 Found 51 hits Enz. Inhib. hit(s) with all data for entry = 50014839
Found 51 hits Enz. Inhib. hit(s) with all data for entry = 50014839 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Nitric oxide synthase, inducible
(Homo sapiens (Human)) | BDBM50091817
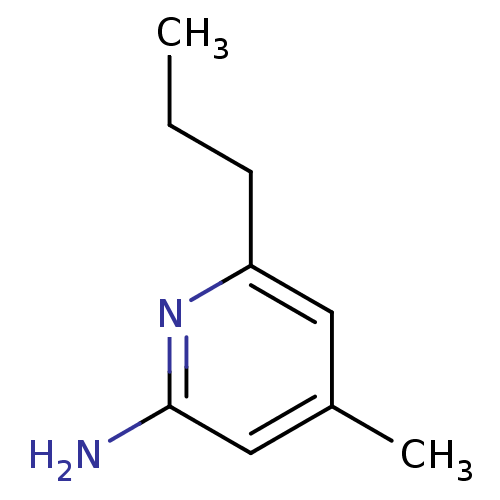
(4-Methyl-6-propyl-pyridin-2-ylamine | 4-Methyl-6-p...)Show InChI InChI=1S/C9H14N2/c1-3-4-8-5-7(2)6-9(10)11-8/h5-6H,3-4H2,1-2H3,(H2,10,11) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca R&D Charnwood
Curated by ChEMBL
| Assay Description
In vitro inhibition of human Inducible nitric oxide synthase. |
J Med Chem 47: 3320-3 (2004)
Article DOI: 10.1021/jm031035n
BindingDB Entry DOI: 10.7270/Q25M656D |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Nitric oxide synthase, brain
(Homo sapiens (Human)) | BDBM50091817

(4-Methyl-6-propyl-pyridin-2-ylamine | 4-Methyl-6-p...)Show InChI InChI=1S/C9H14N2/c1-3-4-8-5-7(2)6-9(10)11-8/h5-6H,3-4H2,1-2H3,(H2,10,11) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca R&D Charnwood
Curated by ChEMBL
| Assay Description
In vitro inhibition of human neuronal nitric oxide synthase. |
J Med Chem 47: 3320-3 (2004)
Article DOI: 10.1021/jm031035n
BindingDB Entry DOI: 10.7270/Q25M656D |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, inducible
(Homo sapiens (Human)) | BDBM50148168
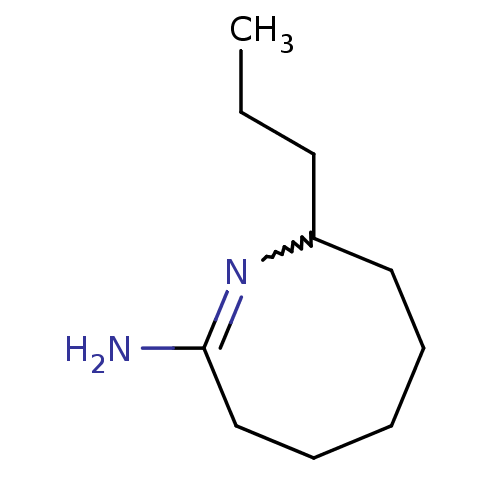
((E)-8-Propyl-3,4,5,6,7,8-hexahydro-azocin-2-ylamin...)Show InChI InChI=1S/C10H20N2/c1-2-6-9-7-4-3-5-8-10(11)12-9/h9H,2-8H2,1H3,(H2,11,12) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 26 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca R&D Charnwood
Curated by ChEMBL
| Assay Description
In vitro inhibition of human Inducible nitric oxide synthase. |
J Med Chem 47: 3320-3 (2004)
Article DOI: 10.1021/jm031035n
BindingDB Entry DOI: 10.7270/Q25M656D |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, inducible
(Homo sapiens (Human)) | BDBM50124535
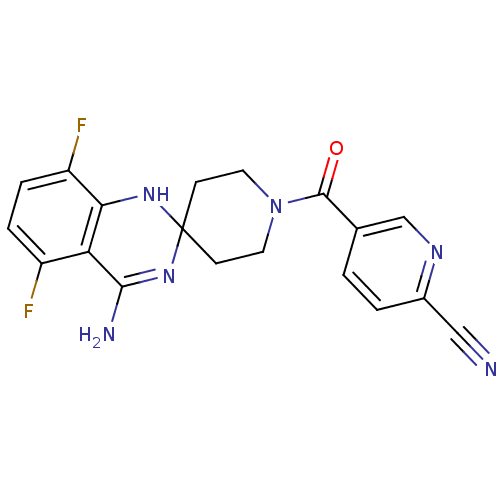
(1-(6-CYANO-3-PYRIDYLCARBONYL)-5',8'-DIFLUOROSPIRO[...)Show SMILES NC1=NC2(CCN(CC2)C(=O)c2ccc(nc2)C#N)Nc2c(F)ccc(F)c12 |t:1| Show InChI InChI=1S/C19H16F2N6O/c20-13-3-4-14(21)16-15(13)17(23)26-19(25-16)5-7-27(8-6-19)18(28)11-1-2-12(9-22)24-10-11/h1-4,10,25H,5-8H2,(H2,23,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 37 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca R&D Charnwood
Curated by ChEMBL
| Assay Description
In vitro inhibition of human Inducible nitric oxide synthase. |
J Med Chem 47: 3320-3 (2004)
Article DOI: 10.1021/jm031035n
BindingDB Entry DOI: 10.7270/Q25M656D |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Nitric oxide synthase, inducible
(Homo sapiens (Human)) | BDBM50091805

(2-amino-4,6-dimethylpyridine | 4,6-Dimethyl-pyridi...)Show InChI InChI=1S/C7H10N2/c1-5-3-6(2)9-7(8)4-5/h3-4H,1-2H3,(H2,8,9) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 45 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca R&D Charnwood
Curated by ChEMBL
| Assay Description
In vitro inhibition of human Inducible nitric oxide synthase. |
J Med Chem 47: 3320-3 (2004)
Article DOI: 10.1021/jm031035n
BindingDB Entry DOI: 10.7270/Q25M656D |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, endothelial
(Homo sapiens (Human)) | BDBM50091817

(4-Methyl-6-propyl-pyridin-2-ylamine | 4-Methyl-6-p...)Show InChI InChI=1S/C9H14N2/c1-3-4-8-5-7(2)6-9(10)11-8/h5-6H,3-4H2,1-2H3,(H2,10,11) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca R&D Charnwood
Curated by ChEMBL
| Assay Description
In vitro inhibition of human endothelial nitric oxide synthase. |
J Med Chem 47: 3320-3 (2004)
Article DOI: 10.1021/jm031035n
BindingDB Entry DOI: 10.7270/Q25M656D |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, inducible
(Homo sapiens (Human)) | BDBM50148169
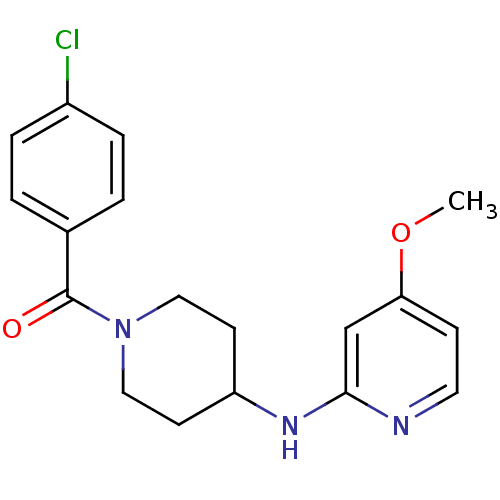
((4-Chloro-phenyl)-[4-(4-methoxy-pyridin-2-ylamino)...)Show InChI InChI=1S/C18H20ClN3O2/c1-24-16-6-9-20-17(12-16)21-15-7-10-22(11-8-15)18(23)13-2-4-14(19)5-3-13/h2-6,9,12,15H,7-8,10-11H2,1H3,(H,20,21) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 53 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca R&D Charnwood
Curated by ChEMBL
| Assay Description
In vitro inhibition of human Inducible nitric oxide synthase. |
J Med Chem 47: 3320-3 (2004)
Article DOI: 10.1021/jm031035n
BindingDB Entry DOI: 10.7270/Q25M656D |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, inducible
(Homo sapiens (Human)) | BDBM50086467
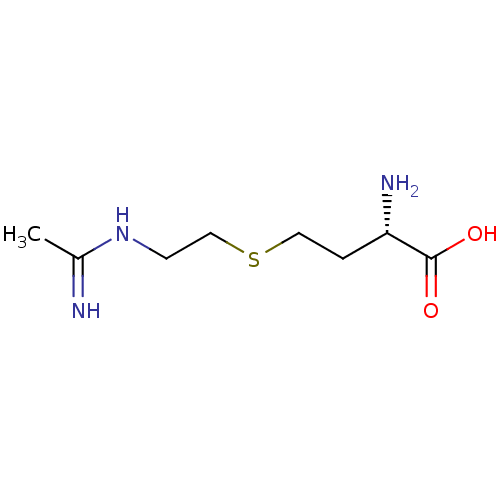
((S)-4-(2-Acetimidoylamino-ethylsulfanyl)-2-amino-b...)Show InChI InChI=1S/C8H17N3O2S/c1-6(9)11-3-5-14-4-2-7(10)8(12)13/h7H,2-5,10H2,1H3,(H2,9,11)(H,12,13)/t7-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 70 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca R&D Charnwood
Curated by ChEMBL
| Assay Description
In vitro inhibition of human Inducible nitric oxide synthase. |
J Med Chem 47: 3320-3 (2004)
Article DOI: 10.1021/jm031035n
BindingDB Entry DOI: 10.7270/Q25M656D |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, inducible
(Homo sapiens (Human)) | BDBM50148167
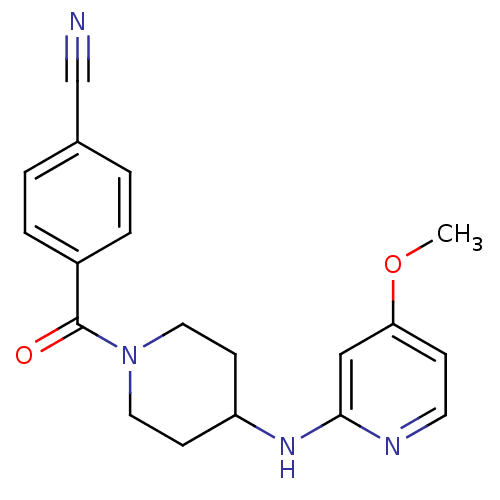
(4-(4-(4-methoxypyridin-2-ylamino)piperidine-1-carb...)Show InChI InChI=1S/C19H20N4O2/c1-25-17-6-9-21-18(12-17)22-16-7-10-23(11-8-16)19(24)15-4-2-14(13-20)3-5-15/h2-6,9,12,16H,7-8,10-11H2,1H3,(H,21,22) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| n/a | n/a | 71 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca R&D Charnwood
Curated by ChEMBL
| Assay Description
In vitro inhibition of human Inducible nitric oxide synthase. |
J Med Chem 47: 3320-3 (2004)
Article DOI: 10.1021/jm031035n
BindingDB Entry DOI: 10.7270/Q25M656D |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Nitric oxide synthase, inducible
(Homo sapiens (Human)) | BDBM50148164
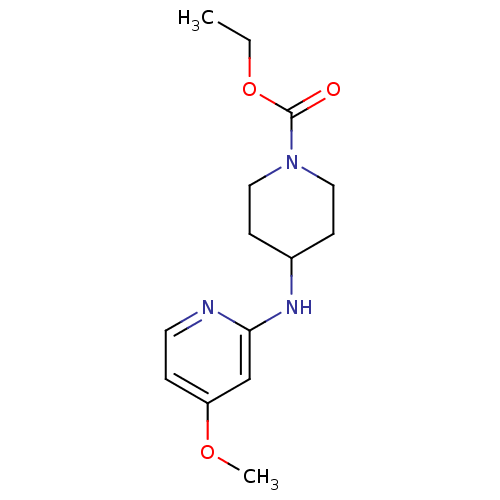
(4-(4-Methoxy-pyridin-2-ylamino)-piperidine-1-carbo...)Show InChI InChI=1S/C14H21N3O3/c1-3-20-14(18)17-8-5-11(6-9-17)16-13-10-12(19-2)4-7-15-13/h4,7,10-11H,3,5-6,8-9H2,1-2H3,(H,15,16) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 89 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca R&D Charnwood
Curated by ChEMBL
| Assay Description
In vitro inhibition of human Inducible nitric oxide synthase. |
J Med Chem 47: 3320-3 (2004)
Article DOI: 10.1021/jm031035n
BindingDB Entry DOI: 10.7270/Q25M656D |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, brain
(Homo sapiens (Human)) | BDBM50091805

(2-amino-4,6-dimethylpyridine | 4,6-Dimethyl-pyridi...)Show InChI InChI=1S/C7H10N2/c1-5-3-6(2)9-7(8)4-5/h3-4H,1-2H3,(H2,8,9) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 98 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca R&D Charnwood
Curated by ChEMBL
| Assay Description
In vitro inhibition of human neuronal nitric oxide synthase. |
J Med Chem 47: 3320-3 (2004)
Article DOI: 10.1021/jm031035n
BindingDB Entry DOI: 10.7270/Q25M656D |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, brain
(Homo sapiens (Human)) | BDBM50091800
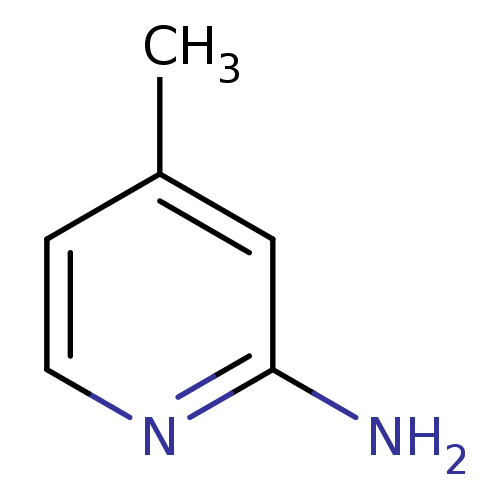
(2-amino-4-methylpyridine | 4-METHYLPYRIDIN-2-AMINE...)Show InChI InChI=1S/C6H8N2/c1-5-2-3-8-6(7)4-5/h2-4H,1H3,(H2,7,8) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca R&D Charnwood
Curated by ChEMBL
| Assay Description
In vitro inhibition of human neuronal nitric oxide synthase. |
J Med Chem 47: 3320-3 (2004)
Article DOI: 10.1021/jm031035n
BindingDB Entry DOI: 10.7270/Q25M656D |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, inducible
(Homo sapiens (Human)) | BDBM50091800

(2-amino-4-methylpyridine | 4-METHYLPYRIDIN-2-AMINE...)Show InChI InChI=1S/C6H8N2/c1-5-2-3-8-6(7)4-5/h2-4H,1H3,(H2,7,8) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 120 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca R&D Charnwood
Curated by ChEMBL
| Assay Description
In vitro inhibition of human Inducible nitric oxide synthase. |
J Med Chem 47: 3320-3 (2004)
Article DOI: 10.1021/jm031035n
BindingDB Entry DOI: 10.7270/Q25M656D |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Nitric oxide synthase, inducible
(Homo sapiens (Human)) | BDBM50063300
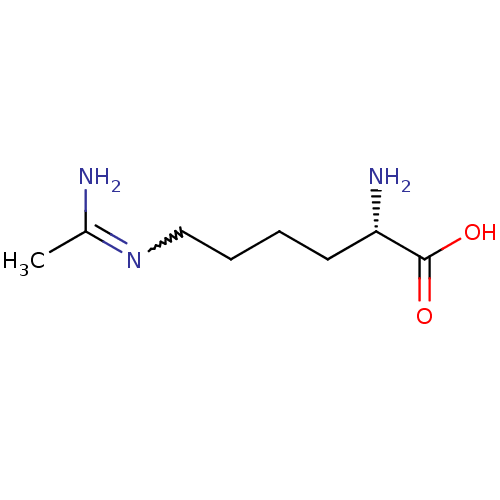
((L-N6-1-iminoethyl)lysine | (S)-6-Acetimidoylamino...)Show InChI InChI=1S/C8H17N3O2/c1-6(9)11-5-3-2-4-7(10)8(12)13/h7H,2-5,10H2,1H3,(H2,9,11)(H,12,13)/t7-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MMDB
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 130 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca R&D Charnwood
Curated by ChEMBL
| Assay Description
In vitro inhibition of human Inducible nitric oxide synthase. |
J Med Chem 47: 3320-3 (2004)
Article DOI: 10.1021/jm031035n
BindingDB Entry DOI: 10.7270/Q25M656D |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, endothelial
(Homo sapiens (Human)) | BDBM50091805

(2-amino-4,6-dimethylpyridine | 4,6-Dimethyl-pyridi...)Show InChI InChI=1S/C7H10N2/c1-5-3-6(2)9-7(8)4-5/h3-4H,1-2H3,(H2,8,9) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 170 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca R&D Charnwood
Curated by ChEMBL
| Assay Description
In vitro inhibition of human endothelial nitric oxide synthase. |
J Med Chem 47: 3320-3 (2004)
Article DOI: 10.1021/jm031035n
BindingDB Entry DOI: 10.7270/Q25M656D |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, brain
(Homo sapiens (Human)) | BDBM50148168

((E)-8-Propyl-3,4,5,6,7,8-hexahydro-azocin-2-ylamin...)Show InChI InChI=1S/C10H20N2/c1-2-6-9-7-4-3-5-8-10(11)12-9/h9H,2-8H2,1H3,(H2,11,12) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 240 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca R&D Charnwood
Curated by ChEMBL
| Assay Description
In vitro inhibition of human neuronal nitric oxide synthase. |
J Med Chem 47: 3320-3 (2004)
Article DOI: 10.1021/jm031035n
BindingDB Entry DOI: 10.7270/Q25M656D |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, endothelial
(Homo sapiens (Human)) | BDBM50091800

(2-amino-4-methylpyridine | 4-METHYLPYRIDIN-2-AMINE...)Show InChI InChI=1S/C6H8N2/c1-5-2-3-8-6(7)4-5/h2-4H,1H3,(H2,7,8) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca R&D Charnwood
Curated by ChEMBL
| Assay Description
In vitro inhibition of human endothelial nitric oxide synthase. |
J Med Chem 47: 3320-3 (2004)
Article DOI: 10.1021/jm031035n
BindingDB Entry DOI: 10.7270/Q25M656D |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, inducible
(Homo sapiens (Human)) | BDBM50148162
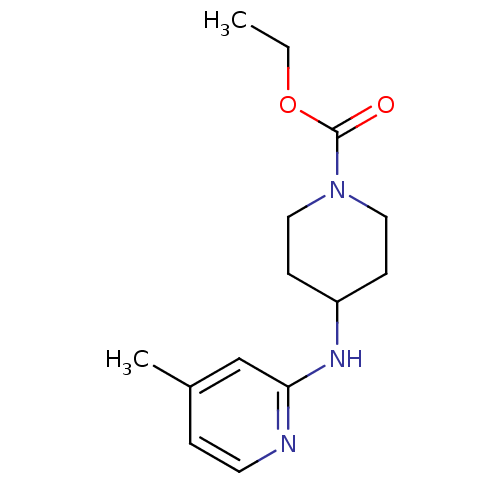
(4-(4-Methyl-pyridin-2-ylamino)-piperidine-1-carbox...)Show InChI InChI=1S/C14H21N3O2/c1-3-19-14(18)17-8-5-12(6-9-17)16-13-10-11(2)4-7-15-13/h4,7,10,12H,3,5-6,8-9H2,1-2H3,(H,15,16) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| MMDB
PDB
Article
PubMed
| n/a | n/a | 350 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca R&D Charnwood
Curated by ChEMBL
| Assay Description
In vitro inhibition of human Inducible nitric oxide synthase. |
J Med Chem 47: 3320-3 (2004)
Article DOI: 10.1021/jm031035n
BindingDB Entry DOI: 10.7270/Q25M656D |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Nitric oxide synthase, inducible
(Homo sapiens (Human)) | BDBM50148163
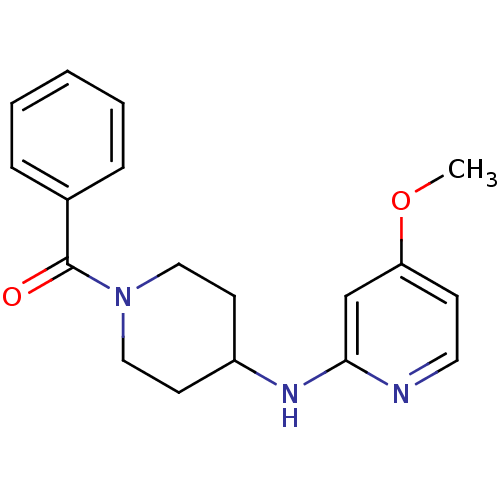
(CHEMBL112747 | [4-(4-Methoxy-pyridin-2-ylamino)-pi...)Show InChI InChI=1S/C18H21N3O2/c1-23-16-7-10-19-17(13-16)20-15-8-11-21(12-9-15)18(22)14-5-3-2-4-6-14/h2-7,10,13,15H,8-9,11-12H2,1H3,(H,19,20) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 530 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca R&D Charnwood
Curated by ChEMBL
| Assay Description
In vitro inhibition of human Inducible nitric oxide synthase. |
J Med Chem 47: 3320-3 (2004)
Article DOI: 10.1021/jm031035n
BindingDB Entry DOI: 10.7270/Q25M656D |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, brain
(Homo sapiens (Human)) | BDBM50124535

(1-(6-CYANO-3-PYRIDYLCARBONYL)-5',8'-DIFLUOROSPIRO[...)Show SMILES NC1=NC2(CCN(CC2)C(=O)c2ccc(nc2)C#N)Nc2c(F)ccc(F)c12 |t:1| Show InChI InChI=1S/C19H16F2N6O/c20-13-3-4-14(21)16-15(13)17(23)26-19(25-16)5-7-27(8-6-19)18(28)11-1-2-12(9-22)24-10-11/h1-4,10,25H,5-8H2,(H2,23,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 740 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca R&D Charnwood
Curated by ChEMBL
| Assay Description
In vitro inhibition of human neuronal nitric oxide synthase. |
J Med Chem 47: 3320-3 (2004)
Article DOI: 10.1021/jm031035n
BindingDB Entry DOI: 10.7270/Q25M656D |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, brain
(Homo sapiens (Human)) | BDBM50148170
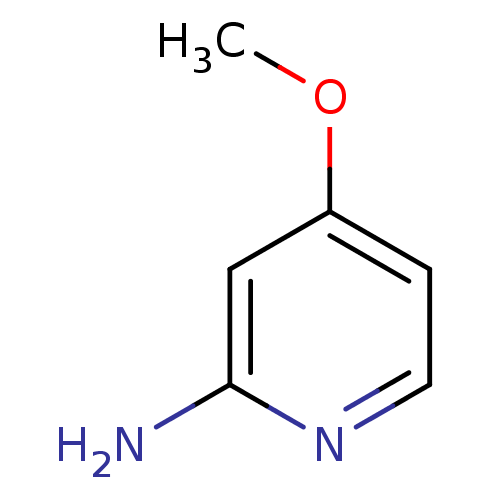
(4-Methoxy-pyridin-2-ylamine | CHEMBL113793)Show InChI InChI=1S/C6H8N2O/c1-9-5-2-3-8-6(7)4-5/h2-4H,1H3,(H2,7,8) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca R&D Charnwood
Curated by ChEMBL
| Assay Description
In vitro inhibition of human neuronal nitric oxide synthase. |
J Med Chem 47: 3320-3 (2004)
Article DOI: 10.1021/jm031035n
BindingDB Entry DOI: 10.7270/Q25M656D |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, endothelial
(Homo sapiens (Human)) | BDBM50148170

(4-Methoxy-pyridin-2-ylamine | CHEMBL113793)Show InChI InChI=1S/C6H8N2O/c1-9-5-2-3-8-6(7)4-5/h2-4H,1H3,(H2,7,8) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca R&D Charnwood
Curated by ChEMBL
| Assay Description
In vitro inhibition of human endothelial nitric oxide synthase. |
J Med Chem 47: 3320-3 (2004)
Article DOI: 10.1021/jm031035n
BindingDB Entry DOI: 10.7270/Q25M656D |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, inducible
(Homo sapiens (Human)) | BDBM50148170

(4-Methoxy-pyridin-2-ylamine | CHEMBL113793)Show InChI InChI=1S/C6H8N2O/c1-9-5-2-3-8-6(7)4-5/h2-4H,1H3,(H2,7,8) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca R&D Charnwood
Curated by ChEMBL
| Assay Description
In vitro inhibition of human Inducible nitric oxide synthase. |
J Med Chem 47: 3320-3 (2004)
Article DOI: 10.1021/jm031035n
BindingDB Entry DOI: 10.7270/Q25M656D |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, inducible
(Homo sapiens (Human)) | BDBM50091813
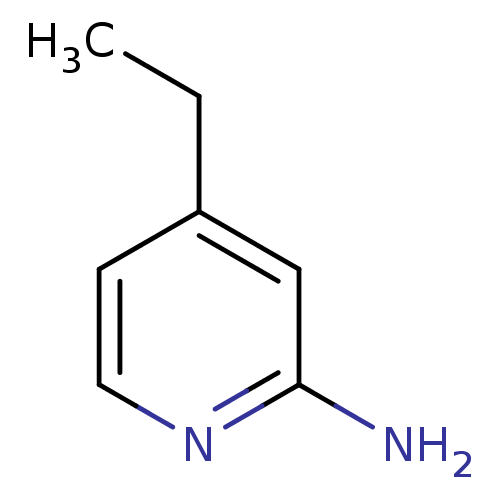
(4-Ethyl-pyridin-2-ylamine | 4-ethylpyridin-2-amine...)Show InChI InChI=1S/C7H10N2/c1-2-6-3-4-9-7(8)5-6/h3-5H,2H2,1H3,(H2,8,9) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca R&D Charnwood
Curated by ChEMBL
| Assay Description
In vitro inhibition of human Inducible nitric oxide synthase. |
J Med Chem 47: 3320-3 (2004)
Article DOI: 10.1021/jm031035n
BindingDB Entry DOI: 10.7270/Q25M656D |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, brain
(Homo sapiens (Human)) | BDBM50063300

((L-N6-1-iminoethyl)lysine | (S)-6-Acetimidoylamino...)Show InChI InChI=1S/C8H17N3O2/c1-6(9)11-5-3-2-4-7(10)8(12)13/h7H,2-5,10H2,1H3,(H2,9,11)(H,12,13)/t7-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MMDB
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca R&D Charnwood
Curated by ChEMBL
| Assay Description
In vitro inhibition of human neuronal nitric oxide synthase. |
J Med Chem 47: 3320-3 (2004)
Article DOI: 10.1021/jm031035n
BindingDB Entry DOI: 10.7270/Q25M656D |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, brain
(Homo sapiens (Human)) | BDBM50091813

(4-Ethyl-pyridin-2-ylamine | 4-ethylpyridin-2-amine...)Show InChI InChI=1S/C7H10N2/c1-2-6-3-4-9-7(8)5-6/h3-5H,2H2,1H3,(H2,8,9) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca R&D Charnwood
Curated by ChEMBL
| Assay Description
In vitro inhibition of human neuronal nitric oxide synthase. |
J Med Chem 47: 3320-3 (2004)
Article DOI: 10.1021/jm031035n
BindingDB Entry DOI: 10.7270/Q25M656D |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, endothelial
(Homo sapiens (Human)) | BDBM50063300

((L-N6-1-iminoethyl)lysine | (S)-6-Acetimidoylamino...)Show InChI InChI=1S/C8H17N3O2/c1-6(9)11-5-3-2-4-7(10)8(12)13/h7H,2-5,10H2,1H3,(H2,9,11)(H,12,13)/t7-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MMDB
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca R&D Charnwood
Curated by ChEMBL
| Assay Description
In vitro inhibition of human endothelial nitric oxide synthase. |
J Med Chem 47: 3320-3 (2004)
Article DOI: 10.1021/jm031035n
BindingDB Entry DOI: 10.7270/Q25M656D |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, inducible
(Homo sapiens (Human)) | BDBM50148166
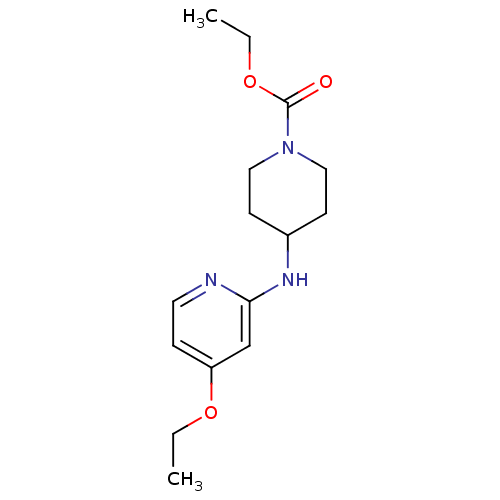
(4-(4-Ethoxy-pyridin-2-ylamino)-piperidine-1-carbox...)Show InChI InChI=1S/C15H23N3O3/c1-3-20-13-5-8-16-14(11-13)17-12-6-9-18(10-7-12)15(19)21-4-2/h5,8,11-12H,3-4,6-7,9-10H2,1-2H3,(H,16,17) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca R&D Charnwood
Curated by ChEMBL
| Assay Description
In vitro inhibition of human Inducible nitric oxide synthase. |
J Med Chem 47: 3320-3 (2004)
Article DOI: 10.1021/jm031035n
BindingDB Entry DOI: 10.7270/Q25M656D |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, endothelial
(Homo sapiens (Human)) | BDBM50091813

(4-Ethyl-pyridin-2-ylamine | 4-ethylpyridin-2-amine...)Show InChI InChI=1S/C7H10N2/c1-2-6-3-4-9-7(8)5-6/h3-5H,2H2,1H3,(H2,8,9) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 4.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca R&D Charnwood
Curated by ChEMBL
| Assay Description
In vitro inhibition of human endothelial nitric oxide synthase. |
J Med Chem 47: 3320-3 (2004)
Article DOI: 10.1021/jm031035n
BindingDB Entry DOI: 10.7270/Q25M656D |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, endothelial
(Homo sapiens (Human)) | BDBM50148168

((E)-8-Propyl-3,4,5,6,7,8-hexahydro-azocin-2-ylamin...)Show InChI InChI=1S/C10H20N2/c1-2-6-9-7-4-3-5-8-10(11)12-9/h9H,2-8H2,1H3,(H2,11,12) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca R&D Charnwood
Curated by ChEMBL
| Assay Description
In vitro inhibition of human endothelial nitric oxide synthase. |
J Med Chem 47: 3320-3 (2004)
Article DOI: 10.1021/jm031035n
BindingDB Entry DOI: 10.7270/Q25M656D |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, brain
(Homo sapiens (Human)) | BDBM50148167

(4-(4-(4-methoxypyridin-2-ylamino)piperidine-1-carb...)Show InChI InChI=1S/C19H20N4O2/c1-25-17-6-9-21-18(12-17)22-16-7-10-23(11-8-16)19(24)15-4-2-14(13-20)3-5-15/h2-6,9,12,16H,7-8,10-11H2,1H3,(H,21,22) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 6.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca R&D Charnwood
Curated by ChEMBL
| Assay Description
In vitro inhibition of human neuronal nitric oxide synthase. |
J Med Chem 47: 3320-3 (2004)
Article DOI: 10.1021/jm031035n
BindingDB Entry DOI: 10.7270/Q25M656D |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, brain
(Homo sapiens (Human)) | BDBM50148169

((4-Chloro-phenyl)-[4-(4-methoxy-pyridin-2-ylamino)...)Show InChI InChI=1S/C18H20ClN3O2/c1-24-16-6-9-20-17(12-16)21-15-7-10-22(11-8-15)18(23)13-2-4-14(19)5-3-13/h2-6,9,12,15H,7-8,10-11H2,1H3,(H,20,21) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 7.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca R&D Charnwood
Curated by ChEMBL
| Assay Description
In vitro inhibition of human neuronal nitric oxide synthase. |
J Med Chem 47: 3320-3 (2004)
Article DOI: 10.1021/jm031035n
BindingDB Entry DOI: 10.7270/Q25M656D |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, inducible
(Homo sapiens (Human)) | BDBM50148171
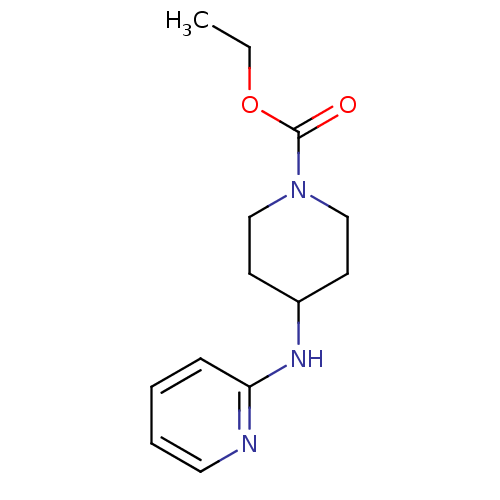
(4-(Pyridin-2-ylamino)-piperidine-1-carboxylic acid...)Show InChI InChI=1S/C13H19N3O2/c1-2-18-13(17)16-9-6-11(7-10-16)15-12-5-3-4-8-14-12/h3-5,8,11H,2,6-7,9-10H2,1H3,(H,14,15) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca R&D Charnwood
Curated by ChEMBL
| Assay Description
In vitro inhibition of human Inducible nitric oxide synthase. |
J Med Chem 47: 3320-3 (2004)
Article DOI: 10.1021/jm031035n
BindingDB Entry DOI: 10.7270/Q25M656D |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, brain
(Homo sapiens (Human)) | BDBM50086467

((S)-4-(2-Acetimidoylamino-ethylsulfanyl)-2-amino-b...)Show InChI InChI=1S/C8H17N3O2S/c1-6(9)11-3-5-14-4-2-7(10)8(12)13/h7H,2-5,10H2,1H3,(H2,9,11)(H,12,13)/t7-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca R&D Charnwood
Curated by ChEMBL
| Assay Description
In vitro inhibition of human neuronal nitric oxide synthase. |
J Med Chem 47: 3320-3 (2004)
Article DOI: 10.1021/jm031035n
BindingDB Entry DOI: 10.7270/Q25M656D |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, brain
(Homo sapiens (Human)) | BDBM50148162

(4-(4-Methyl-pyridin-2-ylamino)-piperidine-1-carbox...)Show InChI InChI=1S/C14H21N3O2/c1-3-19-14(18)17-8-5-12(6-9-17)16-13-10-11(2)4-7-15-13/h4,7,10,12H,3,5-6,8-9H2,1-2H3,(H,15,16) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca R&D Charnwood
Curated by ChEMBL
| Assay Description
In vitro inhibition of human neuronal nitric oxide synthase. |
J Med Chem 47: 3320-3 (2004)
Article DOI: 10.1021/jm031035n
BindingDB Entry DOI: 10.7270/Q25M656D |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, brain
(Homo sapiens (Human)) | BDBM50148163

(CHEMBL112747 | [4-(4-Methoxy-pyridin-2-ylamino)-pi...)Show InChI InChI=1S/C18H21N3O2/c1-23-16-7-10-19-17(13-16)20-15-8-11-21(12-9-15)18(22)14-5-3-2-4-6-14/h2-7,10,13,15H,8-9,11-12H2,1H3,(H,19,20) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca R&D Charnwood
Curated by ChEMBL
| Assay Description
In vitro inhibition of human neuronal nitric oxide synthase. |
J Med Chem 47: 3320-3 (2004)
Article DOI: 10.1021/jm031035n
BindingDB Entry DOI: 10.7270/Q25M656D |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, endothelial
(Homo sapiens (Human)) | BDBM50148165
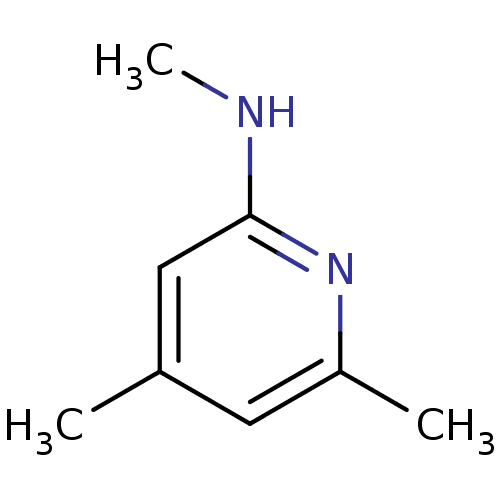
((4,6-Dimethyl-pyridin-2-yl)-methyl-amine | CHEMBL1...)Show InChI InChI=1S/C8H12N2/c1-6-4-7(2)10-8(5-6)9-3/h4-5H,1-3H3,(H,9,10) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca R&D Charnwood
Curated by ChEMBL
| Assay Description
In vitro inhibition of human endothelial nitric oxide synthase. |
J Med Chem 47: 3320-3 (2004)
Article DOI: 10.1021/jm031035n
BindingDB Entry DOI: 10.7270/Q25M656D |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, brain
(Homo sapiens (Human)) | BDBM50148164

(4-(4-Methoxy-pyridin-2-ylamino)-piperidine-1-carbo...)Show InChI InChI=1S/C14H21N3O3/c1-3-20-14(18)17-8-5-11(6-9-17)16-13-10-12(19-2)4-7-15-13/h4,7,10-11H,3,5-6,8-9H2,1-2H3,(H,15,16) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca R&D Charnwood
Curated by ChEMBL
| Assay Description
In vitro inhibition of human neuronal nitric oxide synthase. |
J Med Chem 47: 3320-3 (2004)
Article DOI: 10.1021/jm031035n
BindingDB Entry DOI: 10.7270/Q25M656D |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, endothelial
(Homo sapiens (Human)) | BDBM50086467

((S)-4-(2-Acetimidoylamino-ethylsulfanyl)-2-amino-b...)Show InChI InChI=1S/C8H17N3O2S/c1-6(9)11-3-5-14-4-2-7(10)8(12)13/h7H,2-5,10H2,1H3,(H2,9,11)(H,12,13)/t7-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca R&D Charnwood
Curated by ChEMBL
| Assay Description
In vitro inhibition of human endothelial nitric oxide synthase. |
J Med Chem 47: 3320-3 (2004)
Article DOI: 10.1021/jm031035n
BindingDB Entry DOI: 10.7270/Q25M656D |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, endothelial
(Homo sapiens (Human)) | BDBM50148169

((4-Chloro-phenyl)-[4-(4-methoxy-pyridin-2-ylamino)...)Show InChI InChI=1S/C18H20ClN3O2/c1-24-16-6-9-20-17(12-16)21-15-7-10-22(11-8-15)18(23)13-2-4-14(19)5-3-13/h2-6,9,12,15H,7-8,10-11H2,1H3,(H,20,21) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca R&D Charnwood
Curated by ChEMBL
| Assay Description
In vitro inhibition of human endothelial nitric oxide synthase. |
J Med Chem 47: 3320-3 (2004)
Article DOI: 10.1021/jm031035n
BindingDB Entry DOI: 10.7270/Q25M656D |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, endothelial
(Homo sapiens (Human)) | BDBM50148164

(4-(4-Methoxy-pyridin-2-ylamino)-piperidine-1-carbo...)Show InChI InChI=1S/C14H21N3O3/c1-3-20-14(18)17-8-5-11(6-9-17)16-13-10-12(19-2)4-7-15-13/h4,7,10-11H,3,5-6,8-9H2,1-2H3,(H,15,16) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca R&D Charnwood
Curated by ChEMBL
| Assay Description
In vitro inhibition of human endothelial nitric oxide synthase. |
J Med Chem 47: 3320-3 (2004)
Article DOI: 10.1021/jm031035n
BindingDB Entry DOI: 10.7270/Q25M656D |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, endothelial
(Homo sapiens (Human)) | BDBM50148162

(4-(4-Methyl-pyridin-2-ylamino)-piperidine-1-carbox...)Show InChI InChI=1S/C14H21N3O2/c1-3-19-14(18)17-8-5-12(6-9-17)16-13-10-11(2)4-7-15-13/h4,7,10,12H,3,5-6,8-9H2,1-2H3,(H,15,16) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca R&D Charnwood
Curated by ChEMBL
| Assay Description
In vitro inhibition of human endothelial nitric oxide synthase. |
J Med Chem 47: 3320-3 (2004)
Article DOI: 10.1021/jm031035n
BindingDB Entry DOI: 10.7270/Q25M656D |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Nitric oxide synthase, inducible
(Homo sapiens (Human)) | BDBM50148165

((4,6-Dimethyl-pyridin-2-yl)-methyl-amine | CHEMBL1...)Show InChI InChI=1S/C8H12N2/c1-6-4-7(2)10-8(5-6)9-3/h4-5H,1-3H3,(H,9,10) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 8.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca R&D Charnwood
Curated by ChEMBL
| Assay Description
In vitro inhibition of human Inducible nitric oxide synthase. |
J Med Chem 47: 3320-3 (2004)
Article DOI: 10.1021/jm031035n
BindingDB Entry DOI: 10.7270/Q25M656D |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, brain
(Homo sapiens (Human)) | BDBM50148166

(4-(4-Ethoxy-pyridin-2-ylamino)-piperidine-1-carbox...)Show InChI InChI=1S/C15H23N3O3/c1-3-20-13-5-8-16-14(11-13)17-12-6-9-18(10-7-12)15(19)21-4-2/h5,8,11-12H,3-4,6-7,9-10H2,1-2H3,(H,16,17) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 9.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca R&D Charnwood
Curated by ChEMBL
| Assay Description
In vitro inhibition of human neuronal nitric oxide synthase. |
J Med Chem 47: 3320-3 (2004)
Article DOI: 10.1021/jm031035n
BindingDB Entry DOI: 10.7270/Q25M656D |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, brain
(Homo sapiens (Human)) | BDBM50148171

(4-(Pyridin-2-ylamino)-piperidine-1-carboxylic acid...)Show InChI InChI=1S/C13H19N3O2/c1-2-18-13(17)16-9-6-11(7-10-16)15-12-5-3-4-8-14-12/h3-5,8,11H,2,6-7,9-10H2,1H3,(H,14,15) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca R&D Charnwood
Curated by ChEMBL
| Assay Description
In vitro inhibition of human neuronal nitric oxide synthase. |
J Med Chem 47: 3320-3 (2004)
Article DOI: 10.1021/jm031035n
BindingDB Entry DOI: 10.7270/Q25M656D |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, brain
(Homo sapiens (Human)) | BDBM50148165

((4,6-Dimethyl-pyridin-2-yl)-methyl-amine | CHEMBL1...)Show InChI InChI=1S/C8H12N2/c1-6-4-7(2)10-8(5-6)9-3/h4-5H,1-3H3,(H,9,10) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca R&D Charnwood
Curated by ChEMBL
| Assay Description
In vitro inhibition of human neuronal nitric oxide synthase. |
J Med Chem 47: 3320-3 (2004)
Article DOI: 10.1021/jm031035n
BindingDB Entry DOI: 10.7270/Q25M656D |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, endothelial
(Homo sapiens (Human)) | BDBM50124535

(1-(6-CYANO-3-PYRIDYLCARBONYL)-5',8'-DIFLUOROSPIRO[...)Show SMILES NC1=NC2(CCN(CC2)C(=O)c2ccc(nc2)C#N)Nc2c(F)ccc(F)c12 |t:1| Show InChI InChI=1S/C19H16F2N6O/c20-13-3-4-14(21)16-15(13)17(23)26-19(25-16)5-7-27(8-6-19)18(28)11-1-2-12(9-22)24-10-11/h1-4,10,25H,5-8H2,(H2,23,26) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca R&D Charnwood
Curated by ChEMBL
| Assay Description
In vitro inhibition of human endothelial nitric oxide synthase. |
J Med Chem 47: 3320-3 (2004)
Article DOI: 10.1021/jm031035n
BindingDB Entry DOI: 10.7270/Q25M656D |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, endothelial
(Homo sapiens (Human)) | BDBM50148167

(4-(4-(4-methoxypyridin-2-ylamino)piperidine-1-carb...)Show InChI InChI=1S/C19H20N4O2/c1-25-17-6-9-21-18(12-17)22-16-7-10-23(11-8-16)19(24)15-4-2-14(13-20)3-5-15/h2-6,9,12,16H,7-8,10-11H2,1H3,(H,21,22) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca R&D Charnwood
Curated by ChEMBL
| Assay Description
In vitro inhibition of human endothelial nitric oxide synthase. |
J Med Chem 47: 3320-3 (2004)
Article DOI: 10.1021/jm031035n
BindingDB Entry DOI: 10.7270/Q25M656D |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, endothelial
(Homo sapiens (Human)) | BDBM50148166

(4-(4-Ethoxy-pyridin-2-ylamino)-piperidine-1-carbox...)Show InChI InChI=1S/C15H23N3O3/c1-3-20-13-5-8-16-14(11-13)17-12-6-9-18(10-7-12)15(19)21-4-2/h5,8,11-12H,3-4,6-7,9-10H2,1-2H3,(H,16,17) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca R&D Charnwood
Curated by ChEMBL
| Assay Description
In vitro inhibition of human endothelial nitric oxide synthase. |
J Med Chem 47: 3320-3 (2004)
Article DOI: 10.1021/jm031035n
BindingDB Entry DOI: 10.7270/Q25M656D |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, endothelial
(Homo sapiens (Human)) | BDBM50148171

(4-(Pyridin-2-ylamino)-piperidine-1-carboxylic acid...)Show InChI InChI=1S/C13H19N3O2/c1-2-18-13(17)16-9-6-11(7-10-16)15-12-5-3-4-8-14-12/h3-5,8,11H,2,6-7,9-10H2,1H3,(H,14,15) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca R&D Charnwood
Curated by ChEMBL
| Assay Description
In vitro inhibition of human endothelial nitric oxide synthase. |
J Med Chem 47: 3320-3 (2004)
Article DOI: 10.1021/jm031035n
BindingDB Entry DOI: 10.7270/Q25M656D |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data