

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Oxytocin receptor (Homo sapiens (Human)) | BDBM50326716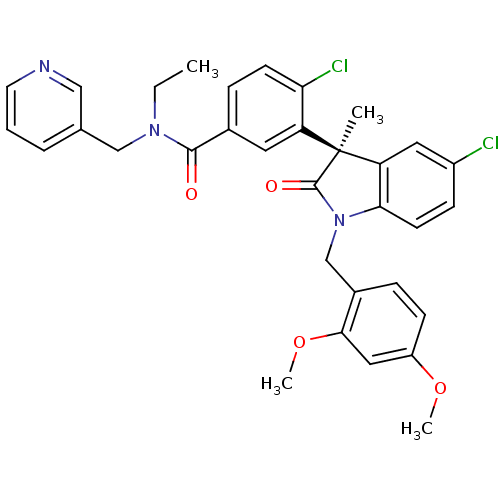 ((R)-4-chloro-3-(5-chloro-1-(2,4-dimethoxybenzyl)-3...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DrugMolDesign Curated by ChEMBL | Assay Description Displacement of [3H]-oxytocin from human oxytocin receptor expressed in CHO cells | J Med Chem 53: 6525-38 (2010) Article DOI: 10.1021/jm901812z BindingDB Entry DOI: 10.7270/Q20R9PMZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxytocin receptor (Homo sapiens (Human)) | BDBM50326714 ((3R,6R)-6-sec-butyl-3-(2,3-dihydro-1H-inden-2-yl)-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.630 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DrugMolDesign Curated by ChEMBL | Assay Description Displacement of [3H]-oxytocin from oxytocin receptor in human uterus tissue | J Med Chem 53: 6525-38 (2010) Article DOI: 10.1021/jm901812z BindingDB Entry DOI: 10.7270/Q20R9PMZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxytocin receptor (Homo sapiens (Human)) | BDBM50326722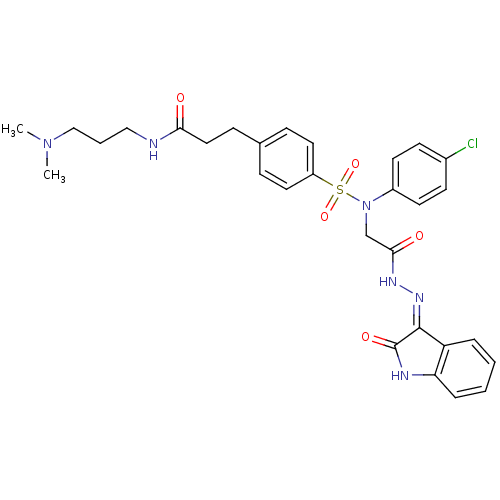 ((Z)-3-(4-(N-(4-chlorophenyl)-N-(2-oxo-2-(2-(2-oxoi...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.650 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DrugMolDesign Curated by ChEMBL | Assay Description Displacement of [3H]-oxytocin from human oxytocin receptor expressed in HEK293-EBNA cells | J Med Chem 53: 6525-38 (2010) Article DOI: 10.1021/jm901812z BindingDB Entry DOI: 10.7270/Q20R9PMZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V1a receptor (Homo sapiens (Human)) | BDBM50326722 ((Z)-3-(4-(N-(4-chlorophenyl)-N-(2-oxo-2-(2-(2-oxoi...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.650 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DrugMolDesign Curated by ChEMBL | Assay Description Displacement of [3H]-vasopressin from vasopressin V1a receptor in human liver tissue | J Med Chem 53: 6525-38 (2010) Article DOI: 10.1021/jm901812z BindingDB Entry DOI: 10.7270/Q20R9PMZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxytocin receptor (RAT) | BDBM50326722 ((Z)-3-(4-(N-(4-chlorophenyl)-N-(2-oxo-2-(2-(2-oxoi...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.670 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DrugMolDesign Curated by ChEMBL | Assay Description Displacement of [3H]-oxytocin from rat oxytocin receptor expressed in HEK293-EBNA cells | J Med Chem 53: 6525-38 (2010) Article DOI: 10.1021/jm901812z BindingDB Entry DOI: 10.7270/Q20R9PMZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V2 receptor (Homo sapiens (Human)) | BDBM50326722 ((Z)-3-(4-(N-(4-chlorophenyl)-N-(2-oxo-2-(2-(2-oxoi...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.670 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DrugMolDesign Curated by ChEMBL | Assay Description Displacement of [3H]-vasopressin from vasopressin V2 receptor in human kidney tissue | J Med Chem 53: 6525-38 (2010) Article DOI: 10.1021/jm901812z BindingDB Entry DOI: 10.7270/Q20R9PMZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxytocin receptor (RAT) | BDBM50326717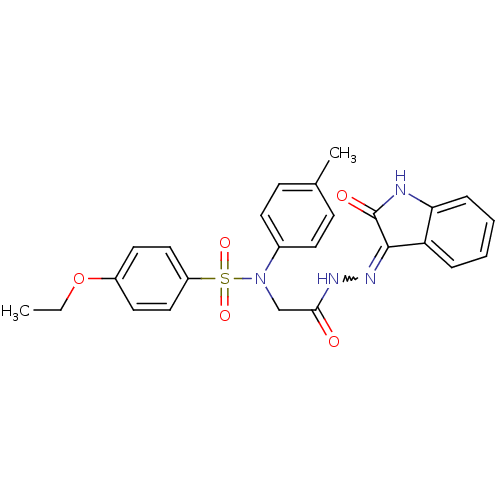 (4-ethoxy-N-(2-oxo-2-(2-(2-oxoindolin-3-ylidene)hyd...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.670 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DrugMolDesign Curated by ChEMBL | Assay Description Displacement of [3H]-oxytocin from rat oxytocin receptor expressed in HEK293-EBNA cells | J Med Chem 53: 6525-38 (2010) Article DOI: 10.1021/jm901812z BindingDB Entry DOI: 10.7270/Q20R9PMZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V1a receptor (RAT) | BDBM50064711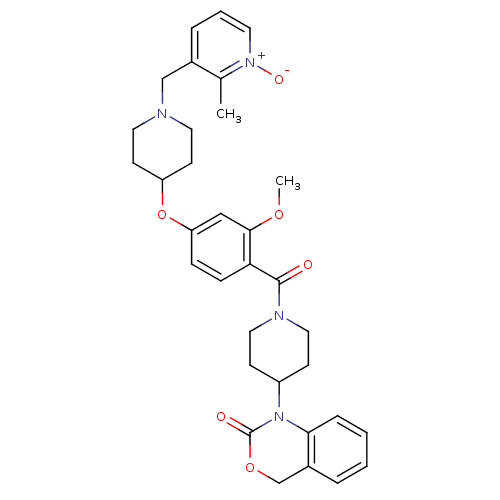 (1-(1-{2-Methoxy-4-[1-(2-methyl-1-oxy-pyridin-3-ylm...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 0.760 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DrugMolDesign Curated by ChEMBL | Assay Description Displacement of [3H]-vasopressin from vasopressin V1a receptor in rat liver tissue | J Med Chem 53: 6525-38 (2010) Article DOI: 10.1021/jm901812z BindingDB Entry DOI: 10.7270/Q20R9PMZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxytocin receptor (Homo sapiens (Human)) | BDBM50372611 (CHEMBL196478) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.26 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DrugMolDesign Curated by ChEMBL | Assay Description Displacement of [3H]-oxytocin from human oxytocin receptor | J Med Chem 53: 6525-38 (2010) Article DOI: 10.1021/jm901812z BindingDB Entry DOI: 10.7270/Q20R9PMZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxytocin receptor (RAT) | BDBM50326716 ((R)-4-chloro-3-(5-chloro-1-(2,4-dimethoxybenzyl)-3...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DrugMolDesign Curated by ChEMBL | Assay Description Displacement of [3H]-oxytocin from rat oxytocin receptor expressed in CHO cells | J Med Chem 53: 6525-38 (2010) Article DOI: 10.1021/jm901812z BindingDB Entry DOI: 10.7270/Q20R9PMZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxytocin receptor (Homo sapiens (Human)) | BDBM50113050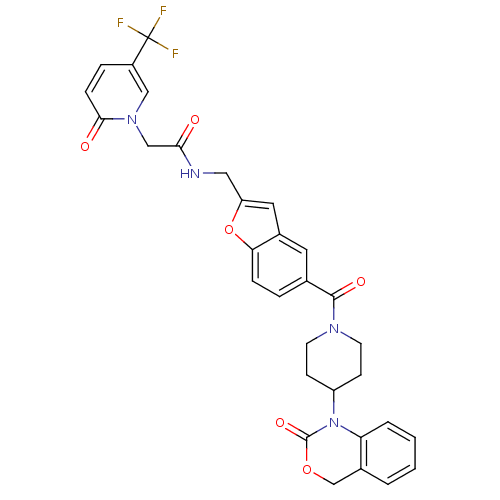 (CHEMBL31065 | N-{5-[4-(2-Oxo-4H-benzo[d][1,3]oxazi...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DrugMolDesign Curated by ChEMBL | Assay Description Displacement of [3H]-oxytocin from human oxytocin receptor expressed in CHO cells | J Med Chem 53: 6525-38 (2010) Article DOI: 10.1021/jm901812z BindingDB Entry DOI: 10.7270/Q20R9PMZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxytocin receptor (Homo sapiens (Human)) | BDBM50113050 (CHEMBL31065 | N-{5-[4-(2-Oxo-4H-benzo[d][1,3]oxazi...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DrugMolDesign Curated by ChEMBL | Assay Description Displacement of [3H]-oxytocin from oxytocin receptor in human uterus tissue | J Med Chem 53: 6525-38 (2010) Article DOI: 10.1021/jm901812z BindingDB Entry DOI: 10.7270/Q20R9PMZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxytocin receptor (Homo sapiens (Human)) | BDBM50416840 (CHEMBL196603) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DrugMolDesign Curated by ChEMBL | Assay Description Displacement of [3H]-oxytocin from human oxytocin receptor | J Med Chem 53: 6525-38 (2010) Article DOI: 10.1021/jm901812z BindingDB Entry DOI: 10.7270/Q20R9PMZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxytocin receptor (RAT) | BDBM50326719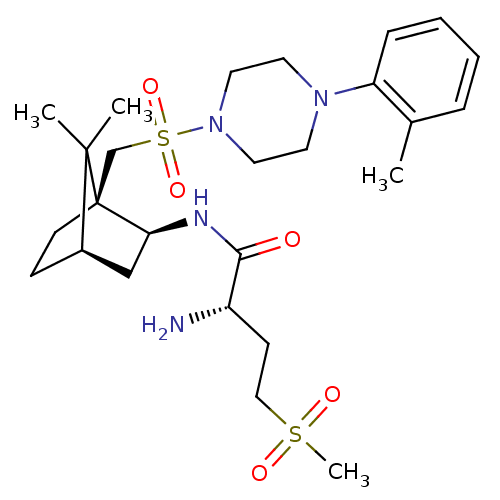 ((S)-2-amino-N-((1S,2S,4R)-7,7-dimethyl-1-((4-o-tol...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DrugMolDesign Curated by ChEMBL | Assay Description Displacement of [3H]-oxytocin from oxytocin receptor in rat uterus tissue | J Med Chem 53: 6525-38 (2010) Article DOI: 10.1021/jm901812z BindingDB Entry DOI: 10.7270/Q20R9PMZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V1a receptor (RAT) | BDBM50029649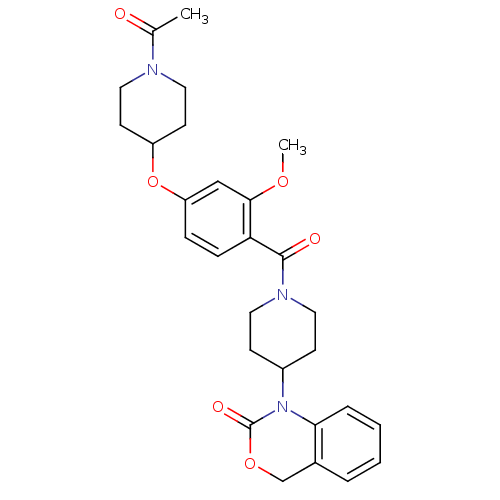 (1-(1-(4-(1-acetylpiperidin-4-yloxy)-2-methoxybenzo...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 3.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DrugMolDesign Curated by ChEMBL | Assay Description Displacement of [3H]-vasopressin from vasopressin V1a receptor in rat liver tissue | J Med Chem 53: 6525-38 (2010) Article DOI: 10.1021/jm901812z BindingDB Entry DOI: 10.7270/Q20R9PMZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxytocin receptor (Homo sapiens (Human)) | BDBM50416839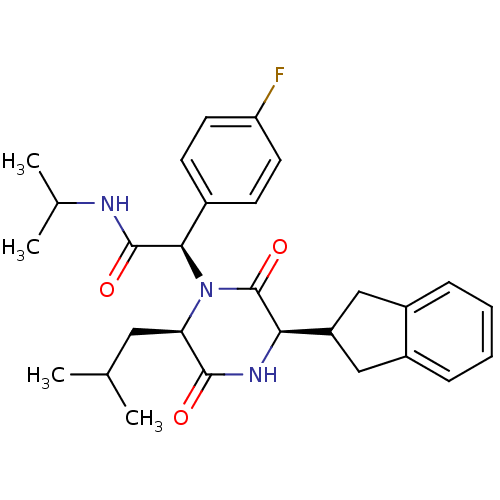 (CHEMBL197793) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.98 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DrugMolDesign Curated by ChEMBL | Assay Description Displacement of [3H]-oxytocin from human oxytocin receptor | J Med Chem 53: 6525-38 (2010) Article DOI: 10.1021/jm901812z BindingDB Entry DOI: 10.7270/Q20R9PMZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxytocin receptor (Homo sapiens (Human)) | BDBM50029649 (1-(1-(4-(1-acetylpiperidin-4-yloxy)-2-methoxybenzo...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 4.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DrugMolDesign Curated by ChEMBL | Assay Description Displacement of [3H]-oxytocin from oxytocin receptor in human uterus tissue | J Med Chem 53: 6525-38 (2010) Article DOI: 10.1021/jm901812z BindingDB Entry DOI: 10.7270/Q20R9PMZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxytocin receptor (Homo sapiens (Human)) | BDBM50064711 (1-(1-{2-Methoxy-4-[1-(2-methyl-1-oxy-pyridin-3-ylm...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 4.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DrugMolDesign Curated by ChEMBL | Assay Description Displacement of [3H]-oxytocin from oxytocin receptor in human uterus tissue | J Med Chem 53: 6525-38 (2010) Article DOI: 10.1021/jm901812z BindingDB Entry DOI: 10.7270/Q20R9PMZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxytocin receptor (Homo sapiens (Human)) | BDBM50262270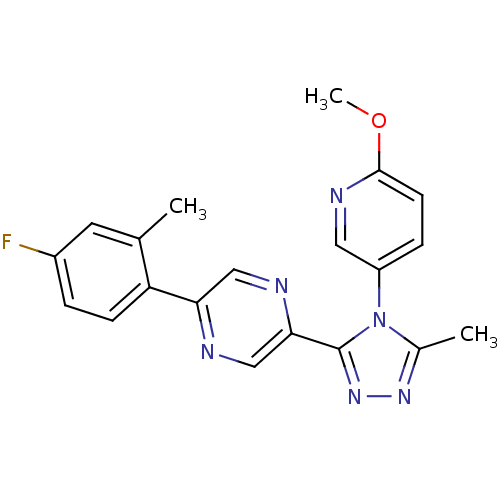 (2-(4-fluoro-2-methylphenyl)-5-(4-(6-methoxypyridin...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DrugMolDesign Curated by ChEMBL | Assay Description Displacement of [3H]-oxytocin from human oxytocin receptor expressed in HEK293-EBNA cells | J Med Chem 53: 6525-38 (2010) Article DOI: 10.1021/jm901812z BindingDB Entry DOI: 10.7270/Q20R9PMZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxytocin receptor (Homo sapiens (Human)) | BDBM50326715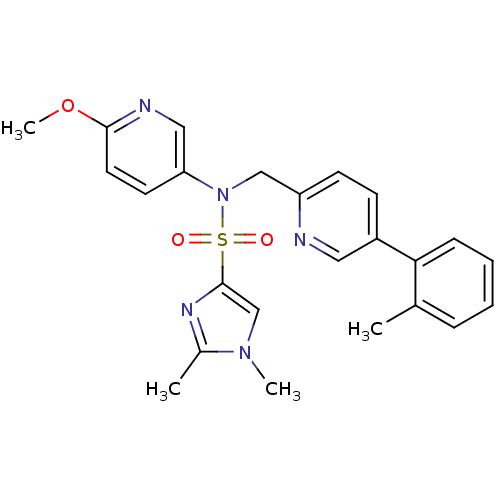 (CHEMBL456263 | N-(6-methoxypyridin-3-yl)-1,2-dimet...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 9.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DrugMolDesign Curated by ChEMBL | Assay Description Displacement of [3H]-oxytocin from oxytocin receptor in human uterus tissue | J Med Chem 53: 6525-38 (2010) Article DOI: 10.1021/jm901812z BindingDB Entry DOI: 10.7270/Q20R9PMZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxytocin receptor (Homo sapiens (Human)) | BDBM50305506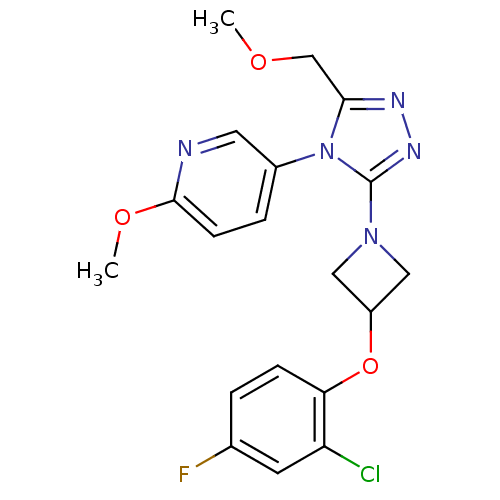 (5-(3-(3-(2-chloro-4-fluorophenoxy)azetidin-1-yl)-5...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 9.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DrugMolDesign Curated by ChEMBL | Assay Description Displacement of [3H]-oxytocin from human oxytocin receptor expressed in HEK293-EBNA cells | J Med Chem 53: 6525-38 (2010) Article DOI: 10.1021/jm901812z BindingDB Entry DOI: 10.7270/Q20R9PMZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxytocin receptor (Homo sapiens (Human)) | BDBM50305506 (5-(3-(3-(2-chloro-4-fluorophenoxy)azetidin-1-yl)-5...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 9.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DrugMolDesign Curated by ChEMBL | Assay Description Displacement of [3H]-oxytocin from oxytocin receptor in human uterus tissue | J Med Chem 53: 6525-38 (2010) Article DOI: 10.1021/jm901812z BindingDB Entry DOI: 10.7270/Q20R9PMZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxytocin receptor (Homo sapiens (Human)) | BDBM50326719 ((S)-2-amino-N-((1S,2S,4R)-7,7-dimethyl-1-((4-o-tol...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DrugMolDesign Curated by ChEMBL | Assay Description Displacement of [3H]-oxytocin from oxytocin receptor in human uterus tissue | J Med Chem 53: 6525-38 (2010) Article DOI: 10.1021/jm901812z BindingDB Entry DOI: 10.7270/Q20R9PMZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxytocin receptor (RAT) | BDBM50064711 (1-(1-{2-Methoxy-4-[1-(2-methyl-1-oxy-pyridin-3-ylm...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DrugMolDesign Curated by ChEMBL | Assay Description Displacement of [3H]-oxytocin from oxytocin receptor in rat uterus tissue | J Med Chem 53: 6525-38 (2010) Article DOI: 10.1021/jm901812z BindingDB Entry DOI: 10.7270/Q20R9PMZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxytocin receptor (Homo sapiens (Human)) | BDBM50326717 (4-ethoxy-N-(2-oxo-2-(2-(2-oxoindolin-3-ylidene)hyd...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DrugMolDesign Curated by ChEMBL | Assay Description Displacement of [3H]-oxytocin from human oxytocin receptor expressed in HEK293-EBNA cells | J Med Chem 53: 6525-38 (2010) Article DOI: 10.1021/jm901812z BindingDB Entry DOI: 10.7270/Q20R9PMZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxytocin receptor (RAT) | BDBM50029649 (1-(1-(4-(1-acetylpiperidin-4-yloxy)-2-methoxybenzo...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 19 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DrugMolDesign Curated by ChEMBL | Assay Description Displacement of [3H]-oxytocin from oxytocin receptor in rat uterus tissue | J Med Chem 53: 6525-38 (2010) Article DOI: 10.1021/jm901812z BindingDB Entry DOI: 10.7270/Q20R9PMZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxytocin receptor (Homo sapiens (Human)) | BDBM50326713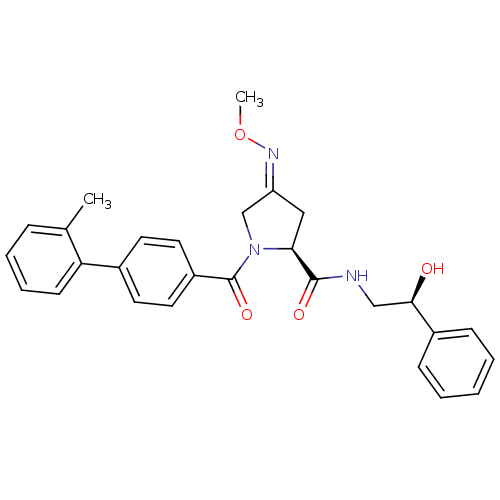 ((2S,4Z)-N-[(2S)-2-hydroxy-2-phenylethyl]-4-(methox...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 28 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DrugMolDesign Curated by ChEMBL | Assay Description Displacement of [3H]-oxytocin from human oxytocin receptor expressed in CHO cells | J Med Chem 53: 6525-38 (2010) Article DOI: 10.1021/jm901812z BindingDB Entry DOI: 10.7270/Q20R9PMZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxytocin receptor (Homo sapiens (Human)) | BDBM50263856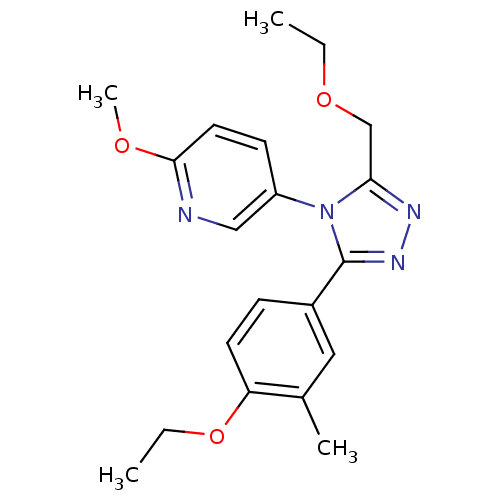 (5-(3-(4-ethoxy-3-methylphenyl)-5-(ethoxymethyl)-4H...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 28 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DrugMolDesign Curated by ChEMBL | Assay Description Displacement of [3H]-oxytocin from human oxytocin receptor expressed in HEK293-EBNA cells | J Med Chem 53: 6525-38 (2010) Article DOI: 10.1021/jm901812z BindingDB Entry DOI: 10.7270/Q20R9PMZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxytocin receptor (Homo sapiens (Human)) | BDBM50326713 ((2S,4Z)-N-[(2S)-2-hydroxy-2-phenylethyl]-4-(methox...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 28 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DrugMolDesign Curated by ChEMBL | Assay Description Displacement of [3H]-oxytocin from oxytocin receptor in human uterus tissue | J Med Chem 53: 6525-38 (2010) Article DOI: 10.1021/jm901812z BindingDB Entry DOI: 10.7270/Q20R9PMZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V1a receptor (Homo sapiens (Human)) | BDBM50262153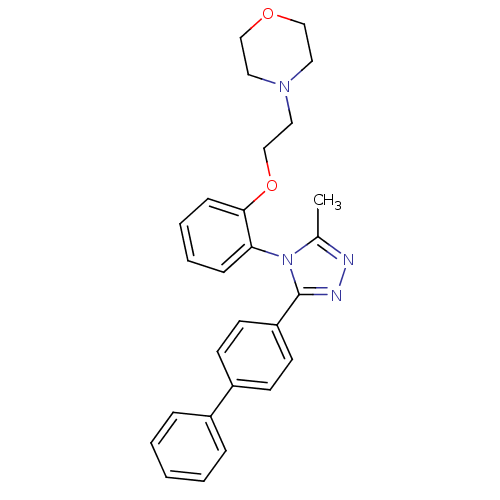 (4-{2-[2-(3-Biphenyl-4-yl-5-methyl-[1,2,4]triazol-4...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 28 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DrugMolDesign Curated by ChEMBL | Assay Description Displacement of [3H]-vasopressin from human vasopressin V1a receptor expressed in CHO cells at 10 uM | J Med Chem 53: 6525-38 (2010) Article DOI: 10.1021/jm901812z BindingDB Entry DOI: 10.7270/Q20R9PMZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V1a receptor (RAT) | BDBM50045130 (CHEMBL60954 | N-(2-{4-[4-(2-Oxo-3,4-dihydro-2H-qui...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 32 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DrugMolDesign Curated by ChEMBL | Assay Description Displacement of [3H]-vasopressin from vasopressin V1a receptor in rat liver tissue | J Med Chem 53: 6525-38 (2010) Article DOI: 10.1021/jm901812z BindingDB Entry DOI: 10.7270/Q20R9PMZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V1b receptor (RAT) | BDBM50326716 ((R)-4-chloro-3-(5-chloro-1-(2,4-dimethoxybenzyl)-3...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 46 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DrugMolDesign Curated by ChEMBL | Assay Description Displacement of [3H]-vasopressin from rat vasopressin V1b receptor expressed in CHO cells | J Med Chem 53: 6525-38 (2010) Article DOI: 10.1021/jm901812z BindingDB Entry DOI: 10.7270/Q20R9PMZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxytocin receptor (Homo sapiens (Human)) | BDBM50326723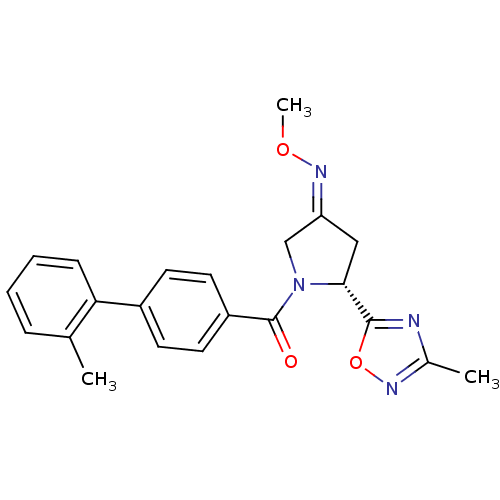 ((R)-(4-(methoxyimino)-2-(3-methyl-1,2,4-oxadiazol-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 50 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DrugMolDesign Curated by ChEMBL | Assay Description Displacement of [3H]-oxytocin from human oxytocin receptor expressed in HEK293-EBNA cells | J Med Chem 53: 6525-38 (2010) Article DOI: 10.1021/jm901812z BindingDB Entry DOI: 10.7270/Q20R9PMZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxytocin receptor (Homo sapiens (Human)) | BDBM50326724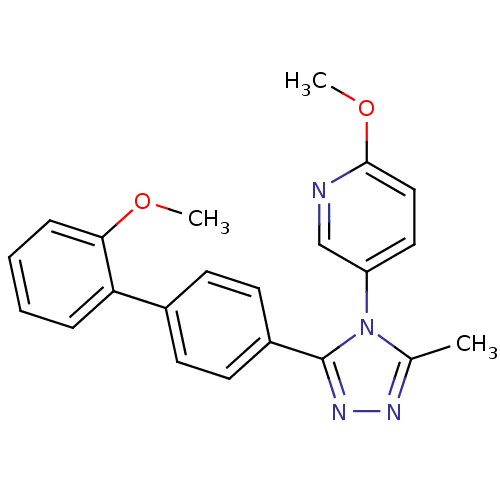 (2-methoxy-5-(3-(2'-methoxybiphenyl-4-yl)-5-methyl-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 56 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DrugMolDesign Curated by ChEMBL | Assay Description Displacement of [3H]-oxytocin from human oxytocin receptor expressed in HEK293-EBNA cells | J Med Chem 53: 6525-38 (2010) Article DOI: 10.1021/jm901812z BindingDB Entry DOI: 10.7270/Q20R9PMZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V1a receptor (Homo sapiens (Human)) | BDBM50326723 ((R)-(4-(methoxyimino)-2-(3-methyl-1,2,4-oxadiazol-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DrugMolDesign Curated by ChEMBL | Assay Description Displacement of [3H]-vasopressin from human vasopressin V1a receptor expressed in CHO cells at 10 uM | J Med Chem 53: 6525-38 (2010) Article DOI: 10.1021/jm901812z BindingDB Entry DOI: 10.7270/Q20R9PMZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxytocin receptor (Homo sapiens (Human)) | BDBM50326718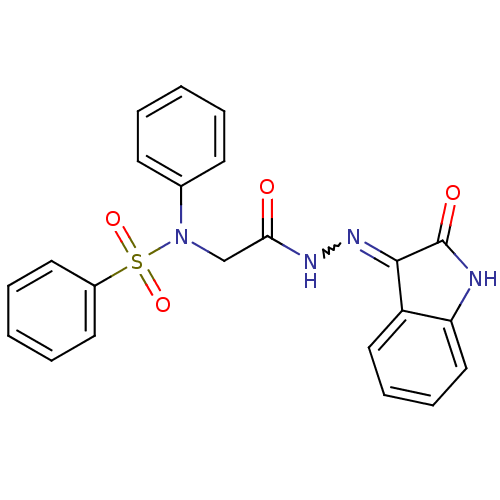 (CHEMBL1254116 | N-(2-oxo-2-(2-(2-oxoindolin-3-ylid...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DrugMolDesign Curated by ChEMBL | Assay Description Displacement of [3H]-oxytocin from human oxytocin receptor expressed in HEK293-EBNA cells | J Med Chem 53: 6525-38 (2010) Article DOI: 10.1021/jm901812z BindingDB Entry DOI: 10.7270/Q20R9PMZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxytocin receptor (Homo sapiens (Human)) | BDBM50326721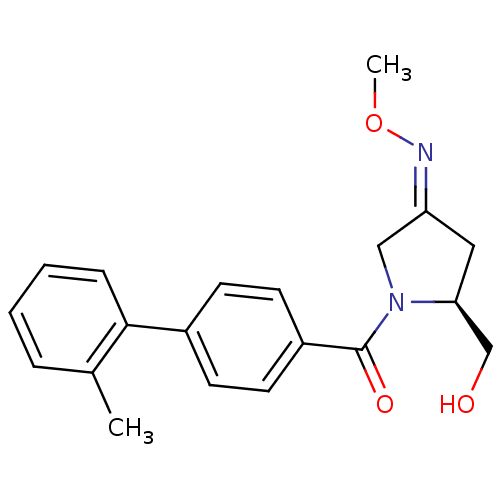 ((S)-(2-(hydroxymethyl)-4-(methoxyimino)pyrrolidin-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents | Article PubMed | 95 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DrugMolDesign Curated by ChEMBL | Assay Description Displacement of [3H]-oxytocin from human oxytocin receptor expressed in CHO cells | J Med Chem 53: 6525-38 (2010) Article DOI: 10.1021/jm901812z BindingDB Entry DOI: 10.7270/Q20R9PMZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V1a receptor (RAT) | BDBM50326716 ((R)-4-chloro-3-(5-chloro-1-(2,4-dimethoxybenzyl)-3...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 99 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DrugMolDesign Curated by ChEMBL | Assay Description Displacement of [3H]-vasopressin from rat vasopressin V1a receptor expressed in CHO cells | J Med Chem 53: 6525-38 (2010) Article DOI: 10.1021/jm901812z BindingDB Entry DOI: 10.7270/Q20R9PMZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V1a receptor (RAT) | BDBM50326719 ((S)-2-amino-N-((1S,2S,4R)-7,7-dimethyl-1-((4-o-tol...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DrugMolDesign Curated by ChEMBL | Assay Description Displacement of [3H]-vasopressin from vasopressin V1a receptor in rat liver tissue | J Med Chem 53: 6525-38 (2010) Article DOI: 10.1021/jm901812z BindingDB Entry DOI: 10.7270/Q20R9PMZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxytocin receptor (RAT) | BDBM50326713 ((2S,4Z)-N-[(2S)-2-hydroxy-2-phenylethyl]-4-(methox...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 135 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DrugMolDesign Curated by ChEMBL | Assay Description Displacement of [3H]-oxytocin from rat oxytocin receptor expressed in CHO cells | J Med Chem 53: 6525-38 (2010) Article DOI: 10.1021/jm901812z BindingDB Entry DOI: 10.7270/Q20R9PMZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V1a receptor (Homo sapiens (Human)) | BDBM50326716 ((R)-4-chloro-3-(5-chloro-1-(2,4-dimethoxybenzyl)-3...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 143 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DrugMolDesign Curated by ChEMBL | Assay Description Displacement of [3H]-vasopressin from human vasopressin V1a receptor expressed in CHO cells | J Med Chem 53: 6525-38 (2010) Article DOI: 10.1021/jm901812z BindingDB Entry DOI: 10.7270/Q20R9PMZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V1a receptor (Homo sapiens (Human)) | BDBM50326713 ((2S,4Z)-N-[(2S)-2-hydroxy-2-phenylethyl]-4-(methox...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DrugMolDesign Curated by ChEMBL | Assay Description Displacement of [3H]-vasopressin from human vasopressin V1a receptor expressed in CHO cells | J Med Chem 53: 6525-38 (2010) Article DOI: 10.1021/jm901812z BindingDB Entry DOI: 10.7270/Q20R9PMZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxytocin receptor (Homo sapiens (Human)) | BDBM50045130 (CHEMBL60954 | N-(2-{4-[4-(2-Oxo-3,4-dihydro-2H-qui...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DrugMolDesign Curated by ChEMBL | Assay Description Displacement of [3H]-oxytocin from oxytocin receptor in human uterus tissue | J Med Chem 53: 6525-38 (2010) Article DOI: 10.1021/jm901812z BindingDB Entry DOI: 10.7270/Q20R9PMZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V1a receptor (Homo sapiens (Human)) | BDBM50326719 ((S)-2-amino-N-((1S,2S,4R)-7,7-dimethyl-1-((4-o-tol...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DrugMolDesign Curated by ChEMBL | Assay Description Displacement of [3H]-vasopressin from vasopressin V1a receptor in human liver tissue | J Med Chem 53: 6525-38 (2010) Article DOI: 10.1021/jm901812z BindingDB Entry DOI: 10.7270/Q20R9PMZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V2 receptor (Rattus norvegicus (Rat)) | BDBM50326719 ((S)-2-amino-N-((1S,2S,4R)-7,7-dimethyl-1-((4-o-tol...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DrugMolDesign Curated by ChEMBL | Assay Description Displacement of [3H]-vasopressin from vasopressin V2 receptor in rat kidney tissue | J Med Chem 53: 6525-38 (2010) Article DOI: 10.1021/jm901812z BindingDB Entry DOI: 10.7270/Q20R9PMZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxytocin receptor (RAT) | BDBM50326721 ((S)-(2-(hydroxymethyl)-4-(methoxyimino)pyrrolidin-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents | Article PubMed | 210 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DrugMolDesign Curated by ChEMBL | Assay Description Displacement of [3H]-oxytocin from rat oxytocin receptor expressed in CHO cells | J Med Chem 53: 6525-38 (2010) Article DOI: 10.1021/jm901812z BindingDB Entry DOI: 10.7270/Q20R9PMZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxytocin receptor (RAT) | BDBM50045130 (CHEMBL60954 | N-(2-{4-[4-(2-Oxo-3,4-dihydro-2H-qui...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 230 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DrugMolDesign Curated by ChEMBL | Assay Description Displacement of [3H]-oxytocin from oxytocin receptor in rat uterus tissue | J Med Chem 53: 6525-38 (2010) Article DOI: 10.1021/jm901812z BindingDB Entry DOI: 10.7270/Q20R9PMZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxytocin receptor (Homo sapiens (Human)) | BDBM50326720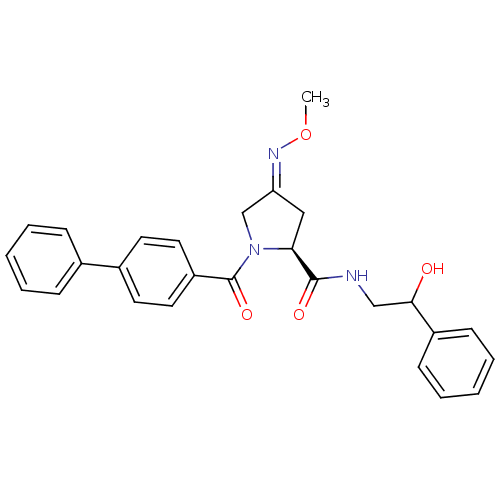 ((2S)-1-(biphenylcarbonyl)-N-(2-hydroxy-2-phenyleth...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 260 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DrugMolDesign Curated by ChEMBL | Assay Description Displacement of [3H]-oxytocin from human oxytocin receptor expressed in CHO cells | J Med Chem 53: 6525-38 (2010) Article DOI: 10.1021/jm901812z BindingDB Entry DOI: 10.7270/Q20R9PMZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxytocin receptor (Homo sapiens (Human)) | BDBM50262153 (4-{2-[2-(3-Biphenyl-4-yl-5-methyl-[1,2,4]triazol-4...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 304 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DrugMolDesign Curated by ChEMBL | Assay Description Displacement of [3H]-oxytocin from human oxytocin receptor expressed in HEK293-EBNA cells | J Med Chem 53: 6525-38 (2010) Article DOI: 10.1021/jm901812z BindingDB Entry DOI: 10.7270/Q20R9PMZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V1a receptor (Homo sapiens (Human)) | BDBM50326721 ((S)-(2-(hydroxymethyl)-4-(methoxyimino)pyrrolidin-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents | Article PubMed | 330 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DrugMolDesign Curated by ChEMBL | Assay Description Displacement of [3H]-vasopressin from human vasopressin V1a receptor expressed in CHO cells | J Med Chem 53: 6525-38 (2010) Article DOI: 10.1021/jm901812z BindingDB Entry DOI: 10.7270/Q20R9PMZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 94 total ) | Next | Last >> |