 Found 33 hits Enz. Inhib. hit(s) with all data for entry = 50045220
Found 33 hits Enz. Inhib. hit(s) with all data for entry = 50045220 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Adenosylhomocysteinase
(Homo sapiens (Human)) | BDBM50050862
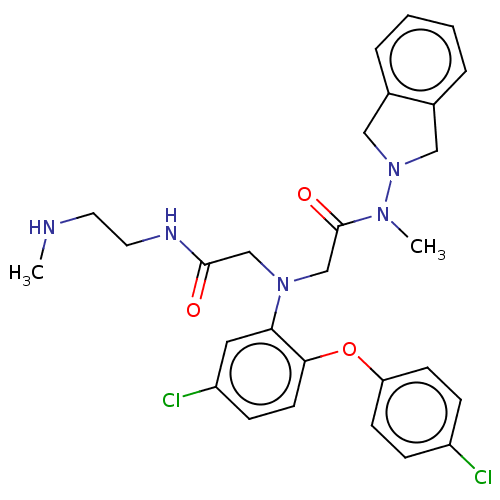
(CHEMBL3322562)Show SMILES Cl.CNCCNC(=O)CN(CC(=O)N(C)N1Cc2ccccc2C1)c1cc(Cl)ccc1Oc1ccc(Cl)cc1 Show InChI InChI=1S/C28H31Cl2N5O3.ClH/c1-31-13-14-32-27(36)18-34(19-28(37)33(2)35-16-20-5-3-4-6-21(20)17-35)25-15-23(30)9-12-26(25)38-24-10-7-22(29)8-11-24;/h3-12,15,31H,13-14,16-19H2,1-2H3,(H,32,36);1H | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Mitsubishi Tanabe Pharma Corporation
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant S-adenosyl-L-homocysteine hydrolase assessed as AdoHcy hydrolysis activity by Lineweaver-Burk plot |
Bioorg Med Chem Lett 24: 4336-40 (2014)
Article DOI: 10.1016/j.bmcl.2014.06.008
BindingDB Entry DOI: 10.7270/Q2377BCP |
More data for this
Ligand-Target Pair | |
Adenosylhomocysteinase
(Homo sapiens (Human)) | BDBM50050862

(CHEMBL3322562)Show SMILES Cl.CNCCNC(=O)CN(CC(=O)N(C)N1Cc2ccccc2C1)c1cc(Cl)ccc1Oc1ccc(Cl)cc1 Show InChI InChI=1S/C28H31Cl2N5O3.ClH/c1-31-13-14-32-27(36)18-34(19-28(37)33(2)35-16-20-5-3-4-6-21(20)17-35)25-15-23(30)9-12-26(25)38-24-10-7-22(29)8-11-24;/h3-12,15,31H,13-14,16-19H2,1-2H3,(H,32,36);1H | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Mitsubishi Tanabe Pharma Corporation
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant S-adenosyl-L-homocysteine hydrolase assessed as AdoHcy hydrolysis activity by HPLC analysis |
Bioorg Med Chem Lett 24: 4336-40 (2014)
Article DOI: 10.1016/j.bmcl.2014.06.008
BindingDB Entry DOI: 10.7270/Q2377BCP |
More data for this
Ligand-Target Pair | |
Adenosylhomocysteinase
(Homo sapiens (Human)) | BDBM50050877
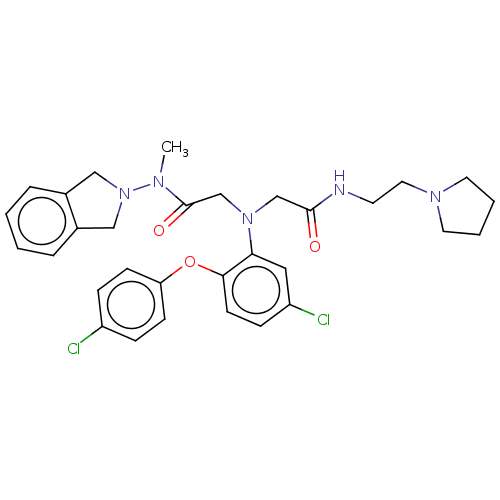
(CHEMBL3322547)Show SMILES CN(N1Cc2ccccc2C1)C(=O)CN(CC(=O)NCCN1CCCC1)c1cc(Cl)ccc1Oc1ccc(Cl)cc1 Show InChI InChI=1S/C31H35Cl2N5O3/c1-35(38-19-23-6-2-3-7-24(23)20-38)31(40)22-37(21-30(39)34-14-17-36-15-4-5-16-36)28-18-26(33)10-13-29(28)41-27-11-8-25(32)9-12-27/h2-3,6-13,18H,4-5,14-17,19-22H2,1H3,(H,34,39) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Mitsubishi Tanabe Pharma Corporation
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant S-adenosyl-L-homocysteine hydrolase assessed as AdoHcy hydrolysis activity by HPLC analysis |
Bioorg Med Chem Lett 24: 4336-40 (2014)
Article DOI: 10.1016/j.bmcl.2014.06.008
BindingDB Entry DOI: 10.7270/Q2377BCP |
More data for this
Ligand-Target Pair | |
Adenosylhomocysteinase
(Homo sapiens (Human)) | BDBM50050874
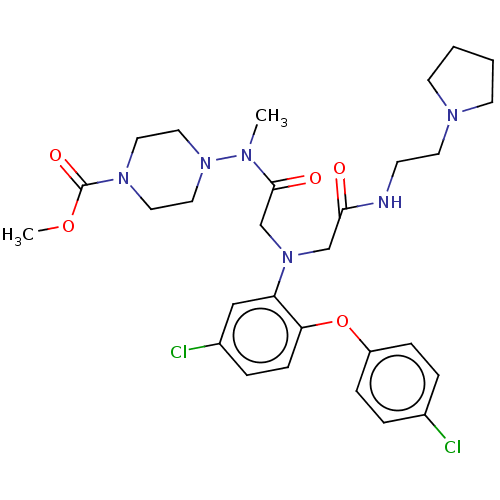
(CHEMBL3322550)Show SMILES COC(=O)N1CCN(CC1)N(C)C(=O)CN(CC(=O)NCCN1CCCC1)c1cc(Cl)ccc1Oc1ccc(Cl)cc1 Show InChI InChI=1S/C29H38Cl2N6O5/c1-33(37-17-15-35(16-18-37)29(40)41-2)28(39)21-36(20-27(38)32-11-14-34-12-3-4-13-34)25-19-23(31)7-10-26(25)42-24-8-5-22(30)6-9-24/h5-10,19H,3-4,11-18,20-21H2,1-2H3,(H,32,38) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 44 | n/a | n/a | n/a | n/a | n/a | n/a |
Mitsubishi Tanabe Pharma Corporation
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant S-adenosyl-L-homocysteine hydrolase assessed as AdoHcy hydrolysis activity by HPLC analysis |
Bioorg Med Chem Lett 24: 4336-40 (2014)
Article DOI: 10.1016/j.bmcl.2014.06.008
BindingDB Entry DOI: 10.7270/Q2377BCP |
More data for this
Ligand-Target Pair | |
Adenosylhomocysteinase
(Homo sapiens (Human)) | BDBM50050875
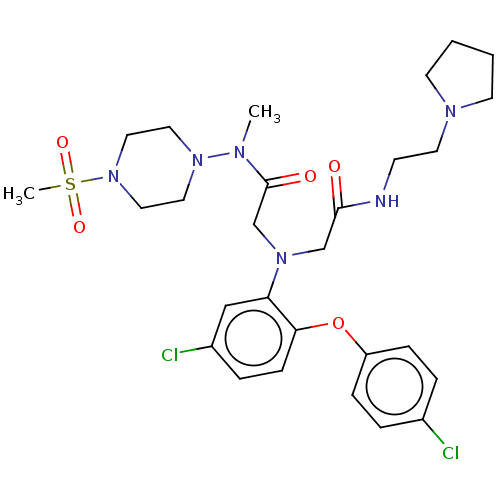
(CHEMBL3322549)Show SMILES CN(N1CCN(CC1)S(C)(=O)=O)C(=O)CN(CC(=O)NCCN1CCCC1)c1cc(Cl)ccc1Oc1ccc(Cl)cc1 Show InChI InChI=1S/C28H38Cl2N6O5S/c1-32(35-15-17-36(18-16-35)42(2,39)40)28(38)21-34(20-27(37)31-11-14-33-12-3-4-13-33)25-19-23(30)7-10-26(25)41-24-8-5-22(29)6-9-24/h5-10,19H,3-4,11-18,20-21H2,1-2H3,(H,31,37) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 49 | n/a | n/a | n/a | n/a | n/a | n/a |
Mitsubishi Tanabe Pharma Corporation
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant S-adenosyl-L-homocysteine hydrolase assessed as AdoHcy hydrolysis activity by HPLC analysis |
Bioorg Med Chem Lett 24: 4336-40 (2014)
Article DOI: 10.1016/j.bmcl.2014.06.008
BindingDB Entry DOI: 10.7270/Q2377BCP |
More data for this
Ligand-Target Pair | |
Adenosylhomocysteinase
(Homo sapiens (Human)) | BDBM50050868
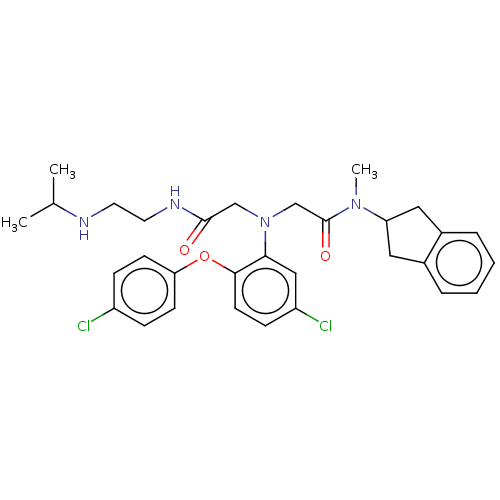
(CHEMBL3322556)Show SMILES CC(C)NCCNC(=O)CN(CC(=O)N(C)C1Cc2ccccc2C1)c1cc(Cl)ccc1Oc1ccc(Cl)cc1 Show InChI InChI=1S/C31H36Cl2N4O3/c1-21(2)34-14-15-35-30(38)19-37(20-31(39)36(3)26-16-22-6-4-5-7-23(22)17-26)28-18-25(33)10-13-29(28)40-27-11-8-24(32)9-12-27/h4-13,18,21,26,34H,14-17,19-20H2,1-3H3,(H,35,38) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 49 | n/a | n/a | n/a | n/a | n/a | n/a |
Mitsubishi Tanabe Pharma Corporation
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant S-adenosyl-L-homocysteine hydrolase assessed as AdoHcy hydrolysis activity by HPLC analysis |
Bioorg Med Chem Lett 24: 4336-40 (2014)
Article DOI: 10.1016/j.bmcl.2014.06.008
BindingDB Entry DOI: 10.7270/Q2377BCP |
More data for this
Ligand-Target Pair | |
Adenosylhomocysteinase
(Homo sapiens (Human)) | BDBM50050884
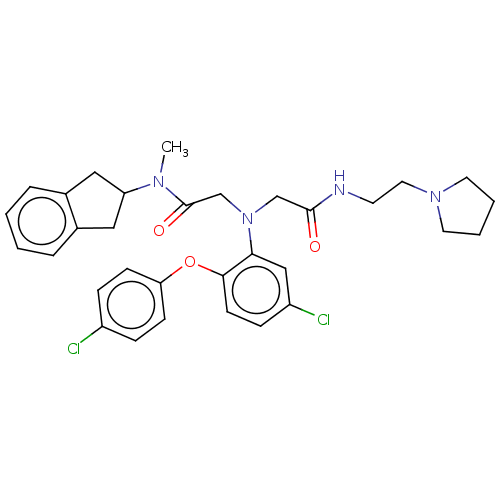
(CHEMBL3322540)Show SMILES CN(C1Cc2ccccc2C1)C(=O)CN(CC(=O)NCCN1CCCC1)c1cc(Cl)ccc1Oc1ccc(Cl)cc1 Show InChI InChI=1S/C32H36Cl2N4O3/c1-36(27-18-23-6-2-3-7-24(23)19-27)32(40)22-38(21-31(39)35-14-17-37-15-4-5-16-37)29-20-26(34)10-13-30(29)41-28-11-8-25(33)9-12-28/h2-3,6-13,20,27H,4-5,14-19,21-22H2,1H3,(H,35,39) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 52 | n/a | n/a | n/a | n/a | n/a | n/a |
Mitsubishi Tanabe Pharma Corporation
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant S-adenosyl-L-homocysteine hydrolase assessed as AdoHcy hydrolysis activity by HPLC analysis |
Bioorg Med Chem Lett 24: 4336-40 (2014)
Article DOI: 10.1016/j.bmcl.2014.06.008
BindingDB Entry DOI: 10.7270/Q2377BCP |
More data for this
Ligand-Target Pair | |
Adenosylhomocysteinase
(Homo sapiens (Human)) | BDBM50050869
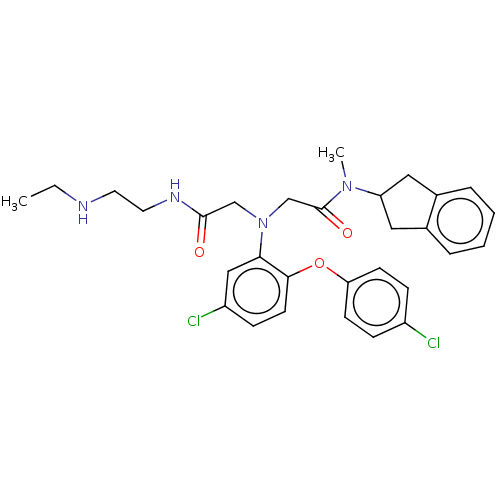
(CHEMBL3322555)Show SMILES CCNCCNC(=O)CN(CC(=O)N(C)C1Cc2ccccc2C1)c1cc(Cl)ccc1Oc1ccc(Cl)cc1 Show InChI InChI=1S/C30H34Cl2N4O3/c1-3-33-14-15-34-29(37)19-36(20-30(38)35(2)25-16-21-6-4-5-7-22(21)17-25)27-18-24(32)10-13-28(27)39-26-11-8-23(31)9-12-26/h4-13,18,25,33H,3,14-17,19-20H2,1-2H3,(H,34,37) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 60 | n/a | n/a | n/a | n/a | n/a | n/a |
Mitsubishi Tanabe Pharma Corporation
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant S-adenosyl-L-homocysteine hydrolase assessed as AdoHcy hydrolysis activity by HPLC analysis |
Bioorg Med Chem Lett 24: 4336-40 (2014)
Article DOI: 10.1016/j.bmcl.2014.06.008
BindingDB Entry DOI: 10.7270/Q2377BCP |
More data for this
Ligand-Target Pair | |
Adenosylhomocysteinase
(Homo sapiens (Human)) | BDBM50050870

(CHEMBL3322554)Show SMILES CNCCNC(=O)CN(CC(=O)N(C)C1Cc2ccccc2C1)c1cc(Cl)ccc1Oc1ccc(Cl)cc1 Show InChI InChI=1S/C29H32Cl2N4O3/c1-32-13-14-33-28(36)18-35(19-29(37)34(2)24-15-20-5-3-4-6-21(20)16-24)26-17-23(31)9-12-27(26)38-25-10-7-22(30)8-11-25/h3-12,17,24,32H,13-16,18-19H2,1-2H3,(H,33,36) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 70 | n/a | n/a | n/a | n/a | n/a | n/a |
Mitsubishi Tanabe Pharma Corporation
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant S-adenosyl-L-homocysteine hydrolase assessed as AdoHcy hydrolysis activity by HPLC analysis |
Bioorg Med Chem Lett 24: 4336-40 (2014)
Article DOI: 10.1016/j.bmcl.2014.06.008
BindingDB Entry DOI: 10.7270/Q2377BCP |
More data for this
Ligand-Target Pair | |
Adenosylhomocysteinase
(Homo sapiens (Human)) | BDBM50050883
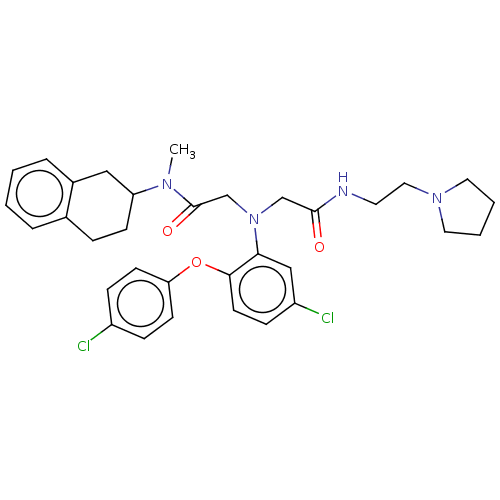
(CHEMBL3322541)Show SMILES CN(C1CCc2ccccc2C1)C(=O)CN(CC(=O)NCCN1CCCC1)c1cc(Cl)ccc1Oc1ccc(Cl)cc1 Show InChI InChI=1S/C33H38Cl2N4O3/c1-37(28-12-8-24-6-2-3-7-25(24)20-28)33(41)23-39(22-32(40)36-16-19-38-17-4-5-18-38)30-21-27(35)11-15-31(30)42-29-13-9-26(34)10-14-29/h2-3,6-7,9-11,13-15,21,28H,4-5,8,12,16-20,22-23H2,1H3,(H,36,40) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 81 | n/a | n/a | n/a | n/a | n/a | n/a |
Mitsubishi Tanabe Pharma Corporation
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant S-adenosyl-L-homocysteine hydrolase assessed as AdoHcy hydrolysis activity by HPLC analysis |
Bioorg Med Chem Lett 24: 4336-40 (2014)
Article DOI: 10.1016/j.bmcl.2014.06.008
BindingDB Entry DOI: 10.7270/Q2377BCP |
More data for this
Ligand-Target Pair | |
Adenosylhomocysteinase
(Homo sapiens (Human)) | BDBM50050865
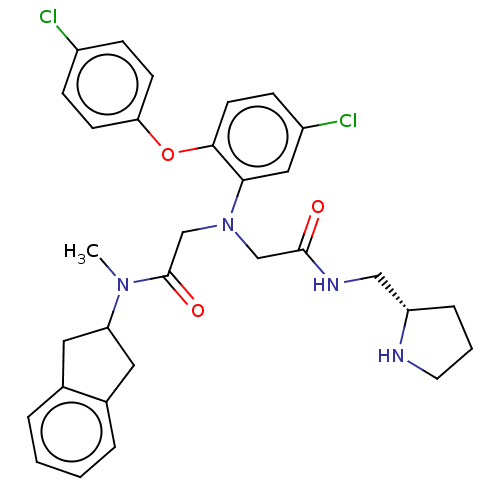
(CHEMBL3322559)Show SMILES CN(C1Cc2ccccc2C1)C(=O)CN(CC(=O)NC[C@@H]1CCCN1)c1cc(Cl)ccc1Oc1ccc(Cl)cc1 |r| Show InChI InChI=1S/C31H34Cl2N4O3/c1-36(26-15-21-5-2-3-6-22(21)16-26)31(39)20-37(19-30(38)35-18-25-7-4-14-34-25)28-17-24(33)10-13-29(28)40-27-11-8-23(32)9-12-27/h2-3,5-6,8-13,17,25-26,34H,4,7,14-16,18-20H2,1H3,(H,35,38)/t25-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
Mitsubishi Tanabe Pharma Corporation
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant S-adenosyl-L-homocysteine hydrolase assessed as AdoHcy hydrolysis activity by HPLC analysis |
Bioorg Med Chem Lett 24: 4336-40 (2014)
Article DOI: 10.1016/j.bmcl.2014.06.008
BindingDB Entry DOI: 10.7270/Q2377BCP |
More data for this
Ligand-Target Pair | |
Adenosylhomocysteinase
(Homo sapiens (Human)) | BDBM50050871
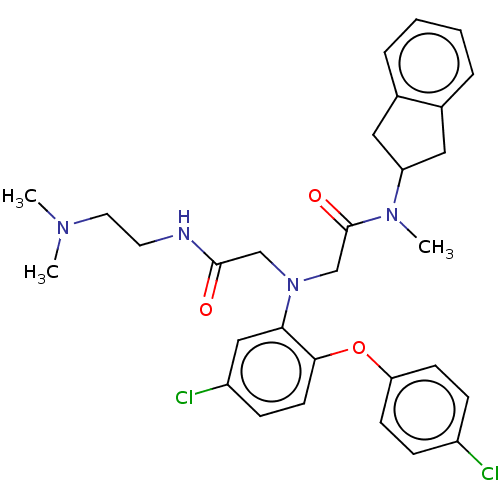
(CHEMBL3322553)Show SMILES CN(C)CCNC(=O)CN(CC(=O)N(C)C1Cc2ccccc2C1)c1cc(Cl)ccc1Oc1ccc(Cl)cc1 Show InChI InChI=1S/C30H34Cl2N4O3/c1-34(2)15-14-33-29(37)19-36(20-30(38)35(3)25-16-21-6-4-5-7-22(21)17-25)27-18-24(32)10-13-28(27)39-26-11-8-23(31)9-12-26/h4-13,18,25H,14-17,19-20H2,1-3H3,(H,33,37) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 130 | n/a | n/a | n/a | n/a | n/a | n/a |
Mitsubishi Tanabe Pharma Corporation
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant S-adenosyl-L-homocysteine hydrolase assessed as AdoHcy hydrolysis activity by HPLC analysis |
Bioorg Med Chem Lett 24: 4336-40 (2014)
Article DOI: 10.1016/j.bmcl.2014.06.008
BindingDB Entry DOI: 10.7270/Q2377BCP |
More data for this
Ligand-Target Pair | |
Adenosylhomocysteinase
(Homo sapiens (Human)) | BDBM50050863
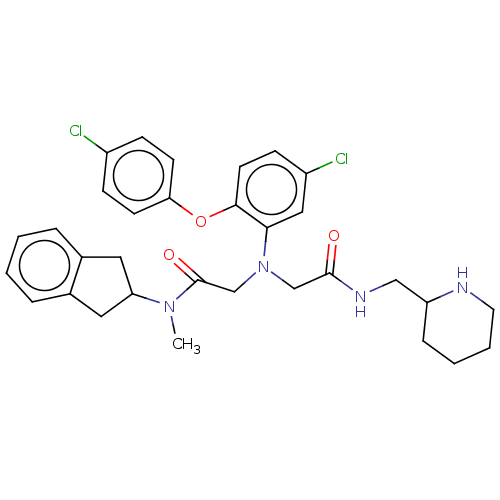
(CHEMBL3322561)Show SMILES CN(C1Cc2ccccc2C1)C(=O)CN(CC(=O)NCC1CCCCN1)c1cc(Cl)ccc1Oc1ccc(Cl)cc1 Show InChI InChI=1S/C32H36Cl2N4O3/c1-37(27-16-22-6-2-3-7-23(22)17-27)32(40)21-38(20-31(39)36-19-26-8-4-5-15-35-26)29-18-25(34)11-14-30(29)41-28-12-9-24(33)10-13-28/h2-3,6-7,9-14,18,26-27,35H,4-5,8,15-17,19-21H2,1H3,(H,36,39) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 150 | n/a | n/a | n/a | n/a | n/a | n/a |
Mitsubishi Tanabe Pharma Corporation
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant S-adenosyl-L-homocysteine hydrolase assessed as AdoHcy hydrolysis activity by HPLC analysis |
Bioorg Med Chem Lett 24: 4336-40 (2014)
Article DOI: 10.1016/j.bmcl.2014.06.008
BindingDB Entry DOI: 10.7270/Q2377BCP |
More data for this
Ligand-Target Pair | |
Adenosylhomocysteinase
(Homo sapiens (Human)) | BDBM50050876
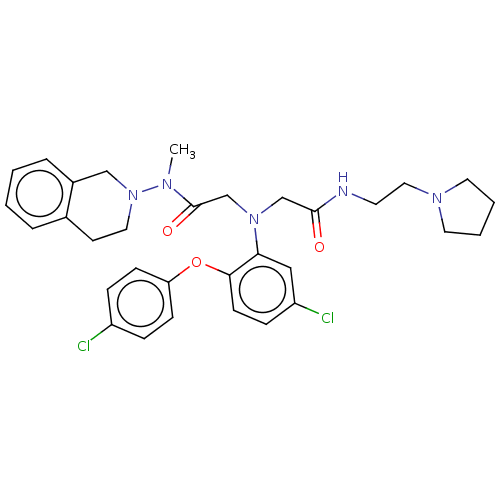
(CHEMBL3322548)Show SMILES CN(N1CCc2ccccc2C1)C(=O)CN(CC(=O)NCCN1CCCC1)c1cc(Cl)ccc1Oc1ccc(Cl)cc1 Show InChI InChI=1S/C32H37Cl2N5O3/c1-36(39-18-14-24-6-2-3-7-25(24)21-39)32(41)23-38(22-31(40)35-15-19-37-16-4-5-17-37)29-20-27(34)10-13-30(29)42-28-11-8-26(33)9-12-28/h2-3,6-13,20H,4-5,14-19,21-23H2,1H3,(H,35,40) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
Mitsubishi Tanabe Pharma Corporation
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant S-adenosyl-L-homocysteine hydrolase assessed as AdoHcy hydrolysis activity by HPLC analysis |
Bioorg Med Chem Lett 24: 4336-40 (2014)
Article DOI: 10.1016/j.bmcl.2014.06.008
BindingDB Entry DOI: 10.7270/Q2377BCP |
More data for this
Ligand-Target Pair | |
Adenosylhomocysteinase
(Homo sapiens (Human)) | BDBM50050879
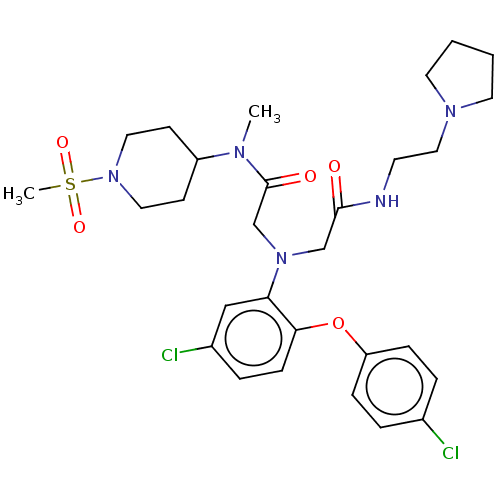
(CHEMBL3322545)Show SMILES CN(C1CCN(CC1)S(C)(=O)=O)C(=O)CN(CC(=O)NCCN1CCCC1)c1cc(Cl)ccc1Oc1ccc(Cl)cc1 Show InChI InChI=1S/C29H39Cl2N5O5S/c1-33(24-11-16-36(17-12-24)42(2,39)40)29(38)21-35(20-28(37)32-13-18-34-14-3-4-15-34)26-19-23(31)7-10-27(26)41-25-8-5-22(30)6-9-25/h5-10,19,24H,3-4,11-18,20-21H2,1-2H3,(H,32,37) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 230 | n/a | n/a | n/a | n/a | n/a | n/a |
Mitsubishi Tanabe Pharma Corporation
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant S-adenosyl-L-homocysteine hydrolase assessed as AdoHcy hydrolysis activity by HPLC analysis |
Bioorg Med Chem Lett 24: 4336-40 (2014)
Article DOI: 10.1016/j.bmcl.2014.06.008
BindingDB Entry DOI: 10.7270/Q2377BCP |
More data for this
Ligand-Target Pair | |
Adenosylhomocysteinase
(Homo sapiens (Human)) | BDBM50050872
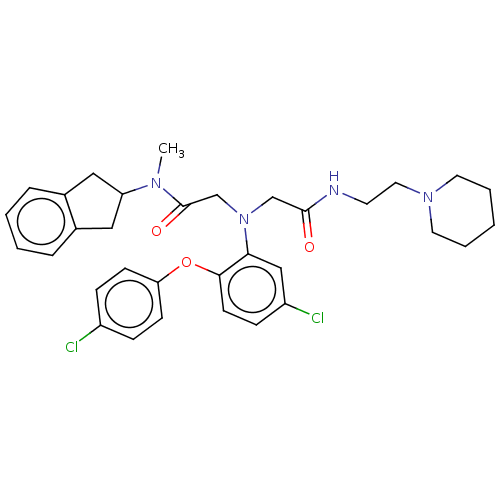
(CHEMBL3322552)Show SMILES CN(C1Cc2ccccc2C1)C(=O)CN(CC(=O)NCCN1CCCCC1)c1cc(Cl)ccc1Oc1ccc(Cl)cc1 Show InChI InChI=1S/C33H38Cl2N4O3/c1-37(28-19-24-7-3-4-8-25(24)20-28)33(41)23-39(22-32(40)36-15-18-38-16-5-2-6-17-38)30-21-27(35)11-14-31(30)42-29-12-9-26(34)10-13-29/h3-4,7-14,21,28H,2,5-6,15-20,22-23H2,1H3,(H,36,40) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 320 | n/a | n/a | n/a | n/a | n/a | n/a |
Mitsubishi Tanabe Pharma Corporation
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant S-adenosyl-L-homocysteine hydrolase assessed as AdoHcy hydrolysis activity by HPLC analysis |
Bioorg Med Chem Lett 24: 4336-40 (2014)
Article DOI: 10.1016/j.bmcl.2014.06.008
BindingDB Entry DOI: 10.7270/Q2377BCP |
More data for this
Ligand-Target Pair | |
Adenosylhomocysteinase
(Homo sapiens (Human)) | BDBM50050873
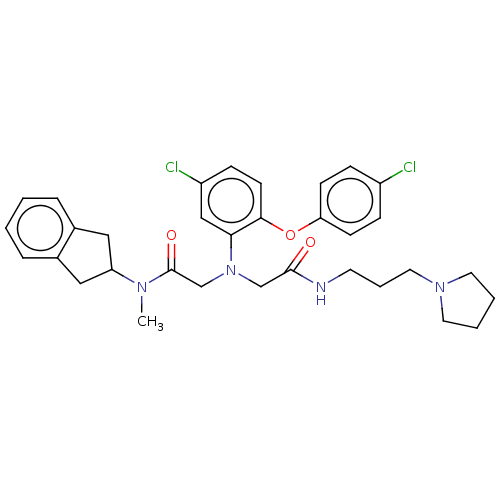
(CHEMBL3322551)Show SMILES CN(C1Cc2ccccc2C1)C(=O)CN(CC(=O)NCCCN1CCCC1)c1cc(Cl)ccc1Oc1ccc(Cl)cc1 Show InChI InChI=1S/C33H38Cl2N4O3/c1-37(28-19-24-7-2-3-8-25(24)20-28)33(41)23-39(22-32(40)36-15-6-18-38-16-4-5-17-38)30-21-27(35)11-14-31(30)42-29-12-9-26(34)10-13-29/h2-3,7-14,21,28H,4-6,15-20,22-23H2,1H3,(H,36,40) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 330 | n/a | n/a | n/a | n/a | n/a | n/a |
Mitsubishi Tanabe Pharma Corporation
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant S-adenosyl-L-homocysteine hydrolase assessed as AdoHcy hydrolysis activity by HPLC analysis |
Bioorg Med Chem Lett 24: 4336-40 (2014)
Article DOI: 10.1016/j.bmcl.2014.06.008
BindingDB Entry DOI: 10.7270/Q2377BCP |
More data for this
Ligand-Target Pair | |
Adenosylhomocysteinase
(Homo sapiens (Human)) | BDBM50050891
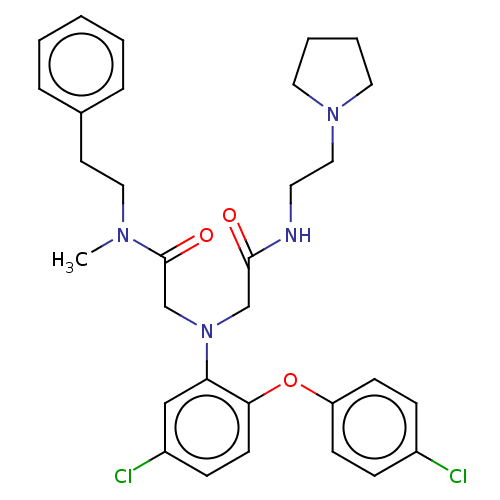
(CHEMBL3322534)Show SMILES CN(CCc1ccccc1)C(=O)CN(CC(=O)NCCN1CCCC1)c1cc(Cl)ccc1Oc1ccc(Cl)cc1 Show InChI InChI=1S/C31H36Cl2N4O3/c1-35(19-15-24-7-3-2-4-8-24)31(39)23-37(22-30(38)34-16-20-36-17-5-6-18-36)28-21-26(33)11-14-29(28)40-27-12-9-25(32)10-13-27/h2-4,7-14,21H,5-6,15-20,22-23H2,1H3,(H,34,38) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 340 | n/a | n/a | n/a | n/a | n/a | n/a |
Mitsubishi Tanabe Pharma Corporation
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant S-adenosyl-L-homocysteine hydrolase assessed as AdoHcy hydrolysis activity by HPLC analysis |
Bioorg Med Chem Lett 24: 4336-40 (2014)
Article DOI: 10.1016/j.bmcl.2014.06.008
BindingDB Entry DOI: 10.7270/Q2377BCP |
More data for this
Ligand-Target Pair | |
Adenosylhomocysteinase
(Homo sapiens (Human)) | BDBM50050892
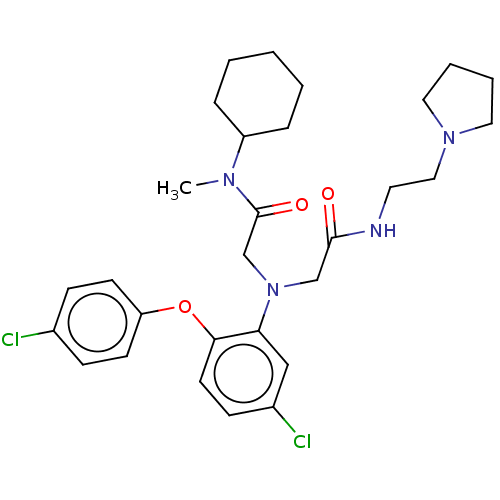
(CHEMBL3322533)Show SMILES CN(C1CCCCC1)C(=O)CN(CC(=O)NCCN1CCCC1)c1cc(Cl)ccc1Oc1ccc(Cl)cc1 Show InChI InChI=1S/C29H38Cl2N4O3/c1-33(24-7-3-2-4-8-24)29(37)21-35(20-28(36)32-15-18-34-16-5-6-17-34)26-19-23(31)11-14-27(26)38-25-12-9-22(30)10-13-25/h9-14,19,24H,2-8,15-18,20-21H2,1H3,(H,32,36) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 380 | n/a | n/a | n/a | n/a | n/a | n/a |
Mitsubishi Tanabe Pharma Corporation
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant S-adenosyl-L-homocysteine hydrolase assessed as AdoHcy hydrolysis activity by HPLC analysis |
Bioorg Med Chem Lett 24: 4336-40 (2014)
Article DOI: 10.1016/j.bmcl.2014.06.008
BindingDB Entry DOI: 10.7270/Q2377BCP |
More data for this
Ligand-Target Pair | |
Adenosylhomocysteinase
(Homo sapiens (Human)) | BDBM50050882
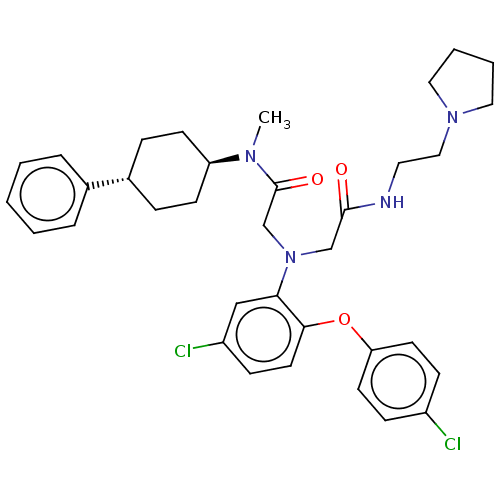
(CHEMBL3322542)Show SMILES CN([C@H]1CC[C@@H](CC1)c1ccccc1)C(=O)CN(CC(=O)NCCN1CCCC1)c1cc(Cl)ccc1Oc1ccc(Cl)cc1 |r,wU:2.1,wD:5.8,(23.46,-26.56,;23.46,-25.02,;24.79,-24.24,;24.77,-22.71,;26.11,-21.93,;27.44,-22.7,;27.44,-24.24,;26.12,-25.01,;28.77,-21.94,;30.1,-22.71,;31.43,-21.94,;31.43,-20.4,;30.09,-19.63,;28.76,-20.4,;22.13,-24.26,;20.8,-25.03,;22.12,-22.72,;20.79,-21.95,;20.78,-20.41,;22.11,-19.64,;23.45,-20.4,;22.1,-18.1,;23.44,-17.32,;24.77,-18.09,;26.1,-17.31,;27.5,-17.93,;28.53,-16.78,;27.76,-15.45,;26.25,-15.78,;19.45,-22.73,;19.46,-24.28,;18.12,-25.05,;18.12,-26.59,;16.79,-24.28,;16.79,-22.74,;18.12,-21.96,;18.11,-20.42,;16.78,-19.66,;16.78,-18.12,;15.45,-17.35,;14.11,-18.13,;12.78,-17.36,;14.12,-19.68,;15.46,-20.44,)| Show InChI InChI=1S/C35H42Cl2N4O3/c1-39(30-14-9-27(10-15-30)26-7-3-2-4-8-26)35(43)25-41(24-34(42)38-19-22-40-20-5-6-21-40)32-23-29(37)13-18-33(32)44-31-16-11-28(36)12-17-31/h2-4,7-8,11-13,16-18,23,27,30H,5-6,9-10,14-15,19-22,24-25H2,1H3,(H,38,42)/t27-,30- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 530 | n/a | n/a | n/a | n/a | n/a | n/a |
Mitsubishi Tanabe Pharma Corporation
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant S-adenosyl-L-homocysteine hydrolase assessed as AdoHcy hydrolysis activity by HPLC analysis |
Bioorg Med Chem Lett 24: 4336-40 (2014)
Article DOI: 10.1016/j.bmcl.2014.06.008
BindingDB Entry DOI: 10.7270/Q2377BCP |
More data for this
Ligand-Target Pair | |
Adenosylhomocysteinase
(Homo sapiens (Human)) | BDBM50050893
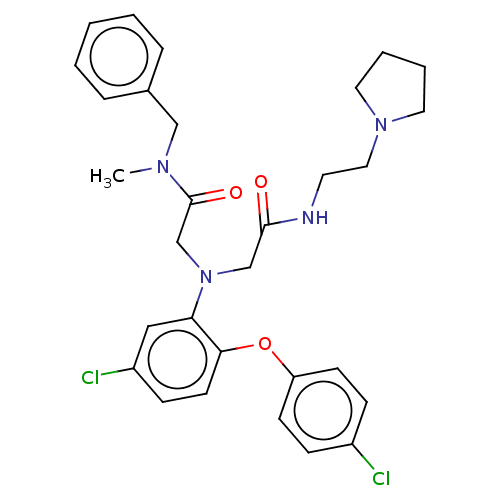
(CHEMBL3322532)Show SMILES CN(Cc1ccccc1)C(=O)CN(CC(=O)NCCN1CCCC1)c1cc(Cl)ccc1Oc1ccc(Cl)cc1 Show InChI InChI=1S/C30H34Cl2N4O3/c1-34(20-23-7-3-2-4-8-23)30(38)22-36(21-29(37)33-15-18-35-16-5-6-17-35)27-19-25(32)11-14-28(27)39-26-12-9-24(31)10-13-26/h2-4,7-14,19H,5-6,15-18,20-22H2,1H3,(H,33,37) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 530 | n/a | n/a | n/a | n/a | n/a | n/a |
Mitsubishi Tanabe Pharma Corporation
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant S-adenosyl-L-homocysteine hydrolase assessed as AdoHcy hydrolysis activity by HPLC analysis |
Bioorg Med Chem Lett 24: 4336-40 (2014)
Article DOI: 10.1016/j.bmcl.2014.06.008
BindingDB Entry DOI: 10.7270/Q2377BCP |
More data for this
Ligand-Target Pair | |
Adenosylhomocysteinase
(Homo sapiens (Human)) | BDBM50050864
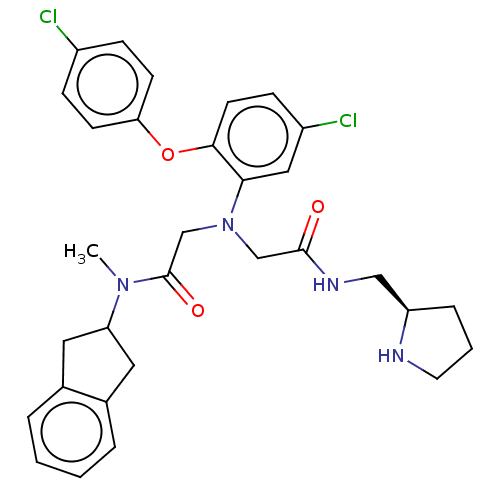
(CHEMBL3322560)Show SMILES CN(C1Cc2ccccc2C1)C(=O)CN(CC(=O)NC[C@H]1CCCN1)c1cc(Cl)ccc1Oc1ccc(Cl)cc1 |r| Show InChI InChI=1S/C31H34Cl2N4O3/c1-36(26-15-21-5-2-3-6-22(21)16-26)31(39)20-37(19-30(38)35-18-25-7-4-14-34-25)28-17-24(33)10-13-29(28)40-27-11-8-23(32)9-12-27/h2-3,5-6,8-13,17,25-26,34H,4,7,14-16,18-20H2,1H3,(H,35,38)/t25-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 600 | n/a | n/a | n/a | n/a | n/a | n/a |
Mitsubishi Tanabe Pharma Corporation
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant S-adenosyl-L-homocysteine hydrolase assessed as AdoHcy hydrolysis activity by HPLC analysis |
Bioorg Med Chem Lett 24: 4336-40 (2014)
Article DOI: 10.1016/j.bmcl.2014.06.008
BindingDB Entry DOI: 10.7270/Q2377BCP |
More data for this
Ligand-Target Pair | |
Adenosylhomocysteinase
(Homo sapiens (Human)) | BDBM50050878
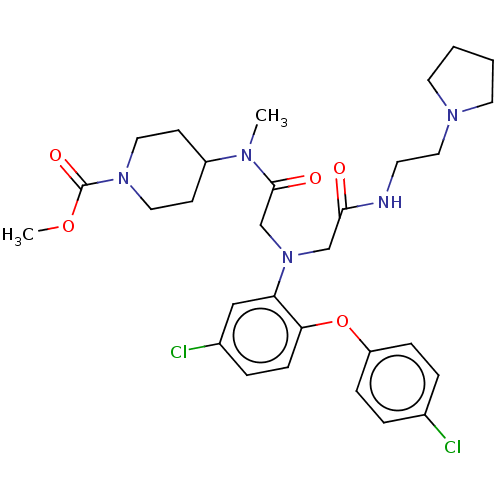
(CHEMBL3322546)Show SMILES COC(=O)N1CCC(CC1)N(C)C(=O)CN(CC(=O)NCCN1CCCC1)c1cc(Cl)ccc1Oc1ccc(Cl)cc1 Show InChI InChI=1S/C30H39Cl2N5O5/c1-34(24-11-16-36(17-12-24)30(40)41-2)29(39)21-37(20-28(38)33-13-18-35-14-3-4-15-35)26-19-23(32)7-10-27(26)42-25-8-5-22(31)6-9-25/h5-10,19,24H,3-4,11-18,20-21H2,1-2H3,(H,33,38) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 890 | n/a | n/a | n/a | n/a | n/a | n/a |
Mitsubishi Tanabe Pharma Corporation
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant S-adenosyl-L-homocysteine hydrolase assessed as AdoHcy hydrolysis activity by HPLC analysis |
Bioorg Med Chem Lett 24: 4336-40 (2014)
Article DOI: 10.1016/j.bmcl.2014.06.008
BindingDB Entry DOI: 10.7270/Q2377BCP |
More data for this
Ligand-Target Pair | |
Adenosylhomocysteinase
(Homo sapiens (Human)) | BDBM50050890
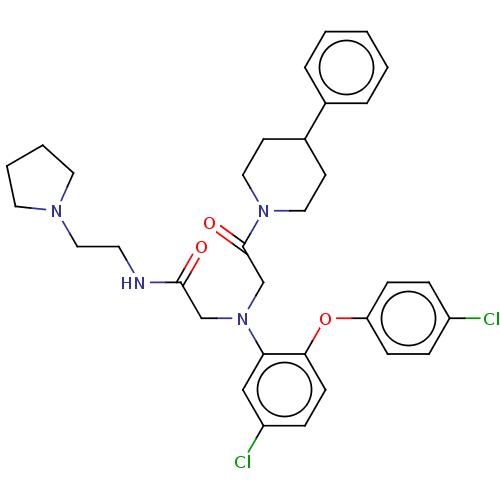
(CHEMBL3322535)Show SMILES Clc1ccc(Oc2ccc(Cl)cc2N(CC(=O)NCCN2CCCC2)CC(=O)N2CCC(CC2)c2ccccc2)cc1 Show InChI InChI=1S/C33H38Cl2N4O3/c34-27-8-11-29(12-9-27)42-31-13-10-28(35)22-30(31)39(23-32(40)36-16-21-37-17-4-5-18-37)24-33(41)38-19-14-26(15-20-38)25-6-2-1-3-7-25/h1-3,6-13,22,26H,4-5,14-21,23-24H2,(H,36,40) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 970 | n/a | n/a | n/a | n/a | n/a | n/a |
Mitsubishi Tanabe Pharma Corporation
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant S-adenosyl-L-homocysteine hydrolase assessed as AdoHcy hydrolysis activity by HPLC analysis |
Bioorg Med Chem Lett 24: 4336-40 (2014)
Article DOI: 10.1016/j.bmcl.2014.06.008
BindingDB Entry DOI: 10.7270/Q2377BCP |
More data for this
Ligand-Target Pair | |
Adenosylhomocysteinase
(Homo sapiens (Human)) | BDBM50050867
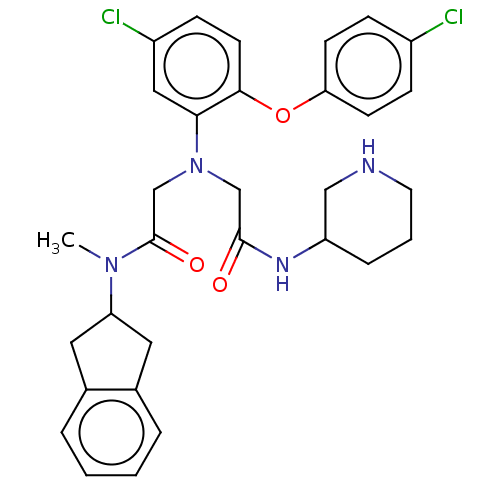
(CHEMBL3322557)Show SMILES CN(C1Cc2ccccc2C1)C(=O)CN(CC(=O)NC1CCCNC1)c1cc(Cl)ccc1Oc1ccc(Cl)cc1 Show InChI InChI=1S/C31H34Cl2N4O3/c1-36(26-15-21-5-2-3-6-22(21)16-26)31(39)20-37(19-30(38)35-25-7-4-14-34-18-25)28-17-24(33)10-13-29(28)40-27-11-8-23(32)9-12-27/h2-3,5-6,8-13,17,25-26,34H,4,7,14-16,18-20H2,1H3,(H,35,38) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Mitsubishi Tanabe Pharma Corporation
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant S-adenosyl-L-homocysteine hydrolase assessed as AdoHcy hydrolysis activity by HPLC analysis |
Bioorg Med Chem Lett 24: 4336-40 (2014)
Article DOI: 10.1016/j.bmcl.2014.06.008
BindingDB Entry DOI: 10.7270/Q2377BCP |
More data for this
Ligand-Target Pair | |
Adenosylhomocysteinase
(Homo sapiens (Human)) | BDBM50050866
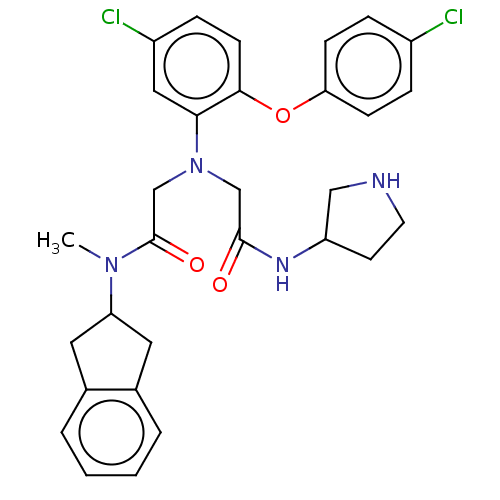
(CHEMBL3322558)Show SMILES CN(C1Cc2ccccc2C1)C(=O)CN(CC(=O)NC1CCNC1)c1cc(Cl)ccc1Oc1ccc(Cl)cc1 Show InChI InChI=1S/C30H32Cl2N4O3/c1-35(25-14-20-4-2-3-5-21(20)15-25)30(38)19-36(18-29(37)34-24-12-13-33-17-24)27-16-23(32)8-11-28(27)39-26-9-6-22(31)7-10-26/h2-11,16,24-25,33H,12-15,17-19H2,1H3,(H,34,37) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Mitsubishi Tanabe Pharma Corporation
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant S-adenosyl-L-homocysteine hydrolase assessed as AdoHcy hydrolysis activity by HPLC analysis |
Bioorg Med Chem Lett 24: 4336-40 (2014)
Article DOI: 10.1016/j.bmcl.2014.06.008
BindingDB Entry DOI: 10.7270/Q2377BCP |
More data for this
Ligand-Target Pair | |
Adenosylhomocysteinase
(Homo sapiens (Human)) | BDBM50050885

(CHEMBL3322539)Show SMILES CN(C(=O)CN(CC(=O)NCCN1CCCC1)c1cc(Cl)ccc1Oc1ccc(Cl)cc1)c1ccccc1 Show InChI InChI=1S/C29H32Cl2N4O3/c1-33(24-7-3-2-4-8-24)29(37)21-35(20-28(36)32-15-18-34-16-5-6-17-34)26-19-23(31)11-14-27(26)38-25-12-9-22(30)10-13-25/h2-4,7-14,19H,5-6,15-18,20-21H2,1H3,(H,32,36) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Mitsubishi Tanabe Pharma Corporation
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant S-adenosyl-L-homocysteine hydrolase assessed as AdoHcy hydrolysis activity by HPLC analysis |
Bioorg Med Chem Lett 24: 4336-40 (2014)
Article DOI: 10.1016/j.bmcl.2014.06.008
BindingDB Entry DOI: 10.7270/Q2377BCP |
More data for this
Ligand-Target Pair | |
Adenosylhomocysteinase
(Homo sapiens (Human)) | BDBM50050880
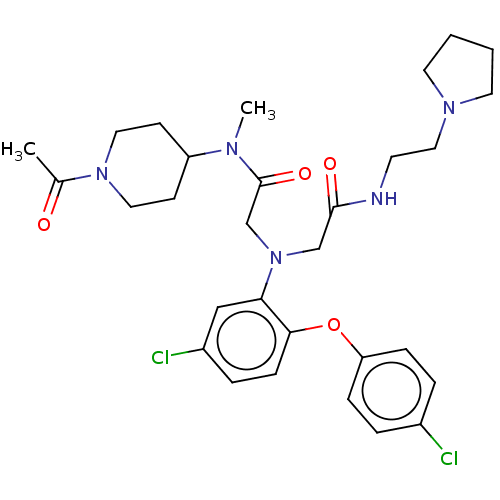
(CHEMBL3322544)Show SMILES CN(C1CCN(CC1)C(C)=O)C(=O)CN(CC(=O)NCCN1CCCC1)c1cc(Cl)ccc1Oc1ccc(Cl)cc1 Show InChI InChI=1S/C30H39Cl2N5O4/c1-22(38)36-16-11-25(12-17-36)34(2)30(40)21-37(20-29(39)33-13-18-35-14-3-4-15-35)27-19-24(32)7-10-28(27)41-26-8-5-23(31)6-9-26/h5-10,19,25H,3-4,11-18,20-21H2,1-2H3,(H,33,39) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Mitsubishi Tanabe Pharma Corporation
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant S-adenosyl-L-homocysteine hydrolase assessed as AdoHcy hydrolysis activity by HPLC analysis |
Bioorg Med Chem Lett 24: 4336-40 (2014)
Article DOI: 10.1016/j.bmcl.2014.06.008
BindingDB Entry DOI: 10.7270/Q2377BCP |
More data for this
Ligand-Target Pair | |
Adenosylhomocysteinase
(Homo sapiens (Human)) | BDBM50050889
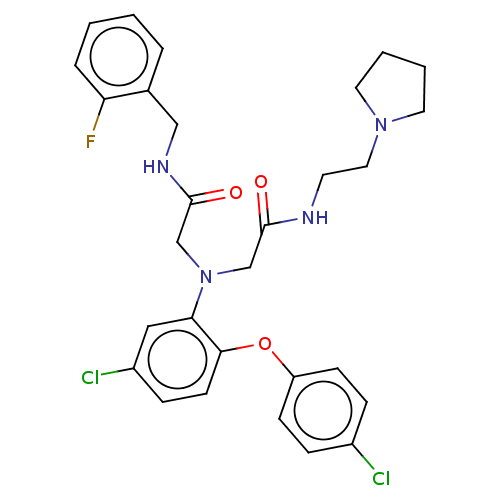
(CHEMBL3321765)Show SMILES Fc1ccccc1CNC(=O)CN(CC(=O)NCCN1CCCC1)c1cc(Cl)ccc1Oc1ccc(Cl)cc1 Show InChI InChI=1S/C29H31Cl2FN4O3/c30-22-7-10-24(11-8-22)39-27-12-9-23(31)17-26(27)36(19-28(37)33-13-16-35-14-3-4-15-35)20-29(38)34-18-21-5-1-2-6-25(21)32/h1-2,5-12,17H,3-4,13-16,18-20H2,(H,33,37)(H,34,38) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8.78E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Mitsubishi Tanabe Pharma Corporation
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant S-adenosyl-L-homocysteine hydrolase assessed as AdoHcy hydrolysis activity by HPLC analysis |
Bioorg Med Chem Lett 24: 4336-40 (2014)
Article DOI: 10.1016/j.bmcl.2014.06.008
BindingDB Entry DOI: 10.7270/Q2377BCP |
More data for this
Ligand-Target Pair | |
Adenosylhomocysteinase
(Homo sapiens (Human)) | BDBM50050888

(CHEMBL3322536)Show SMILES Clc1ccc(Oc2ccc(Cl)cc2N(CC(=O)NCCN2CCCC2)CC(=O)NC2Cc3ccccc3C2)cc1 Show InChI InChI=1S/C31H34Cl2N4O3/c32-24-7-10-27(11-8-24)40-29-12-9-25(33)19-28(29)37(20-30(38)34-13-16-36-14-3-4-15-36)21-31(39)35-26-17-22-5-1-2-6-23(22)18-26/h1-2,5-12,19,26H,3-4,13-18,20-21H2,(H,34,38)(H,35,39) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.14E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Mitsubishi Tanabe Pharma Corporation
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant S-adenosyl-L-homocysteine hydrolase assessed as AdoHcy hydrolysis activity by HPLC analysis |
Bioorg Med Chem Lett 24: 4336-40 (2014)
Article DOI: 10.1016/j.bmcl.2014.06.008
BindingDB Entry DOI: 10.7270/Q2377BCP |
More data for this
Ligand-Target Pair | |
Adenosylhomocysteinase
(Homo sapiens (Human)) | BDBM50050886
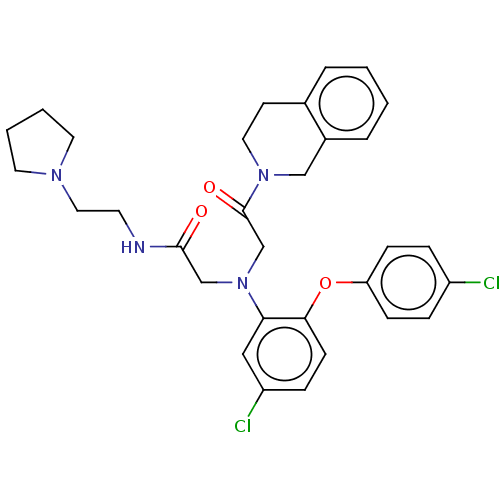
(CHEMBL3322538)Show SMILES Clc1ccc(Oc2ccc(Cl)cc2N(CC(=O)NCCN2CCCC2)CC(=O)N2CCc3ccccc3C2)cc1 Show InChI InChI=1S/C31H34Cl2N4O3/c32-25-7-10-27(11-8-25)40-29-12-9-26(33)19-28(29)37(21-30(38)34-14-18-35-15-3-4-16-35)22-31(39)36-17-13-23-5-1-2-6-24(23)20-36/h1-2,5-12,19H,3-4,13-18,20-22H2,(H,34,38) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Mitsubishi Tanabe Pharma Corporation
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant S-adenosyl-L-homocysteine hydrolase assessed as AdoHcy hydrolysis activity by HPLC analysis |
Bioorg Med Chem Lett 24: 4336-40 (2014)
Article DOI: 10.1016/j.bmcl.2014.06.008
BindingDB Entry DOI: 10.7270/Q2377BCP |
More data for this
Ligand-Target Pair | |
Adenosylhomocysteinase
(Homo sapiens (Human)) | BDBM50050881
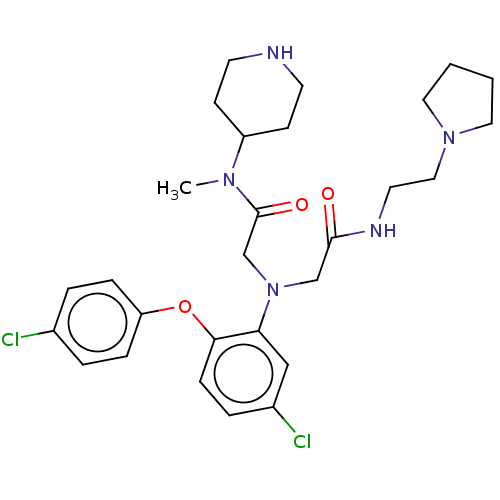
(CHEMBL3322543)Show SMILES CN(C1CCNCC1)C(=O)CN(CC(=O)NCCN1CCCC1)c1cc(Cl)ccc1Oc1ccc(Cl)cc1 Show InChI InChI=1S/C28H37Cl2N5O3/c1-33(23-10-12-31-13-11-23)28(37)20-35(19-27(36)32-14-17-34-15-2-3-16-34)25-18-22(30)6-9-26(25)38-24-7-4-21(29)5-8-24/h4-9,18,23,31H,2-3,10-17,19-20H2,1H3,(H,32,36) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Mitsubishi Tanabe Pharma Corporation
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant S-adenosyl-L-homocysteine hydrolase assessed as AdoHcy hydrolysis activity by HPLC analysis |
Bioorg Med Chem Lett 24: 4336-40 (2014)
Article DOI: 10.1016/j.bmcl.2014.06.008
BindingDB Entry DOI: 10.7270/Q2377BCP |
More data for this
Ligand-Target Pair | |
Adenosylhomocysteinase
(Homo sapiens (Human)) | BDBM50050887
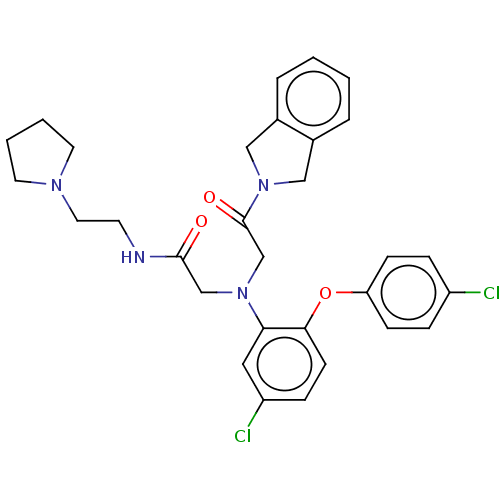
(CHEMBL3322537)Show SMILES Clc1ccc(Oc2ccc(Cl)cc2N(CC(=O)NCCN2CCCC2)CC(=O)N2Cc3ccccc3C2)cc1 Show InChI InChI=1S/C30H32Cl2N4O3/c31-24-7-10-26(11-8-24)39-28-12-9-25(32)17-27(28)35(20-29(37)33-13-16-34-14-3-4-15-34)21-30(38)36-18-22-5-1-2-6-23(22)19-36/h1-2,5-12,17H,3-4,13-16,18-21H2,(H,33,37) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Mitsubishi Tanabe Pharma Corporation
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant S-adenosyl-L-homocysteine hydrolase assessed as AdoHcy hydrolysis activity by HPLC analysis |
Bioorg Med Chem Lett 24: 4336-40 (2014)
Article DOI: 10.1016/j.bmcl.2014.06.008
BindingDB Entry DOI: 10.7270/Q2377BCP |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data