

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sodium-dependent serotonin transporter (Rattus norvegicus (rat)) | BDBM50295066 ((2S)-8-Methyl-2-{[4-(6-nitroquinolin-2-yl)piperazi...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research Curated by ChEMBL | Assay Description Displacement of [3H]paroxetine from 5HT transporter in rat cortical membrane | J Med Chem 52: 4955-9 (2009) Article DOI: 10.1021/jm900374r BindingDB Entry DOI: 10.7270/Q2CZ376D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Rattus norvegicus (rat)) | BDBM50295064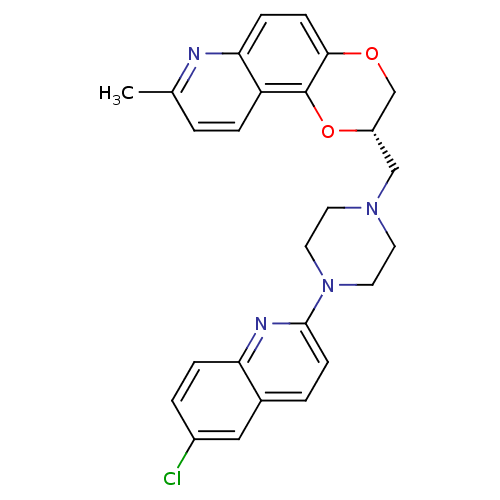 ((2S)-2-{[4-(6-Chloroquinolin-2-yl)piperazin-1-yl]m...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.880 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research Curated by ChEMBL | Assay Description Displacement of [3H]paroxetine from 5HT transporter in rat cortical membrane | J Med Chem 52: 4955-9 (2009) Article DOI: 10.1021/jm900374r BindingDB Entry DOI: 10.7270/Q2CZ376D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM86708 (CAS_146714-97-8 | CHEMBL31354 | CHEMBL514874 | CHE...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research Curated by ChEMBL | Assay Description Displacement of [3H]8-OH-DPAT from human 5HT1A receptor expressed in CHO cells | J Med Chem 52: 4955-9 (2009) Article DOI: 10.1021/jm900374r BindingDB Entry DOI: 10.7270/Q2CZ376D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Rattus norvegicus (rat)) | BDBM50295055 (2-(4-{[(2S)-8-Methyl-2,3-dihydro[1,4]dioxino[2,3-f...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research Curated by ChEMBL | Assay Description Displacement of [3H]paroxetine from 5HT transporter in rat cortical membrane | J Med Chem 52: 4955-9 (2009) Article DOI: 10.1021/jm900374r BindingDB Entry DOI: 10.7270/Q2CZ376D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50295054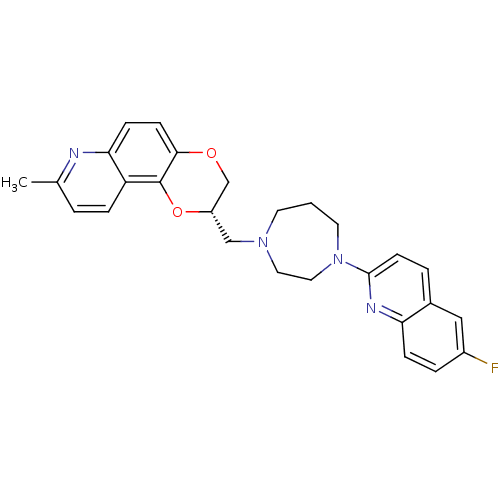 ((2S)-2-{[4-(6-Fluoroquinolin-2-yl)-1,4-diazepan-1-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research Curated by ChEMBL | Assay Description Displacement of [3H]8-OH-DPAT from human 5HT1A receptor expressed in CHO cells | J Med Chem 52: 4955-9 (2009) Article DOI: 10.1021/jm900374r BindingDB Entry DOI: 10.7270/Q2CZ376D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50295055 (2-(4-{[(2S)-8-Methyl-2,3-dihydro[1,4]dioxino[2,3-f...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research Curated by ChEMBL | Assay Description Displacement of [3H]8-OH-DPAT from human 5HT1A receptor expressed in CHO cells | J Med Chem 52: 4955-9 (2009) Article DOI: 10.1021/jm900374r BindingDB Entry DOI: 10.7270/Q2CZ376D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Rattus norvegicus (rat)) | BDBM50295053 ((S)-2-((4-(1H-Indol-3-yl)-5,6-dihydropyridin-1(2H)...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | 2.34 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research Curated by ChEMBL | Assay Description Displacement of [3H]paroxetine from 5HT transporter in rat cortical membrane | J Med Chem 52: 4955-9 (2009) Article DOI: 10.1021/jm900374r BindingDB Entry DOI: 10.7270/Q2CZ376D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Rattus norvegicus (rat)) | BDBM30130 (CHEMBL1201082 | CHEMBL41 | Fluoxetin | Fluoxetine ...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 2.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research Curated by ChEMBL | Assay Description Displacement of [3H]paroxetine from 5HT transporter in rat cortical membrane | J Med Chem 52: 4955-9 (2009) Article DOI: 10.1021/jm900374r BindingDB Entry DOI: 10.7270/Q2CZ376D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50295057 (2-((3R)-3-Methyl-4-{[(2S)-8-methyl-2,3-dihydro[1,4...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research Curated by ChEMBL | Assay Description Displacement of [3H]8-OH-DPAT from human 5HT1A receptor expressed in CHO cells | J Med Chem 52: 4955-9 (2009) Article DOI: 10.1021/jm900374r BindingDB Entry DOI: 10.7270/Q2CZ376D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50295053 ((S)-2-((4-(1H-Indol-3-yl)-5,6-dihydropyridin-1(2H)...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | 3.02 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research Curated by ChEMBL | Assay Description Displacement of [3H]8-OH-DPAT from human 5HT1A receptor expressed in CHO cells | J Med Chem 52: 4955-9 (2009) Article DOI: 10.1021/jm900374r BindingDB Entry DOI: 10.7270/Q2CZ376D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50295052 (2-(4-{[(2S)-8-Methyl-2,3-dihydro[1,4]dioxino[2,3-f...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research Curated by ChEMBL | Assay Description Displacement of [3H]8-OH-DPAT from human 5HT1A receptor expressed in CHO cells | J Med Chem 52: 4955-9 (2009) Article DOI: 10.1021/jm900374r BindingDB Entry DOI: 10.7270/Q2CZ376D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Rattus norvegicus (rat)) | BDBM50295054 ((2S)-2-{[4-(6-Fluoroquinolin-2-yl)-1,4-diazepan-1-...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 3.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research Curated by ChEMBL | Assay Description Displacement of [3H]paroxetine from 5HT transporter in rat cortical membrane | J Med Chem 52: 4955-9 (2009) Article DOI: 10.1021/jm900374r BindingDB Entry DOI: 10.7270/Q2CZ376D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50295058 ((2S)-8-Methyl-2-{[(2R)-2-methyl-4-quinolin-2-ylpip...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 5.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research Curated by ChEMBL | Assay Description Displacement of [3H]8-OH-DPAT from human 5HT1A receptor expressed in CHO cells | J Med Chem 52: 4955-9 (2009) Article DOI: 10.1021/jm900374r BindingDB Entry DOI: 10.7270/Q2CZ376D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50295056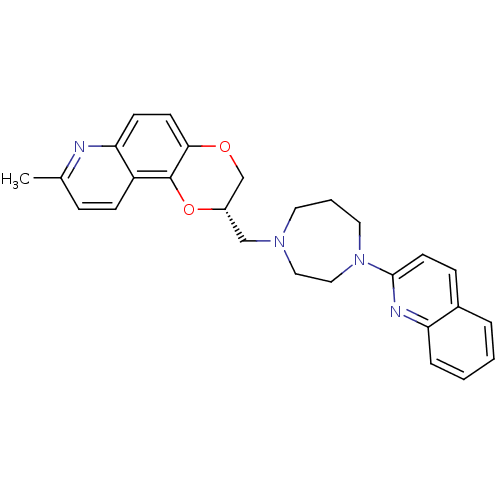 ((2S)-8-Methyl-2-[(4-quinolin-2-yl-1,4-diazepan-1-y...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 6.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research Curated by ChEMBL | Assay Description Displacement of [3H]8-OH-DPAT from human 5HT1A receptor expressed in CHO cells | J Med Chem 52: 4955-9 (2009) Article DOI: 10.1021/jm900374r BindingDB Entry DOI: 10.7270/Q2CZ376D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50295062 (2-(4-{[(2S)-8-Methyl-2,3-dihydro[1,4]dioxino[2,3-f...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 7.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research Curated by ChEMBL | Assay Description Displacement of [3H]8-OH-DPAT from human 5HT1A receptor expressed in CHO cells | J Med Chem 52: 4955-9 (2009) Article DOI: 10.1021/jm900374r BindingDB Entry DOI: 10.7270/Q2CZ376D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Rattus norvegicus (rat)) | BDBM50295056 ((2S)-8-Methyl-2-[(4-quinolin-2-yl-1,4-diazepan-1-y...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 7.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research Curated by ChEMBL | Assay Description Displacement of [3H]paroxetine from 5HT transporter in rat cortical membrane | J Med Chem 52: 4955-9 (2009) Article DOI: 10.1021/jm900374r BindingDB Entry DOI: 10.7270/Q2CZ376D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Rattus norvegicus (rat)) | BDBM50295059 ((2S)-8-Methyl-2-{[(2S)-2-methyl-4-quinolin-2-ylpip...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 10.3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research Curated by ChEMBL | Assay Description Displacement of [3H]paroxetine from 5HT transporter in rat cortical membrane | J Med Chem 52: 4955-9 (2009) Article DOI: 10.1021/jm900374r BindingDB Entry DOI: 10.7270/Q2CZ376D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Rattus norvegicus (rat)) | BDBM50295063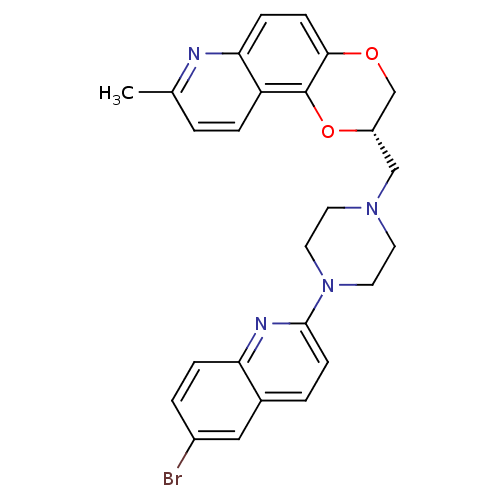 ((2S)-2-{[4-(6-Bromoquinolin-2-yl)piperazin-1-yl]me...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 12.7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research Curated by ChEMBL | Assay Description Displacement of [3H]paroxetine from 5HT transporter in rat cortical membrane | J Med Chem 52: 4955-9 (2009) Article DOI: 10.1021/jm900374r BindingDB Entry DOI: 10.7270/Q2CZ376D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Rattus norvegicus (rat)) | BDBM50295052 (2-(4-{[(2S)-8-Methyl-2,3-dihydro[1,4]dioxino[2,3-f...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 13.8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research Curated by ChEMBL | Assay Description Displacement of [3H]paroxetine from 5HT transporter in rat cortical membrane | J Med Chem 52: 4955-9 (2009) Article DOI: 10.1021/jm900374r BindingDB Entry DOI: 10.7270/Q2CZ376D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Rattus norvegicus (rat)) | BDBM50295062 (2-(4-{[(2S)-8-Methyl-2,3-dihydro[1,4]dioxino[2,3-f...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 17 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research Curated by ChEMBL | Assay Description Displacement of [3H]paroxetine from 5HT transporter in rat cortical membrane | J Med Chem 52: 4955-9 (2009) Article DOI: 10.1021/jm900374r BindingDB Entry DOI: 10.7270/Q2CZ376D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50295063 ((2S)-2-{[4-(6-Bromoquinolin-2-yl)piperazin-1-yl]me...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 17.2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research Curated by ChEMBL | Assay Description Displacement of [3H]8-OH-DPAT from human 5HT1A receptor expressed in CHO cells | J Med Chem 52: 4955-9 (2009) Article DOI: 10.1021/jm900374r BindingDB Entry DOI: 10.7270/Q2CZ376D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50295066 ((2S)-8-Methyl-2-{[4-(6-nitroquinolin-2-yl)piperazi...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research Curated by ChEMBL | Assay Description Displacement of [3H]8-OH-DPAT from human 5HT1A receptor expressed in CHO cells | J Med Chem 52: 4955-9 (2009) Article DOI: 10.1021/jm900374r BindingDB Entry DOI: 10.7270/Q2CZ376D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Rattus norvegicus (rat)) | BDBM50295058 ((2S)-8-Methyl-2-{[(2R)-2-methyl-4-quinolin-2-ylpip...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 32.8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research Curated by ChEMBL | Assay Description Displacement of [3H]paroxetine from 5HT transporter in rat cortical membrane | J Med Chem 52: 4955-9 (2009) Article DOI: 10.1021/jm900374r BindingDB Entry DOI: 10.7270/Q2CZ376D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50295065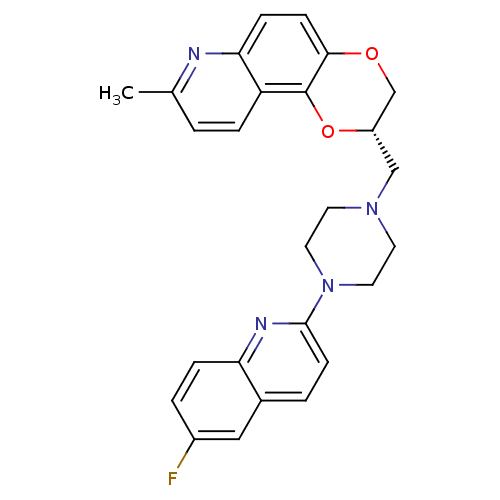 ((2S)-2-{[4-(6-Fluoroquinolin-2-yl)piperazin-1-yl]m...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 32.9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research Curated by ChEMBL | Assay Description Displacement of [3H]8-OH-DPAT from human 5HT1A receptor expressed in CHO cells | J Med Chem 52: 4955-9 (2009) Article DOI: 10.1021/jm900374r BindingDB Entry DOI: 10.7270/Q2CZ376D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50295060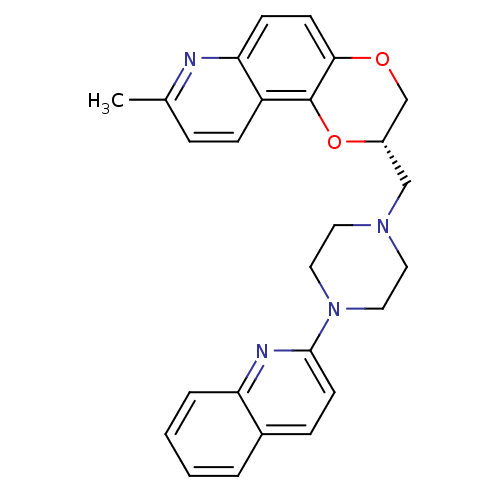 ((2S)-8-Methyl-2-[(4-quinolin-2-ylpiperazin-1-yl)me...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 47.3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research Curated by ChEMBL | Assay Description Displacement of [3H]8-OH-DPAT from human 5HT1A receptor expressed in CHO cells | J Med Chem 52: 4955-9 (2009) Article DOI: 10.1021/jm900374r BindingDB Entry DOI: 10.7270/Q2CZ376D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Rattus norvegicus (rat)) | BDBM50295065 ((2S)-2-{[4-(6-Fluoroquinolin-2-yl)piperazin-1-yl]m...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 66 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research Curated by ChEMBL | Assay Description Displacement of [3H]paroxetine from 5HT transporter in rat cortical membrane | J Med Chem 52: 4955-9 (2009) Article DOI: 10.1021/jm900374r BindingDB Entry DOI: 10.7270/Q2CZ376D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Rattus norvegicus (rat)) | BDBM50295060 ((2S)-8-Methyl-2-[(4-quinolin-2-ylpiperazin-1-yl)me...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 75 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research Curated by ChEMBL | Assay Description Displacement of [3H]paroxetine from 5HT transporter in rat cortical membrane | J Med Chem 52: 4955-9 (2009) Article DOI: 10.1021/jm900374r BindingDB Entry DOI: 10.7270/Q2CZ376D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Rattus norvegicus (rat)) | BDBM50295057 (2-((3R)-3-Methyl-4-{[(2S)-8-methyl-2,3-dihydro[1,4...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 89.6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research Curated by ChEMBL | Assay Description Displacement of [3H]paroxetine from 5HT transporter in rat cortical membrane | J Med Chem 52: 4955-9 (2009) Article DOI: 10.1021/jm900374r BindingDB Entry DOI: 10.7270/Q2CZ376D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Rattus norvegicus (rat)) | BDBM50295061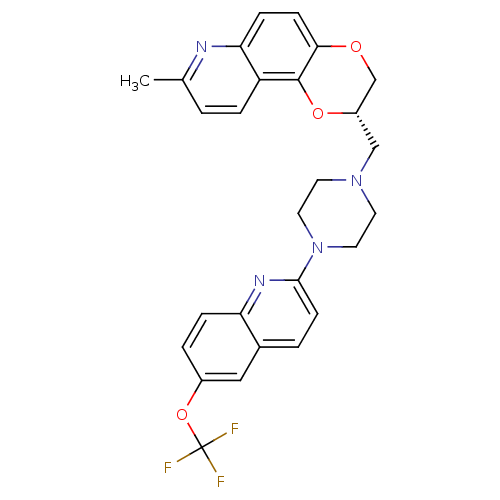 ((2S)-8-Methyl-2-({4-[6-(trifluoromethoxy)quinolin-...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 149 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research Curated by ChEMBL | Assay Description Displacement of [3H]paroxetine from 5HT transporter in rat cortical membrane | J Med Chem 52: 4955-9 (2009) Article DOI: 10.1021/jm900374r BindingDB Entry DOI: 10.7270/Q2CZ376D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50295059 ((2S)-8-Methyl-2-{[(2S)-2-methyl-4-quinolin-2-ylpip...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 167 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research Curated by ChEMBL | Assay Description Displacement of [3H]8-OH-DPAT from human 5HT1A receptor expressed in CHO cells | J Med Chem 52: 4955-9 (2009) Article DOI: 10.1021/jm900374r BindingDB Entry DOI: 10.7270/Q2CZ376D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50295061 ((2S)-8-Methyl-2-({4-[6-(trifluoromethoxy)quinolin-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 172 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research Curated by ChEMBL | Assay Description Displacement of [3H]8-OH-DPAT from human 5HT1A receptor expressed in CHO cells | J Med Chem 52: 4955-9 (2009) Article DOI: 10.1021/jm900374r BindingDB Entry DOI: 10.7270/Q2CZ376D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50295064 ((2S)-2-{[4-(6-Chloroquinolin-2-yl)piperazin-1-yl]m...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 214 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research Curated by ChEMBL | Assay Description Displacement of [3H]8-OH-DPAT from human 5HT1A receptor expressed in CHO cells | J Med Chem 52: 4955-9 (2009) Article DOI: 10.1021/jm900374r BindingDB Entry DOI: 10.7270/Q2CZ376D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM50295052 (2-(4-{[(2S)-8-Methyl-2,3-dihydro[1,4]dioxino[2,3-f...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.31E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research Curated by ChEMBL | Assay Description Displacement of [3H]spiperone from human dopamine D2 receptor expressed in CHO cells | J Med Chem 52: 4955-9 (2009) Article DOI: 10.1021/jm900374r BindingDB Entry DOI: 10.7270/Q2CZ376D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50295052 (2-(4-{[(2S)-8-Methyl-2,3-dihydro[1,4]dioxino[2,3-f...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.32E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research Curated by ChEMBL | Assay Description Displacement of [3H]spiperone from human dopamine D3 receptor expressed in CHO cells | J Med Chem 52: 4955-9 (2009) Article DOI: 10.1021/jm900374r BindingDB Entry DOI: 10.7270/Q2CZ376D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(4) dopamine receptor (Homo sapiens (Human)) | BDBM50295052 (2-(4-{[(2S)-8-Methyl-2,3-dihydro[1,4]dioxino[2,3-f...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.42E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research Curated by ChEMBL | Assay Description Displacement of [3H]spiperone from human dopamine D4 receptor expressed in CHO cells | J Med Chem 52: 4955-9 (2009) Article DOI: 10.1021/jm900374r BindingDB Entry DOI: 10.7270/Q2CZ376D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50295053 ((S)-2-((4-(1H-Indol-3-yl)-5,6-dihydropyridin-1(2H)...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 56.7 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research Curated by ChEMBL | Assay Description Antagonist activity at human 5HT1A receptor expressed in CHO cells assessed as stimulation of [35S]GTPgammaS binding | J Med Chem 52: 4955-9 (2009) Article DOI: 10.1021/jm900374r BindingDB Entry DOI: 10.7270/Q2CZ376D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50295055 (2-(4-{[(2S)-8-Methyl-2,3-dihydro[1,4]dioxino[2,3-f...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 58 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research Curated by ChEMBL | Assay Description Antagonist activity at human 5HT1A receptor expressed in CHO cells assessed as stimulation of [35S]GTPgammaS binding | J Med Chem 52: 4955-9 (2009) Article DOI: 10.1021/jm900374r BindingDB Entry DOI: 10.7270/Q2CZ376D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50295054 ((2S)-2-{[4-(6-Fluoroquinolin-2-yl)-1,4-diazepan-1-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 70 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research Curated by ChEMBL | Assay Description Antagonist activity at human 5HT1A receptor expressed in CHO cells assessed as stimulation of [35S]GTPgammaS binding | J Med Chem 52: 4955-9 (2009) Article DOI: 10.1021/jm900374r BindingDB Entry DOI: 10.7270/Q2CZ376D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50295056 ((2S)-8-Methyl-2-[(4-quinolin-2-yl-1,4-diazepan-1-y...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 70 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research Curated by ChEMBL | Assay Description Antagonist activity at human 5HT1A receptor expressed in CHO cells assessed as stimulation of [35S]GTPgammaS binding | J Med Chem 52: 4955-9 (2009) Article DOI: 10.1021/jm900374r BindingDB Entry DOI: 10.7270/Q2CZ376D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50295052 (2-(4-{[(2S)-8-Methyl-2,3-dihydro[1,4]dioxino[2,3-f...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 102 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research Curated by ChEMBL | Assay Description Antagonist activity at human 5HT1A receptor expressed in CHO cells assessed as stimulation of [35S]GTPgammaS binding | J Med Chem 52: 4955-9 (2009) Article DOI: 10.1021/jm900374r BindingDB Entry DOI: 10.7270/Q2CZ376D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50295058 ((2S)-8-Methyl-2-{[(2R)-2-methyl-4-quinolin-2-ylpip...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 117 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research Curated by ChEMBL | Assay Description Antagonist activity at human 5HT1A receptor expressed in CHO cells assessed as stimulation of [35S]GTPgammaS binding | J Med Chem 52: 4955-9 (2009) Article DOI: 10.1021/jm900374r BindingDB Entry DOI: 10.7270/Q2CZ376D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Homo sapiens (Human)) | BDBM50295053 ((S)-2-((4-(1H-Indol-3-yl)-5,6-dihydropyridin-1(2H)...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 145 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research Curated by ChEMBL | Assay Description Antagonist activity at human 5HT transporter expressed in staurosporine treated human JAR cells | J Med Chem 52: 4955-9 (2009) Article DOI: 10.1021/jm900374r BindingDB Entry DOI: 10.7270/Q2CZ376D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50295063 ((2S)-2-{[4-(6-Bromoquinolin-2-yl)piperazin-1-yl]me...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 156 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research Curated by ChEMBL | Assay Description Antagonist activity at human 5HT1A receptor expressed in CHO cells assessed as stimulation of [35S]GTPgammaS binding | J Med Chem 52: 4955-9 (2009) Article DOI: 10.1021/jm900374r BindingDB Entry DOI: 10.7270/Q2CZ376D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50295062 (2-(4-{[(2S)-8-Methyl-2,3-dihydro[1,4]dioxino[2,3-f...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 164 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research Curated by ChEMBL | Assay Description Antagonist activity at human 5HT1A receptor expressed in CHO cells assessed as stimulation of [35S]GTPgammaS binding | J Med Chem 52: 4955-9 (2009) Article DOI: 10.1021/jm900374r BindingDB Entry DOI: 10.7270/Q2CZ376D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50295057 (2-((3R)-3-Methyl-4-{[(2S)-8-methyl-2,3-dihydro[1,4...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 180 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research Curated by ChEMBL | Assay Description Antagonist activity at human 5HT1A receptor expressed in CHO cells assessed as stimulation of [35S]GTPgammaS binding | J Med Chem 52: 4955-9 (2009) Article DOI: 10.1021/jm900374r BindingDB Entry DOI: 10.7270/Q2CZ376D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50295066 ((2S)-8-Methyl-2-{[4-(6-nitroquinolin-2-yl)piperazi...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 294 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research Curated by ChEMBL | Assay Description Antagonist activity at human 5HT1A receptor expressed in CHO cells assessed as stimulation of [35S]GTPgammaS binding | J Med Chem 52: 4955-9 (2009) Article DOI: 10.1021/jm900374r BindingDB Entry DOI: 10.7270/Q2CZ376D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50295061 ((2S)-8-Methyl-2-({4-[6-(trifluoromethoxy)quinolin-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 588 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research Curated by ChEMBL | Assay Description Antagonist activity at human 5HT1A receptor expressed in CHO cells assessed as stimulation of [35S]GTPgammaS binding | J Med Chem 52: 4955-9 (2009) Article DOI: 10.1021/jm900374r BindingDB Entry DOI: 10.7270/Q2CZ376D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50295064 ((2S)-2-{[4-(6-Chloroquinolin-2-yl)piperazin-1-yl]m...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 781 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research Curated by ChEMBL | Assay Description Antagonist activity at human 5HT1A receptor expressed in CHO cells assessed as stimulation of [35S]GTPgammaS binding | J Med Chem 52: 4955-9 (2009) Article DOI: 10.1021/jm900374r BindingDB Entry DOI: 10.7270/Q2CZ376D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50295059 ((2S)-8-Methyl-2-{[(2S)-2-methyl-4-quinolin-2-ylpip...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research Curated by ChEMBL | Assay Description Antagonist activity at human 5HT1A receptor expressed in CHO cells assessed as stimulation of [35S]GTPgammaS binding | J Med Chem 52: 4955-9 (2009) Article DOI: 10.1021/jm900374r BindingDB Entry DOI: 10.7270/Q2CZ376D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50295065 ((2S)-2-{[4-(6-Fluoroquinolin-2-yl)piperazin-1-yl]m...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.17E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research Curated by ChEMBL | Assay Description Antagonist activity at human 5HT1A receptor expressed in CHO cells assessed as stimulation of [35S]GTPgammaS binding | J Med Chem 52: 4955-9 (2009) Article DOI: 10.1021/jm900374r BindingDB Entry DOI: 10.7270/Q2CZ376D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 55 total ) | Next | Last >> |