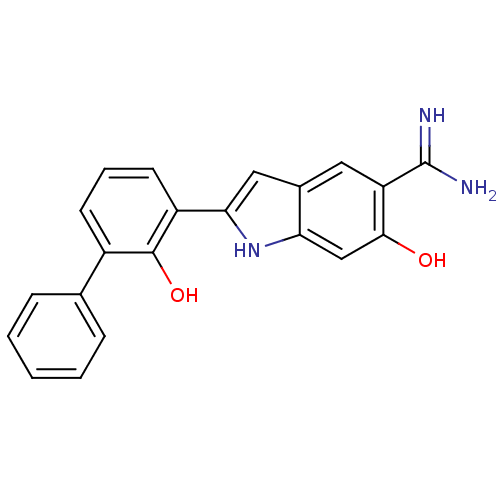 (6-Hydroxy-2-(2-hydroxy-biphenyl-3-yl)-1H-indole-5-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Axys Pharmaceuticals Inc. Curated by ChEMBL | Assay Description The compound was tested for inhibition of human urokinase type plasminogen activator (microPa) | J Med Chem 44: 3856-71 (2001) BindingDB Entry DOI: 10.7270/Q22R3QXR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tissue-type plasminogen activator (Homo sapiens (Human)) | BDBM50106238 (4-Chloro-2-(2-hydroxy-biphenyl-3-yl)-1H-indole-5-c...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Axys Pharmaceuticals Inc. Curated by ChEMBL | Assay Description The compound was tested for inhibition of human plasmin | J Med Chem 44: 3856-71 (2001) BindingDB Entry DOI: 10.7270/Q22R3QXR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tissue-type plasminogen activator (Homo sapiens (Human)) | BDBM50106238 (4-Chloro-2-(2-hydroxy-biphenyl-3-yl)-1H-indole-5-c...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 3.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Axys Pharmaceuticals Inc. Curated by ChEMBL | Assay Description The compound was tested for inhibition of human tissue plasminogen activator | J Med Chem 44: 3856-71 (2001) BindingDB Entry DOI: 10.7270/Q22R3QXR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tissue-type plasminogen activator (Homo sapiens (Human)) | BDBM50106239 (6-Fluoro-2-(2-hydroxy-biphenyl-3-yl)-1H-indole-5-c...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 3.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Axys Pharmaceuticals Inc. Curated by ChEMBL | Assay Description The compound was tested for inhibition of human tissue plasminogen activator | J Med Chem 44: 3856-71 (2001) BindingDB Entry DOI: 10.7270/Q22R3QXR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tissue-type plasminogen activator (Homo sapiens (Human)) | BDBM50106241 (2-(2-Hydroxy-biphenyl-3-yl)-6-methoxy-1H-indole-5-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 3.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Axys Pharmaceuticals Inc. Curated by ChEMBL | Assay Description The compound was tested for inhibition of human plasmin | J Med Chem 44: 3856-71 (2001) BindingDB Entry DOI: 10.7270/Q22R3QXR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease 1/Trypsin-2 (Homo sapiens (Human)) | BDBM50106238 (4-Chloro-2-(2-hydroxy-biphenyl-3-yl)-1H-indole-5-c...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 4.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Axys Pharmaceuticals Inc. Curated by ChEMBL | Assay Description The compound was tested for inhibition of human trypsin | J Med Chem 44: 3856-71 (2001) BindingDB Entry DOI: 10.7270/Q22R3QXR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM50106238 (4-Chloro-2-(2-hydroxy-biphenyl-3-yl)-1H-indole-5-c...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 5.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Axys Pharmaceuticals Inc. Curated by ChEMBL | Assay Description Binding affinity against human coagulation factor X | J Med Chem 44: 3856-71 (2001) BindingDB Entry DOI: 10.7270/Q22R3QXR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor VII (Homo sapiens (Human)) | BDBM50106238 (4-Chloro-2-(2-hydroxy-biphenyl-3-yl)-1H-indole-5-c...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 5.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Axys Pharmaceuticals Inc. Curated by ChEMBL | Assay Description The compound was tested for inhibition of human coagulation factor VII | J Med Chem 44: 3856-71 (2001) BindingDB Entry DOI: 10.7270/Q22R3QXR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor VII (Homo sapiens (Human)) | BDBM50106243 (2-(2-Hydroxy-biphenyl-3-yl)-6-methyl-1H-indole-5-c...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 7.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Axys Pharmaceuticals Inc. Curated by ChEMBL | Assay Description The compound was tested for inhibition of human coagulation factor VII | J Med Chem 44: 3856-71 (2001) BindingDB Entry DOI: 10.7270/Q22R3QXR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50106239 (6-Fluoro-2-(2-hydroxy-biphenyl-3-yl)-1H-indole-5-c...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 7.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Axys Pharmaceuticals Inc. Curated by ChEMBL | Assay Description The compound was tested for inhibition of human thrombin | J Med Chem 44: 3856-71 (2001) BindingDB Entry DOI: 10.7270/Q22R3QXR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tissue-type plasminogen activator (Homo sapiens (Human)) | BDBM50106240 (6-Chloro-2-(2-hydroxy-biphenyl-3-yl)-1H-indole-5-c...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 8.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Axys Pharmaceuticals Inc. Curated by ChEMBL | Assay Description The compound was tested for inhibition of human tissue plasminogen activator | J Med Chem 44: 3856-71 (2001) BindingDB Entry DOI: 10.7270/Q22R3QXR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease 1/Trypsin-2 (Homo sapiens (Human)) | BDBM50106241 (2-(2-Hydroxy-biphenyl-3-yl)-6-methoxy-1H-indole-5-...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Axys Pharmaceuticals Inc. Curated by ChEMBL | Assay Description The compound was tested for inhibition of human trypsin | J Med Chem 44: 3856-71 (2001) BindingDB Entry DOI: 10.7270/Q22R3QXR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor VII (Homo sapiens (Human)) | BDBM50106241 (2-(2-Hydroxy-biphenyl-3-yl)-6-methoxy-1H-indole-5-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Axys Pharmaceuticals Inc. Curated by ChEMBL | Assay Description The compound was tested for inhibition of human coagulation factor VII | J Med Chem 44: 3856-71 (2001) BindingDB Entry DOI: 10.7270/Q22R3QXR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tissue-type plasminogen activator (Homo sapiens (Human)) | BDBM50106241 (2-(2-Hydroxy-biphenyl-3-yl)-6-methoxy-1H-indole-5-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Axys Pharmaceuticals Inc. Curated by ChEMBL | Assay Description The compound was tested for inhibition of human tissue plasminogen activator | J Med Chem 44: 3856-71 (2001) BindingDB Entry DOI: 10.7270/Q22R3QXR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tissue-type plasminogen activator (Homo sapiens (Human)) | BDBM50106242 (6-Hydroxy-2-(2-hydroxy-biphenyl-3-yl)-1H-indole-5-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.90E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Axys Pharmaceuticals Inc. Curated by ChEMBL | Assay Description The compound was tested for inhibition of human plasmin | J Med Chem 44: 3856-71 (2001) BindingDB Entry DOI: 10.7270/Q22R3QXR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM50106240 (6-Chloro-2-(2-hydroxy-biphenyl-3-yl)-1H-indole-5-c...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 1.90E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Axys Pharmaceuticals Inc. Curated by ChEMBL | Assay Description Binding affinity against human coagulation factor X | J Med Chem 44: 3856-71 (2001) BindingDB Entry DOI: 10.7270/Q22R3QXR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM50106241 (2-(2-Hydroxy-biphenyl-3-yl)-6-methoxy-1H-indole-5-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 2.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Axys Pharmaceuticals Inc. Curated by ChEMBL | Assay Description Binding affinity against human coagulation factor X | J Med Chem 44: 3856-71 (2001) BindingDB Entry DOI: 10.7270/Q22R3QXR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tissue-type plasminogen activator (Homo sapiens (Human)) | BDBM50106243 (2-(2-Hydroxy-biphenyl-3-yl)-6-methyl-1H-indole-5-c...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 3.90E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Axys Pharmaceuticals Inc. Curated by ChEMBL | Assay Description The compound was tested for inhibition of human tissue plasminogen activator | J Med Chem 44: 3856-71 (2001) BindingDB Entry DOI: 10.7270/Q22R3QXR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tissue-type plasminogen activator (Homo sapiens (Human)) | BDBM50106242 (6-Hydroxy-2-(2-hydroxy-biphenyl-3-yl)-1H-indole-5-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 4.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Axys Pharmaceuticals Inc. Curated by ChEMBL | Assay Description The compound was tested for inhibition of human tissue plasminogen activator | J Med Chem 44: 3856-71 (2001) BindingDB Entry DOI: 10.7270/Q22R3QXR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease 1/Trypsin-2 (Homo sapiens (Human)) | BDBM50106242 (6-Hydroxy-2-(2-hydroxy-biphenyl-3-yl)-1H-indole-5-...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Axys Pharmaceuticals Inc. Curated by ChEMBL | Assay Description The compound was tested for inhibition of human trypsin | J Med Chem 44: 3856-71 (2001) BindingDB Entry DOI: 10.7270/Q22R3QXR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50106240 (6-Chloro-2-(2-hydroxy-biphenyl-3-yl)-1H-indole-5-c...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 6.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Axys Pharmaceuticals Inc. Curated by ChEMBL | Assay Description The compound was tested for inhibition of human thrombin | J Med Chem 44: 3856-71 (2001) BindingDB Entry DOI: 10.7270/Q22R3QXR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50106238 (4-Chloro-2-(2-hydroxy-biphenyl-3-yl)-1H-indole-5-c...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 6.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Axys Pharmaceuticals Inc. Curated by ChEMBL | Assay Description The compound was tested for inhibition of human thrombin | J Med Chem 44: 3856-71 (2001) BindingDB Entry DOI: 10.7270/Q22R3QXR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50106241 (2-(2-Hydroxy-biphenyl-3-yl)-6-methoxy-1H-indole-5-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | >7.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Axys Pharmaceuticals Inc. Curated by ChEMBL | Assay Description The compound was tested for inhibition of human thrombin | J Med Chem 44: 3856-71 (2001) BindingDB Entry DOI: 10.7270/Q22R3QXR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50106242 (6-Hydroxy-2-(2-hydroxy-biphenyl-3-yl)-1H-indole-5-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | >7.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Axys Pharmaceuticals Inc. Curated by ChEMBL | Assay Description The compound was tested for inhibition of human thrombin | J Med Chem 44: 3856-71 (2001) BindingDB Entry DOI: 10.7270/Q22R3QXR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor VII (Homo sapiens (Human)) | BDBM50106242 (6-Hydroxy-2-(2-hydroxy-biphenyl-3-yl)-1H-indole-5-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | >7.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Axys Pharmaceuticals Inc. Curated by ChEMBL | Assay Description The compound was tested for inhibition of human coagulation factor VII | J Med Chem 44: 3856-71 (2001) BindingDB Entry DOI: 10.7270/Q22R3QXR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM50106242 (6-Hydroxy-2-(2-hydroxy-biphenyl-3-yl)-1H-indole-5-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | >7.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Axys Pharmaceuticals Inc. Curated by ChEMBL | Assay Description Binding affinity against human coagulation factor X | J Med Chem 44: 3856-71 (2001) BindingDB Entry DOI: 10.7270/Q22R3QXR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50106243 (2-(2-Hydroxy-biphenyl-3-yl)-6-methyl-1H-indole-5-c...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | >7.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Axys Pharmaceuticals Inc. Curated by ChEMBL | Assay Description The compound was tested for inhibition of human thrombin | J Med Chem 44: 3856-71 (2001) BindingDB Entry DOI: 10.7270/Q22R3QXR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM50106243 (2-(2-Hydroxy-biphenyl-3-yl)-6-methyl-1H-indole-5-c...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | >7.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Axys Pharmaceuticals Inc. Curated by ChEMBL | Assay Description Binding affinity against human coagulation factor X | J Med Chem 44: 3856-71 (2001) BindingDB Entry DOI: 10.7270/Q22R3QXR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||